Strategies for Solubility and Bioavailability Enhancement and Toxicity Reduction of Norcantharidin
Abstract
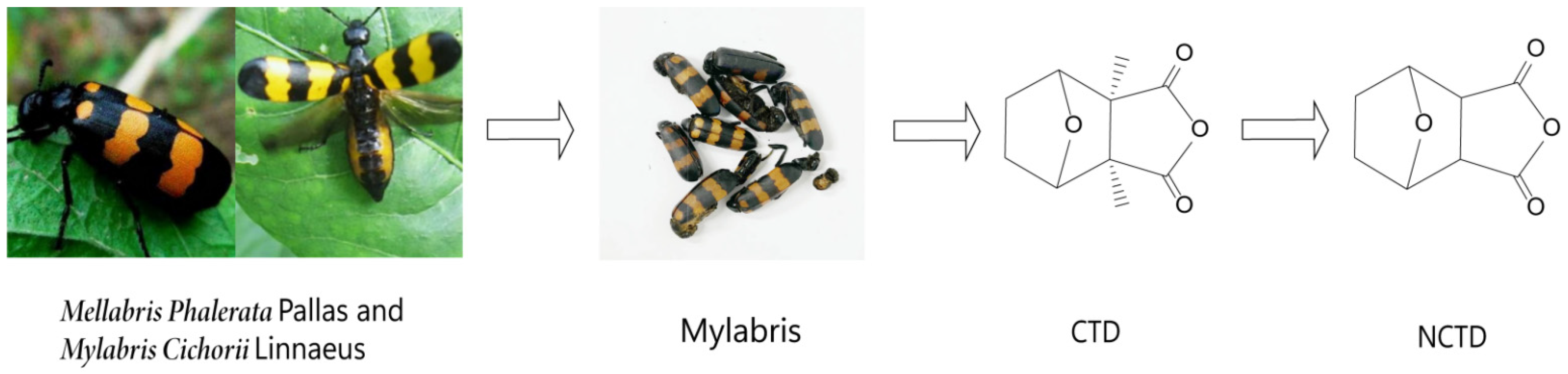
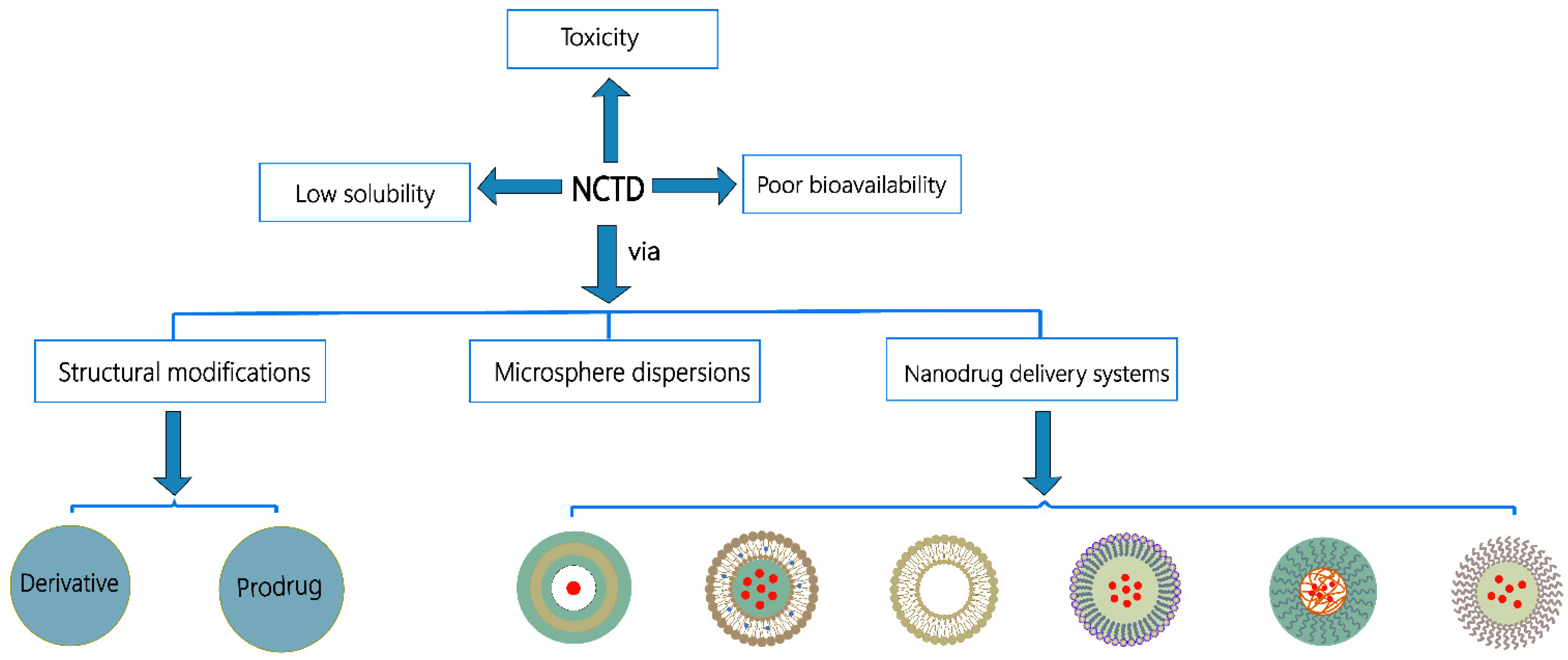
1. Introduction
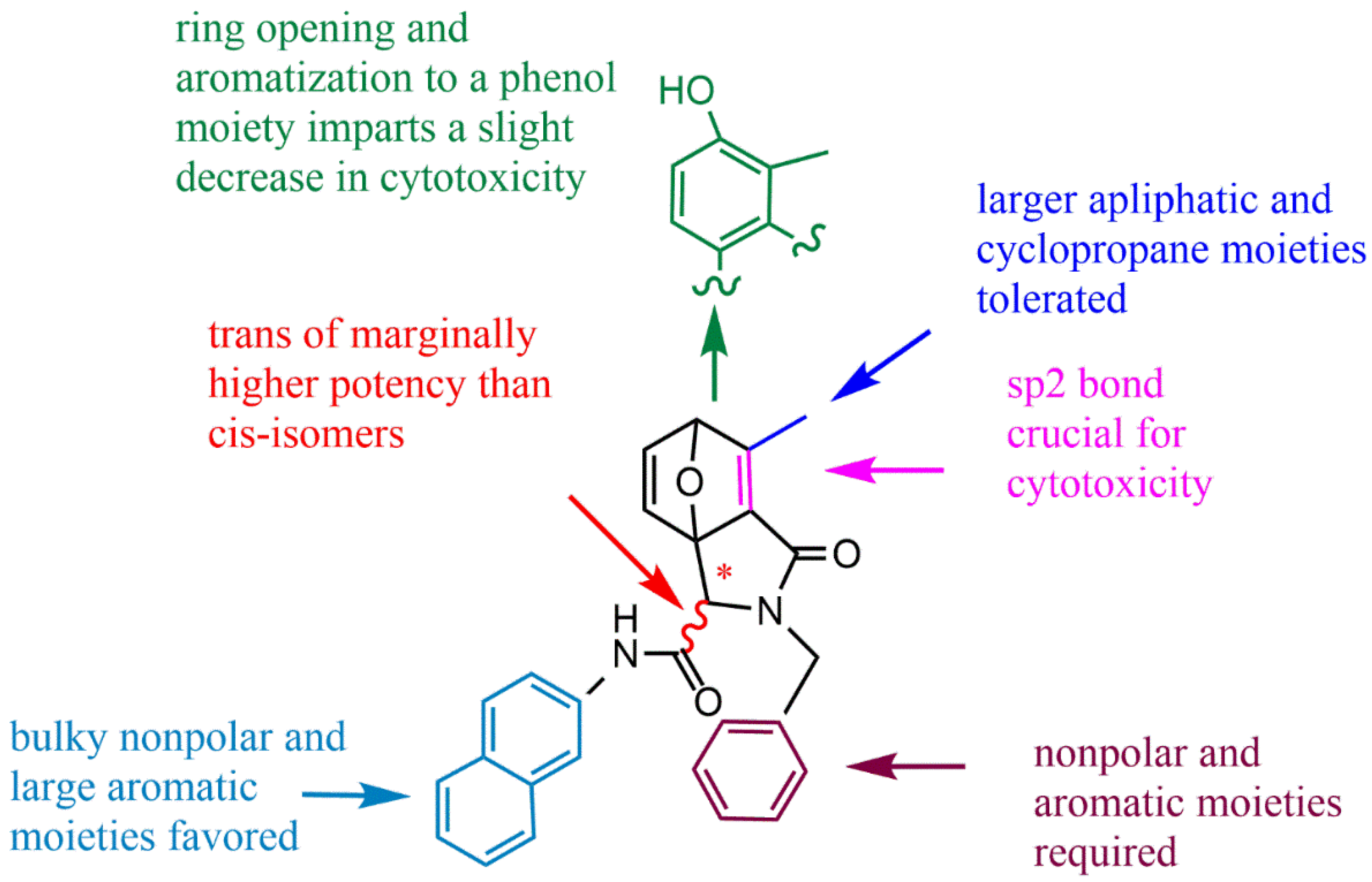
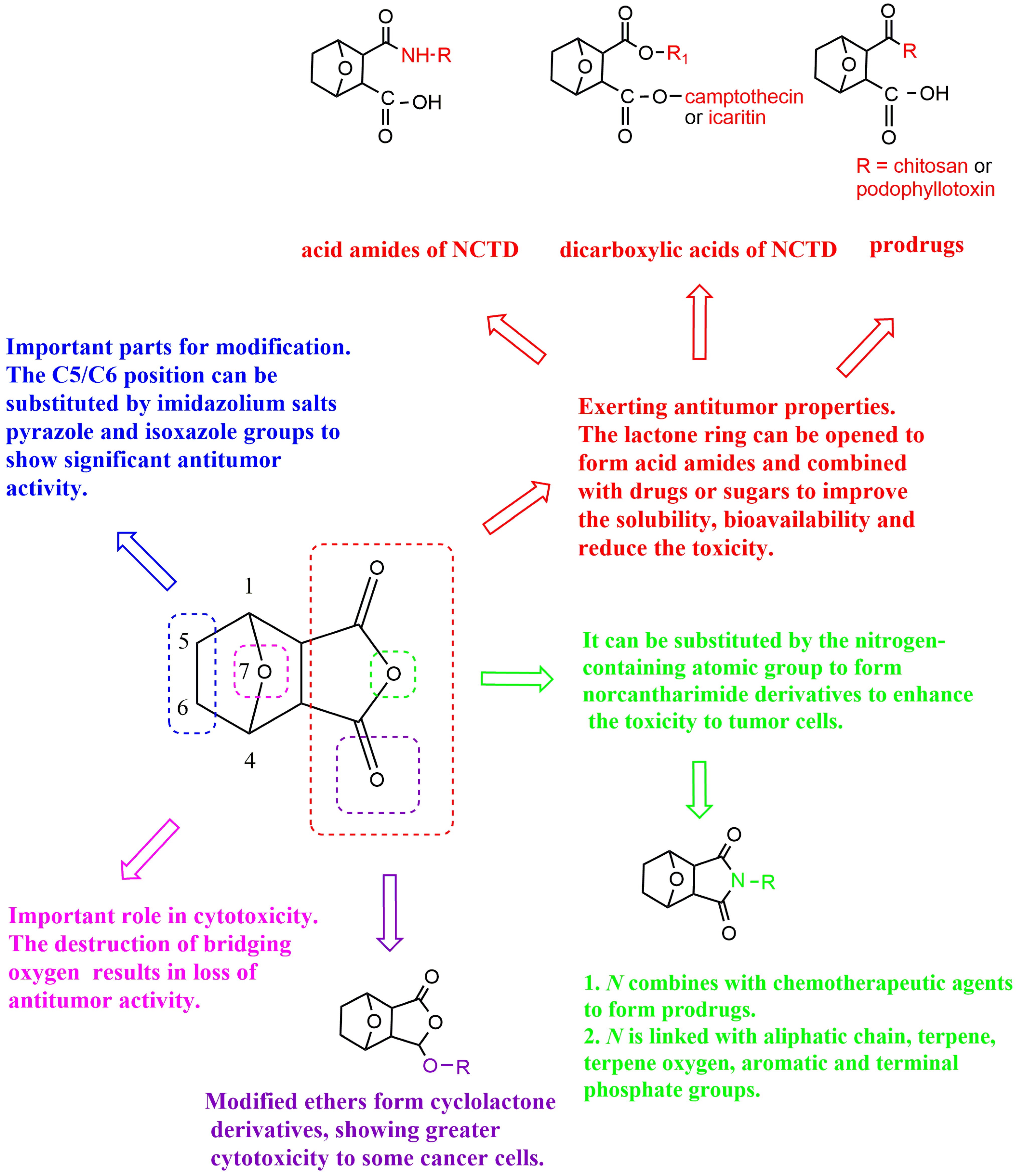
2. The Structure–Activity Relationship of NCTD
3. Chemical Structure Changes
3.1. Derivatives
3.1.1. Structural Modification of Dicarboxylate Anhydride
Cyclolactone Derivatives of NCTD
Norcantharimide Derivatives
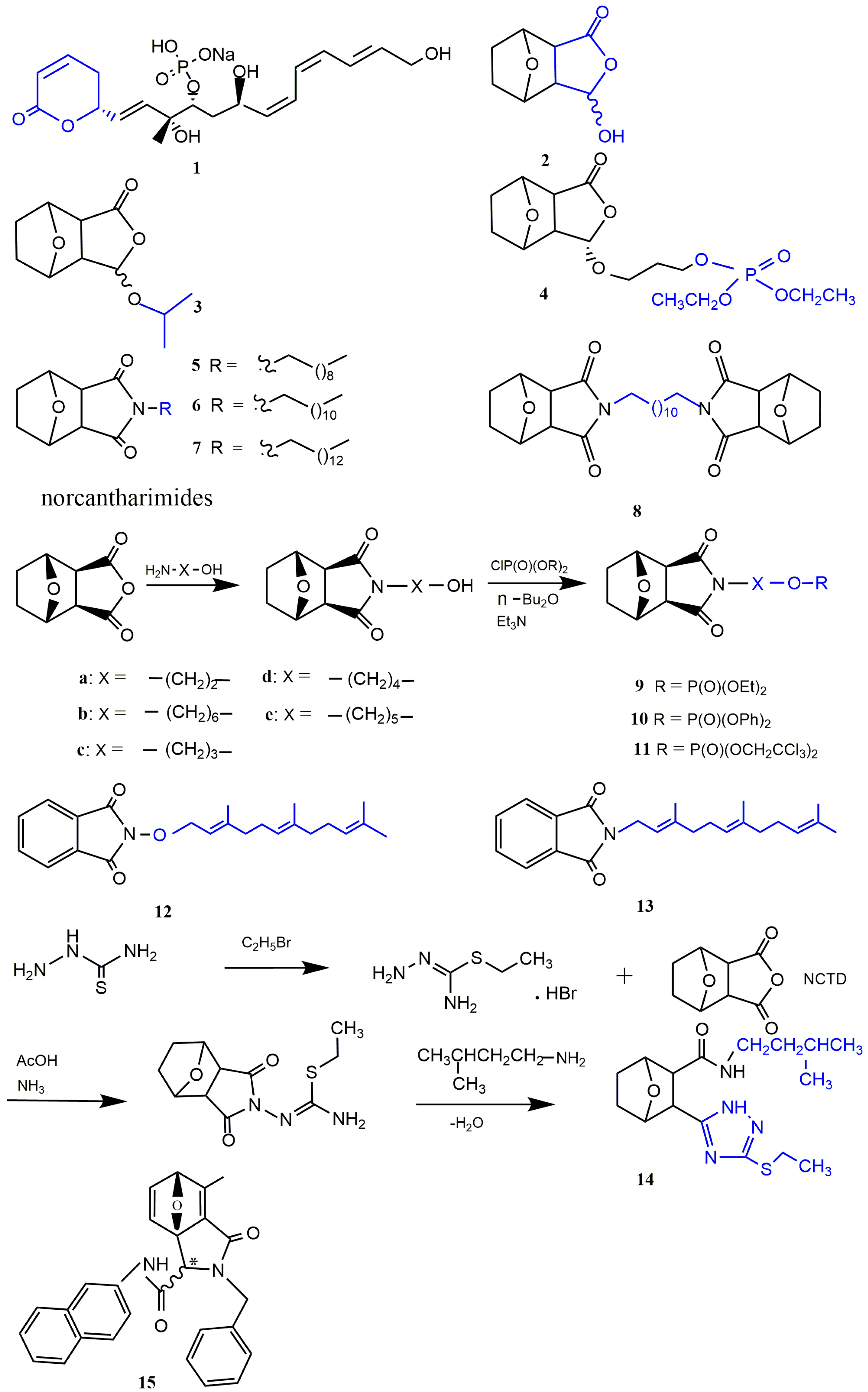
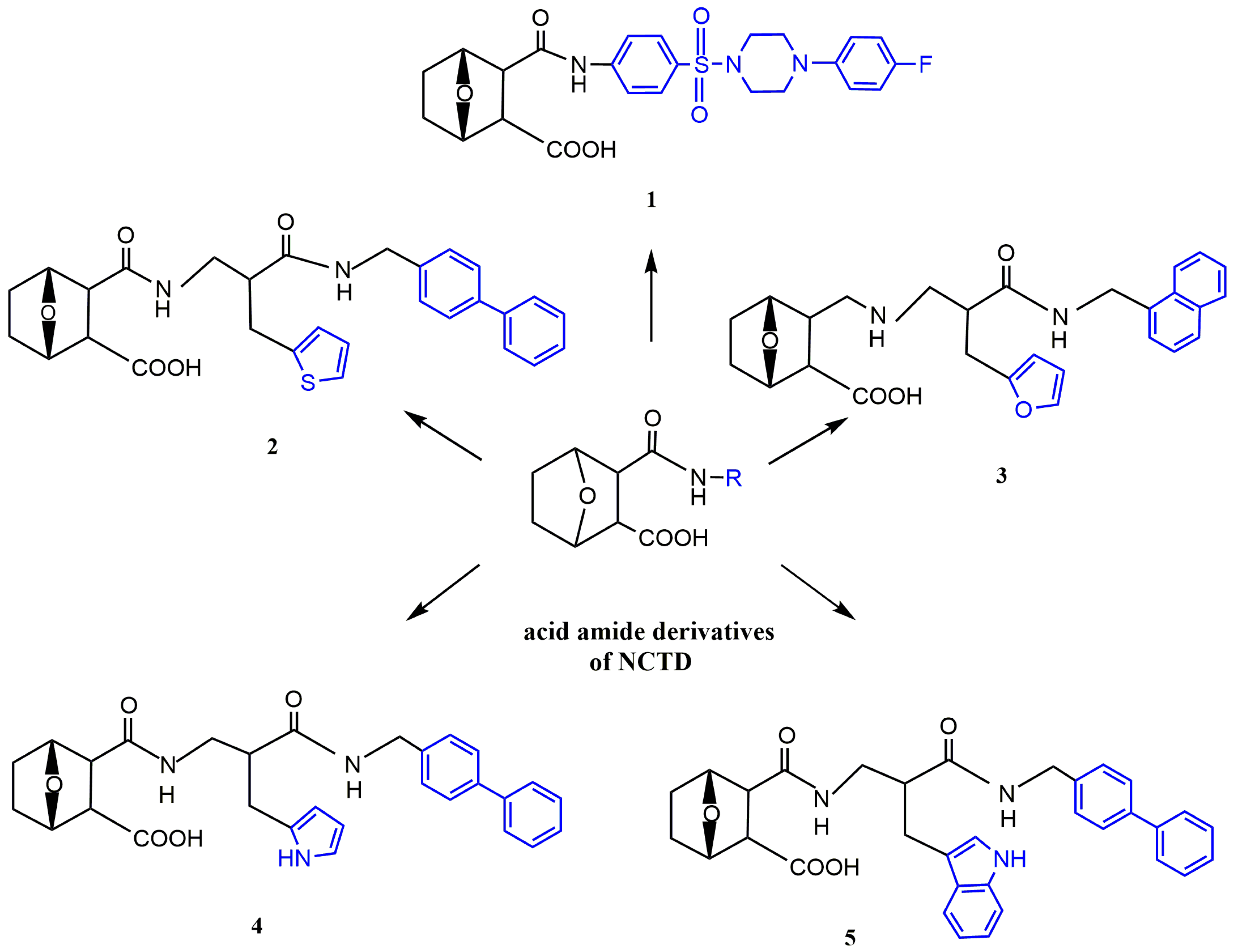
Acid Amide Derivatives of NCTD
Dicarboxylic Acid Derivative of NCTD
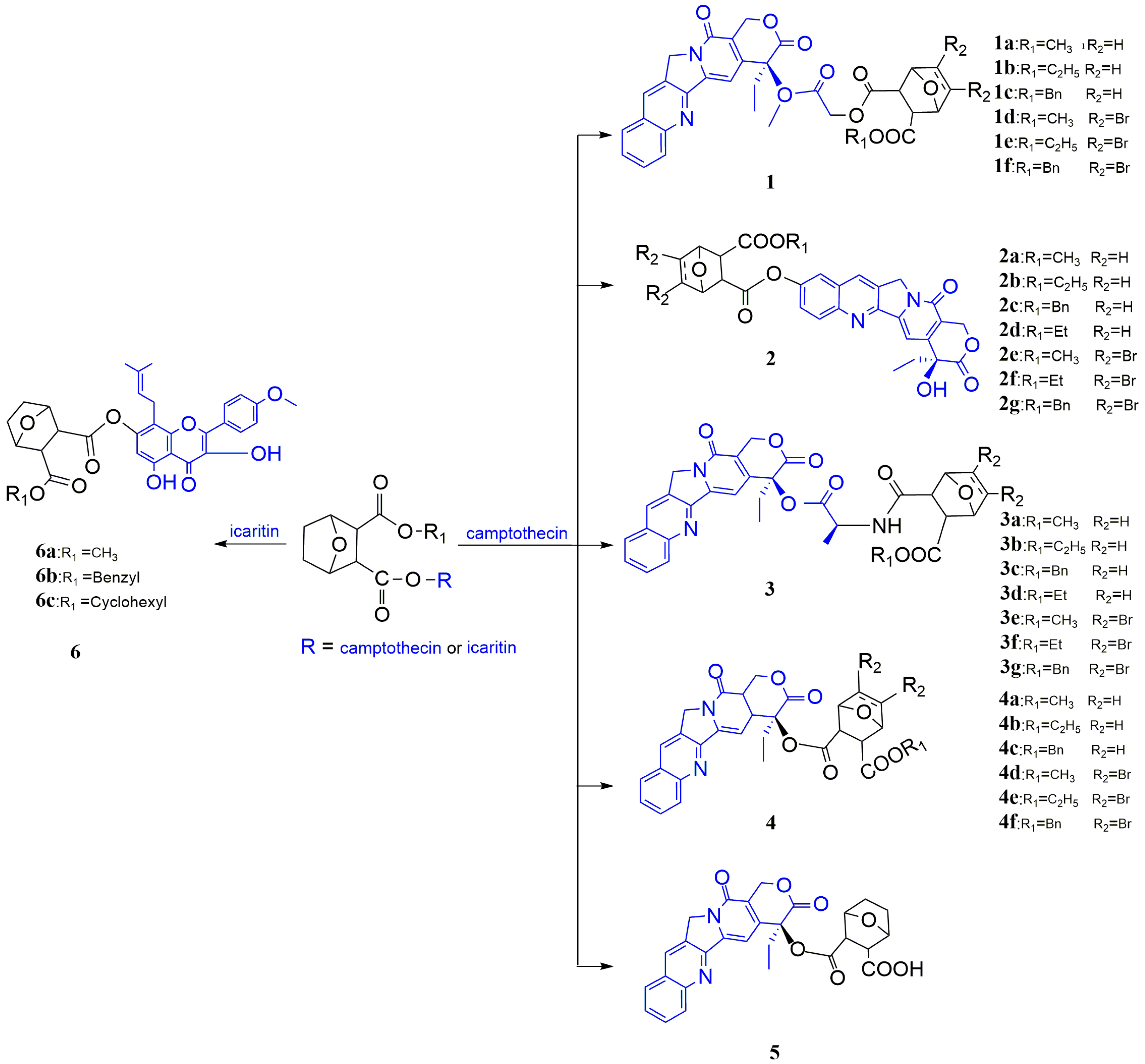
3.1.2. Modification at the C5/C6 position

3.2. Prodrug Strategies
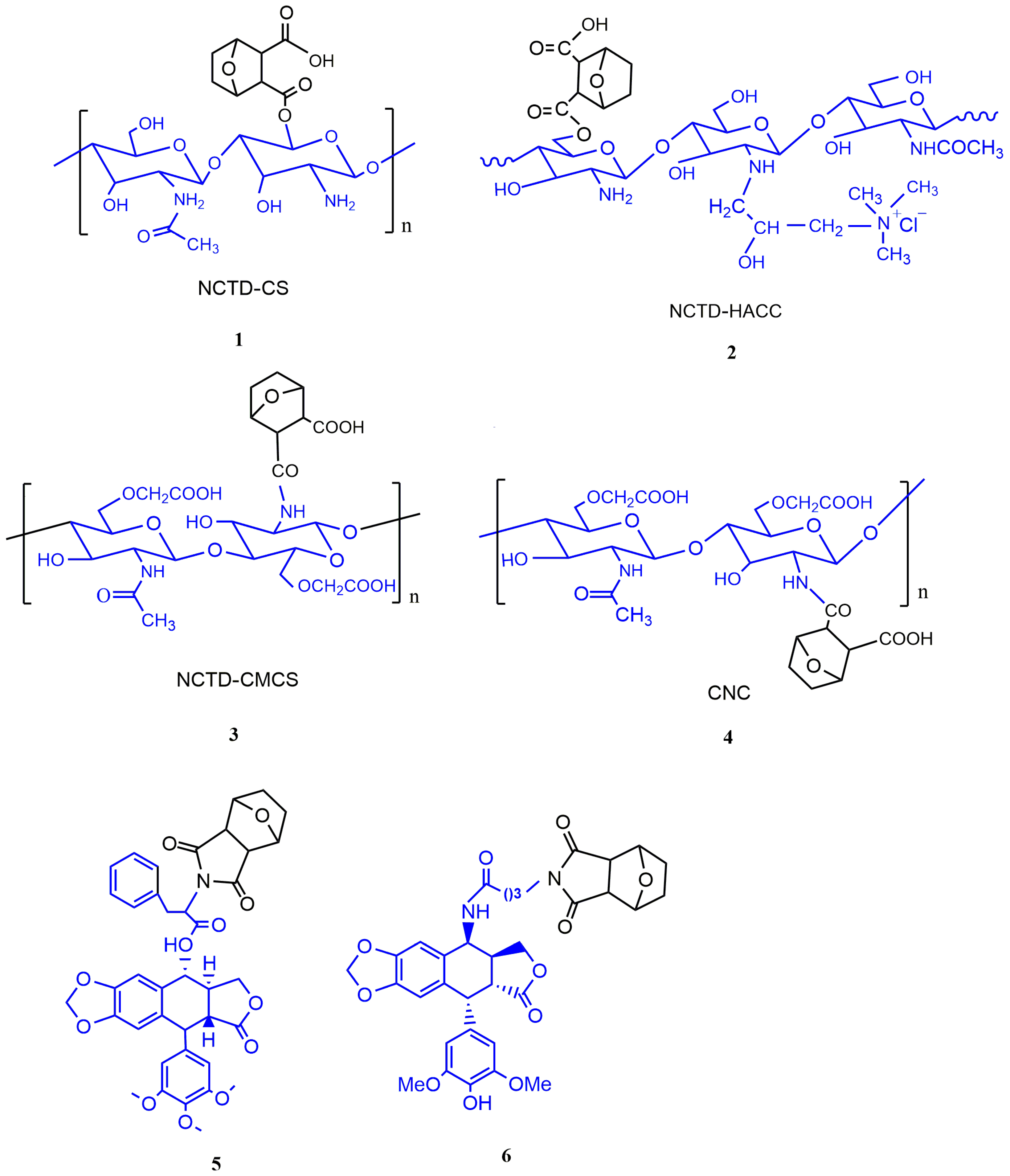
3.2.1. The Conjugates of NCTD with Chitosan and Its Derivatives
3.2.2. The Conjugates of NCTD with Phostophyllotoxin
4. Microsphere Dispersion Systems
5. Nanodrug Delivery Systems
5.1. Nanoparticles
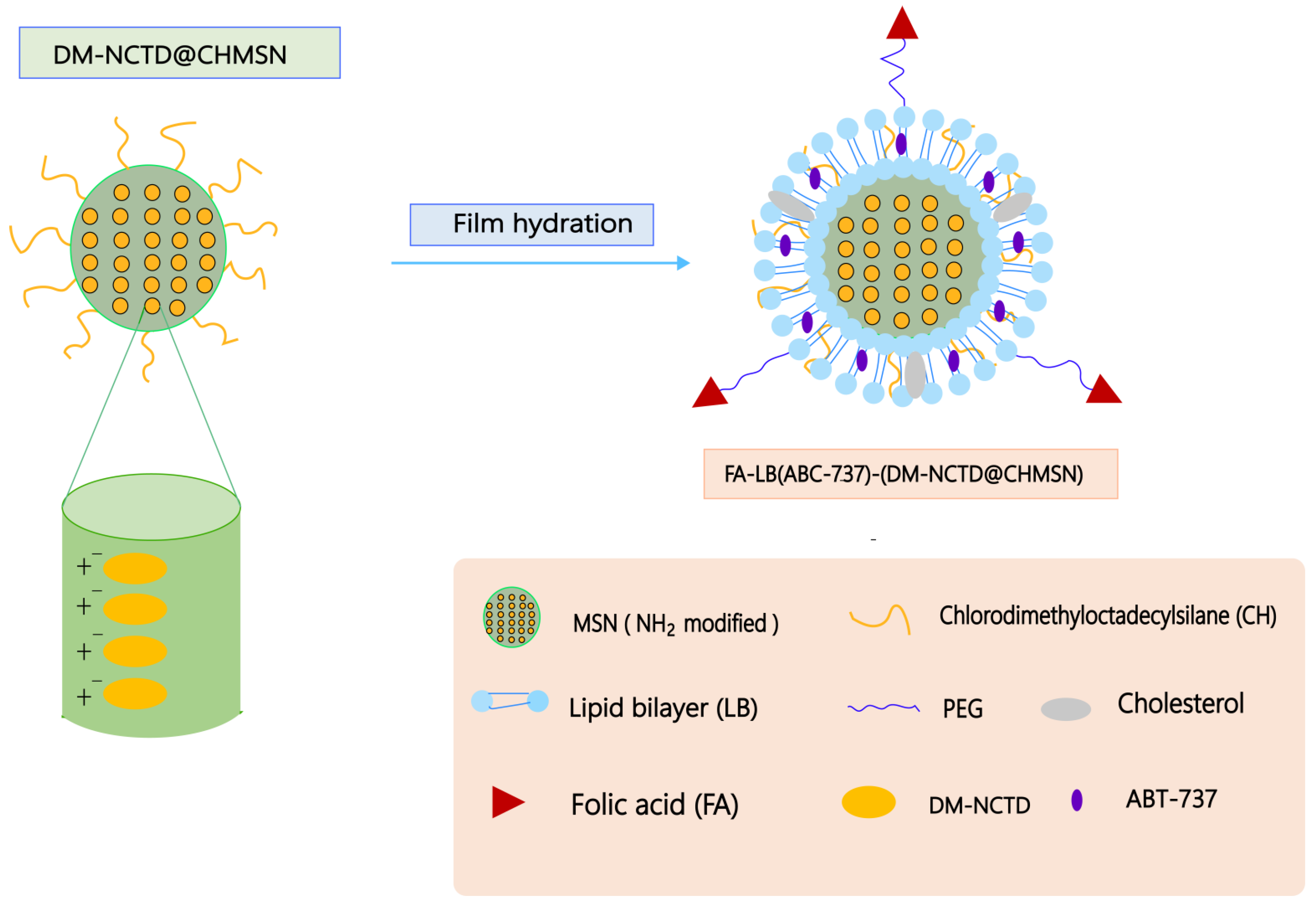
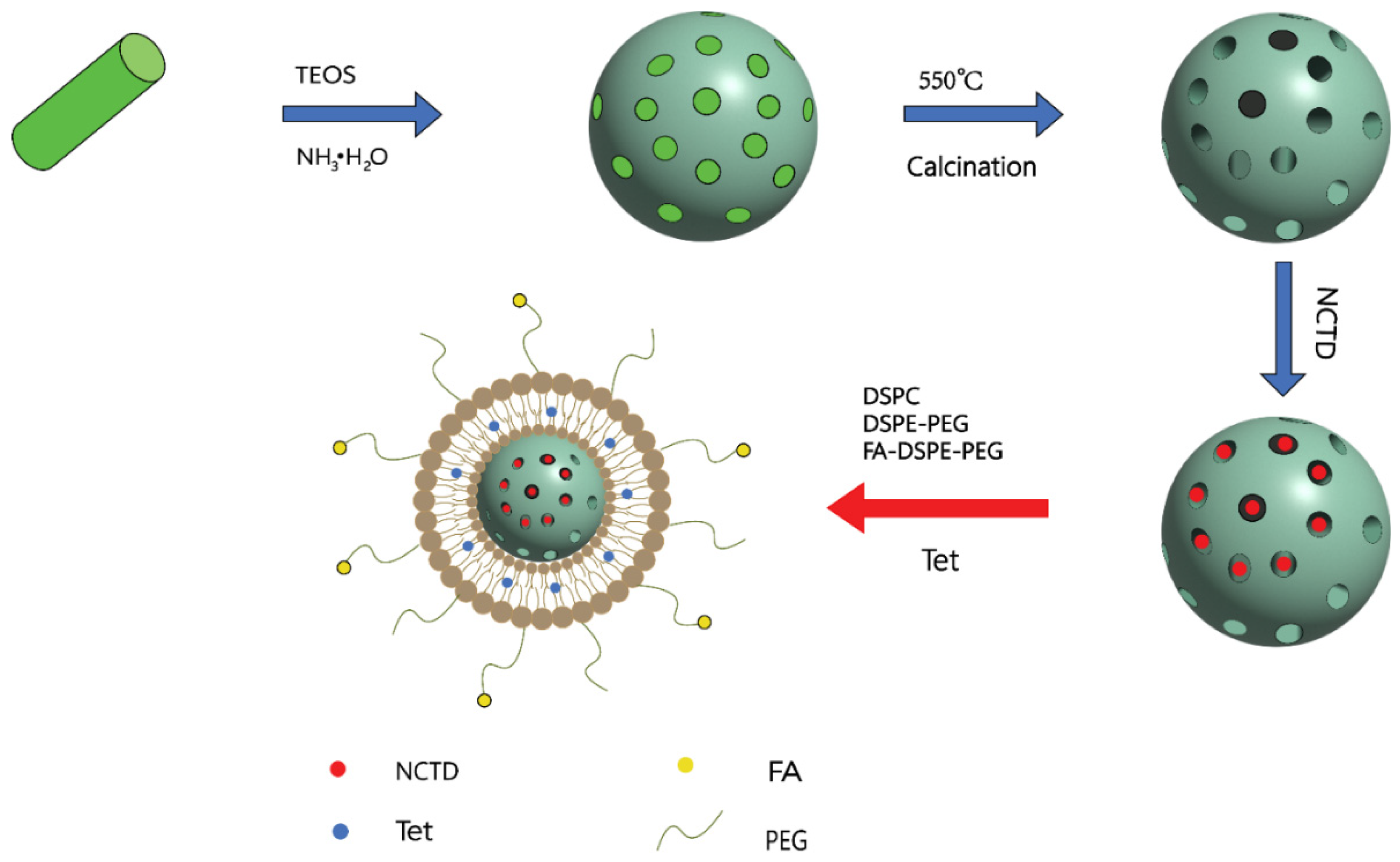
5.2. Liposomes
5.3. Lipid Microspheres
5.4. Polymer Micelles
5.5. Thermosensitive Gel

6. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chinese Pharmacopoeia Commission. Pharmacopoeia of the People’s Republic of China; China Medical Science Press: Beijing, China, 2020; Volume 1, p. 402. [Google Scholar]
- Wang, G.S. Medical uses of mylabris in ancient China and recent studies. J. Ethnopharmacol. 1989, 26, 147–162. [Google Scholar] [CrossRef]
- Gu, X.D.; Xu, L.L.; Zhao, H.; Gu, J.Z.; Xie, X.H. Cantharidin suppressed breast cancer MDA-MB-231 cell growth and migration by inhibiting MAPK signaling pathway. Braz. J. Med. Biol. Res. Rev. Bras. De Pesqui. Med. E Biol. 2017, 50, e5920. [Google Scholar] [CrossRef] [PubMed]
- Nazim, U.M.; Yin, H.; Park, S.Y. Downregulation of c-FLIP and upregulation of DR-5 by cantharidin sensitizes TRAIL-mediated apoptosis in prostate cancer cells via autophagy flux. Int. J. Mol. Med. 2020, 46, 280–288. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.P.; Li, L.; Xu, L.; Dai, E.N.; Chen, W.D. Cantharidin suppresses cell growth and migration, and activates autophagy in human non-small cell lung cancer cells. Oncol. Lett. 2018, 15, 6527–6532. [Google Scholar] [CrossRef]
- Li, H.C.; Xia, Z.H.; Chen, Y.F.; Yang, F.; Feng, W.; Cai, H.; Mei, Y.; Jiang, Y.M.; Xu, K.; Feng, D.X. Cantharidin Inhibits the Growth of Triple-Negative Breast Cancer Cells by Suppressing Autophagy and Inducing Apoptosis in Vitro and in Vivo. Cell. Physiol. Biochem. Int. J. Exp. Cell. Physiol. Biochem. Pharmacol. 2017, 43, 1829–1840. [Google Scholar] [CrossRef]
- Zhu, M.; Shi, X.; Gong, Z.; Su, Q.; Yu, R.; Wang, B.; Yang, T.; Dai, B.; Zhan, Y.; Zhang, D.; et al. Cantharidin treatment inhibits hepatocellular carcinoma development by regulating the JAK2/STAT3 and PI3K/Akt pathways in an EphB4-dependent manner. Pharmacol. Res. 2020, 158, 104868. [Google Scholar] [CrossRef]
- Xiao, Z.; Wen, L.; Zeng, D.; Yin, D.; Zhou, X.; Tang, C.; Li, Y. Protein Phosphatase 2A Inhibiting β-Catenin Phosphorylation Contributes Critically to the Anti-renal Interstitial Fibrotic Effect of Norcantharidin. Inflammation 2020, 43, 878–891. [Google Scholar] [CrossRef]
- Yu, Y.; Zhang, Y.; Zhang, J.; Guan, C.; Liu, L.; Ren, L. Cantharidin-induced acute hepatotoxicity: The role of TNF-α, IKK-α, Bcl-2, Bax and caspase3. J. Appl. Toxicol. JAT 2020, 40, 1526–1533. [Google Scholar] [CrossRef]
- He, T.; Wang, Q.; Ao, J.; Chen, K.; Li, X.; Zhang, J.; Duan, C. Endoplasmic reticulum stress contributes to autophagy and apoptosis in cantharidin-induced nephrotoxicity. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2022, 163, 112986. [Google Scholar] [CrossRef]
- Du, L.J.; Feng, Y.X.; He, Z.X.; Huang, L.; Wang, Q.; Wen, C.P.; Zhang, Y. Norcantharidin ameliorates the development of murine lupus via inhibiting the generation of IL-17 producing cells. Acta Pharmacol. Sin. 2022, 43, 1521–1533. [Google Scholar] [CrossRef]
- Hsia, C.H.; Lu, W.J.; Lin, K.H.; Chou, D.S.; Geraldine, P.; Jayakuma, T.; Chang, N.C.; Sheu, J.R. Norcantharidin, a clinical used chemotherapeutic agent, acts as a powerful inhibitor by interfering with fibrinogen-integrin α(II)(b) β(3) binding in human platelets. J. Cell. Mol. Med. 2018, 22, 2142–2152. [Google Scholar] [CrossRef]
- Xiao, H.; Liao, Y.; Tang, C.; Xiao, Z.; Luo, H.; Li, J.; Liu, H.; Sun, L.; Zeng, D.; Li, Y. RNA-Seq analysis of potential lncRNAs and genes for the anti-renal fibrotic effect of norcantharidin. J. Cell. Biochem. 2019, 120, 17354–17367. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.W.; Yin, D.D.; Xiao, Z.; Wen, L.; Liao, Y.J.; Tang, C.Y.; Zeng, D.; Xiao, H.T.; Li, Y. Anti-renal interstitial fibrosis effect of norcantharidin is exerted through inhibition of PP2Ac-mediated C-terminal phosphorylation of Smad3. Chem. Biol. Drug Des. 2021, 97, 293–304. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Xiao, Z.; Wei, J.; Shan, Y.; Zeng, D.; Tao, Y.; Fang, X.; Tang, C.; Chen, X.; Li, Y. NCTD Prevents Renal Interstitial Fibrosis via Targeting Sp1/lncRNA Gm26669 Axis. Int. J. Biol. Sci. 2021, 17, 3118–3132. [Google Scholar] [CrossRef]
- Pan, M.S.; Cao, J.; Fan, Y.Z. Insight into norcantharidin, a small-molecule synthetic compound with potential multi-target anticancer activities. Chin. Med. 2020, 15, 55. [Google Scholar] [CrossRef]
- Wang, Y.; Jiang, W.; Li, C.; Xiong, X.; Guo, H.; Tian, Q.; Li, X. Autophagy Suppression Accelerates Apoptosis Induced by Norcantharidin in Cholangiocarcinoma. Pathol. Oncol. Res. POR 2020, 26, 1697–1707. [Google Scholar] [CrossRef] [PubMed]
- Tsauer, W.; Lin, J.G.; Lin, P.Y.; Hsu, F.L.; Chiang, H.C. The effects of cantharidin analogues on xanthine oxidase. Anticancer. Res. 1997, 17, 2095–2098. [Google Scholar]
- Chen, Y.J.; Tsai, Y.M.; Kuo, C.D.; Ku, K.L.; Shie, H.S.; Liao, H.F. Norcantharidin is a small-molecule synthetic compound with anti-angiogenesis effect. Life Sci. 2009, 85, 642–651. [Google Scholar] [CrossRef]
- Yang, P.Y.; Hu, D.N.; Kao, Y.H.; Lin, I.C.; Chou, C.Y.; Wu, Y.C. Norcantharidin induces apoptosis in human prostate cancer cells through both intrinsic and extrinsic pathways. Pharmacol. Rep. PR 2016, 68, 874–880. [Google Scholar] [CrossRef]
- Qiu, P.; Wang, S.; Liu, M.; Ma, H.; Zeng, X.; Zhang, M.; Xu, L.; Cui, Y.; Xu, H.; Tang, Y.; et al. Norcantharidin Inhibits cell growth by suppressing the expression and phosphorylation of both EGFR and c-Met in human colon cancer cells. BMC Cancer 2017, 17, 55. [Google Scholar] [CrossRef]
- Yang, P.Y.; Chen, M.F.; Kao, Y.H.; Hu, D.N.; Chang, F.R.; Wu, Y.C. Norcantharidin induces apoptosis of breast cancer cells: Involvement of activities of mitogen activated protein kinases and signal transducers and activators of transcription. Toxicol. Vitr. Int. J. Publ. Assoc. BIBRA 2011, 25, 699–707. [Google Scholar] [CrossRef] [PubMed]
- Han, Z.; Li, B.; Wang, J.; Zhang, X.; Li, Z.; Dai, L.; Cao, M.; Jiang, J. Norcantharidin Inhibits SK-N-SH Neuroblastoma Cell Growth by Induction of Autophagy and Apoptosis. Technol. Cancer Res. Treat. 2017, 16, 33–44. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Mi, Y.; Wang, Z.; Jia, X.; Jin, Z. Norcantharidin inhibits viability and induces cell cycle arrest and apoptosis in osteosarcoma. Oncol. Lett. 2019, 17, 456–461. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Xu, X.; Lu, F.; Guo, S. Primary in vitro and in vivo evaluation of norcantharidin-chitosan/poly (vinyl alcohol) for cancer treatment. Drug Deliv. 2014, 21, 293–301. [Google Scholar] [CrossRef]
- Wang, J.; Huang, X.; Li, H.; Yan, D.; Huang, W. Two Zn(II) coordination polymers with anticancer drug norcantharidin as ligands for cancer chemotherapy. Dalton Trans. 2022, 51, 5624–5634. [Google Scholar] [CrossRef]
- Chi, J.; Jiang, Z.; Chen, X.; Peng, Y.; Liu, W.; Han, B.; Han, B. Studies on anti-hepatocarcinoma effect, pharmacokinetics and tissue distribution of carboxymethyl chitosan based norcantharidin conjugates. Carbohydr. Polym. 2019, 226, 115297. [Google Scholar] [CrossRef]
- Ding, X.Y.; Hong, C.J.; Liu, Y.; Gu, Z.L.; Xing, K.L.; Zhu, A.J.; Chen, W.L.; Shi, L.S.; Zhang, X.N.; Zhang, Q. Pharmacokinetics, tissue distribution, and metabolites of a polyvinylpyrrolidone-coated norcantharidin chitosan nanoparticle formulation in rats and mice, using LC-MS/MS. Int. J. Nanomed. 2012, 7, 1723–1735. [Google Scholar]
- Lixin, W.; Haibing, H.; Xing, T.; Ruiying, S.; Dawei, C. A less irritant norcantharidin lipid microspheres: Formulation and drug distribution. Int. J. Pharm. 2006, 323, 161–167. [Google Scholar] [CrossRef]
- Thaqi, A.; Scott, J.L.; Gilbert, J.; Sakoff, J.A.; McCluskey, A. Synthesis and biological activity of Delta-5,6-norcantharimides: Importance of the 5,6-bridge. Eur. J. Med. Chem. 2010, 45, 1717–1723. [Google Scholar] [CrossRef]
- Yeh, C.B.; Su, C.J.; Hwang, J.M.; Chou, M.C. Therapeutic effects of cantharidin analogues without bridging ether oxygen on human hepatocellular carcinoma cells. Eur. J. Med. Chem. 2010, 45, 3981–3985. [Google Scholar] [CrossRef]
- Hill, T.A.; Stewart, S.G.; Ackland, S.P.; Gilbert, J.; Sauer, B.; Sakoff, J.A.; McCluskey, A. Norcantharimides, synthesis and anticancer activity: Synthesis of new norcantharidin analogues and their anticancer evaluation. Bioorg. Med. Chem. 2007, 15, 6126–6134. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, S.; Dai, M.; Nai, J.; Zhu, L.; Sheng, H. Solubility and Bioavailability Enhancement of Oridonin: A Review. Molecules 2020, 25, 332. [Google Scholar] [CrossRef] [PubMed]
- Shao, H.; Li, B.; Li, H.; Gao, L.; Zhang, C.; Sheng, H.; Zhu, L. Novel Strategies for Solubility and Bioavailability Enhancement of Bufadienolides. Molecules 2021, 27, 51. [Google Scholar] [CrossRef] [PubMed]
- de Jong, R.S.; Mulder, N.H.; Uges, D.R.; Sleijfer, D.T.; Höppener, F.J.; Groen, H.J.; Willemse, P.H.; van der Graaf, W.T.; de Vries, E.G. Phase I and pharmacokinetic study of the topoisomerase II catalytic inhibitor fostriecin. Br. J. Cancer 1999, 79, 882–887. [Google Scholar] [CrossRef]
- Swingle, M.R.; Amable, L.; Lawhorn, B.G.; Buck, S.B.; Burke, C.P.; Ratti, P.; Fischer, K.L.; Boger, D.L.; Honkanen, R.E. Structure-activity relationship studies of fostriecin, cytostatin, and key analogs, with PP1, PP2A, PP5, and(beta12-beta13)-chimeras (PP1/PP2A and PP5/PP2A), provide further insight into the inhibitory actions of fostriecin family inhibitors. J. Pharmacol. Exp. Ther. 2009, 331, 45–53. [Google Scholar] [CrossRef]
- Tarleton, M.; Gilbert, J.; Sakoff, J.A.; McCluskey, A. Synthesis and anticancer activity of a series of norcantharidin analogues. Eur. J. Med. Chem. 2012, 54, 573–581. [Google Scholar] [CrossRef]
- Robertson, M.J.; Gordon, C.P.; Gilbert, J.; McCluskey, A.; Sakoff, J.A. Norcantharimide analogues possessing terminal phosphate esters and their anti-cancer activity. Bioorg. Med. Chem. 2011, 19, 5734–5741. [Google Scholar] [CrossRef]
- Wu, J.Y.; Kuo, C.D.; Chu, C.Y.; Chen, M.S.; Lin, J.H.; Chen, Y.J.; Liao, H.F. Synthesis of novel lipophilic N-substituted norcantharimide derivatives and evaluation of their anticancer activities. Molecules 2014, 19, 6911–6928. [Google Scholar] [CrossRef]
- Pachuta-Stec, A.; Szuster-Ciesielska, A. New Norcantharidin Analogs: Synthesis and Anticancer Activity. Arch. Pharm. 2015, 348, 897–907. [Google Scholar] [CrossRef]
- Chang, M.C.; Tsai, E.T.; Wu, J.Y.; Liao, H.F.; Chen, Y.J.; Kuo, C.D. N-Farnesyloxy-norcantharimide and N-farnesyl-norcantharimide inhibit the progression of leukemia and increase survival days in a syngeneic mouse leukemia model. Anticancer Drugs 2015, 26, 508–517. [Google Scholar] [CrossRef]
- Chang, M.C.; Wu, J.Y.; Liao, H.F.; Chen, Y.J.; Kuo, C.D. N-Farnesyloxy-norcantharimide inhibits progression of human leukemic Jurkat T cells through regulation of mitogen-activated protein kinase and interleukin-2 production. Anticancer Drugs 2015, 26, 1034–1042. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.C.; Wu, J.Y.; Liao, H.F.; Chen, Y.J.; Kuo, C.D. Comparative assessment of therapeutic safety of norcantharidin, N-farnesyloxy-norcantharimide, and N-farnesyl-norcantharimide against Jurkat T cells relative to human normal lymphoblast: A quantitative pilot study. Medicine 2016, 95, e4467. [Google Scholar] [CrossRef] [PubMed]
- Spare, L.K.; Falsetta, P.; Gilbert, J.; Harman, D.G.; Baker, M.A.; Li, F.; McCluskey, A.; Clegg, J.K.; Sakoff, J.A.; Aldrich-Wright, J.R.; et al. Cytotoxicity of a Series of Norcantharidin-Inspired Tetrahydroepoxyisoindole Carboxamides. ChemMedChem 2017, 12, 130–145. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Guan, X.W.; Chen, S.W.; Hui, L. Synthesis and biological evaluation of norcantharidin derivatives as protein phosphatase-1 inhibitors. Bioorg. Med. Chem. Lett. 2015, 25, 363–366. [Google Scholar] [CrossRef] [PubMed]
- Hizartzidis, L.; Tarleton, M.; Gordon, C.P.; McCluskey, A. Chemoselective flow hydrogenation approaches to isoindole-7-carboxylic acids and 7-oxa-bicyclio [2.2.1] heptanes. RSC Adv. 2014, 4, 9709–9722. [Google Scholar] [CrossRef]
- Hizartzidis, L.; Gilbert, J.; Gordon, C.P.; Sakoff, J.A.; McCluskey, A. Synthesis and Cytotoxicity of Octahydroepoxyisoindole-7-carboxylic Acids and Norcantharidin-Amide Hybrids as Norcantharidin Analogues. ChemMedChem 2019, 14, 1152–1161. [Google Scholar] [CrossRef]
- Wang, X.H.; Huang, M.; Zhao, C.K.; Li, C.; Xu, L. Design, synthesis, and biological activity evaluation of campthothecin-HAA-Norcantharidin conjugates as antitumor agents in vitro. Chem. Biol. Drug Des. 2019, 93, 986–992. [Google Scholar] [CrossRef]
- Zhao, C.K.; Li, C.; Wang, X.H.; Bao, Y.J.; Yang, F.H.; Huang, M. The regio-selective synthesis of 10-hydroxy camptothecin norcantharidin conjugates and their biological activity evaluation in vitro. R. Soc. Open Sci. 2018, 5, 172317. [Google Scholar] [CrossRef]
- Zhao, C.K.; Xu, L.; Wang, X.H.; Bao, Y.J.; Wang, Y. Synthesis of Dual Target CPT-Ala-Nor Conjugates and Their Biological Activity Evaluation. Anticancer Agents Med. Chem. 2019, 19, 502–508. [Google Scholar] [CrossRef]
- Zhao, C.; Wang, X.; Yang, F.; Gao, L.; Wang, Y. A simple route to a novel acid-sensitive 20(S)-O-linked camptothecin norcantharidin acid ester derivative. R. Soc. Open Sci. 2018, 5, 170842. [Google Scholar] [CrossRef]
- Wang, X.H.; Yang, F.H.; Zhao, C.K.; Gao, L.; Li, C. Sealed tube promoted coupling of camptothecin and norcantharidin acid ester and their preliminary biological activity evaluation in vitro. Med. Chem. Res. 2017, 27, 406–411. [Google Scholar] [CrossRef]
- Dong, W.W.; Wang, X.H.; Qian, S.; Wang, Y.H.; Zhao, C.K. Regio-selective synthesis and activity research on 7-icaritin norcantharidin conjugates. Nat. Prod. Res. 2022, 1–9. [Google Scholar] [CrossRef]
- Stromyer, M.L.; Southerland, M.R.; Satyal, U.; Sikder, R.K.; Weader, D.J.; Baughman, J.A.; Youngs, W.J.; Abbosh, P.H. Synthesis, characterization, and biological activity of a triphenylphosphonium-containing imidazolium salt against select bladder cancer cell lines. Eur. J. Med. Chem. 2020, 185, 111832. [Google Scholar] [CrossRef] [PubMed]
- Sawicka, D.; Hryniewicka, A.; Gohal, S.; Sadowska, A.; Pryczynicz, A.; Guzińska-Ustymowicz, K.; Sokołowska, E.; Morzycki, J.W.; Car, H. Establishment of In Vitro and In Vivo Anticolorectal Cancer Efficacy of Lithocholic Acid-Based Imidazolium Salts. Int. J. Mol. Sci. 2022, 23, 7019. [Google Scholar] [CrossRef] [PubMed]
- Sun, R.-R.; Guo, J.-H.; Yang, C.; Yang, L.-J.; Huang, C. Synthesis and Antitumor Evaluation of Novel N-substituted Norcantharidin Imidazolium Derivatives. Curr. Org. Synth. 2018, 15, 237–245. [Google Scholar] [CrossRef]
- Wang, W.; Deng, L.; Tang, S.; Qian, Q. Synthesis of Pyrazole-linked Norcantharidin Analogues of Substituted Chromones. J. Heterocycl. Chem. 2016, 53, 1631–1634. [Google Scholar] [CrossRef]
- Wang, W.; Deng, L.P.; Hu, C.Q.; Zhang, Y.H.; Li, Y.; Zuo, S.F. Synthesis of Isoxazole-Linked Norcantharidin Analogues of Substituted Chromones. J. Heterocycl. Chem. 2017, 54, 1806–1811. [Google Scholar] [CrossRef]
- Shariatinia, Z. Pharmaceutical applications of chitosan. Adv. Colloid Interface Sci. 2019, 263, 131–194. [Google Scholar] [CrossRef]
- Xu, X.; Li, Y.; Shen, Y.; Guo, S. Synthesis and in vitro cellular evaluation of novel anti-tumor norcantharidin-conjugated chitosan derivatives. Int. J. Biol. Macromol. 2013, 62, 418–425. [Google Scholar] [CrossRef]
- Xu, X.; Li, Y.; Wang, F.; Lv, L.; Liu, J.; Li, M.; Guo, A.; Jiang, J.; Shen, Y.; Guo, S. Synthesis, in vitro and in vivo evaluation of new norcantharidin-conjugated hydroxypropyltrimethyl ammonium chloride chitosan derivatives as polymer therapeutics. Int. J. Pharm. 2013, 453, 610–619. [Google Scholar] [CrossRef]
- Shariatinia, Z. Carboxymethyl chitosan: Properties and biomedical applications. Int. J. Biol. Macromol. 2018, 120 Pt B, 1406–1419. [Google Scholar] [CrossRef]
- Jiang, Z.; Chi, J.; Han, B.; Liu, W. Preparation and pharmacological evaluation of norcantharidin-conjugated carboxymethyl chitosan in mice bearing hepatocellular carcinoma. Carbohydr. Polym. 2017, 174, 282–290. [Google Scholar] [CrossRef] [PubMed]
- Chi, J.; Jiang, Z.; Qiao, J.; Peng, Y.; Liu, W.; Han, B. Synthesis and anti-metastasis activities of norcantharidin-conjugated carboxymethyl chitosan as a novel drug delivery system. Carbohydr. Polym. 2019, 214, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Chi, J.; Jiang, Z.; Qiao, J.; Zhang, W.; Peng, Y.; Liu, W.; Han, B. Antitumor evaluation of carboxymethyl chitosan based norcantharidin conjugates against gastric cancer as novel polymer therapeutics. Int. J. Biol. Macromol. 2019, 136, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Han, H.W.; Qiu, H.Y.; Hu, C.; Sun, W.X.; Yang, R.W.; Qi, J.L.; Wang, X.M.; Lu, G.H.; Yang, Y.H. Design, synthesis and anti-cancer activity evaluation of podophyllotoxin-norcantharidin hybrid drugs. Bioorg. Med. Chem. Lett. 2016, 26, 3237–3242. [Google Scholar] [CrossRef]
- Tang, Z.B.; Chen, Y.Z.; Zhao, J.; Guan, X.W.; Bo, Y.X.; Chen, S.W.; Hui, L. Conjugates of podophyllotoxin and norcantharidin as dual inhibitors of topoisomeraseⅡ and protein phosphatase 2A. Eur. J. Med. Chem. 2016, 123, 568–576. [Google Scholar] [CrossRef]
- Liu, X.; Heng, W.S.; Paul; Li, Q.; Chan, L.W. Novel polymeric microspheres containing norcantharidin for chemoembolization. J. Control. Release 2006, 116, 35–41. [Google Scholar] [CrossRef]
- Pekarek, K.J.; Jacob, J.S.; Mathiowitz, E. Double-walled polymer microspheres for controlled drug release. Nature 1994, 367, 258–260. [Google Scholar] [CrossRef]
- Jeong, B.; Bae, Y.H.; Lee, D.S.; Kim, S.W. Biodegradable block copolymers as injectable drug-delivery systems. Nature 1997, 388, 860–862. [Google Scholar] [CrossRef]
- Dasan, K.P.; Rekha, C. Polymer blend microspheres for controlled drug release: The techniques for preparation and characterization: A review article. Curr. Drug Deliv. 2012, 9, 588–595. [Google Scholar] [CrossRef]
- Yan, F.; Li, B.; Shen, F.; Fu, Q. Formulation and characterization of albumin microspheres containing norcantharidate for liver tumor targeting. Drug Deliv. 2015, 22, 862–868. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.Y.; Zhou, X.F.; Zhou, X.Y.; Wen, Q.Y.; You, B.G.; Liu, Y.; Zhang, X.N.; Jin, Y. Effect of alginate-chitosan sustained release microcapsules for transhepatic arterial embolization in VX2 rabbit liver cancer model. J. Biomed. Mater. Res. A 2013, 101, 3192–3200. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Guo, S.; Cheng, L. Disodium norcantharidate loaded poly(epsilon-caprolactone) microspheres I. Preparation and evaluation. Int. J. Pharm. 2008, 350, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Guo, S. Disodium norcantharidate-loaded poly(epsilon-caprolactone) microspheres II. Modification of morphology and release behavior. Int. J. Pharm. 2008, 353, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Guo, S. Formation mechanism and release behavior of poly(epsilon-caprolactone) microspheres containing disodium norcantharidate. Eur. J. Pharm. Biopharm. Off. J. Arb. Fur Pharm. Verfahr. e.V 2008, 69, 1176–1181. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Hu, K.; Di, L.; Wang, P.; Liu, Z.; Zhang, J.; Yue, P.; Song, W.; Zhang, J.; Chen, T.; et al. Traditional herbal medicine and nanomedicine: Converging disciplines to improve therapeutic efficacy and human health. Adv. Drug Deliv. Rev. 2021, 178, 113964. [Google Scholar] [CrossRef] [PubMed]
- Dahiya, S.; Dahiya, R.; Hernández, E. Nanocarriers for Anticancer Drug Targeting: Recent Trends and Challenges. Crit. Rev. Ther. Drug Carr. Syst. 2021, 38, 49–103. [Google Scholar] [CrossRef]
- Guo, L.; Zhang, Y.; Al-Jamal, K.T. Recent progress in nanotechnology-based drug carriers for celastrol delivery. Biomater. Sci. 2021, 9, 6355–6380. [Google Scholar] [CrossRef]
- Samadzadeh, S.; Mousazadeh, H.; Ghareghomi, S.; Dadashpour, M.; Babazadeh, M.; Zarghami, N. In vitro anticancer efficacy of Metformin-loaded PLGA nanofibers towards the post-surgical therapy of lung cancer. J. Drug Deliv. Sci. Tec. 2021, 61, 102318. [Google Scholar] [CrossRef]
- Chen, J.; Gao, C.; Zhang, Y.; Wang, T.; Qian, Y.; Yang, B.; Dong, P.; Zhang, Y. Inorganic Nano-Targeted Drugs Delivery System and Its Application of Platinum-Based Anticancer Drugs. J. Nanosci. Nanotechnol. 2017, 17, 1–17. [Google Scholar] [CrossRef]
- Kim, H.; Park, Y.; Stevens, M.M.; Kwon, W.; Hahn, S.K. Multifunctional hyaluronate-nanoparticle hybrid systems for diagnostic, therapeutic and theranostic applications. J. Control. Release 2019, 303, 55–66. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Wang, X.; Gong, F.; Liu, T.; Liu, Z. 2D Nanomaterials for Cancer Theranostic Applications. Adv. Mater. 2020, 32, e1902333. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Beack, S.; Han, S.; Shin, M.; Lee, T.; Park, Y.; Kim, K.S.; Yetisen, A.K.; Yun, S.H.; Kwon, W.; et al. Multifunctional Photonic Nanomaterials for Diagnostic, Therapeutic, and Theranostic Applications. Adv. Mater. 2018, 30, 1701460. [Google Scholar] [CrossRef] [PubMed]
- Muhamad, N.; Plengsuriyakarn, T.; Na-Bangchang, K. Application of active targeting nanoparticle delivery system for chemotherapeutic drugs and traditional/herbal medicines in cancer therapy: A systematic review. Int. J. Nanomed. 2018, 13, 3921–3935. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Jia, C.; Yang, J. Designing Nanoparticle-based Drug Delivery Systems for Precision Medicine. Int. J. Med. Sci. 2021, 18, 2943–2949. [Google Scholar] [CrossRef]
- Adlravan, E.; Jalilzadeh-Razin, S.; Nejati, K.; Karimi, M.A.; Mousazadeh, H.; Abbasi, A.; Dadashpour, M. Potential activity of free and PLGA/PEG nanoencapsulated nasturtium officinale extract in inducing cytotoxicity and apoptosis in human lung carcinoma A549 cells. J. Drug Deliv. Sci. Tec. 2021, 61, 102256. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, L.; Hu, W.; Hu, Z.H.; Bei, Y.Y.; Xu, J.Y.; Wang, W.J.; Zhang, X.N.; Zhang, Q. Norcantharidin-associated galactosylated chitosan nanoparticles for hepatocyte-targeted delivery. Nanomedicine 2010, 6, 371–381. [Google Scholar] [CrossRef]
- Bei, Y.Y.; Chen, X.Y.; Liu, Y.; Xu, J.Y.; Wang, W.J.; Gu, Z.L.; Xing, K.L.; Zhu, A.J.; Chen, W.L.; Shi, L.S.; et al. Novel norcantharidin-loaded liver targeting chitosan nanoparticles to enhance intestinal absorption. Int. J. Nanomed. 2012, 7, 1819–1827. [Google Scholar]
- Guan, M.; Zhou, Y.; Zhu, Q.L.; Liu, Y.; Bei, Y.Y.; Zhang, X.N.; Zhang, Q. N-trimethyl chitosan nanoparticle-encapsulated lactosyl-norcantharidin for liver cancer therapy with high targeting efficacy. Nanomedicine 2012, 8, 1172–1181. [Google Scholar] [CrossRef]
- Cheng, M.; Xu, H.; Wang, Y.; Chen, H.; He, B.; Gao, X.; Li, Y.; Han, J.; Zhang, Z. Glycyrrhetinic acid-modified chitosan nanoparticles enhanced the effect of 5-fluorouracil in murine liver cancer model via regulatory T-cells. Drug Des. Dev. Ther. 2013, 7, 1287–1299. [Google Scholar]
- Qi, W.W.; Yu, H.Y.; Guo, H.; Lou, J.; Wang, Z.M.; Liu, P.; Sapin-Minet, A.; Maincent, P.; Hong, X.C.; Hu, X.M.; et al. Doxorubicin-loaded glycyrrhetinic acid modified recombinant human serum albumin nanoparticles for targeting liver tumor chemotherapy. Mol. Pharm. 2015, 12, 675–683. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Jiang, Y.; Ni, X.; Chen, L.; Wu, M.; Liu, J.; Yang, B.; Shan, X.; Yang, L.; Fan, J.; et al. Glycyrrhetinic Acid-Modified Norcantharidin Nanoparticles for Active Targeted Therapy of Hepatocellular Carcinoma. J. Biomed. Nanotechnol. 2018, 14, 114–126. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xiao, Y.; Lin, H.P.; Reichel, D.; Bae, Y.; Lee, E.Y.; Jiang, Y.; Huang, X.; Yang, C.; Wang, Z. In vivo β-catenin attenuation by the integrin α5-targeting nano-delivery strategy suppresses triple negative breast cancer stemness and metastasis. Biomaterials 2019, 188, 160–172. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Sun, H.; Lu, Y.; Zhao, F.; Liu, C.; Wang, Q.; Zheng, C.; Lu, R.; Song, K. Strontium/Chitosan/Hydroxyapatite/Norcantharidin Composite That Inhibits Osteosarcoma and Promotes Osteogenesis In Vitro. BioMed Res. Int. 2020, 2020, 9825073. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Yang, K.; Tang, X.; Bi, Y.; Ding, Y.; Deng, M.; Xia, D.; Zhao, Y.; Chen, T. Norcantharidin Nanostructured Lipid Carrier (NCTD-NLC) Suppresses the Viability of Human Hepatocellular Carcinoma HepG2 Cells and Accelerates the Apoptosis. J. Immunol. Res. 2022, 2022, 3851604. [Google Scholar] [CrossRef] [PubMed]
- Cheng, R.; Liu, X.; Wang, Z.; Tang, K. ABT-737, a Bcl-2 family inhibitor, has a synergistic effect with apoptosis by inducing urothelial carcinoma cell necroptosis. Mol. Med. Rep. 2021, 23, 412. [Google Scholar] [CrossRef]
- Zhang, S.; Li, G.; Ma, X.; Wang, Y.; Liu, G.; Feng, L.; Zhao, Y.; Zhang, G.; Wu, Y.; Ye, X.; et al. Norcantharidin enhances ABT-737-induced apoptosis in hepatocellular carcinoma cells by transcriptional repression of Mcl-1. Cell. Signal. 2012, 24, 1803–1809. [Google Scholar] [CrossRef]
- Ren, J.; Li, G.; Zhao, W.; Lin, L.; Ye, T. Norcantharidin combined with ABT-737 for hepatocellular carcinoma: Therapeutic effects and molecular mechanisms. World J. Gastroenterol. 2016, 22, 3962–3968. [Google Scholar] [CrossRef]
- Liu, M.; Tu, J.; Feng, Y.; Zhang, J.; Wu, J. Synergistic co-delivery of diacid metabolite of norcantharidin and ABT-737 based on folate-modified lipid bilayer-coated mesoporous silica nanoparticle against hepatic carcinoma. J. Nanobiotechnology 2020, 18, 114. [Google Scholar] [CrossRef]
- Xiong, Y.X.; Tang, H.X.; Liu, W.H.; Zhang, T.T.; Ma, R.; Mu, C.F.; Zhu, Z.H.; Li, F.Z. Characterization and Evaluation of a Folic Acid Receptor-Targeted Norcantharidin/Tetrandrine Dual-Drug Loaded Delivery System. J. Nanomater. 2019, 2019, 7683791. [Google Scholar] [CrossRef]
- Xie, M.H.; Fu, Z.L.; Hua, A.L.; Zhou, J.F.; Chen, Q.; Li, J.B.; Yao, S.; Cai, X.J.; Ge, M.; Zhou, L.; et al. A new core-shell-type nanoparticle loaded with paclitaxel/norcantharidin and modified with APRPG enhances anti-tumor effects in hepatocellular carcinoma. Front. Oncol. 2022, 12, 932156. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; Sun, C.; Xu, K.; Wang, C.; Guo, J. Immobilization of ALA-Zn(II) Coordination Polymer Pro-photosensitizers on Magnetite Colloidal Supraparticles for Target Photodynamic Therapy of Bladder Cancer. Small 2015, 11, 6338–6346. [Google Scholar] [CrossRef] [PubMed]
- Paul, M.; Sarkar, K.; Deb, J.; Dastidar, P. Hand-Ground Nanoscale Zn(II) -Based Coordination Polymers Derived from NSAIDs: Cell Migration Inhibition of Human Breast Cancer Cells. Chemistry 2017, 23, 5736–5747. [Google Scholar] [CrossRef]
- Mukherjee, S.; Ganguly, S.; Manna, K.; Mondal, S.; Mahapatra, S.; Das, D. Green Approach To Synthesize Crystalline Nanoscale Zn(II)-Coordination Polymers: Cell Growth Inhibition and Immunofluorescence Study. Inorg. Chem. 2018, 57, 4050–4060. [Google Scholar] [CrossRef] [PubMed]
- Torchilin, V. Antibody-modified liposomes for cancer chemotherapy. Expert Opin. Drug Deliv. 2008, 5, 1003–1025. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, K.S.; Hussein, S.A.; Ali, A.H.; Korma, S.A.; Lipeng, Q.; Jinghua, C. Liposome: Composition, characterisation, preparation, and recent innovation in clinical applications. J. Drug Target. 2019, 27, 742–761. [Google Scholar] [CrossRef]
- Yan, W.; Leung, S.S.; To, K.K. Updates on the use of liposomes for active tumor targeting in cancer therapy. Nanomedicine 2020, 15, 303–318. [Google Scholar] [CrossRef] [PubMed]
- Pereira, S.; Egbu, R.; Jannati, G.; Al-Jamal, W.T. Docetaxel-loaded liposomes: The effect of lipid composition and purification on drug encapsulation and in vitro toxicity. Int. J. Pharm. 2016, 514, 150–159. [Google Scholar] [CrossRef]
- Duong, T.T.; Isomäki, A.; Paaver, U.; Laidmäe, I.; Tõnisoo, A.; Yen, T.T.H.; Kogermann, K.; Raal, A.; Heinämäki, J.; Pham, T.M. Nanoformulation and Evaluation of Oral Berberine-Loaded Liposomes. Molecules 2021, 26, 2591. [Google Scholar] [CrossRef]
- Zhang, J.; Tang, Y.; Qian, B.; Sheng, H. Preparation and evaluation of norcantharidin-encapsulated liposomes modified with a novel CD19 monoclonal antibody 2E8. J. Huazhong Univ. Sci. Technolog. Med. Sci. 2010, 30, 240–247. [Google Scholar] [CrossRef]
- Zhang, J.; Tang, Y.; Li, S.; Liao, C.; Guo, X. Targeting of the B-lineage leukemia stem cells and their progeny with norcantharidin encapsulated liposomes modified with a novel CD19 monoclonal antibody 2E8 in vitro. J. Drug Target. 2010, 18, 675–687. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Shen, D.; Jia, M.; Zhao, H.; Tang, Y. The targeting effect of Hm2E8b-NCTD-liposomes on B-lineage leukaemia stem cells is associated with the HLF-SLUG axis. J. Drug Target. 2018, 26, 55–65. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Zhang, W.; Wang, D.; Li, S.; Wu, W. Preparation and characterization of norcantharidin liposomes modified with stearyl glycyrrhetinate. Exp. Ther. Med. 2018, 16, 1639–1646. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhao, L.; Tan, X.; Wu, Z.; Zhou, N.; Dong, N.; Zhang, Y.; Yin, T.; He, H.; Gou, J.; et al. Preclinical evaluations of Norcantharidin liposome and emulsion hybrid delivery system with improved encapsulation efficiency and enhanced antitumor activity. Expert Opin. Drug Deliv. 2022, 19, 451–464. [Google Scholar] [CrossRef]
- Venugopalan, P.; Jain, S.; Sankar, S.; Singh, P.; Rawat, A.; Vyas, S.P. pH-sensitive liposomes: Mechanism of triggered release to drug and gene delivery prospects. Die Pharm. 2002, 57, 659–671. [Google Scholar]
- Ferreira Ddos, S.; Lopes, S.C.; Franco, M.S.; Oliveira, M.C. pH-sensitive liposomes for drug delivery in cancer treatment. Ther. Deliv. 2013, 4, 1099–1123. [Google Scholar] [CrossRef]
- Qiao-ling, Z.; Yi, Z.; Min, G.; Di-jia, Y.; Xiao-feng, Z.; Yang, L.; Jing-yu, X.; Ying, W.; Zong-lin, G.; Kong-lang, X.; et al. Hepatocyte-targeted delivery using ph-sensitive liposomes loaded with lactosylnorcantharidin phospholipid complex: Preparation, characterization, and therapeutic evaluation in vivo and in vitro. Curr. Med. Chem. 2012, 19, 5754–5763. [Google Scholar] [CrossRef]
- Liu, M.; Ma, X.; Jin, Z.; Li, W.; Guo, M.; Li, F. Determination and pharmacokinetic study of the diacid metabolite of norcantharidin in beagle plasma by use of liquid chromatography-tandem mass spectrometry. Anal. Bioanal. Chem. 2013, 405, 9273–9283. [Google Scholar] [CrossRef]
- Liu, M.C.; Ma, X.Q.; Xu, Y.; Peng, L.H.; Han, M.; Gao, J.Q. Liquid chromatography-tandem mass spectrometry evaluation of the pharmacokinetics of a diacid metabolite of norcantharidin loaded in folic acid-targeted liposomes in mice. J. Pharm. Biomed. Anal. 2016, 119, 76–83. [Google Scholar] [CrossRef]
- Liu, X.; Han, M.; Xu, J.; Geng, S.; Zhang, Y.; Ye, X.; Gou, J.; Yin, T.; He, H.; Tang, X. Asialoglycoprotein receptor-targeted liposomes loaded with a norcantharimide derivative for hepatocyte-selective targeting. Int. J. Pharm. 2017, 520, 98–110. [Google Scholar] [CrossRef]
- Jiang, Y.; Liu, X.; Tan, X.; Hou, Y.; Sun, W.; Gou, J.; Yin, T.; He, H.; Zhang, Y.; Tang, X. In Vitro and In Vivo Evaluation of SP94 Modified Liposomes Loaded with N-14NCTDA, a Norcantharimide Derivative for Hepatocellular Carcinoma-Targeting. AAPS PharmSciTech 2020, 21, 277. [Google Scholar] [CrossRef] [PubMed]
- Nasirideen, S.; Kaş, H.S.; Oner, F.; Alpar, R.; Hincal, A.A. Naproxen incorporated lipid emulsions. I. Formulation and stability studies. J. Clin. Pharm. Ther. 1998, 23, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.J.; Wang, J.; Zhang, H.Y.; He, H.B.; Tang, X. Formulation, preparation and evaluation of flunarizine-loaded lipid microspheres. J. Pharm. Pharmacol. 2007, 59, 351–357. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Zhang, B.; Zhang, K.; Zhang, Y.; Wang, J.; Qi, N.; Yang, S.; He, H.; Tang, X. Preclinical evaluations of norcantharidin-loaded intravenous lipid microspheres with low toxicity. Expert Opin. Drug Deliv. 2012, 9, 1449–1462. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Teng, H.; Wang, J.; Zhang, Y.; Ren, T.; Tang, X.; Cai, C. A highly stable norcantharidin loaded lipid microspheres: Preparation, biodistribution and targeting evaluation. Int. J. Pharm. 2014, 473, 475–484. [Google Scholar] [CrossRef]
- Pan, Z.D.; Niu, Y.T.; Wang, Y.Q.; Tang, Y.L.; Tang, X.; Cai, C.F. Intravenous lipid microspheres loaded with alkylated norcantharidin derivative norcantharimide: Improved stability and prolonged half-life. Eur. J. Lipid Sci. Technol. 2015, 117, 55–64. [Google Scholar] [CrossRef]
- Croy, S.R.; Kwon, G.S. Polymeric micelles for drug delivery. Curr. Pharm. Des. 2006, 12, 4669–4684. [Google Scholar] [CrossRef]
- Jiang, S.; Li, M.; Hu, Y.; Zhang, Z.; Lv, H. Multifunctional self-assembled micelles of galactosamine-hyaluronic acid-vitamin E succinate for targeting delivery of norcantharidin to hepatic carcinoma. Carbohydr. Polym. 2018, 197, 194–203. [Google Scholar] [CrossRef]
- Zhang, Z.; Yang, L.; Hou, J.; Xia, X.; Wang, J.; Ning, Q.; Jiang, S. Promising positive liver targeting delivery system based on arabinogalactan-anchored polymeric micelles of norcantharidin. Artif. Cells Nanomed. Biotechnol. 2018, 46 (Suppl. S3), S630–S640. [Google Scholar] [CrossRef]
- Svirskis, D.; Chandramouli, K.; Bhusal, P.; Wu, Z.; Alphonso, J.; Chow, J.; Patel, D.; Ramakrishna, R.; Yeo, S.J.; Stowers, R.; et al. Injectable thermosensitive gelling delivery system for the sustained release of lidocaine. Ther. Deliv. 2016, 7, 359–368. [Google Scholar] [CrossRef]
- Xie, M.H.; Ge, M.; Peng, J.B.; Jiang, X.R.; Wang, D.S.; Ji, L.Q.; Ying, Y.; Wang, Z. In-vivo anti-tumor activity of a novel poloxamer-based thermosensitive in situ gel for sustained delivery of norcantharidin. Pharm. Dev. Technol. 2019, 24, 623–629. [Google Scholar] [CrossRef] [PubMed]
- Li, X.Y.; Guan, Q.X.; Shang, Y.Z.; Wang, Y.H.; Lv, S.W.; Yang, Z.X.; Wang, R.; Feng, Y.F.; Li, W.N.; Li, Y.J. Metal-organic framework IRMOFs coated with a temperature-sensitive gel delivering norcantharidin to treat liver cancer. World J. Gastroenterol. 2021, 27, 4208–4220. [Google Scholar] [CrossRef] [PubMed]
- Gao, B.; Luo, J.; Liu, Y.; Su, S.; Fu, S.; Yang, X.; Li, B. Intratumoral Administration of Thermosensitive Hydrogel Co-Loaded with Norcantharidin Nanoparticles and Doxorubicin for the Treatment of Hepatocellular Carcinoma. Int. J. Nanomed. 2021, 16, 4073–4085. [Google Scholar] [CrossRef] [PubMed]
- Xiao, S.; Wang, Y.; Ma, W.; Zhou, P.; Wang, B.; Wu, Z.; Wen, Q.; Xiong, K.; Liu, Y.; Fu, S. Intraperitoneal administration of thermosensitive hydrogel Co-loaded with norcantharidin nanoparticles and oxaliplatin inhibits malignant ascites of hepatocellular carcinoma. Drug Deliv. 2022, 29, 2713–2722. [Google Scholar] [CrossRef]
- Li, B.; Shao, H.; Gao, L.; Li, H.; Sheng, H.; Zhu, L. Nano-drug co-delivery system of natural active ingredients and chemotherapy drugs for cancer treatment: A review. Drug Deliv. 2022, 29, 2130–2161. [Google Scholar] [CrossRef]
| Carrier Types | Materials | Experimental Subject | Properties | Ref. |
|---|---|---|---|---|
| Nanoparticles | PVP K30-coated NCTD chitosan | SD rats, ICR mice | Prolonged half-life and improved tissue distribution in mice. | [28] |
| NCTD-loaded GC | SMMC-7721 and HepG2 cells, H22 mice | Enhanced toxicity to liver cancer cells, reduced the toxicity, and improved the intestinal absorption of NCTD. | [88,89] | |
| N-Trimethyl chitosan-encapsulated Lac-NCTD | HepG2 cells, H22 nude mice | Showed high liver targeting and strong antitumor effects in nude mice. | [90] | |
| NCTD-loaded mPEG-PCL-PEI-GA | HepG2 cells, tumor-bearing mice | Showed higher cytotoxicity and liver targeting, inhibited tumor growth, and prolonged the survival of tumor-bearing mice. | [93] | |
| NCTD-loaded RGD-LPH | TNBC cells, nude mouse | Inhibited TNBC tumor growth and metastasis. | [94] | |
| Strontium/Chitosan/Hydroxyapatite/NCTD Composite | MG-63 and MC3T3-E1 cells | Promoted osteogenesis and inhibited the proliferation of bone tumor cells. | [95] | |
| DMCA-Zn1 and DMCA-Zn2 nanoparticles | HepG2, Hep3B, and L927 cells | Inhibited cancer cell growth and proliferation and reduced the toxicity. | [26] | |
| NCTD-loaded modified lipid nanoparticles | HepG2 cells, SD rats | Inhibited HepG2 cell proliferation and induced apoptosis. | [96] | |
| DM-NCTD loaded in CHMSN and ABT-737 in lipid bilayer | H22 tumor-bearing mice | Showed stronger antitumor activity and reduced the toxicity in vivo. | [100] | |
| NCTD/Tet dual drug-loaded lipid nanoparticles | LO2, HepG2, HepG2/Adr and MCF-7cells | Showed strong antitumor effect by reversing the multidrug resistance and reduced the toxicity. | [101] | |
| NCTD/PTX-loaded core-shell lipid nanoparticles modified with APRPG | HepG2 cells, tumor-bearing mice | Remarkably inhibited the proliferation and migration of HCC cells. | [102] | |
| Liposomes | NCTD liposomes modified with a novel mouse anti-human CD19 monoclonal antibody 2E8 | BALB/c mice, Nalm-6 and Molt-3 cells | Specifically killed Nalm-6 cells and reduced the toxicity and was an effective method for treating B lineage hematopoietic malignancies. | [111] |
| Nalm-6, Raji, Molt-3, and K562 cells | Specifically targeted B-LSCs and induced apoptosis. | [112] | ||
| NCTD liposomes modified with a novel mouse anti-human CD19 monoclonal antibody 2E8b | HAL-01 and Molt-3 cells | Had the potential to specifically kill the B-LSCs and reduced the toxicity. | [113] | |
| NCTD-loaded SG-NCTD-LIP | HepG2 cells | Increased cytotoxicity to HepG2 cells. | [114] | |
| NCTD liposome–emulsion hybrid | H22 cells, SD rats, and H22 heterotopic or H22/Luc orthotopic tumor-bearing C57BL/6 mice | Controlled drug release, enhanced tumor-targeted accumulation, and reduced cardiotoxicity and nephrotoxicity. | [115] | |
| PH-sensitive liposomes loaded with Lac-NCTD phospholipid complex | HepG2 cells, H22 tumor-bearing mice | Showed better membrane permeability, higher capture rate, and stronger tumor-suppressive effects. | [118] | |
| DM-NCTD-loaded PEG/FA-PEG-liposomes | Kunming mice | Prolonged the circulation time of DM-NCTD in the blood and improved the bioavailability. | [120] | |
| Asialoglycoprotein receptor-targeted, galactosylated liposomes loaded with N-14NCTDA | HepG2 cells, SD rats, mice | Improved bioavailability and anticancer activity, reduced hepatorenal toxicity. | [121] | |
| N-14NCTDA-loaded liposomes modified with SP94 | HepG2 cells, H22 tumor-bearing mice | Increased the liver-targeted accumulation of drug, improved the efficacy, and reduced the toxicity. | [122] | |
| Lipid Microspheres | NCTD-loaded lipid microspheres | Male and female rats | Reduced cardiotoxicity and nephrotoxicity by avoiding the direct contact between NCTD and body fluids. | [125] |
| NCTD–phospholipid complex | Kunming strain mice | Improved tumor-targeting ability and reduced nephrotoxicity. | [126] | |
| N-14NCTD-loaded lipid microspheres | SD rats | Improved stability, prolonged the half-life, and reduced the toxicity. | [127] | |
| Polymer Micelles | NCTD-loaded self-assembled micelles | MCF-7, MCF-7/Adr and HepG2 cells, BALB/c nude mice | Showed high cytotoxicity in vitro, high liver targeting, strong antitumor effects, and low toxicity in vivo. | [129] |
| arabinogalactan (AG)-modified N- (4-methimidazole) -hydroxyethyl (chitosan (MHC) | Male athymic nude mice | Increased the targeting of active liver drugs, improved the antitumor efficacy, and reduced the toxicity. | [130] | |
| Thermosensitive Gel | NCTD-loaded metal–organic framework IRMOF-3 coated with a temperature-sensitive gel | Hepa1–6 cells | Showed sustained-release effects and inhibited the proliferation and induced apoptosis of Hepa1–6 cells. | [133] |
| A thermosensitive hydrogel system co-encapsulated NCTD-NPs and Dox | H22 and HepG2 cells, Kunming female mice | Had significant anti-proliferative activity to HepG2 cells, inhibited tumor growth, and prolonged survival in H22 tumor mice. | [134] | |
| A thermosensitive N/O/hydrogel delivery system co-encapsulated NCTD-NPs and L-OHP | H22 and Huh7 cells, Kunming white rat | Showed stronger pro-apoptotic ability, prolonged the survival of tumor-bearing mice, and reduced systemic toxicity. | [135] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Q.; Sun, H.; Li, X.; Sheng, H.; Zhu, L. Strategies for Solubility and Bioavailability Enhancement and Toxicity Reduction of Norcantharidin. Molecules 2022, 27, 7740. https://doi.org/10.3390/molecules27227740
Liu Q, Sun H, Li X, Sheng H, Zhu L. Strategies for Solubility and Bioavailability Enhancement and Toxicity Reduction of Norcantharidin. Molecules. 2022; 27(22):7740. https://doi.org/10.3390/molecules27227740
Chicago/Turabian StyleLiu, Qian, Henglai Sun, Xinyu Li, Huagang Sheng, and Liqiao Zhu. 2022. "Strategies for Solubility and Bioavailability Enhancement and Toxicity Reduction of Norcantharidin" Molecules 27, no. 22: 7740. https://doi.org/10.3390/molecules27227740
APA StyleLiu, Q., Sun, H., Li, X., Sheng, H., & Zhu, L. (2022). Strategies for Solubility and Bioavailability Enhancement and Toxicity Reduction of Norcantharidin. Molecules, 27(22), 7740. https://doi.org/10.3390/molecules27227740





