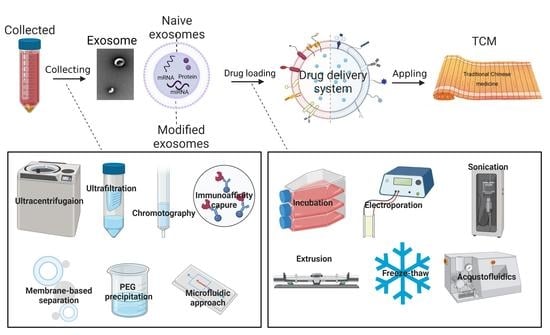
Exosomes as Novel Delivery Systems for Application in Traditional Chinese Medicine
Abstract
:1. Introduction
2. Classification of Exosomes
2.1. Naive Exosomes
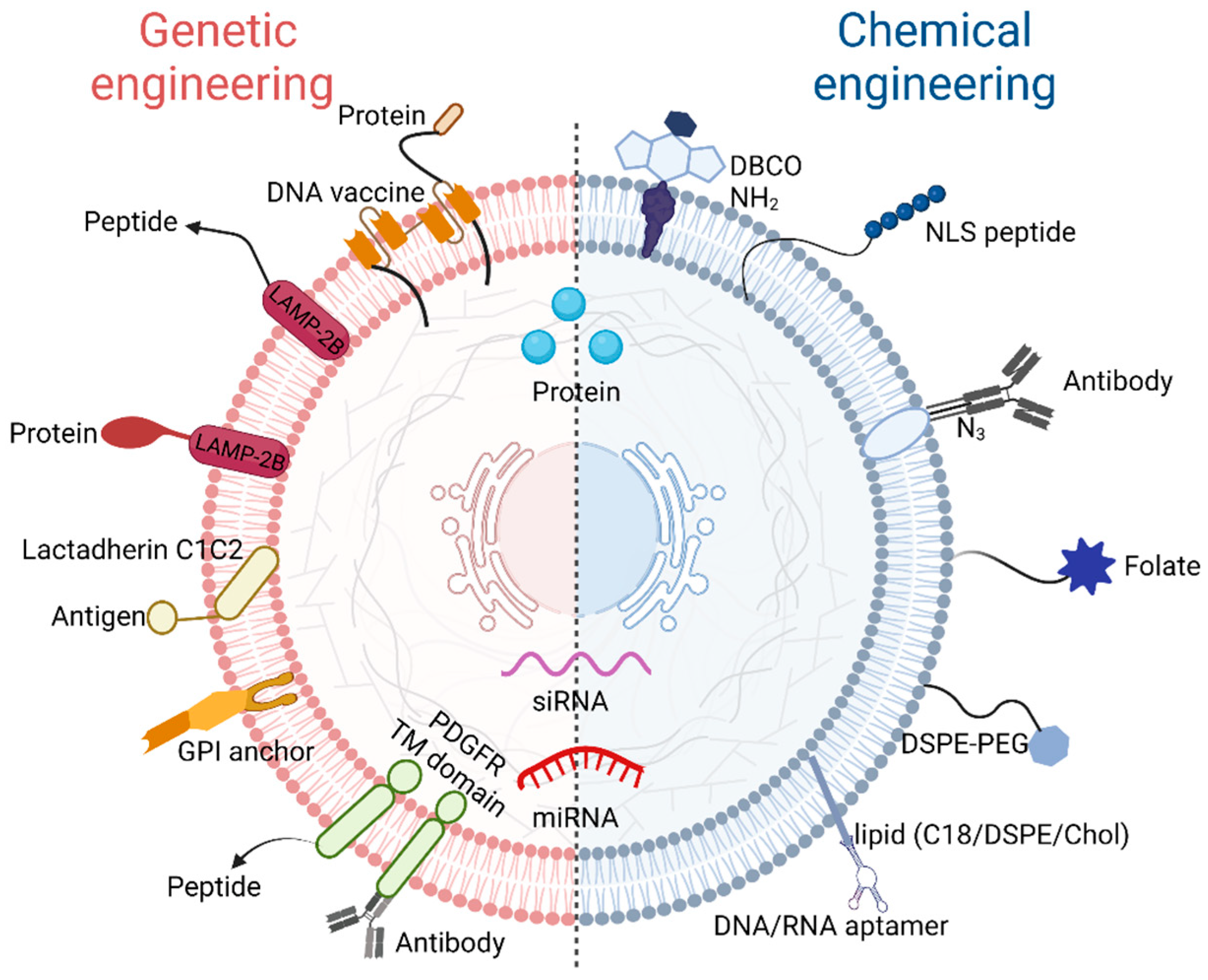
2.2. Modified Exosomes
3. Construction of Exosomes as Drug Delivery Systems
3.1. Exosomes Isolation and Modification
3.2. Drug Loading Methods
4. The Application in Chinese Medicine
4.1. Oncology
4.2. Cardiovascular Diseases
4.3. Others
5. Conclusions
5.1. Conclusive Remarks
5.2. Future Recommendations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kalluri, R.; LeBleu, V. The biology, function, and biomedical applications of exosomes. Science 2020, 367, eaau6977. [Google Scholar] [CrossRef]
- Théry, C.; Zitvogel, L.; Amigorena, S. Exosomes: Composition, biogenesis and function. Nat. Rev. Immunol. 2002, 2, 569–579. [Google Scholar] [CrossRef] [PubMed]
- Thakur, A.; Parra, D.; Motallebnejad, P.; Brocchi, M.; Chen, H. Exosomes: Small vesicles with big roles in cancer, vaccine development, and therapeutics. Bioact. Mater. 2022, 10, 281–294. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Man, Q.; Gao, X.; Lin, H.; Wang, J.; Su, F.; Wang, H.; Bu, L.; Liu, B.; Chen, G. Tissue-derived extracellular vesicles in cancers and non-cancer diseases: Present and future. J. Extracell. Vesicles 2021, 10, e12175. [Google Scholar] [CrossRef] [PubMed]
- Cai, P.; Zheng, Y.; Zhou, Y.; Wang, W.; Li, M.; Shi, Y.; Lin, H.; Lin, S. Research progress on the role of exosomes in obstructive sleep apnea-hypopnea syndrome-related atherosclerosis. Sleep Med. Rev. 2022, 66, 101696. [Google Scholar] [CrossRef]
- Bano, A.; Vats, R.; Yadav, P.; Bhardwaj, R. Exosomics in oral cancer diagnosis, prognosis, and therapeutics—An emergent and imperative non-invasive natural nanoparticle-based approach. Crit. Rev. Oncol. Hematol. 2022, 178, 103799. [Google Scholar] [CrossRef]
- Liu, C.; Wang, Y.; Li, L.; He, D.; Chi, J.; Li, Q.; Wu, Y.; Zhao, Y.; Zhang, S.; Wang, L.; et al. Engineered extracellular vesicles and their mimetics for cancer immunotherapy. J. Control. Release 2022, 349, 679–698. [Google Scholar] [CrossRef]
- Song, J.; Song, B.; Yuan, L.; Yang, G. Multiplexed strategies toward clinical translation of extracellular vesicles. Theranostics 2022, 12, 6740–6761. [Google Scholar] [CrossRef]
- Qian, K.; Fu, W.; Li, T.; Zhao, J.; Lei, C.; Hu, S. The roles of small extracellular vesicles in cancer and immune regulation and translational potential in cancer therapy. J. Exp. Clin. Cancer Res. 2022, 41, 286. [Google Scholar] [CrossRef]
- Wang, X.; Huang, J.; Chen, W.; Li, G.; Li, Z.; Lei, J. The updated role of exosomal proteins in the diagnosis, prognosis, and treatment of cancer. Exp. Mol. Med. 2022, 54, 1390–1400. [Google Scholar] [CrossRef]
- Al-Masawa, M.; Alshawsh, M.; Ng, C.; Ng, A.; Foo, J.; Vijakumaran, U.; Subramaniam, R.; Ghani, N.; Witwer, K.; Law, J. Efficacy and safety of small extracellular vesicle interventions in wound healing and skin regeneration: A systematic review and meta-analysis of animal studies. Theranostics 2022, 12, 6455–6508. [Google Scholar] [CrossRef] [PubMed]
- Vaz, M.; Martins, T.; Henriques, A. Extracellular vesicles in the study of Alzheimer’s and Parkinson’s diseases: Methodologies applied from cells to biofluids. J. Neurochem. 2022, 00, 1–44. [Google Scholar] [CrossRef] [PubMed]
- Park, A.; Tsunoda, I. Helicobacter pylori infection in the stomach induces neuroinflammation: The potential roles of bacterial outer membrane vesicles in an animal model of Alzheimer’s disease. Inflamm. Regen. 2022, 42, 39. [Google Scholar] [CrossRef] [PubMed]
- D’Acunzo, P.; Kim, Y.; Ungania, J.; Pérez-González, R.; Goulbourne, C.; Levy, E. Isolation of mitochondria-derived mitovesicles and subpopulations of microvesicles and exosomes from brain tissues. Nat. Protoc. 2022, 17, 2517–2549. [Google Scholar] [CrossRef]
- Chen, X.; Feng, J.; Chen, W.; Shao, S.; Chen, L.; Wan, H. Small extracellular vesicles: From promoting pre-metastatic niche formation to therapeutic strategies in breast cancer. Cell Commun. Signal 2022, 20, 141. [Google Scholar] [CrossRef]
- Rethi, L.; Mutalik, C.; Anurogo, D.; Lu, L.; Chu, H.; Yougbaré, S.; Kuo, T.; Cheng, T.; Chen, F. Lipid-based nanomaterials for drug delivery systems in breast cancer therapy. Nanomaterials 2022, 12, 2948. [Google Scholar] [CrossRef]
- Mo, C.; Zhao, J.; Liang, J.; Wang, H.; Chen, Y.; Huang, G. Exosomes: A novel insight into traditional Chinese medicine. Front. Pharmacol. 2022, 13, 844782. [Google Scholar] [CrossRef]
- Ji, L.; Li, Q.; He, Y.; Zhang, X.; Zhou, Z.; Gao, Y.; Fang, M.; Yu, Z.; Rodrigues, R.; Cao, Y.; et al. Therapeutic potential of traditional Chinese medicine for the treatment of NAFLD: A promising drug Potentilla discolor Bunge. Acta Pharm. Sin. B 2022, 12, 3529–3547. [Google Scholar] [CrossRef]
- Granchi, C. Biological activity of natural and synthetic compounds. Molecules 2022, 27, 3652. [Google Scholar] [CrossRef]
- Baker, M. Deceptive curcumin offers cautionary tale for chemists. Nature 2017, 541, 144–145. [Google Scholar] [CrossRef]
- Zheng, B.; Zhou, J.; Wang, H. Host microRNAs and exosomes that modulate influenza virus infection. Virus Res. 2020, 279, 197885. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Li, Y.; Tang, Y.; Hao, X.; Xu, W.; Xiang, D.; Wu, J. Apoptotic bodies for advanced drug delivery and therapy. J. Control. Release 2022, 351, 394–406. [Google Scholar] [CrossRef] [PubMed]
- Thakur, A.; Ke, X.; Chen, Y.W.; Motallebnejad, P.; Zhang, K.; Lian, Q.; Chen, H.J. The mini player with diverse functions: Extracellular vesicles in cell biology, disease, and therapeutics. Protein Cell 2022, 13, 631–654. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, Y.; Liu, H.; Tang, W.H. Exosomes: Biogenesis, biologic function and clinical potential. Cell Biosci. 2019, 9, 19. [Google Scholar] [CrossRef] [PubMed]
- Qi, H.; Liu, C.; Long, L.; Ren, Y.; Zhang, S.; Chang, X.; Qian, X.; Jia, H.; Zhao, J.; Sun, J.; et al. Blood exosomes endowed with magnetic and targeting properties for cancer therapy. ACS Nano 2016, 10, 3323–3333. [Google Scholar] [CrossRef] [PubMed]
- Blanc, L.; De Gassart, A.; Géminard, C.; Bette-Bobillo, P.; Vidal, M. Exosome release by reticulocytes—An integral part of the red blood cell differentiation system. Blood Cell Mol. Dis. 2005, 35, 21–26. [Google Scholar] [CrossRef]
- Du, J.; Wan, Z.; Wang, C.; Lu, F.; Wei, M.; Wang, D.; Hao, Q. Designer exosomes for targeted and efficient ferroptosis induction in cancer via chemo-photodynamic therapy. Theranostics 2021, 11, 8185–8196. [Google Scholar] [CrossRef]
- Qu, M.; Lin, Q.; Huang, L.; Fu, Y.; Wang, L.; He, S.; Fu, Y.; Yang, S.; Zhang, Z.; Zhang, L.; et al. Dopamine-loaded blood exosomes targeted to brain for better treatment of Parkinson’s disease. J. Control. Release 2018, 287, 156–166. [Google Scholar] [CrossRef]
- Zhao, Z.; Liu, M.; Xu, Z.; Cai, Y.; Peng, B.; Liang, Q.; Yan, Y.; Liu, W.; Kang, F.; He, Q.; et al. Identification of ACSF gene family as therapeutic targets and immune-associated biomarkers in hepatocellular carcinoma. Aging 2022, 14, 7926–7940. [Google Scholar] [CrossRef]
- Yuan, D.; Zhao, Y.; Banks, W.; Bullock, K.; Haney, M.; Batrakova, E.; Kabanov, A. Macrophage exosomes as natural nanocarriers for protein delivery to inflamed brain. Biomaterials 2017, 142, 1–12. [Google Scholar] [CrossRef]
- Zhu, J.; Liu, B.; Wang, Z.; Wang, D.; Ni, H.; Zhang, L.; Wang, Y. Exosomes from nicotine-stimulated macrophages accelerate atherosclerosis through miR-21-3p/PTEN-mediated VSMC migration and proliferation. Theranostics 2019, 9, 6901–6919. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Yu, D. Exosomes in cancer development, metastasis, and immunity. Biochim. Biophys. Acta Rev. Cancer 2019, 1871, 455–468. [Google Scholar] [CrossRef] [PubMed]
- Dai, S.; Zhou, X.; Wang, B.; Wang, Q.; Fu, Y.; Chen, T.; Wan, T.; Yu, Y.; Cao, X. Enhanced induction of dendritic cell maturation and HLA-A*0201-restricted CEA-specific CD8+ CTL response by exosomes derived from IL-18 gene-modified CEA-positive tumor cells. J. Mol. Med. 2006, 84, 1067–1076. [Google Scholar] [CrossRef] [PubMed]
- Wolfers, J.; Lozier, A.; Raposo, G.; Regnault, A.; Théry, C.; Masurier, C.; Flament, C.; Pouzieux, S.; Faure, F.; Tursz, T.; et al. Tumor-derived exosomes are a source of shared tumor rejection antigens for CTL cross-priming. Nat. Med. 2001, 7, 297–303. [Google Scholar] [CrossRef]
- Zhang, H.; Zhuang, X.; Sun, D.; Liu, Y.; Xiang, X.; Grizzle, W.E. Exosomes and immune surveillance of neoplastic lesions: A review. Biotech. Histochem. 2012, 87, 161–168. [Google Scholar] [CrossRef]
- Zhou, W.; Zhou, Y.; Chen, X.; Ning, T.; Chen, H.; Guo, Q.; Zhang, Y.; Liu, P.; Zhang, Y.; Li, C.; et al. Pancreatic cancer-targeting exosomes for enhancing immunotherapy and reprogramming tumor microenvironment. Biomaterials 2021, 268, 120546. [Google Scholar] [CrossRef]
- Ji, Y.; Ji, J.; Yin, H.; Chen, X.; Zhao, P.; Lu, H.; Wang, T. Exosomes derived from microRNA-129-5p-modified tumor cells selectively enhanced suppressive effect in malignant behaviors of homologous colon cancer cells. Bioengineered 2021, 12, 12148–12156. [Google Scholar] [CrossRef]
- Brahmbhatt, M.; Gundala, S.R.; Asif, G.; Shamsi, S.A.; Aneja, R. Ginger phytochemicals exhibit synergy to inhibit prostate cancer cell proliferation. Nutr. Cancer 2013, 65, 263–272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Q.; Ren, Y.; Mu, J.; Egilmez, N.; Zhuang, X.; Deng, Z.; Zhang, L.; Yan, J.; Miller, D.; Zhang, H. Grapefruit-derived nanovectors use an activated leukocyte trafficking pathway to deliver therapeutic agents to inflammatory tumor sites. Cancer Res. 2015, 75, 2520–2529. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.; Zhuang, X.; Mu, J.; Deng, Z.; Jiang, H.; Zhang, L.; Xiang, X.; Wang, B.; Yan, J.; Miller, D.; et al. Delivery of therapeutic agents by nanoparticles made of grapefruit-derived lipids. Nat. Commun. 2013, 4, 1867. [Google Scholar] [CrossRef]
- Kim, D.; Rhee, W. Antioxidative effects of carrot-derived nanovesicles in cardiomyoblast neuroblastoma cells. Pharmaceutics 2021, 13, 1203. [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.; Rong, Y.; Teng, Y.; Mu, J.; Zhuang, X.; Tseng, M.; Samykutty, A.; Zhang, L.; Yan, J.; Miller, D.; et al. Broccoli-derived nanoparticle inhibits mouse colitis by activating dendritic cell AMP-activated protein kinase. Mol. Ther. 2017, 25, 1641–1654. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiao, J.; Feng, S.; Wang, X.; Long, K.; Luo, Y.; Wang, Y.; Ma, J.; Tang, Q.; Jin, L.; Li, X.; et al. Identification of exosome-like nanoparticle-derived microRNAs from 11 edible fruits and vegetables. PeerJ 2018, 6, e5186. [Google Scholar] [CrossRef]
- Fujita, D.; Arai, T.; Komori, H.; Shirasaki, Y.; Wakayama, T.; Nakanishi, T.; Tamai, I. Apple-derived nanoparticles modulate expression of organic-anion-transporting polypeptide (OATP) 2B1 in caco-2 cells. Mol. Pharm. 2018, 15, 5772–5780. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, S.; Koo, Y.; Yang, A.; Dai, Y.; Khant, H.; Osman, S.; Chowdhury, M.; Wei, H.; Li, Y.; et al. Characterization of and isolation methods for plant leaf nanovesicles and small extracellular vesicles. Nanomedicine 2020, 29, 102271. [Google Scholar] [CrossRef]
- Mao, Y.; Han, M.; Chen, C.; Wang, X.; Han, J.; Gao, Y.; Wang, S. A biomimetic nanocomposite made of a ginger-derived exosome and an inorganic framework for high-performance delivery of oral antibodies. Nanoscale 2021, 13, 20157–20169. [Google Scholar] [CrossRef]
- Zhang, M.; Xiao, B.; Wang, H.; Han, M.; Zhang, Z.; Viennois, E.; Xu, C.; Merlin, D. Edible ginger-derived nano-lipids loaded with doxorubicin as a novel drug-delivery approach for colon cancer therapy. Mol. Ther. 2016, 24, 1783–1796. [Google Scholar] [CrossRef] [Green Version]
- Sundaram, K.; Miller, D.; Kumar, A.; Teng, Y.; Sayed, M.; Mu, J.; Lei, C.; Sriwastva, M.; Zhang, L.; Yan, J.; et al. Plant-Derived exosomal nanoparticles inhibit pathogenicity of Porphyromonas gingivalis. iScience 2019, 21, 308–327. [Google Scholar] [CrossRef] [Green Version]
- Kulp, A.; Kuehn, M. Biological functions and biogenesis of secreted bacterial outer membrane vesicles. Annu. Rev. Microbiol. 2010, 64, 163–184. [Google Scholar] [CrossRef] [Green Version]
- Schwechheimer, C.; Kuehn, M. Outer-membrane vesicles from Gram-negative bacteria: Biogenesis and functions. Nat. Rev. Microbiol. 2015, 13, 605–619. [Google Scholar] [CrossRef]
- Briaud, P.; Carroll Ronan, K. Extracellular vesicle biogenesis and functions in gram-positive bacteria. Infect. Immun. 2020, 88, e00433-20. [Google Scholar] [CrossRef]
- Thoma, J.; Manioglu, S.; Kalbermatter, D.; Bosshart, P.; Fotiadis, D.; Müller, D. Protein-enriched outer membrane vesicles as a native platform for outer membrane protein studies. Commun. Biol. 2018, 1, 23. [Google Scholar] [CrossRef] [Green Version]
- Huang, W.; Wang, S.; Yao, Y.; Xia, Y.; Yang, X.; Li, K.; Sun, P.; Liu, C.; Sun, W.; Bai, H.; et al. Employing Escherichia coli-derived outer membrane vesicles as an antigen delivery platform elicits protective immunity against Acinetobacter baumannii infection. Sci. Rep. 2016, 6, 37242. [Google Scholar] [CrossRef] [Green Version]
- Kim, O.; Dinh, N.; Park, H.; Choi, S.; Hong, K.; Gho, Y. Bacterial protoplast-derived nanovesicles for tumor targeted delivery of chemotherapeutics. Biomaterials 2017, 113, 68–79. [Google Scholar] [CrossRef] [PubMed]
- Gujrati, V.; Kim, S.; Kim, S.; Min, J.; Choy, H.; Kim, S.; Jon, S. Bioengineered bacterial outer membrane vesicles as cell-specific drug-delivery vehicles for cancer therapy. ACS Nano 2014, 8, 1525–1537. [Google Scholar] [CrossRef]
- Shetgaonkar, G.; Marques, S.; DCruz, C.; Vibhavari, R.; Kumar, L.; Shirodkar, R. Exosomes as cell-derivative carriers in the diagnosis and treatment of central nervous system diseases. Drug Deliv. Transl. Res. 2022, 12, 1047–1079. [Google Scholar] [CrossRef]
- Yáñez-Mó, M.; Siljander, P.; Andreu, Z.; Zavec, A.; Borràs, F.; Buzas, E.; Buzas, K.; Casal, E.; Cappello, F.; Carvalho, J.; et al. Biological properties of extracellular vesicles and their physiological functions. J. Extracell. Vesicles 2015, 4, 1. [Google Scholar] [CrossRef] [Green Version]
- Gurung, S.; Perocheau, D.; Touramanidou, L.; Baruteau, J. The exosome journey: From biogenesis to uptake and intracellular signalling. Cell Commun. Signal 2021, 19, 47. [Google Scholar] [CrossRef]
- Rezaie, J.; Feghhi, M.; Etemadi, T. A review on exosomes application in clinical trials: Perspective, questions, and challenges. Cell Commun. Signal 2022, 20, 145. [Google Scholar] [CrossRef]
- Sung, B.; Lersner, A.; Guerrero, J.; Krystofiak, E.; Inman, D.; Pelletier, R.; Zijlstra, A.; Ponik, S.; Weaver, A. A live cell reporter of exosome secretion and uptake reveals pathfinding behavior of migrating cells. Nat. Commun. 2020, 11, 2092. [Google Scholar] [CrossRef]
- Bellavia, D.; Raimondo, S.; Calabrese, G.; Forte, S.; Cristaldi, M.; Patinella, A.; Memeo, L.; Manno, M.; Raccosta, S.; Diana, P.; et al. Interleukin 3- receptor targeted exosomes inhibit in vitro and in vivo Chronic Myelogenous Leukemia cell growth. Theranostics 2017, 7, 1333–1345. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Q.; Shi, X.; Han, M.; Smbatyan, G.; Lenz, H.; Zhang, Y. Reprogramming exosomes as nanoscale controllers of cellular immunity. J. Am. Chem. Soc. 2018, 140, 16413–16417. [Google Scholar] [CrossRef]
- Ran, N.; Gao, X.; Dong, X.; Li, J.; Lin, C.; Geng, M.; Yin, H. Effects of exosome-mediated delivery of myostatin propeptide on functional recovery of mdx mice. Biomaterials 2020, 236, 119826. [Google Scholar] [CrossRef] [PubMed]
- Kanuma, T.; Yamamoto, T.; Kobiyama, K.; Moriishi, E.; Masuta, Y.; Kusakabe, T.; Ozasa, K.; Kuroda, E.; Jounai, N.; Ishii, K. CD63-mediated antigen delivery into extracellular vesicles via DNA vaccination results in robust CD8+ T cell responses. J. Immunol. 2017, 198, 4707. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilke, S.; Krausze, J.; Büssow, K. Crystal structure of the conserved domain of the DC lysosomal associated membrane protein: Implications for the lysosomal glycocalyx. BMC Biol. 2012, 10, 62. [Google Scholar] [CrossRef] [Green Version]
- Tian, Y.; Li, S.; Song, J.; Ji, T.; Zhu, M.; Anderson, G.J.; Wei, J.; Nie, G. A doxorubicin delivery platform using engineered natural membrane vesicle exosomes for targeted tumor therapy. Biomaterials 2014, 35, 2383–2390. [Google Scholar] [CrossRef]
- Ohno, S.; Takanashi, M.; Sudo, K.; Ueda, S.; Ishikawa, A.; Matsuyama, N.; Fujita, K.; Mizutani, T.; Ohgi, T.; Ochiya, T.; et al. Systemically injected exosomes targeted to EGFR deliver antitumor microRNA to breast cancer cells. Mol. Ther. 2013, 21, 185–191. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Sobral, M.; Snyder, T.; Brudno, Y.; Gorantla, V.; Mooney, D. Clickable, acid labile immunosuppressive prodrugs for in vivo targeting. Biomater. Sci. 2020, 8, 266–277. [Google Scholar] [CrossRef]
- Xu, L.; Raabe, M.; Zegota, M.; Nogueira, J.; Chudasama, V.; Kuan, S.; Weil, T. Site-selective protein modification via disulfide rebridging for fast tetrazine/trans-cyclooctene bioconjugation. Org. Biomol. Chem. 2020, 18, 1140–1147. [Google Scholar] [CrossRef] [Green Version]
- Shee, M.; Singh, N. Chemical versatility of azide radical: Journey from a transient species to synthetic accessibility in organic transformations. Chem. Soc. Rev. 2022, 51, 2255–2312. [Google Scholar] [CrossRef]
- Mansfeld, U.; Pietsch, C.; Hoogenboom, R.; Becer, C.; Schubert, U. Clickable initiators, monomers and polymers in controlled radical polymerizations—A prospective combination in polymer science. Polym. Chem. 2010, 1, 1560–1598. [Google Scholar] [CrossRef]
- Wang, P.; Na, Z.; Fu, J.; Tan, C.; Zhang, H.; Yao, S.; Sun, H. Microarray immobilization of biomolecules using a fast trans-cyclooctene (TCO)–tetrazine reaction. Chem. Commun. 2014, 50, 11818–11821. [Google Scholar] [CrossRef] [PubMed]
- Ravasco, J.; Monteiro, C.; Trindade, A. Cyclopropenes: A new tool for the study of biological systems. Org. Chem. Front. 2017, 4, 1167–1198. [Google Scholar] [CrossRef]
- Xue, Y.; Bai, H.; Peng, B.; Fang, B.; Baell, J.; Li, L.; Huang, W.; Voelcker, N. Stimulus-cleavable chemistry in the field of controlled drug delivery. Chem. Soc. Rev. 2021, 50, 4872–4931. [Google Scholar] [CrossRef]
- Nie, W.; Wu, G.; Zhang, J.; Huang, L.; Ding, J.; Jiang, A.; Zhang, Y.; Liu, Y.; Li, J.; Pu, K.; et al. Responsive exosome nano-bioconjugates for synergistic cancer therapy. Angew. Chem. Int. Ed. 2020, 59, 2018–2022. [Google Scholar] [CrossRef]
- Li, Y.; Wu, J.; Liu, J.; Xu, W.; Qiu, X.; Huang, S.; Hu, X.; Xiang, D. Artificial exosomes for translational nanomedicine. J. Nanobiotechnology 2021, 19, 242. [Google Scholar] [CrossRef]
- Pi, F.; Binzel, D.; Lee, T.; Li, Z.; Sun, M.; Rychahou, P.; Li, H.; Haque, F.; Wang, S.; Croce, C.; et al. Nanoparticle orientation to control RNA loading and ligand display on extracellular vesicles for cancer regression. Nat. Nanotechnol. 2018, 13, 82–89. [Google Scholar] [CrossRef]
- Wang, J.; Li, W.; Lu, Z.; Zhang, L.; Hu, Y.; Li, Q.; Du, W.; Feng, X.; Jia, H.; Liu, B. The use of RGD-engineered exosomes for enhanced targeting ability and synergistic therapy toward angiogenesis. Nanoscale 2017, 9, 15598–15605. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Guo, Y.; Kong, L.; Shi, J.; Liu, P.; Li, R.; Geng, Y.; Gao, W.; Zhang, Z.; Fu, D. A bone-targeted engineered exosome platform delivering siRNA to treat osteoporosis. Bioact. Mater. 2022, 10, 207–221. [Google Scholar] [CrossRef]
- Hu, S.; Wang, X.; Li, Z.; Zhu, D.; Cores, J.; Wang, Z.; Li, J.; Mei, X.; Cheng, X.; Su, T.; et al. Platelet membrane and stem cell exosome hybrids enhance cellular uptake and targeting to heart injury. Nano Today 2021, 39, 101210. [Google Scholar] [CrossRef]
- Liang, C.; Xu, L.; Song, G.; Liu, Z. Emerging nanomedicine approaches fighting tumor metastasis: Animal models, metastasis-targeted drug delivery, phototherapy, and immunotherapy. Chem. Soc. Rev. 2016, 45, 6250–6269. [Google Scholar] [CrossRef] [PubMed]
- Piffoux, M.; Silva, A.; Wilhelm, C.; Gazeau, F.; Tareste, D. Modification of extracellular vesicles by fusion with liposomes for the design of personalized biogenic drug delivery systems. ACS Nano 2018, 12, 6830–6842. [Google Scholar] [CrossRef] [PubMed]
- Mentkowski, K.; Lang, J. Exosomes engineered to express a cardiomyocyte binding peptide demonstrate improved cardiac retention in vivo. Sci. Rep. 2019, 9, 10041. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ou, Y.; Liang, J.; Chng, W.; Muthuramalingam, R.; Ng, Z.; Lee, C.; Neupane, Y.; Yau, J.; Zhang, S.; Lou, C.; et al. Investigations on cellular uptake mechanisms and immunogenicity profile of novel bio-hybrid nanovesicles. Pharmaceutics 2022, 14, 1738. [Google Scholar] [CrossRef]
- Nielsen, T.; Kristensen, A.; Pedersen, S.; Christiansen, G.; Kristensen, S. Investigation of procoagulant activity in extracellular vesicles isolated by differential ultracentrifugation. J. Extracell. Vesicles 2018, 7, 1454777. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, H.; Kim, J.; Park, J. Methods to isolate extracellular vesicles for diagnosis. Micro Nano Syst. Lett. 2017, 5, 15. [Google Scholar] [CrossRef] [Green Version]
- Arab, T.; Raffo-Romero, A.; Camp, C.; Lemaire, Q.; Marrec-Croq, F.; Drago, F.; Aboulouard, S.; Slomianny, C.; Lacoste, A.; Guigon, I.; et al. Proteomic characterisation of leech microglia extracellular vesicles (EVs): Comparison between differential ultracentrifugation and Optiprep™ density gradient isolation. J. Extracell. Vesicles 2019, 8, 1603048. [Google Scholar] [CrossRef] [Green Version]
- Liang, B.; Peng, P.; Chen, S.; Li, L.; Zhang, M.; Cao, D.; Yang, J.; Li, H.; Gui, T.; Li, X.; et al. Characterization and proteomic analysis of ovarian cancer-derived exosomes. J. Proteom. 2013, 80, 171–182. [Google Scholar] [CrossRef]
- Nam, G.; Choi, Y.; Kim, G.; Kim, S.; Kim, S.; Kim, I. Emerging prospects of exosomes for cancer treatment: From conventional therapy to immunotherapy. Adv. Mater. 2020, 32, 2002440. [Google Scholar] [CrossRef]
- Cheruvanky, A.; Zhou, H.; Pisitkun, T.; Kopp, J.; Knepper, M.; Yuen, P.; Star, R. Rapid isolation of urinary exosomal biomarkers using a nanomembrane ultrafiltration concentrator. Am. J. Physiol. Ren. Physiol. 2007, 292, F1657–F1661. [Google Scholar] [CrossRef]
- Yang, D.; Zhang, W.; Zhang, H.; Zhang, F.; Chen, L.; Ma, L.; Larcher, L.; Chen, S.; Liu, N.; Zhao, Q.; et al. Progress, opportunity, and perspective on exosome isolation—efforts for efficient exosome-based theranostics. Theranostics 2020, 10, 3684–3707. [Google Scholar] [CrossRef] [PubMed]
- Gercel-Taylor, C.; Atay, S.; Tullis, R.; Kesimer, M.; Taylor, D. Nanoparticle analysis of circulating cell-derived vesicles in ovarian cancer patients. Anal. Biochem. 2012, 428, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, J.; Leonard, P.; Darcy, E.; Sharma, S.; O’Kennedy, R. Immunoaffinity chromatography: Concepts and applications. Methods Mol. Biol. 2017, 1485, 27–51. [Google Scholar] [CrossRef] [PubMed]
- Tauro, B.; Greening, D.; Mathias, R.; Ji, H.; Mathivanan, S.; Scott, A.; Simpson, R. Comparison of ultracentrifugation, density gradient separation, and immunoaffinity capture methods for isolating human colon cancer cell line LIM1863-derived exosomes. Methods 2012, 56, 293–304. [Google Scholar] [CrossRef] [PubMed]
- Yoo, Y.; Lee, J.; Kim, H.; Hwang, K.; Yoon, D.; Lee, J. Toward exosome-based neuronal diagnostic devices. Micromachines 2018, 9, 634. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, F.; Jiao, F.; Xia, C.; Zhao, Y.; Ying, W.; Xie, Y.; Guan, X.; Tao, M.; Zhang, Y.; Qin, W.; et al. A novel strategy for facile serum exosome isolation based on specific interactions between phospholipid bilayers and TiO2. Chem. Sci. 2019, 10, 1579–1588. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Li, P.; Zhang, T.; Xu, Z.; Huang, X.; Wang, R.; Du, L. Review on strategies and technologies for exosome isolation and purification. Front. Bioeng. Biotechnol. 2022, 9, 811971. [Google Scholar] [CrossRef]
- Tian, J.; Casella, G.; Zhang, Y.; Rostami, A.; Li, X. Potential roles of extracellular vesicles in the pathophysiology, diagnosis, and treatment of autoimmune diseases. Int. J. Biol. Sci. 2020, 16, 620–632. [Google Scholar] [CrossRef] [Green Version]
- Son, K.; Rahimian, A.; Shin, D.; Siltanen, C.; Patel, T.; Revzin, A. Microfluidic compartments with sensing microbeads for dynamic monitoring of cytokine and exosome release from single cells. Analyst 2016, 141, 679–688. [Google Scholar] [CrossRef]
- Wang, Y.; Yuan, W.; Kimber, M.; Lu, M.; Dong, L. Rapid differentiation of host and parasitic exosome vesicles using microfluidic photonic crystal biosensor. ACS Sens. 2018, 3, 1616–1621. [Google Scholar] [CrossRef]
- Lin, S.; Yu, Z.; Chen, D.; Wang, Z.; Miao, J.; Li, Q.; Zhang, D.; Song, J.; Cui, D. Progress in microfluidics-based exosome separation and detection technologies for diagnostic applications. Small 2020, 16, 1903916. [Google Scholar] [CrossRef] [PubMed]
- Stam, J.; Bartel, S.; Bischoff, R.; Wolters, J.C. Isolation of extracellular vesicles with combined enrichment methods. J. Chromatogr. B 2021, 1169, 122604. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Bi, J.; Huang, J.; Tang, Y.; Du, S.; Li, P. Exosome: A review of its classification, isolation techniques, storage, diagnostic and targeted therapy applications. Int. J. Nanomed. 2020, 15, 6917–6934. [Google Scholar] [CrossRef]
- Fu, S.; Wang, Y.; Xia, X.; Zheng, J. Exosome engineering: Current progress in cargo loading and targeted delivery. Nanoimpact 2020, 20, 100261. [Google Scholar] [CrossRef]
- Mehryab, F.; Rabbani, S.; Shahhosseini, S.; Shekari, F.; Fatahi, Y.; Baharvand, H.; Haeri, A. Exosomes as a next-generation drug delivery system: An update on drug loading approaches, characterization, and clinical application challenges. Acta Biomater. 2020, 113, 42–62. [Google Scholar] [CrossRef] [PubMed]
- Saari, H.; Lázaro-Ibáñez, E.; Viitala, T.; Vuorimaa-Laukkanen, E.; Siljander, P.; Yliperttula, M. Microvesicle- and exosome-mediated drug delivery enhances the cytotoxicity of Paclitaxel in autologous prostate cancer cells. J. Control. Release 2015, 220, 727–737. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garofalo, M.; Saari, H.; Somersalo, P.; Crescenti, D.; Kuryk, L.; Aksela, L.; Capasso, C.; Madetoja, M.; Koskinen, K.; Oksanen, T.; et al. Antitumor effect of oncolytic virus and paclitaxel encapsulated in extracellular vesicles for lung cancer treatment. J. Control. Release 2018, 283, 223–234. [Google Scholar] [CrossRef]
- Li, Y.; Gao, Y.; Gong, C.; Wang, Z.; Xia, Q.; Gu, F.; Hu, C.; Zhang, L.; Guo, H.; Gao, S. A33 antibody-functionalized exosomes for targeted delivery of doxorubicin against colorectal cancer. Nanomedicine 2018, 14, 1973–1985. [Google Scholar] [CrossRef]
- Wu, T.; Qi, Y.; Zhang, D.; Song, Q.; Yang, C.; Hu, X.; Bao, Y.; Zhao, Y.; Zhang, Z. Bone marrow dendritic cells derived microvesicles for combinational immunochemotherapy against tumor. Adv. Funct. Mater. 2017, 27, 1703191. [Google Scholar] [CrossRef]
- Hood, J.; Scott, M.; Wickline, S. Maximizing exosome colloidal stability following electroporation. Anal. Biochem. 2014, 448, 41–49. [Google Scholar] [CrossRef]
- Nakase, I.; Futaki, S. Combined treatment with a pH-sensitive fusogenic peptide and cationic lipids achieves enhanced cytosolic delivery of exosomes. Sci. Rep. 2015, 5, 10112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, H.; Park, H.; Noh, G.; Lee, E. pH-responsive hyaluronate-anchored extracellular vesicles to promote tumor-targeted drug delivery. Carbohydr. Polym. 2018, 202, 323–333. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Haney, M.; Zhao, Y.; Mahajan, V.; Deygen, I.; Klyachko, N.; Inskoe, E.; Piroyan, A.; Sokolsky, M.; Okolie, O.; et al. Development of exosome-encapsulated paclitaxel to overcome MDR in cancer cells. Nanomedicine 2016, 12, 655–664. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lamichhane, T.; Jeyaram, A.; Patel, D.; Parajuli, B.; Livingston, N.; Arumugasaamy, N.; Schardt, J.; Jay, S. Oncogene knockdown via active loading of small RNAs into extracellular vesicles by sonication. Cell Mol. Bioeng. 2016, 9, 315–324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Q.; Bai, H.; Wu, W.; Huang, G.; Li, Y.; Wu, M.; Tang, G.; Ping, Y. Bioengineering bacterial vesicle-coated polymeric nanomedicine for enhanced cancer immunotherapy and metastasis prevention. Nano Lett. 2020, 20, 11–21. [Google Scholar] [CrossRef]
- Cheng, G.; Li, W.; Ha, L.; Han, X.; Hao, S.; Wan, Y.; Wang, Z.; Dong, F.; Zou, X.; Mao, Y.; et al. Self-assembly of extracellular vesicle-like metal–organic framework nanoparticles for protection and intracellular delivery of biofunctional proteins. J. Am. Chem. Soc. 2018, 140, 7282–7291. [Google Scholar] [CrossRef]
- Cheng, L.; Zhang, X.; Tang, J.; Lv, Q.; Liu, J. Gene-engineered exosomes-thermosensitive liposomes hybrid nanovesicles by the blockade of CD47 signal for combined photothermal therapy and cancer immunotherapy. Biomaterials 2021, 275, 120964. [Google Scholar] [CrossRef]
- Wang, Z.; Rich, J.; Hao, N.; Gu, Y.; Chen, C.; Yang, S.; Zhang, P.; Huang, T. Acoustofluidics for simultaneous nanoparticle-based drug loading and exosome encapsulation. Microsyst. Nanoeng. 2022, 8, 45. [Google Scholar] [CrossRef]
- Wei, H.; Chen, J.; Wang, S.; Fu, F.; Zhu, X.; Wu, C.; Liu, Z.; Zhong, G.; Lin, J. A nanodrug consisting of Doxorubicin and exosome derived from mesenchymal stem cells for osteosarcoma treatment in vitro. Int. J. Nanomed. 2019, 14, 8603–8610. [Google Scholar] [CrossRef] [Green Version]
- Jeyaram, A.; Lamichhane, T.N.; Wang, S.; Zou, L.; Dahal, E.; Kronstadt, S.M.; Levy, D.; Parajuli, B.; Knudsen, D.R.; Chao, W.; et al. Enhanced loading of functional miRNA cargo via pH gradient modification of extracellular vesicles. Mol. Ther. 2020, 28, 975–985. [Google Scholar] [CrossRef]
- Jeyaram, A.; Jay, S. Preservation and storage stability of extracellular vesicles for therapeutic applications. AAPS J. 2018, 20, 1. [Google Scholar] [CrossRef] [PubMed]
- Théry, C.; Witwer, K.; Aikawa, E.; Alcaraz, M.; Anderson, J.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 2018, 7, 1. [Google Scholar] [CrossRef] [Green Version]
- Yuan, F.; Li, Y.; Wang, Z. Preserving extracellular vesicles for biomedical applications: Consideration of storage stability before and after isolation. Drug Deliv. 2021, 28, 1501–1509. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Feng, K.; Zhao, H.; Di, L.; Wang, L.; Wang, R. Tumor-derived extracellular vesicles as messengers of natural products in cancer treatment. Theranostics 2022, 12, 1683–1714. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Sawyers, C. Targeted cancer therapy. Nature 2004, 432, 294–297. [Google Scholar] [CrossRef]
- Nastiuk, K.; Krolewski, J. Opportunities and challenges in combination gene cancer therapy. Adv. Drug Deliv. Rev. 2016, 98, 35–40. [Google Scholar] [CrossRef] [Green Version]
- Yao, C.; Zhang, J.; Li, J.; Wei, W.; Wu, S.; Guo, D. Traditional Chinese medicine (TCM) as a source of new anticancer drugs. Nat. Prod. Rep. 2021, 38, 1618–1633. [Google Scholar] [CrossRef]
- Davies, H. Synthetic lessons from nature. Nature 2009, 459, 786–787. [Google Scholar] [CrossRef]
- De Furia, M. Paclitaxel (Taxol®): A new natural product with major anticancer activity. Phytomedicine 1997, 4, 273–282. [Google Scholar] [CrossRef]
- Meng, Z.; Lv, Q.; Lu, J.; Yao, H.; Lv, X.; Jiang, F.; Lu, A.; Zhang, G. Prodrug strategies for Paclitaxel. Int. J. Mol. Sci. 2016, 17, 796. [Google Scholar] [CrossRef] [Green Version]
- Zhu, L.; Chen, L. Progress in research on paclitaxel and tumor immunotherapy. Cell Mol. Biol. Lett. 2019, 24, 40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pascucci, L.; Coccè, V.; Bonomi, A.; Ami, D.; Ceccarelli, P.; Ciusani, E.; Viganò, L.; Locatelli, A.; Sisto, F.; Doglia, S.; et al. Paclitaxel is incorporated by mesenchymal stromal cells and released in exosomes that inhibit in vitro tumor growth: A new approach for drug delivery. J. Control. Release 2014, 192, 262–270. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Wang, H.; Huang, Q.; Peng, C.; Yao, L.; Chen, H.; Qiu, Z.; Wu, Y.; Wang, L.; Chen, W. Exosomes from M1-polarized macrophages enhance paclitaxel antitumor activity by activating macrophages-mediated inflammation. Theranostics 2019, 9, 1714–1727. [Google Scholar] [CrossRef] [PubMed]
- Shishodia, S.; Sethi, G.; Aggarwal, B. Curcumin: Getting back to the roots. Ann. N. Y. Acad. Sci. 2005, 1056, 206–217. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Zhang, T. Targeting cancer stem cells by curcumin and clinical applications. Cancer Lett. 2014, 346, 197–205. [Google Scholar] [CrossRef]
- Sordillo, P.; Helson, L. Curcumin and cancer stem cells: Curcumin has asymmetrical effects on cancer and normal stem cells. Anticancer Res. 2015, 35, 599–614. [Google Scholar]
- Du, W.; Feng, Y.; Wang, X.; Piao, X.; Cui, Y.; Chen, L.; Lei, X.; Sun, X.; Liu, X.; Wang, H.; et al. Curcumin suppresses malignant glioma cells growth and induces apoptosis by inhibition of SHH/GLI1 signaling pathway in vitro and vivo. CNS Neurosci. Ther. 2013, 19, 926–936. [Google Scholar] [CrossRef]
- De, D.; Das, C.; Mandal, D.; Mandal, M.; Pawar, N.; Chandra, A.; Gupta, A. Curcumin complexed with graphene derivative for breast cancer therapy. ACS Appl. Bio. Mater. 2020, 3, 6284–6296. [Google Scholar] [CrossRef]
- Kabir, M.; Rahman, M.; Akter, R.; Behl, T.; Kaushik, D.; Mittal, V.; Pandey, P.; Akhtar, M.; Saleem, A.; Albadrani, G.; et al. Potential role of curcumin and its nanoformulations to treat various types of cancers. Biomolecules 2021, 11, 392. [Google Scholar] [CrossRef]
- Ravindran, J.; Prasad, S.; Aggarwal, B. Curcumin and cancer cells: How many ways can curry kill tumor cells selectively? AAPS J. 2009, 11, 495–510. [Google Scholar] [CrossRef] [PubMed]
- Jia, G.; Han, Y.; An, Y.; Ding, Y.; He, C.; Wang, X.; Tang, Q. NRP-1 targeted and cargo-loaded exosomes facilitate simultaneous imaging and therapy of glioma in vitro and in vivo. Biomaterials 2018, 178, 302–316. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Liu, L.; Ma, F.; Wong, C.; Guo, X.; Chacko, J.; Farhoodi, H.; Zhang, S.; Zimak, J.; Ségaliny, A.; et al. Elucidation of exosome migration across the blood–brain barrier model in vitro. Cell Mol. Bioeng. 2016, 9, 509–529. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teesalu, T.; Sugahara, K.; Kotamraju, V.; Ruoslahti, E. C-end rule peptides mediate neuropilin-1-dependent cell, vascular, and tissue penetration. Proc. Natl. Acad. Sci. USA 2009, 106, 16157–16162. [Google Scholar] [CrossRef] [Green Version]
- Wu, B.; Deng, K.; Lu, S.; Zhang, C.; Ao, Y.; Wang, H.; Mei, H.; Wang, C.; Xu, H.; Hu, B.; et al. Reduction-active Fe3O4-loaded micelles with aggregation- enhanced MRI contrast for differential diagnosis of Neroglioma. Biomaterials 2021, 268, 120531. [Google Scholar] [CrossRef]
- Sun, D.; Zhuang, X.; Xiang, X.; Liu, Y.; Zhang, S.; Liu, C.; Barness, S.; Grizzle, W.; Miller, D.; Zhang, H. A novel nanoparticle drug delivery system: The Anti-inflammatory activity of curcumin is enhanced when encapsulated in exosomes. Mol. Ther. 2010, 18, 1606–1614. [Google Scholar] [CrossRef]
- Writing Committee of the Report on Cardiovascular Health and Diseases in China. Report on cardiovascular health and diseases in China 2021: An updated summary. Biomed. Environ. Sci. 2022, 35, 573–603. [Google Scholar] [CrossRef]
- Goldfarb, M.; De Hert, M.; Detraux, J.; Di Palo, K.; Munir, H.; Music, S.; Piña, I.; Ringen, P. Severe mental illness and cardiovascular disease: JACC state-of-the-art review. J. Am. Coll. Cardiol. 2022, 80, 918–933. [Google Scholar] [CrossRef]
- Wang, H.; Liou, K.; Wang, Y.; Lu, C.; Lin, Y.; Lee, I.; Huang, S.; Tsai, Y.; Cheng, Y.; Lin, H.; et al. Deciphering the neuroprotective mechanisms of Bu-yang Huan-wu decoction by an integrative neurofunctional and genomic approach in ischemic stroke mice. J. Ethnopharmacol. 2011, 138, 22–33. [Google Scholar] [CrossRef]
- Yang, J.; Gao, F.; Zhang, Y.; Liu, Y.; Zhang, D. Buyang huanwu decoction (BYHWD) enhances angiogenic effect of mesenchymal stem cell by upregulating VEGF expression after focal cerebral ischemia. J. Mol. Neurosci. 2015, 56, 898–906. [Google Scholar] [CrossRef]
- Lu, Y.; Ji, J.; Chu, S.; Shen, F.; Yang, W.; Lei, W.; Jiang, M.; Bai, G. CaMKII, that binds with ligustilide, as a potential drug target of Suxiao jiuxin pill, a traditional Chinese medicine to dilate thoracic aorta. Clin. Transl. Med. 2022, 12, e907. [Google Scholar] [CrossRef]
- Lei, W.; Deng, Y.; Hu, X.; Ni, J.; Jiang, M.; Bai, G. Phthalides, senkyunolide A and ligustilide, show immunomodulatory effect in improving atherosclerosis, through inhibiting AP-1 and NF-κB expression. Biomed. Pharmacother. 2019, 117, 109074. [Google Scholar] [CrossRef]
- Ruan, X.; Li, Y.; Ju, C.; Shen, Y.; Lei, W.; Chen, C.; Li, Y.; Yu, H.; Liu, Y.; Kim, I.; et al. Exosomes from Suxiao Jiuxin pill-treated cardiac mesenchymal stem cells decrease H3K27 demethylase UTX expression in mouse cardiomyocytes in vitro. Acta Pharmacol. Sin. 2018, 39, 579–586. [Google Scholar] [CrossRef] [Green Version]
- Ai, S.; Yu, X.; Li, Y.; Peng, Y.; Li, C.; Yue, Y.; Tao, G.; Li, C.; Pu, W.; He, A. Divergent requirements for EZH1 in heart development versus regeneration. Circ. Res. 2017, 121, 106–112. [Google Scholar] [CrossRef]
- Juan, A.; Wang, S.; Ko, K.; Zare, H.; Tsai, P.; Feng, X.; Vivanco, K.; Ascoli, A.; Gutierrez-Cruz, G.; Krebs, J.; et al. Roles of H3K27me2 and H3K27me3 examined during fate specification of embryonic stem cells. Cell Rep. 2016, 17, 1369–1382. [Google Scholar] [CrossRef] [Green Version]
- Zhang, M.; Hu, W.; Cai, C.; Wu, Y.; Li, J.; Dong, S. Advanced application of stimuli-responsive drug delivery system for inflammatory arthritis treatment. Mater. Today Bio. 2022, 14, 100223. [Google Scholar] [CrossRef]
- Cronstein, B.; Aune, T. Methotrexate and its mechanisms of action in inflammatory arthritis. Nat. Rev. Rheumatol. 2020, 16, 145–154. [Google Scholar] [CrossRef]
- Beringer, A.; Miossec, P. Systemic effects of IL-17 in inflammatory arthritis. Nat. Rev. Rheumatol. 2019, 15, 491–501. [Google Scholar] [CrossRef]
- Gottlieb, A.; Merola, J. Axial psoriatic arthritis: An update for dermatologists. J. Am. Acad. Dermatol. 2021, 84, 92–101. [Google Scholar] [CrossRef]
- Geenen, R.; Overman, C.; Christensen, R.; Åsenlöf, P.; Capela, S.; Huisinga, K.; Husebø, M.; Köke, A.; Paskins, Z.; Pitsillidou, A.; et al. EULAR recommendations for the health professional’s approach to pain management in inflammatory arthritis and osteoarthritis. Ann. Rheum. Dis. 2018, 77, 797. [Google Scholar] [CrossRef] [Green Version]
- Bruscoli, S.; Febo, M.; Riccardi, C.; Migliorati, G. Glucocorticoid therapy in inflammatory bowel disease: Mechanisms and clinical practice. Front. Immunol. 2021, 12, 691480. [Google Scholar] [CrossRef]
- Smolen, J.; Aletaha, D. Rheumatoid arthritis therapy reappraisal: Strategies, opportunities and challenges. Nat. Rev. Rheumatol. 2015, 11, 276–289. [Google Scholar] [CrossRef]
- Vandewalle, J.; Luypaert, A.; De Bosscher, K.; Libert, C. Therapeutic mechanisms of glucocorticoids. Trends Endocrinol. Metab. 2018, 29, 42–54. [Google Scholar] [CrossRef]
- Barnes, P. Glucocorticosteroids: Current and future directions. Br. J. Pharmacol. 2011, 163, 29–43. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Liu, X.; Zhang, N.; Yin, M.; Dong, J.; Zeng, Q.; Mao, G.; Song, D.; Liu, L.; Deng, H. Berberine diminishes cancer cell PD-L1 expression and facilitates antitumor immunity via inhibiting the deubiquitination activity of CSN5. Acta Pharm. Sin. B 2020, 10, 2299–2312. [Google Scholar] [CrossRef]
- Li, C.; Ai, G.; Wang, Y.; Lu, Q.; Luo, C.; Tan, L.; Lin, G.; Liu, Y.; Li, Y.; Zeng, H.; et al. Oxyberberine, a novel gut microbiota-mediated metabolite of berberine, possesses superior anti-colitis effect: Impact on intestinal epithelial barrier, gut microbiota profile and TLR4-MyD88-NF-κB pathway. Pharmacol. Res. 2020, 152, 104603. [Google Scholar] [CrossRef]
- Ma, Q.; Bai, J.; Xu, J.; Dai, H.; Fan, Q.; Fei, Z.; Chu, J.; Yao, C.; Shi, H.; Zhou, X.; et al. Reshaping the inflammatory environment in rheumatoid arthritis joints by targeting delivery of berberine with platelet-derived extracellular vesicles. Adv. Nanobiomed. Res. 2021, 1, 2100071. [Google Scholar] [CrossRef]
- McDonald, J.; Sadowsky, C. Spinal-cord injury. Lancet 2002, 359, 417–425. [Google Scholar] [CrossRef]
- Ahuja, C.; Wilson, J.; Nori, S.; Kotter, M.; Druschel, C.; Curt, A.; Fehlings, M. Traumatic spinal cord injury. Nat. Rev. Dis. Prim. 2017, 3, 17018. [Google Scholar] [CrossRef] [Green Version]
- Ramer, L.; Ramer, M.; Bradbury, E. Restoring function after spinal cord injury: Towards clinical translation of experimental strategies. Lancet Neurol. 2014, 13, 1241–1256. [Google Scholar] [CrossRef]
- Gao, Z.; Zhang, C.; Xia, N.; Tian, H.; Li, D.; Lin, J.; Mei, X.; Wu, C. Berberine-loaded M2 macrophage-derived exosomes for spinal cord injury therapy. Acta Biomater. 2021, 126, 211–223. [Google Scholar] [CrossRef]
- Hodson, R. Inflammatory bowel disease. Nature 2016, 540, S97. [Google Scholar] [CrossRef] [Green Version]
- Liu, D.; Saikam, V.; Skrada, K.; Merlin, D.; Iyer, S. Inflammatory bowel disease biomarkers. Med. Res. Rev. 2022, 42, 1856–1887. [Google Scholar] [CrossRef]
- Seyedian, S.; Nokhostin, F.; Malamir, M. A review of the diagnosis, prevention, and treatment methods of inflammatory bowel disease. J. Med. Life 2019, 12, 113–122. [Google Scholar] [CrossRef]
- Le Berre, C.; Ananthakrishnan, A.; Danese, S.; Singh, S.; Peyrin-Biroulet, L. Ulcerative colitis and crohn’s disease have similar burden and goals for treatment. Clin. Gastroenterol. Hepatol. 2020, 18, 14–23. [Google Scholar] [CrossRef]
- Berends, S.; Strik, A.; Löwenberg, M.; D’Haens, G.; Mathôt, R. Clinical pharmacokinetic and pharmacodynamic considerations in the treatment of ulcerative colitis. Clin. Pharmacokinet. 2019, 58, 15–37. [Google Scholar] [CrossRef] [Green Version]
- Scarozza, P.; Schmitt, H.; Monteleone, G.; Neurath, M.; Atreya, R. Oligonucleotides—A novel promising therapeutic option for IBD. Front. Pharmacol. 2019, 10, 314. [Google Scholar] [CrossRef]
- Zu, M.; Xie, D.; Canup, B.; Chen, N.; Wang, Y.; Sun, R.; Zhang, Z.; Fu, Y.; Dai, F.; Xiao, B. ‘Green’ nanotherapeutics from tea leaves for orally targeted prevention and alleviation of colon diseases. Biomaterials 2021, 279, 121178. [Google Scholar] [CrossRef]




| Methods | Advantages | Disadvantages | Ref. |
|---|---|---|---|
| Differential ultracentrifugation and density gradient ultracentrifugation | Easy operation, and high purity | Dependence on expensive instruments, low automation, limited production, time consuming | [85,86,87,88,89] |
| Ultrafiltration | Simple and efficient without affecting the biological activity of exosomes | Uneconomical, ultrafiltration tubes required | [90,91] |
| Size exclusion chromatography | Good homogeneity of exosomes, less time consuming, more convenient, and more automatic | Need special equipment, not widely used | [92] |
| Immunoaffinity capture | High specificity, simple operation, and does not affect the integrity of exosome morphology | The biological activity is easily affected by pH and salt concentration | [93,94,95] |
| Membrane-based separation | High yield, and high speed | Impurity | [96,97] |
| Polyethylene glycol precipitation | Easy operation | More impurity proteins, non-uniform particle size | [98] |
| Microfluidic approach | Small consumption of samples and reagents, and a significant reduction in assay time | High cost | [99,100,101] |
| Loading Methods | Loading Drugs | Exosomes Source | Ref. |
|---|---|---|---|
| Incubation | PTX | Prostate cancer cell | [106] |
| PTX and oncolytic adenovirus | Human lung cancer cell | [107] | |
| DOX | Human CRC cell | [108] | |
| DOX | BMDCs | [109] | |
| Electroporation | DOX | Immature DCs | [66] |
| SPIONs | B16-F10 melanoma cells | [110] | |
| Dextran | HeLa cervical cancer cells | [111] | |
| Sonication | DOX | Raw 264.7 macrophages | [112] |
| PTX/DOX | Raw 264.7 macrophages | [113] | |
| Small RNAs | HEK293T and MCF-7 | [114] | |
| Extrusion | Tegafur | Attenuated Salmonella | [115] |
| MOF-protein | MDA-MB-231 tumor cells | [116] | |
| Freeze-thaw | ICG and R837 | CD47-overexpressed CT26 cells | [117] |
| Acoustofluidics | DOX | Human plasma | [118] |
| Hypotonic dialysis | DOX Nucleic acid | BM-MSCs HEK293T | [119] [120] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Q.; Wu, D.; Wang, Y.; Chen, Z. Exosomes as Novel Delivery Systems for Application in Traditional Chinese Medicine. Molecules 2022, 27, 7789. https://doi.org/10.3390/molecules27227789
Chen Q, Wu D, Wang Y, Chen Z. Exosomes as Novel Delivery Systems for Application in Traditional Chinese Medicine. Molecules. 2022; 27(22):7789. https://doi.org/10.3390/molecules27227789
Chicago/Turabian StyleChen, Qi, Di Wu, Yi Wang, and Zhong Chen. 2022. "Exosomes as Novel Delivery Systems for Application in Traditional Chinese Medicine" Molecules 27, no. 22: 7789. https://doi.org/10.3390/molecules27227789