Glycosphingolipids (GSLs) from Sphingomonas paucimobilis Increase the Efficacy of Liposome-Based Nanovaccine against Acinetobacter baumannii-Associated Pneumonia in Immunocompetent and Immunocompromised Mice
Abstract
:1. Introduction
2. Results
2.1. Analysis of the PDI, Size, and Zeta Potential of Liposomes
2.2. BMDCs Treated with GSLs-Liposomes Stimulate the Splenocytes to Secrete Higher Levels of IFN-γ
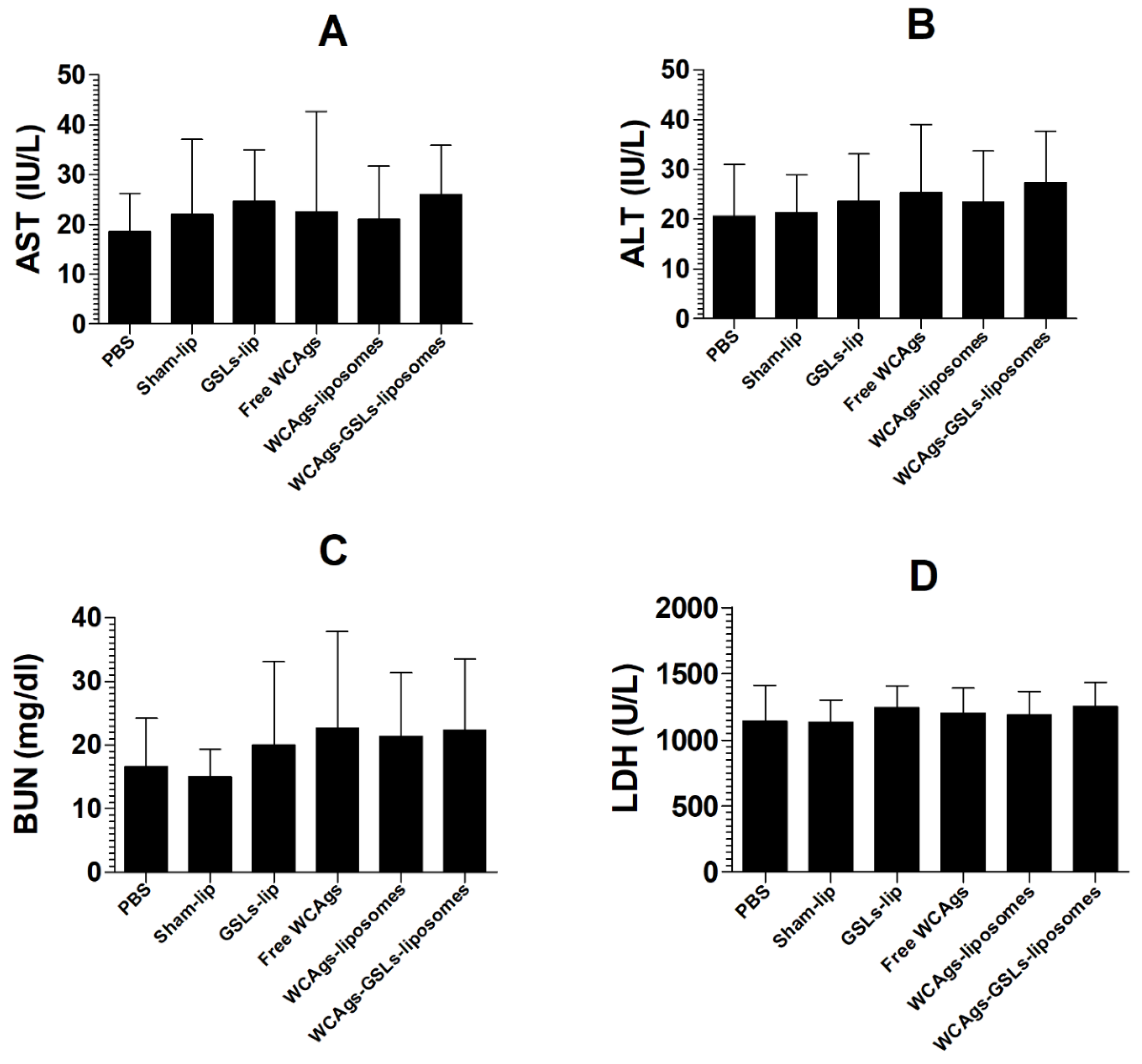
2.3. Immunization with WCAgs-GSLs-Liposomes or WCAgs-Liposomes Induced No Toxicity
2.4. WCAgs-GSLs-Liposomes Induced the Higher Secretion of IgG and Lymphocyte Proliferation
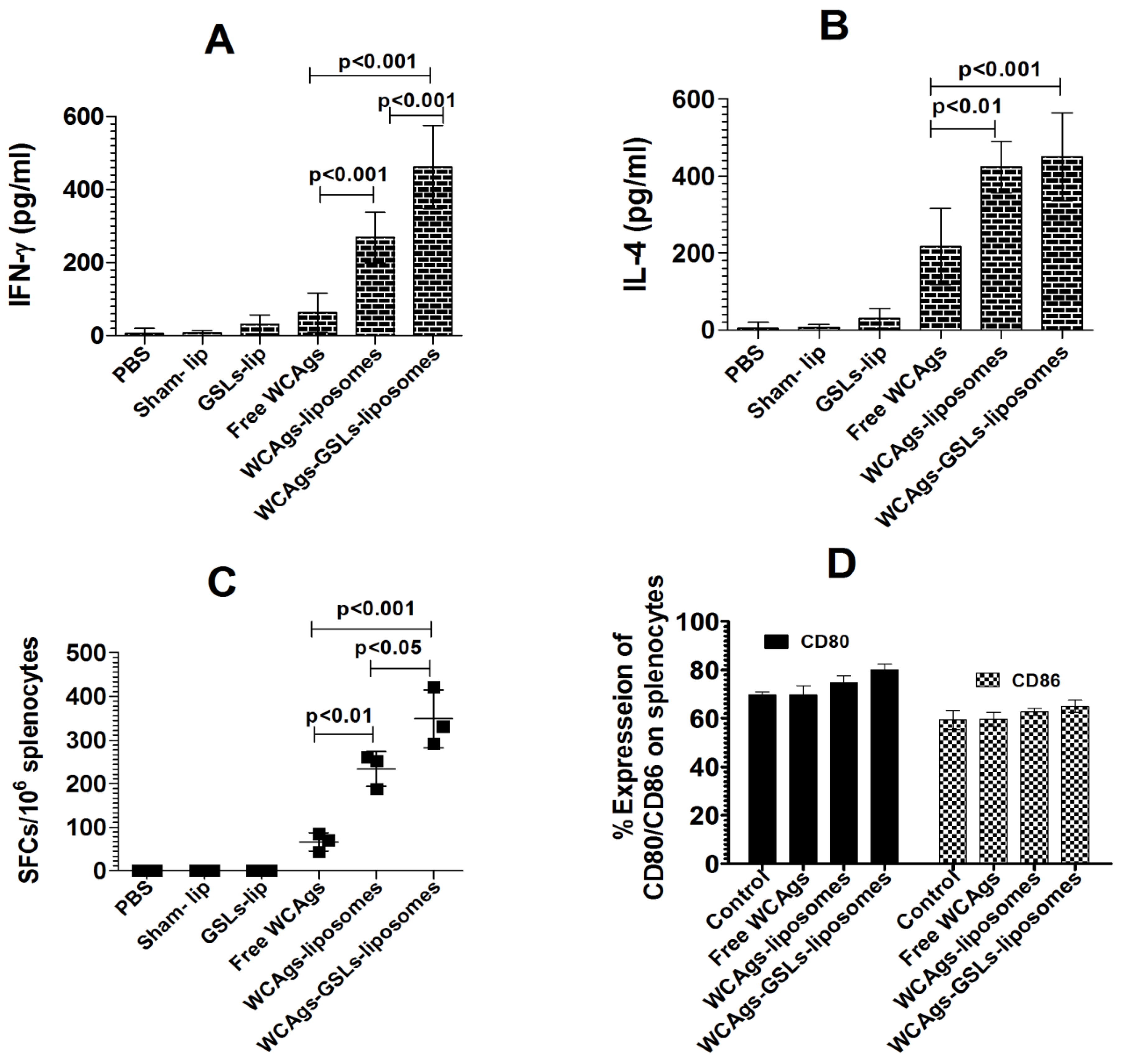
2.5. WCAgs-GSLs-Liposomes-Immunized Mice Had Stimulated Immune Responses, in Terms of Cytokines and Co-Stimulatory Molecules
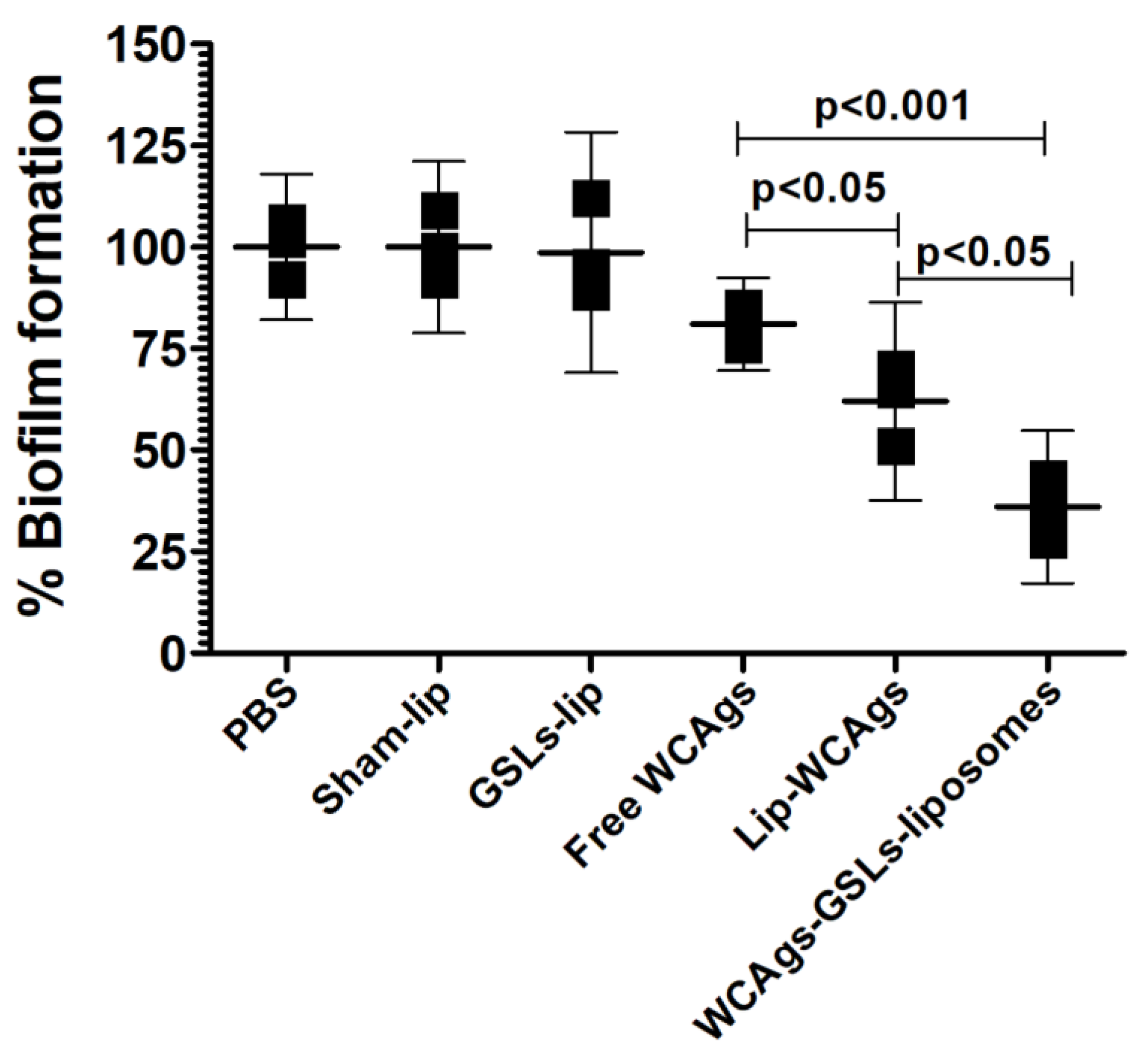
2.6. Anti-sera from WCAgs-GSLs-Liposomes-Immunized Mice Effectively Inhibited the Formation of Biofilm
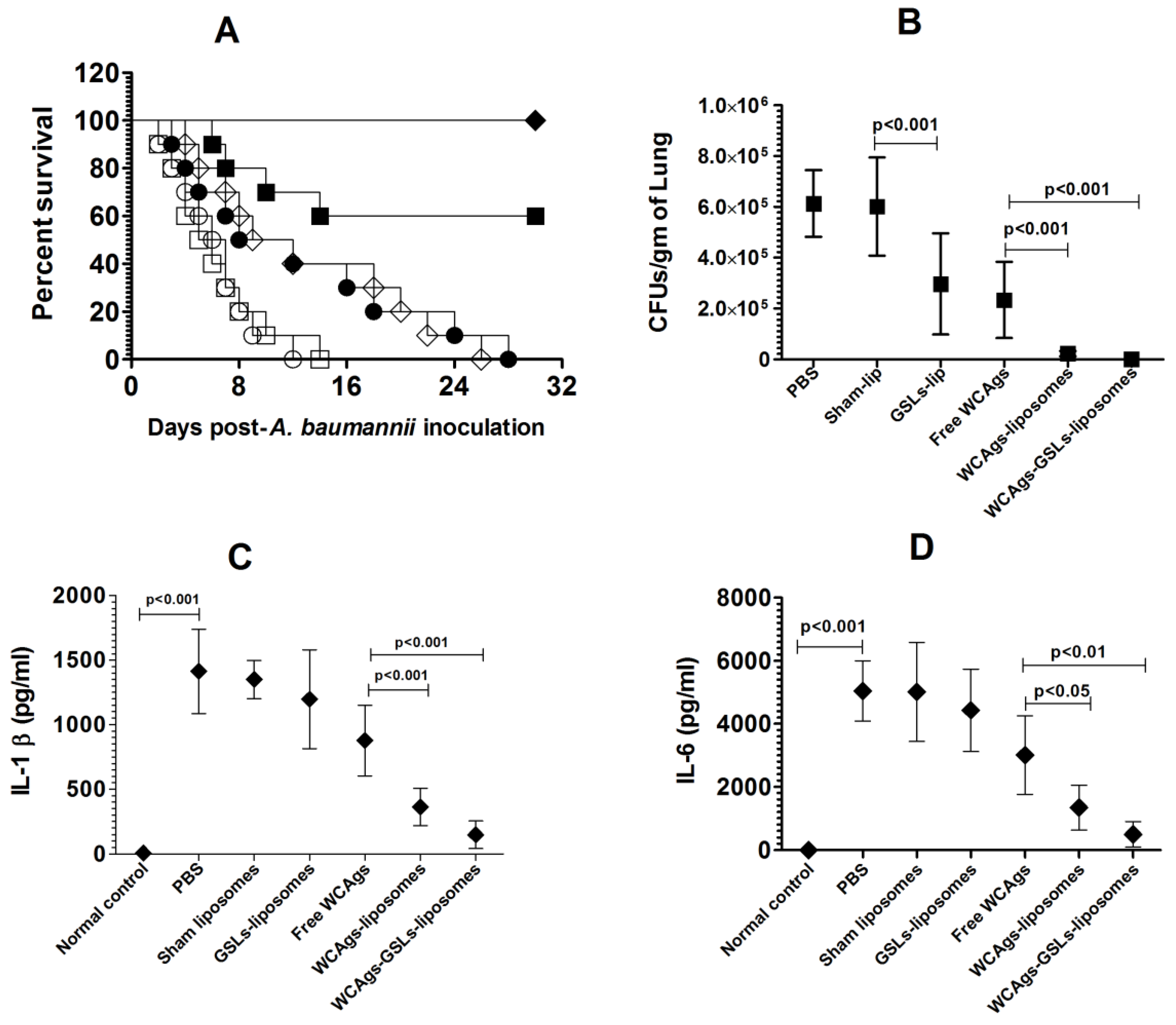
2.7. WCAgs-GSLs-Liposomes-Immunized Mice Showed Higher Resistance to A. baumannii
2.8. Immunization with WCAgs-GSLs-Liposomes Showed Greater Efficacy against A. baumannii Infection in Immunocompromised mice
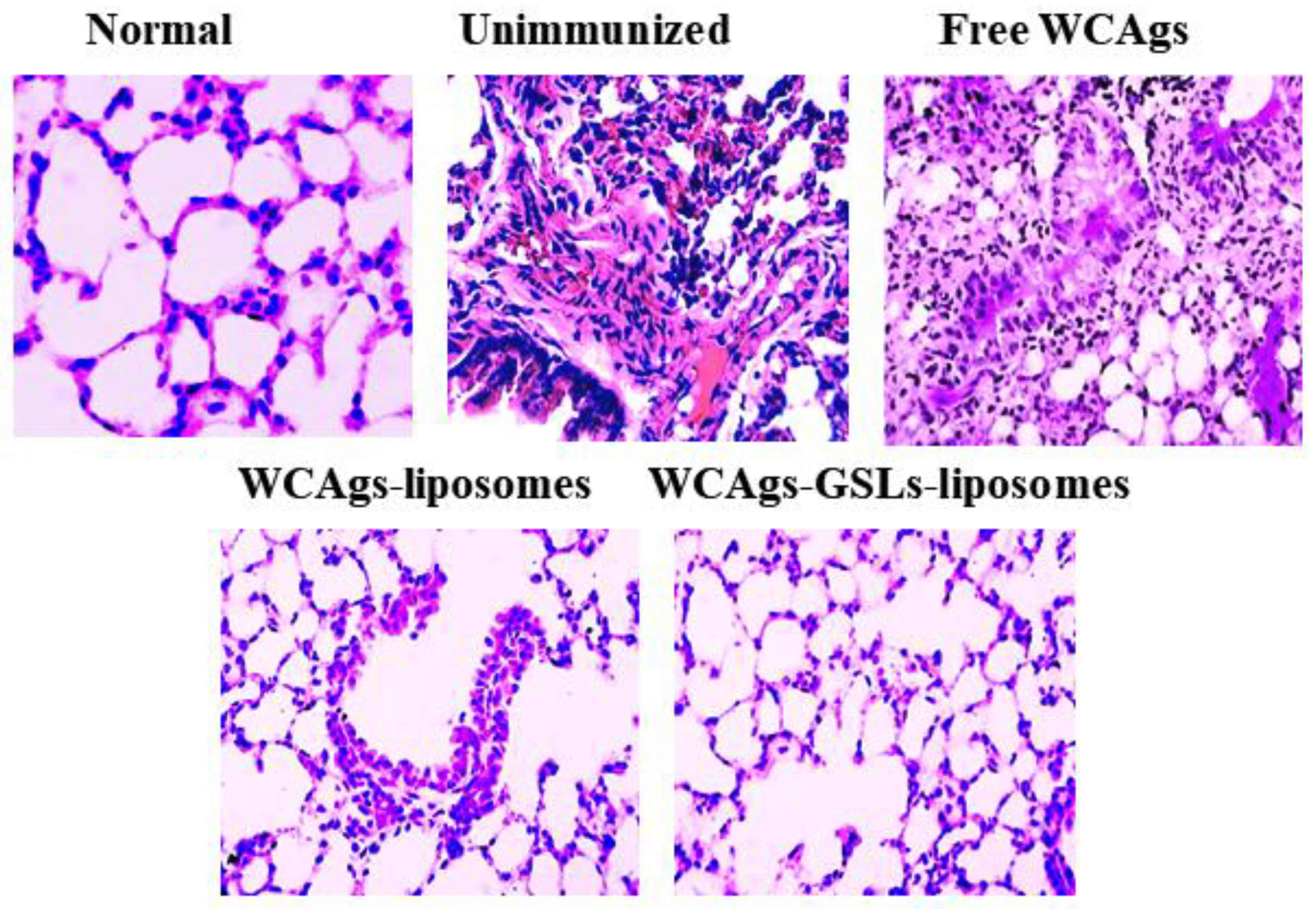
2.9. Mice Immunized with WCAgs-GSLs-Liposomes Exhibited Less Severe Complications in the Lung Tissues
3. Materials and Methods
3.1. Materials
3.2. Sphingomonas paucimobilis
3.3. Preparation of Whole Cell Antigens (WCAgs) from A. baumannii
3.4. Isolation of GSLs from S. paucimobilis
3.5. Preparation of Antigen-Loaded GSLs-Free and GSLs-Bearing Liposomes
3.6. Mice
3.7. Isolation of Bone Marrow-Derived Dendritic Cells (BMDCs) and Loading with GSLs-Liposomes
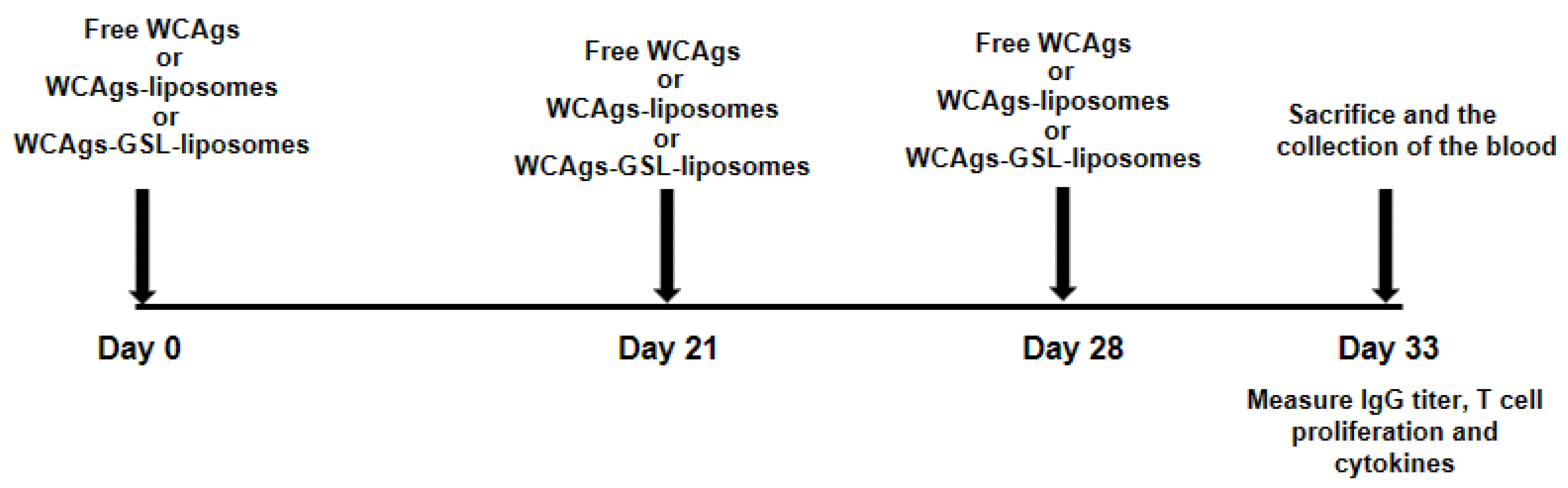
3.8. Immunization of Mice with WCAgs-Liposomes and WCAgs-GSLs-Liposomes
- PBS;
- Sham liposomes;
- GSLs-liposomes;
- Free WCAgs;
- WCAgs-liposomes;
- WCAgs-GSLs-liposomes.
3.9. Analysis of Total IgG and Lymphocyte Proliferation
3.10. ELISPOT Assay
3.11. Analysis of IFN-γ and IL-4
3.12. Determination of the Expression of Co-Stimulatory Molecules and antigen Presenting Molecules in Splenocytes
3.13. To Determine the Effect of Anti-Sera on the Formation of Biofilm by A. baumannii
3.14. Infection of Mice with A. baumannii
3.15. To Determine the Prophylactic Efficacy of Vaccine Formulations against A. baumannii
3.16. Immunization of Immune-Compromised Mice
3.17. Infection of Immunocompromised Mice with A. baumannii
3.18. Effectiveness of Vaccine Formulations in Protection against A. baumannii in Immunocompromised Mice
3.19. Histological Analysis of Lung Tissues
3.20. Statistical Analyses
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Ibrahim, S.; Al-Saryi, N.; Al-Kadmy, I.M.S.; Aziz, S.N. Multidrug-resistant Acinetobacter baumannii as an emerging concern in hospitals. Mol. Biol. Rep. 2021, 48, 6987–6998. [Google Scholar] [CrossRef] [PubMed]
- Peleg, A.Y.; Seifert, H.; Paterson, D.L. Acinetobacter baumannii: Emergence of a successful pathogen. Clin. Microbiol. Rev. 2008, 21, 538–582. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ibrahim, M.E. Prevalence of Acinetobacter baumannii in Saudi Arabia: Risk factors, antimicrobial resistance patterns and mechanisms of carbapenem resistance. Ann. Clin. Microbiol. Antimicrob. 2019, 18, 1. [Google Scholar] [CrossRef]
- Al-Hamad, A.; Pal, T.; Leskafi, H.; Abbas, H.; Hejles, H.; Alsubikhy, F.; Darwish, D.; Ghazawi, A.; Sonnevend, A. Molecular characterization of clinical and environmental carbapenem resistant Acinetobacter baumannii isolates in a hospital of the Eastern Region of Saudi Arabia. J. Infect. Public Health 2020, 13, 632–636. [Google Scholar] [CrossRef]
- Aly, M.; Tayeb, H.T.; Al Johani, S.M.; Alyamani, E.J.; Aldughaishem, F.; Alabdulkarim, I.; Balkhy, H.H. Genetic diversity of OXA-51-like genes among multidrug-resistant Acinetobacter baumannii in Riyadh, Saudi Arabia. Eur. J. Clin. Microbiol. Infect. Dis. 2014, 33, 1223–1228. [Google Scholar] [CrossRef] [PubMed]
- Saeed, N.K.; Kambal, A.M.; El-Khizzi, N.A. Antimicrobial-resistant bacteria in a general intensive care unit in Saudi Arabia. Saudi Med. J. 2010, 31, 1341–1349. [Google Scholar]
- Gedefie, A.; Demsis, W.; Ashagrie, M.; Kassa, Y.; Tesfaye, M.; Tilahun, M.; Bisetegn, H.; Sahle, Z. Acinetobacter baumannii Biofilm Formation and Its Role in Disease Pathogenesis: A Review. Infect. Drug. Resist. 2021, 14, 3711–3719. [Google Scholar] [CrossRef] [PubMed]
- Dijkshoorn, L.; Nemec, A.; Seifert, H. An increasing threat in hospitals: Multidrug-resistant Acinetobacter baumannii. Nat. Rev. Microbiol. 2007, 5, 939–951. [Google Scholar] [CrossRef]
- Gellings, P.S.; Wilkins, A.A.; Morici, L.A. Recent Advances in the Pursuit of an Effective Acinetobacter baumannii Vaccine. Pathogens 2020, 9, 1066. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yang, T.; Cao, J.; Sun, J.; Dai, W.; Zhang, L. Mucosal immunization with purified OmpA elicited protective immunity against infections caused by multidrug-resistant Acinetobacter baumannii. Microb. Pathog. 2016, 96, 20–25. [Google Scholar] [CrossRef]
- Huang, W.; Yao, Y.; Wang, S.; Xia, Y.; Yang, X.; Long, Q.; Sun, W.; Liu, C.; Li, Y.; Chu, X.; et al. Immunization with a 22-kDa outer membrane protein elicits protective immunity to multidrug-resistant Acinetobacter baumannii. Sci. Rep. 2016, 6, 207240. [Google Scholar] [CrossRef] [Green Version]
- Du, X.; Xue, J.; Jiang, M.; Lin, S.; Huang, Y.; Deng, K.; Shu, L.; Xu, H.; Li, Z.; Yao, J.; et al. A Multiepitope Peptide, rOmp22, Encapsulated in Chitosan-PLGA Nanoparticles as a Candidate Vaccine Against Acinetobacter baumannii Infection. Int. J. Nanomed. 2021, 16, 1819–1836. [Google Scholar] [CrossRef] [PubMed]
- Lei, L.; Yang, F.; Zou, J.; Jing, H.; Zhang, J.; Xu, W.; Zou, Q.; Zhang, J.; Wang, X. DNA vaccine encoding OmpA and Pal from Acinetobacter baumannii efficiently protects mice against pulmonary infection. Mol. Biol. Rep. 2019, 46, 5397–5408. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Pan, C.; Liu, Z.; Sun, P.; Hua, X.; Feng, E.; Yu, Y.; Wu, J.; Zhu, L.; Wang, H. Safety and immunogenicity of a new glycoengineered vaccine against Acinetobacter baumannii in mice. Microb. Biotechnol. 2022, 15, 703–716. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.; Khan, A. Role of NKT Cells during Viral Infection and the Development of NKT Cell-Based Nanovaccines. Vaccines 2021, 9, 949. [Google Scholar] [CrossRef]
- Nisini, R.; Poerio, N.; Mariotti, S.; De Santis, F.; Fraziano, M. The multirole of liposomes in therapy and prevention of infectious diseases. Front. Immunol. 2018, 9, 155. [Google Scholar] [CrossRef] [Green Version]
- Ramakrishnan, A.; Schumack, N.M.; Gariepy, C.L.; Eggleston, H.; Nunez, G.; Espinoza, N.; Nieto, M.; Castillo, R.; Rojas, J.; McCoy, A.J.; et al. Enhanced Immunogenicity and Protective Efficacy of a Campylobacter jejuni Conjugate Vaccine Coadministered with Liposomes Containing Monophosphoryl Lipid A and QS-21. Msphere 2019, 4, e00101-19. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, N.; Masood, A.K.; Owais, M. Fusogenic potential of prokaryotic membrane lipids. Implication in vaccine development. Eur. J. Biochem. 2001, 268, 5667–5675. [Google Scholar] [CrossRef]
- Kawahara, K.; Seydel, U.; Matsuura, M.; Danbara, H.; Rietschel, E.T.; Zahringer, U. Chemical structure of glycosphingolipids isolated from Sphingomonas paucimobilis. FEBS Lett. 1991, 292, 107–110. [Google Scholar]
- Busse, H.J.; Kämpfer, P.; Denner, E.B. Chemotaxonomic characterisation of Sphingomonas. J. Ind. Microbiol. Biotechnol. 1999, 23, 242–251. [Google Scholar] [CrossRef]
- Inoue, J.; Ideue, R.; Takahashi, D.; Kubota, M.; Kumazawa, Y. Liposomal glycosphingolipids activate natural killer T cell-mediated immune responses through the endosomal pathway. J. Control. Release 2009, 133, 18–230. [Google Scholar] [CrossRef] [PubMed]
- Krziwon, C.; Zähringer, U.; Kawahara, K.; Weidemann, B.; Kusumoto, S.; Rietschel, E.T.; Flad, H.D.; Ulmer, A.J. Glycosphingolipids from Sphingomonas paucimobilis induce monokine production in human mononuclear cells. Infect. Immun. 1995, 63, 2899–2905. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.; Aljarbou, A.N.; Aldebasi, Y.H.; Alorainy, M.S.; Rahmani, A.H.; Younus, H.; Khan, A. Liposomal formulation of glycosphingolipids from Sphingomonas paucimobilis induces antitumour immunity in mice. J. Drug Target. 2018, 26, 709–7190. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.; Malik, A.; Alruwetei, A.M.; Alzohairy, M.A.; Alhatlani, B.Y.; Al Rugaie, O.; Alhumaydhi, F.A.; Khan, A. Delivery of MERS antigen encapsulated in α-GalCer-bearing liposomes elicits stronger antigen-specific immune responses. J. Drug Target 2022, 22, 1–100. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.; Allemailem, K.S.; Maswadeh, H.; Younus, H. Safety and Prophylactic Efficacy of Liposome-Based Vaccine against the Drug-Resistant Acinetobacter baumannii in Mice. Pharmaceutics 2022, 14, 1357. [Google Scholar] [CrossRef] [PubMed]
- Allemailem, K.S.; Almatroudi, A.; Alrumaihi, F.; Aljaghwani, A.; Alnuqaydan, A.M.; Khalilullah, H.; Younus, H.; El-Kady, A.M.; Aldakheel, F.M.; Khan, A.A.; et al. Antimicrobial, Immunomodulatory and Anti-Inflammatory Potential of Liposomal Thymoquinone: Implications in the Treatment of Bacterial Pneumonia in Immunocompromised Mice. Biomedicines 2021, 9, 1673. [Google Scholar] [CrossRef]
- Alrumaihi, F.; Almatroudi, A.; Allemailem, K.S.; Rahmani, A.H.; Khan, A.; Khan, M.A. Therapeutic Effect of Bilsaan, Sambucus nigra Stem Exudate, on the OVA-Induced Allergic Asthma in Mice. Oxid. Med. Cell Longev. 2020, 2020, 3620192. [Google Scholar] [CrossRef]
- Murray, C.J.; Ikuta, K.S.; Sharara, F.; Swetschinski, L.; Aguilar, G.R.; Gray, A.; Han, C.; Bisignano, C.; Rao, P.; Wool, E.; et al. Antimicrobial Resistance Collaborators. Global Burden of Bacterial Antimicrobial Resistance in 2019: A Systematic Analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef]
- McConnell, M.J.; Domínguez-Herrera, J.; Smani, Y.; López-Rojas, R.; Docobo-Pérez, F.; Pachón, J. Vaccination with outer membrane complexes elicits rapid protective immunity to multidrug-resistant Acinetobacter baumannii. Infect. Immun. 2011, 79, 518–526. [Google Scholar] [CrossRef] [Green Version]
- McConnell, M.J.; Pachón, J. Active and passive immunization against Acinetobacter baumannii using an inactivated whole cell vaccine. Vaccine 2010, 29, 1–5. [Google Scholar] [CrossRef]
- Bentancor, L.V.; O’Malley, J.M.; Bozkurt-Guzel, C.; Pier, G.B.; Maira-Litrán, T. Poly-N-acetyl-β-(1-6)-glucosamine is a target for protective immunity against Acinetobacter baumannii infections. Infect. Immun. 2012, 80, 651–656. [Google Scholar] [CrossRef] [Green Version]
- McConnell, M.J.; Rumbo, C.; Bou, G.; Pachón, J. Outer membrane vesicles as an acellular vaccine against Acinetobacter baumannii. Vaccine 2011, 29, 5705–5710. [Google Scholar] [CrossRef]
- Bentancor, L.V.; Routray, A.; Bozkurt-Guzel, C.; Camacho-Peiro, A.; Pier, G.B.; Maira-Litrán, T. Evaluation of the trimeric autotransporter Ata as a vaccine candidate against Acinetobacter baumannii infections. Infect. Immun. 2012, 80, 3381–3388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, G.; Lin, L.; Ibrahim, A.S.; Baquir, B.; Pantapalangkoor, P.; Bonomo, R.A.; Doi, Y.; Adams, M.D.; Russo, T.A.; Spellberg, B. Active and passive immunization protects against lethal, extreme drug resistant-Acinetobacter baumannii infection. PLoS ONE 2012, 7, e29446. [Google Scholar] [CrossRef] [Green Version]
- Russo, T.A.; Beanan, J.M.; Olson, R.; MacDonald, U.; Cox, A.D.; St Michael, F.; Vinogradov, E.V.; Spellberg, B.; Luke-Marshall, N.R.; Campagnari, A.A. The K1 capsular polysaccharide from Acinetobacter baumannii is a potential therapeutic target via passive immunization. Infect. Immun. 2013, 81, 915–922. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, L.; Tan, B.; Pantapalangkoor, P.; Ho, T.; Hujer, A.M.; Taracila, M.A.; Bonomo, R.A.; Spellberg, B. Acinetobacter baumannii rOmpA vaccine dose alters immune polarization and immunodominant epitopes. Vaccine 2013, 31, 313–318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Syed, F.M.; Khan, M.A.; Nasti, T.H.; Ahmad, N.; Mohammad, O. Antigen entrapped in the escheriosomes leads to the generation of CD4(+) helper and CD8(+) cytotoxic T cell response. Vaccine 2003, 21, 2383–2393. [Google Scholar] [CrossRef]
- Schwendener, R.A. Liposomes as vaccine delivery systems: A review of the recent advances. Adv. Vaccines 2014, 2, 159–182. [Google Scholar] [CrossRef]
- Wang, N.; Chen, M.; Wang, T. Liposomes used as a vaccine adjuvant-delivery system: From basics to clinical immunization. J. Control. Release 2019, 303, 130–150. [Google Scholar] [CrossRef]
- Goel, V.K.; Kapil, A. Monoclonal antibodies against the iron regulated outer membrane Proteins of Acinetobacter baumannii are bactericidal. BMC Microbiol. 2001, 1, 16. [Google Scholar] [CrossRef]
- Singh, R.; Capalash, N.; Sharma, P. Immunoprotective potential of BamA, the outer membrane protein assembly factor, against MDR Acinetobacter baumannii. Sci. Rep. 2017, 7, 12411. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, L.; Flies, D.B. Molecular mechanisms of T cell co-stimulation and co-inhibition. Nat. Rev. Immunol. 2013, 13, 227–242. [Google Scholar] [CrossRef] [PubMed]
- Cohn, L.; Delamarre, L. Dendritic cell-targeted vaccines. Front. Immunol. 2014, 5, 255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harding, C.M.; Hennon, S.W.; Feldman, M.F. Uncovering the mechanisms of Acinetobacter baumannii virulence. Nat. Rev. Microbiol. 2018, 16, 91–102. [Google Scholar] [CrossRef]
- Upmanyu, K.; Haq, Q.M.R.; Singh, R. Factors mediating Acinetobacter baumannii biofilm formation: Opportunities for developing therapeutics. Curr. Res. Microb. Sci. 2022, 3, 100131. [Google Scholar] [CrossRef]
- Alav, I.; Sutton, J.M.; Rahman, K.M. Role of bacterial efflux pumps in biofilm formation. J. Antimicrob. Chemother. 2018, 73, 2003–2020. [Google Scholar] [CrossRef] [Green Version]
- Pakharukova, N.; Tuittila, M.; Paavilainen, S.; Malmi, H.; Parilova, O.; Teneberg, S.; Knight, S.D.; Zavialov, A.V. Structural basis for Acinetobacter baumannii biofilm formation. Proc. Natl. Acad. Sci. USA 2018, 115, 5558–5563. [Google Scholar] [CrossRef] [Green Version]
- Huang, W.; Zhang, Q.; Li, W.; Chen, Y.; Shu, C.; Li, Q.; Zhou, J.; Ye, C.; Bai, H.; Sun, W.; et al. Anti-outer Membrane Vesicle Antibodies Increase Antibiotic Sensitivity of Pan-Drug-Resistant Acinetobacter baumannii. Front. Microbiol. 2019, 10, 1379. [Google Scholar] [CrossRef] [Green Version]
- Nielsen, T.B.; Yan, J.; Slarve, M.; Lu, P.; Li, R.; Ruiz, J.; Lee, B.; Burk, E.; Talyansky, Y.; Oelschlaeger, P.; et al. Monoclonal Antibody Therapy against Acinetobacter baumannii. Infect. Immun. 2021, 89, e0016221. [Google Scholar] [CrossRef]
- Kang, M.J.; Jo, S.G.; Kim, D.J.; Park, J.H. NLRP3 inflammasome mediates interleukin-1β production in immune cells in response to Acinetobacter baumannii and contributes to pulmonary inflammation in mice. Immunology 2017, 150, 495–505. [Google Scholar] [CrossRef]
- Agustí, A.; Edwards, L.D.; Rennard, S.I.; MacNee, W.; Tal-Singer, R.; Miller, B.E.; Vestbo, J.; Lomas, D.A.; Calverley, P.M.; Wouters, E.; et al. Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints (ECLIPSE) Investigators. Persistent systemic inflammation is associated with poor clinical outcomes in COPD: A novel phenotype. PLoS ONE 2012, 7, e37483. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harris, G.; KuoLee, R.; Xu, H.H.; Chen, W. Mouse Models of Acinetobacter baumannii Infection. Curr. Protoc. Microbiol. 2017, 46, 6G.3.1–6G.3.23. [Google Scholar] [CrossRef] [PubMed]





| Liposomal Formulations | PDI | Size (nm) | Zeta Potential |
|---|---|---|---|
| Lip-WCAgs | 0.286 | 110 | −12.4 |
| GSLs-lip-WCAgs | 0.312 | 128 | −16.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, M.A.; Allemailem, K.S.; Maswadeh, H.; Younus, H. Glycosphingolipids (GSLs) from Sphingomonas paucimobilis Increase the Efficacy of Liposome-Based Nanovaccine against Acinetobacter baumannii-Associated Pneumonia in Immunocompetent and Immunocompromised Mice. Molecules 2022, 27, 7790. https://doi.org/10.3390/molecules27227790
Khan MA, Allemailem KS, Maswadeh H, Younus H. Glycosphingolipids (GSLs) from Sphingomonas paucimobilis Increase the Efficacy of Liposome-Based Nanovaccine against Acinetobacter baumannii-Associated Pneumonia in Immunocompetent and Immunocompromised Mice. Molecules. 2022; 27(22):7790. https://doi.org/10.3390/molecules27227790
Chicago/Turabian StyleKhan, Masood Alam, Khaled S. Allemailem, Hamzah Maswadeh, and Hina Younus. 2022. "Glycosphingolipids (GSLs) from Sphingomonas paucimobilis Increase the Efficacy of Liposome-Based Nanovaccine against Acinetobacter baumannii-Associated Pneumonia in Immunocompetent and Immunocompromised Mice" Molecules 27, no. 22: 7790. https://doi.org/10.3390/molecules27227790
APA StyleKhan, M. A., Allemailem, K. S., Maswadeh, H., & Younus, H. (2022). Glycosphingolipids (GSLs) from Sphingomonas paucimobilis Increase the Efficacy of Liposome-Based Nanovaccine against Acinetobacter baumannii-Associated Pneumonia in Immunocompetent and Immunocompromised Mice. Molecules, 27(22), 7790. https://doi.org/10.3390/molecules27227790









