Hydrogels from the Assembly of SAA/Elastin-Inspired Peptides Reveal Non-Canonical Nanotopologies
Abstract
:1. Introduction
2. Results and Discussion
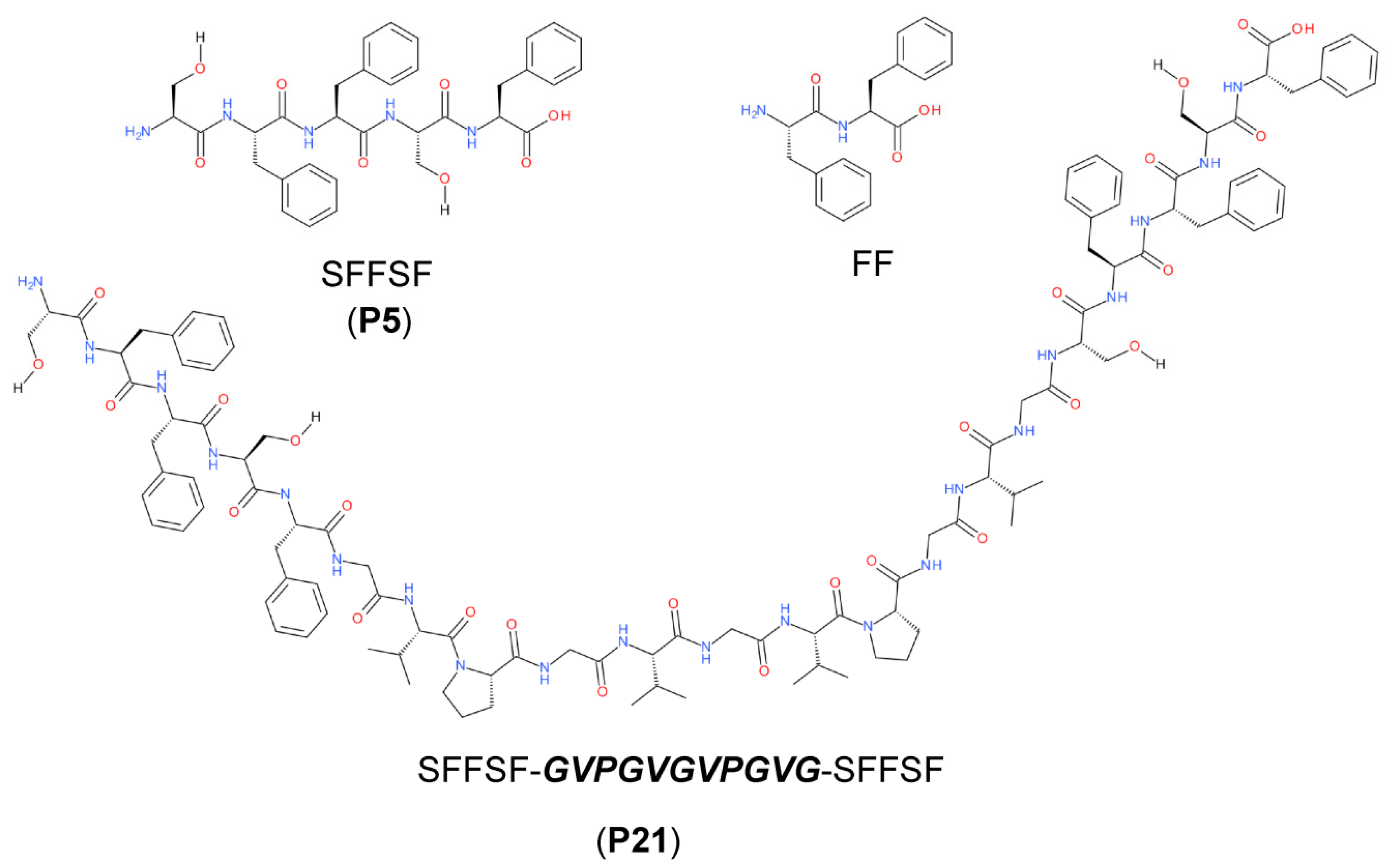
2.1. P5 Peptide Assembly
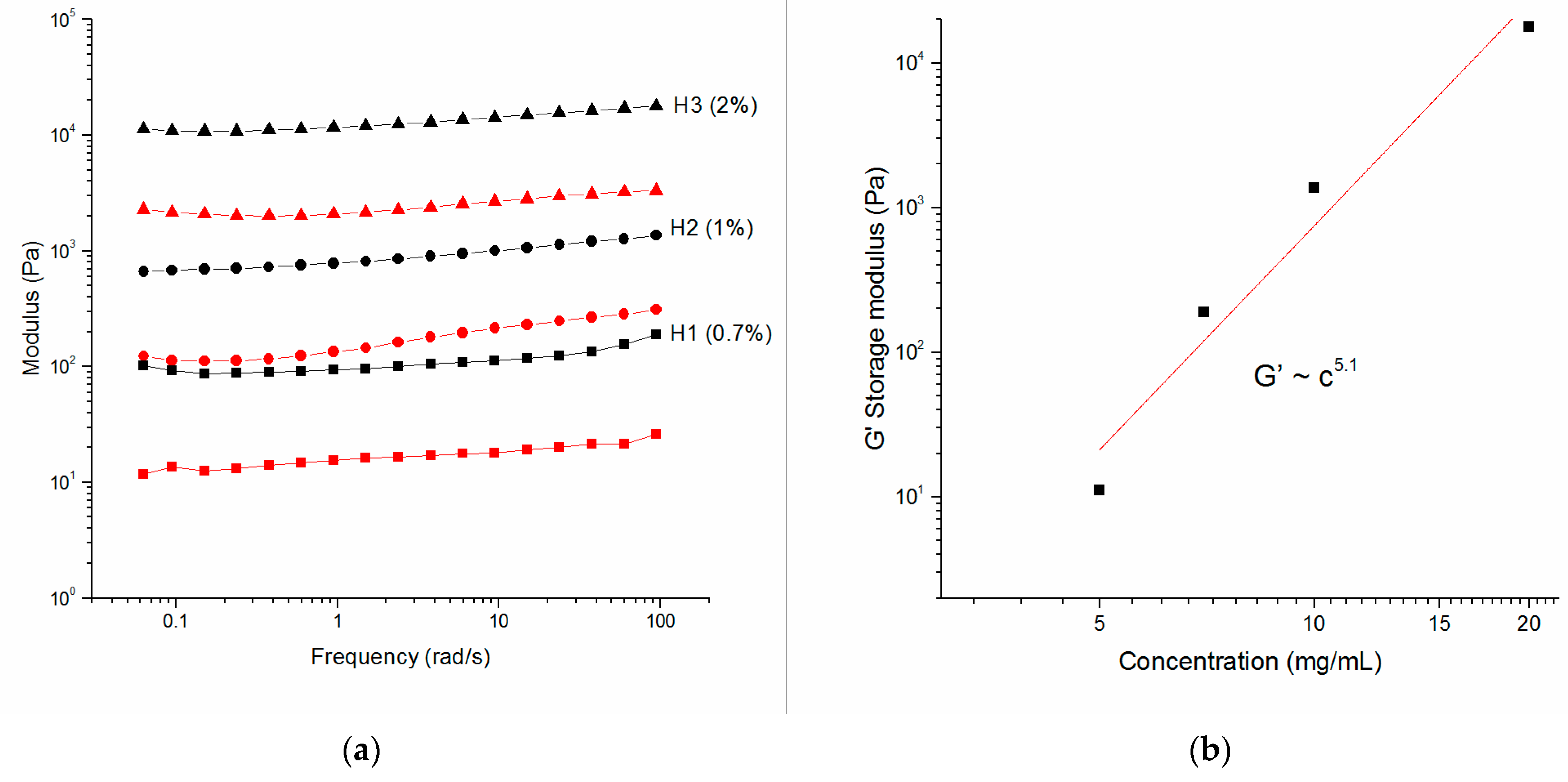
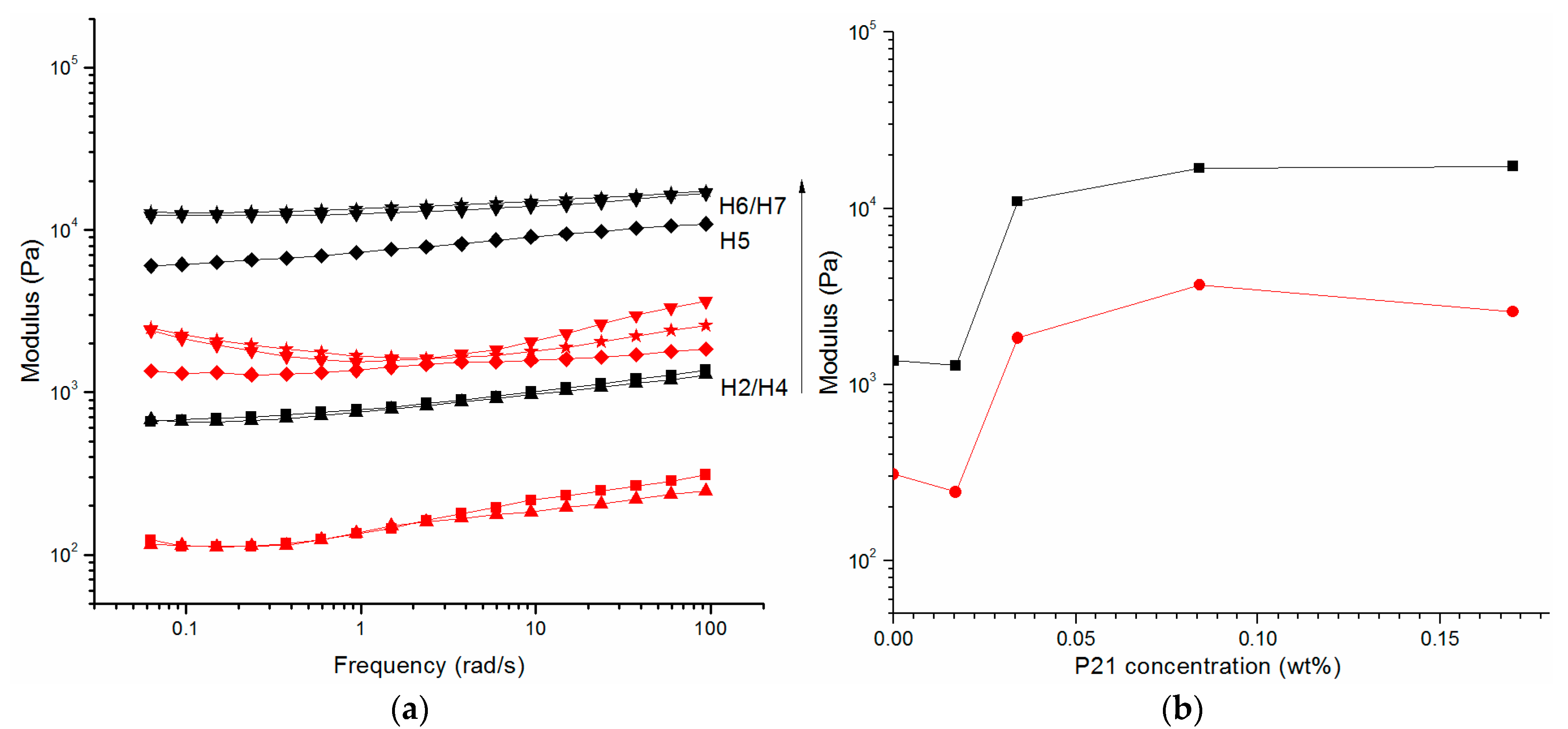
2.2. Oscillatory Rheology
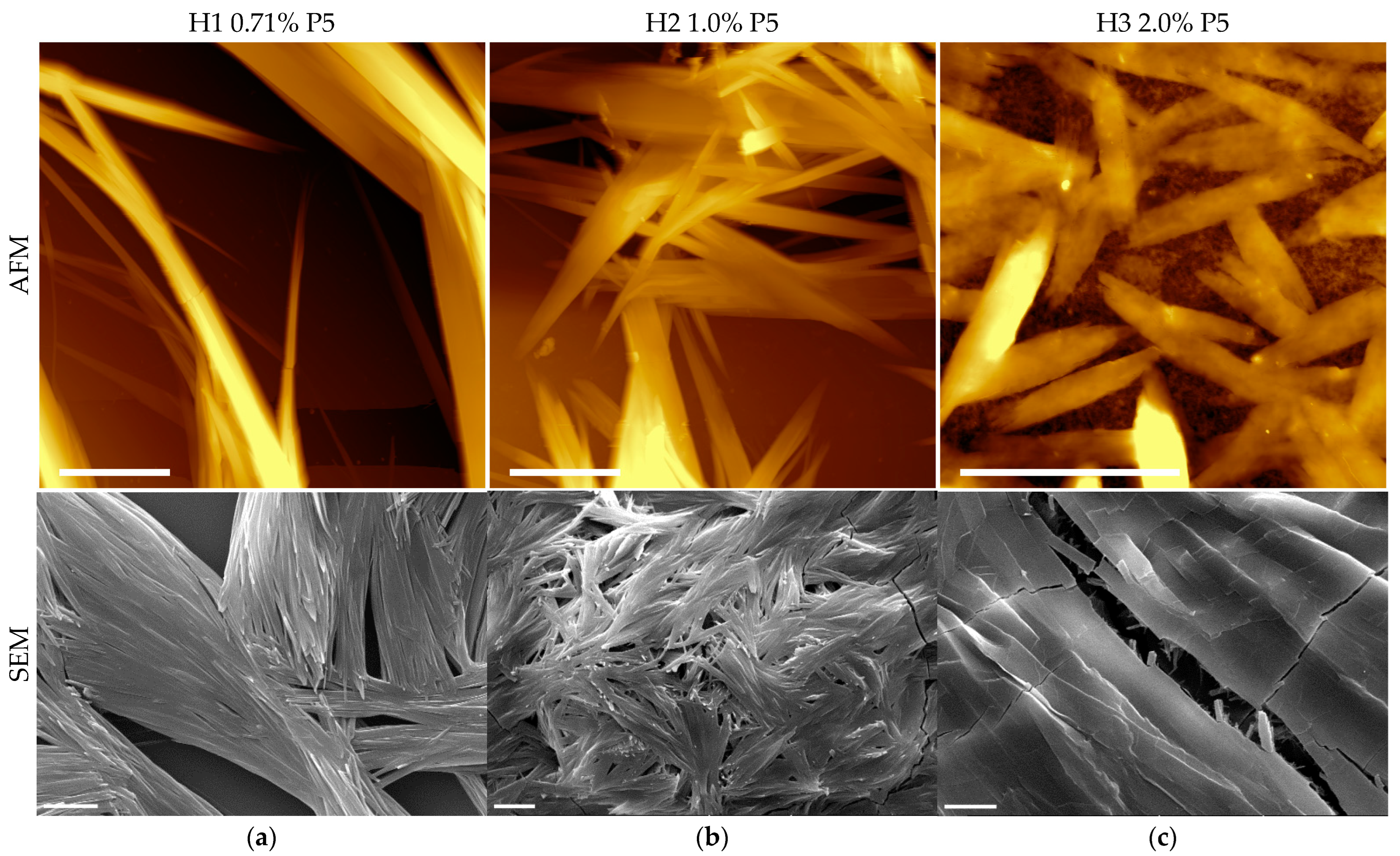
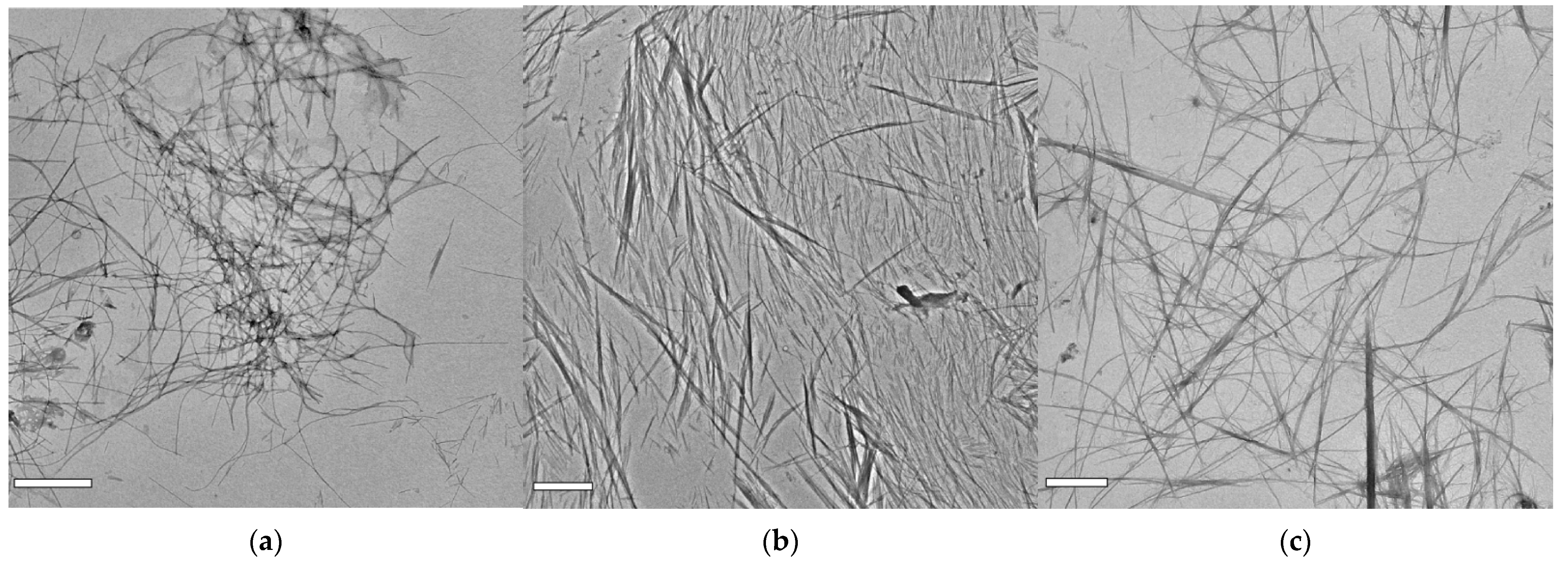
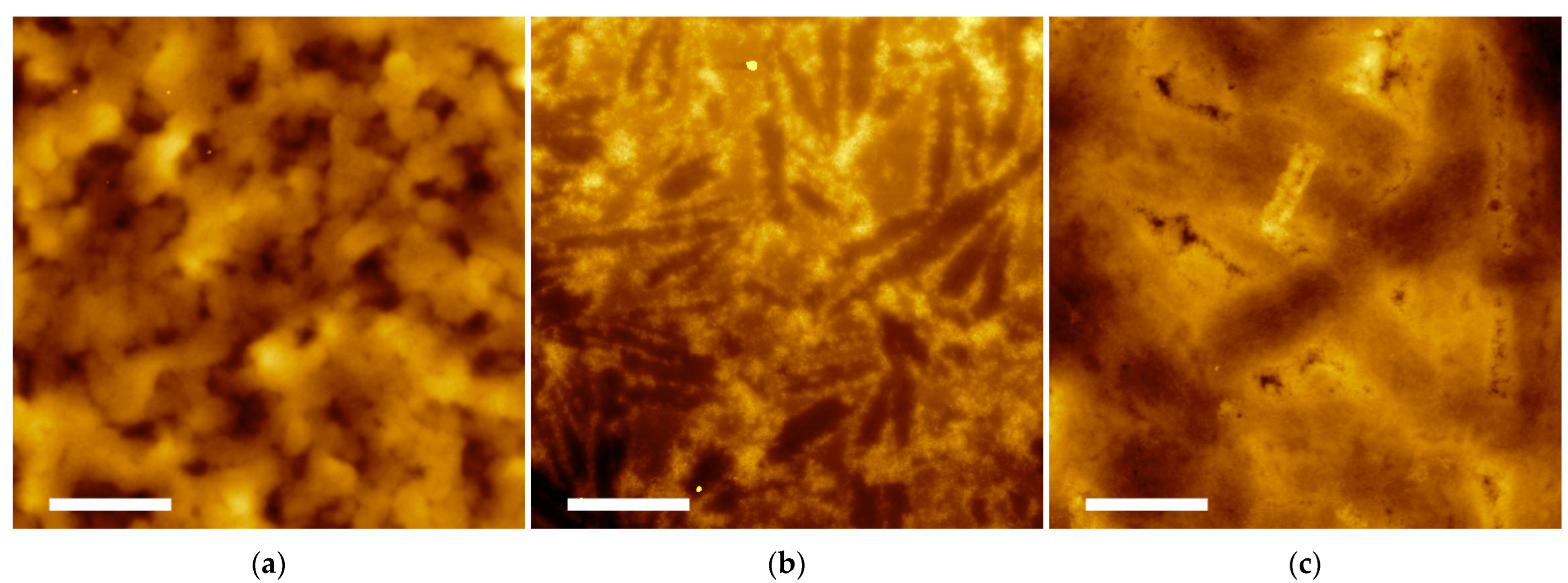
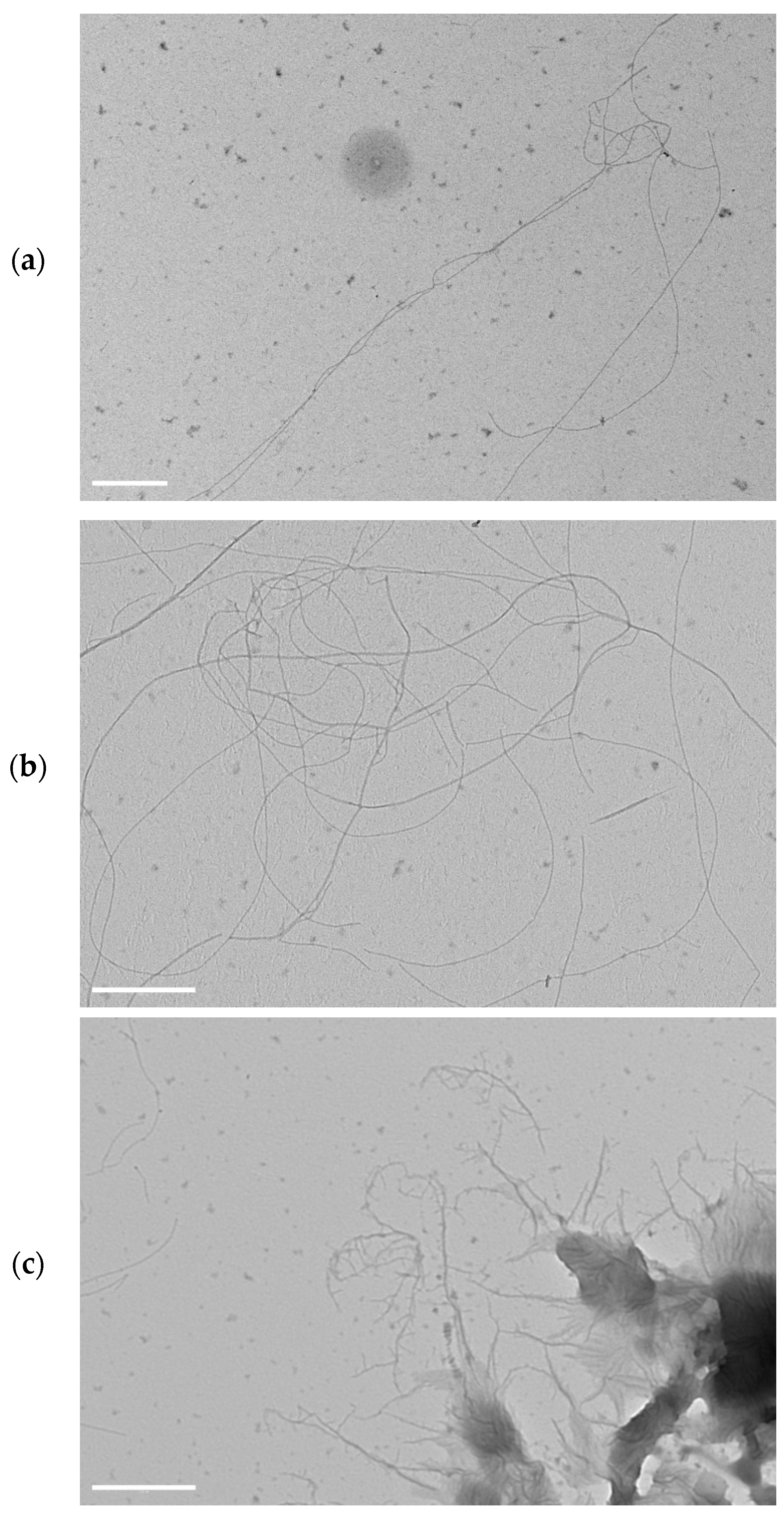
2.3. Supramolecular Nanostructures of Hydrogel as a Function of Concentration
2.4. Assembly of P5 and P21 Peptides
2.5. Alternative Nanotopologies of Peptide Assembly in 10% Acetic Acid (AcOH)
2.6. Assembly of SA5 and FF Peptides in Different Ratios
3. Materials and Methods
3.1. Peptide Synthesis
3.2. CD Spectroscopy
3.3. Hydrogel Preparation
3.4. Oscillatory Rheology
3.5. Atomic Force Microscopy (AFM)
3.6. Transmission Electron Microscopy (TEM)
3.7. Scanning Electron Microscopy (SEM)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Bashir, A.; Awan, T.I.; Tehseen, A.; Tahir, M.B.; Ijaz, M. Chapter 3—Interfaces and Surfaces. In Chemistry of Nanomaterials; Awan, T.I., Bashir, A., Tehseen, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 51–87. [Google Scholar] [CrossRef]
- Philp, D.; Stoddart, J.F. Self-Assembly in Natural and Unnatural Systems. Angew. Chem. Int. Ed. Engl. 1996, 35, 1154–1196. [Google Scholar] [CrossRef]
- Whitesides, G.M.; Grzybowski, B. Self-assembly at all scales. Science 2002, 295, 2418–2421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elsawy, M.A.; Smith, A.M.; Hodson, N.; Squires, A.; Miller, A.F.; Saiani, A. Modification of beta-Sheet Forming Peptide Hydrophobic Face: Effect on Self-Assembly and Gelation. Langmuir ACS J. Surf. Colloids 2016, 32, 4917–4923. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamley, I.W. Self-assembly of amphiphilic peptides. Soft Matter 2011, 7, 4122–4138. [Google Scholar] [CrossRef] [Green Version]
- Dong, H.; Paramonov, S.E.; Hartgerink, J.D. Self-assembly of alpha-helical coiled coil nanofibers. J. Am. Chem. Soc. 2008, 130, 13691–13695. [Google Scholar] [CrossRef] [PubMed]
- Banwell, E.F.; Abelardo, E.S.; Adams, D.J.; Birchall, M.A.; Corrigan, A.; Donald, A.M.; Kirkland, M.; Serpell, L.C.; Butler, M.F.; Woolfson, D.N. Rational design and application of responsive alpha-helical peptide hydrogels. Nat. Mater. 2009, 8, 596–600. [Google Scholar] [CrossRef] [Green Version]
- Bowerman, C.J.; Nilsson, B.L. Self-assembly of amphipathic beta-sheet peptides: Insights and applications. Biopolymers 2012, 98, 169–184. [Google Scholar] [CrossRef]
- Collier, J.H.; Messersmith, P.B. Self-assembling polymer-peptide conjugates: Nanostructural tailoring. Adv. Mater. 2004, 16, 907–910. [Google Scholar] [CrossRef]
- De Leon Rodriguez, L.M.; Hemar, Y.; Cornish, J.; Brimble, M.A. Structure-mechanical property correlations of hydrogel forming beta-sheet peptides. Chem. Soc. Rev. 2016, 45, 4797–4824. [Google Scholar] [CrossRef]
- Nagarkar, R.P.; Schneider, J.P. Synthesis and primary characterization of self-assembled peptide-based hydrogels. Methods Mol. Biol. 2008, 474, 61–77. [Google Scholar] [CrossRef] [Green Version]
- Koutsopoulos, S. Self-assembling peptide nanofiber hydrogels in tissue engineering and regenerative medicine: Progress, design guidelines, and applications. J. Biomed. Mater. Res. A 2016, 104, 1002–1016. [Google Scholar] [CrossRef] [PubMed]
- Boothroyd, S.; Saiani, A.; Miller, A.F. Controlling network topology and mechanical properties of co-assembling peptide hydrogels. Biopolymers 2014, 101, 669–680. [Google Scholar] [CrossRef] [PubMed]
- Colquhoun, C.; Draper, E.R.; Eden, E.G.; Cattoz, B.N.; Morris, K.L.; Chen, L.; McDonald, T.O.; Terry, A.E.; Griffiths, P.C.; Serpell, L.C.; et al. The effect of self-sorting and co-assembly on the mechanical properties of low molecular weight hydrogels. Nanoscale 2014, 6, 13719–13725. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Horgan, C.C.; Rodriguez, A.L.; Li, R.; Bruggeman, K.F.; Stupka, N.; Raynes, J.K.; Day, L.; White, J.W.; Williams, R.J.; Nisbet, D.R. Characterisation of minimalist co-assembled fluorenylmethyloxycarbonyl self-assembling peptide systems for presentation of multiple bioactive peptides. Acta Biomater. 2016, 38, 11–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Collier, J.H.; Rudra, J.S.; Gasiorowski, J.Z.; Jung, J.P. Multi-component extracellular matrices based on peptide self-assembly. Chem. Soc. Rev. 2010, 39, 3413–3424. [Google Scholar] [CrossRef]
- Raymond, D.M.; Nilsson, B.L. Multicomponent peptide assemblies. Chem. Soc. Rev. 2018, 47, 3659–3720. [Google Scholar] [CrossRef]
- Makam, P.; Gazit, E. Minimalistic peptide supramolecular co-assembly: Expanding the conformational space for nanotechnology. Chem. Soc. Rev. 2018, 47, 3406–3420. [Google Scholar] [CrossRef]
- Jung, J.P.; Nagaraj, A.K.; Fox, E.K.; Rudra, J.S.; Devgun, J.M.; Collier, J.H. Co-assembling peptides as defined matrices for endothelial cells. Biomaterials 2009, 30, 2400–2410. [Google Scholar] [CrossRef] [Green Version]
- Frederix, P.W.; Scott, G.G.; Abul-Haija, Y.M.; Kalafatovic, D.; Pappas, C.G.; Javid, N.; Hunt, N.T.; Ulijn, R.V.; Tuttle, T. Exploring the sequence space for (tri-)peptide self-assembly to design and discover new hydrogels. Nat. Chem. 2015, 7, 30–37. [Google Scholar] [CrossRef]
- Scelsi, A.; Bochicchio, B.; Smith, A.; Workman, V.L.; Castillo Diaz, L.A.; Saiani, A.; Pepe, A. Tuning of hydrogel stiffness using a two-component peptide system for mammalian cell culture. J. Biomed. Mater. Res. A 2019, 107, 535–544. [Google Scholar] [CrossRef] [Green Version]
- Rubin, N.; Perugia, E.; Wolf, S.G.; Klein, E.; Fridkin, M.; Addadi, L. Relation between serum amyloid A truncated peptides and their suprastructure chirality. J. Am. Chem. Soc. 2010, 132, 4242–4248. [Google Scholar] [CrossRef] [PubMed]
- Westermark, G.T.; Engstrom, U.; Westermark, P. The N-terminal segment of protein AA determines its fibrillogenic property. Biochem. Biophys. Res. Commun. 1992, 182, 27–33. [Google Scholar] [CrossRef]
- Scelsi, A.; Bochicchio, B.; Smith, A.; Saiani, A.; Pepe, A. Nanospheres from the self-assembly of an elastin-inspired triblock peptide. Soft Matter 2015, 5, 95007–95013. [Google Scholar] [CrossRef]
- Krysmann, M.J.; Castelletto, V.; Kelarakis, A.; Hamley, I.W.; Hule, R.A.; Pochan, D.J. Self-assembly and hydrogelation of an amyloid peptide fragment. Biochemistry 2008, 47, 4597–4605. [Google Scholar] [CrossRef]
- Krysmann, M.J.; Castelletto, V.; Hamley, I.W. Fibrillisation of hydrophobically modified amyloid peptide fragments in an organic solvent. Soft Matter 2007, 3, 1401–1406. [Google Scholar] [CrossRef] [Green Version]
- Reches, M.; Porat, Y.; Gazit, E. Amyloid fibril formation by pentapeptide and tetrapeptide fragments of human calcitonin. J. Biol. Chem. 2002, 277, 35475–35480. [Google Scholar] [CrossRef] [Green Version]
- Semerdzhiev, S.A.; Lindhoud, S.; Stefanovic, A.; Subramaniam, V.; van der Schoot, P.; Claessens, M. Hydrophobic-Interaction-Induced Stiffening of alpha-Synuclein Fibril Networks. Phys. Rev. Lett. 2018, 120, 208102. [Google Scholar] [CrossRef] [Green Version]
- MacKintosh, F.C.; Käs, J.; Janmey, P.A. Elasticity of Semiflexible Biopolymer Networks. Phys. Rev. Lett. 1995, 75, 4425–4428. [Google Scholar] [CrossRef] [Green Version]
- De Gennes, P.G. Scaling Concepts in Polymer Physics; Cornell University Press: London, UK, 1979. [Google Scholar]
- Ruter, A.; Kuczera, S.; Gentile, L.; Olsson, U. Arrested dynamics in a model peptide hydrogel system. Soft Matter 2020, 16, 2642–2651. [Google Scholar] [CrossRef]
- Lee, J.; Song, B.; Subbiah, R.; Chung, J.J.; Choi, U.H.; Park, K.; Kim, S.H.; Oh, S.J. Effect of chain flexibility on cell adhesion: Semi-flexible model-based analysis of cell adhesion to hydrogels. Sci. Rep. 2019, 9, 2463. [Google Scholar] [CrossRef] [Green Version]
- Yuran, S.; Razvag, Y.; Reches, M. Coassembly of aromatic dipeptides into biomolecular necklaces. ACS Nano 2012, 6, 9559–9566. [Google Scholar] [CrossRef] [PubMed]
- Reches, M.; Gazit, E. Casting metal nanowires within discrete self-assembled peptide nanotubes. Science 2003, 300, 625–627. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kol, N.; Adler-Abramovich, L.; Barlam, D.; Shneck, R.Z.; Gazit, E.; Rousso, I. Self-assembled peptide nanotubes are uniquely rigid bioinspired supramolecular structures. Nano Lett. 2005, 5, 1343–1346. [Google Scholar] [CrossRef] [PubMed]
- Tjernberg, L.O.; Naslund, J.; Lindqvist, F.; Johansson, J.; Karlstrom, A.R.; Thyberg, J.; Terenius, L.; Nordstedt, C. Arrest of beta-amyloid fibril formation by a pentapeptide ligand. J. Biol. Chem. 1996, 271, 8545–8548. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bochicchio, B.; Lorusso, M.; Pepe, A.; Tamburro, A.M. On enhancers and inhibitors of elastin-derived amyloidogenesis. Nanomedicine 2009, 4, 31–46. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Challa, S.R.; Medforth, C.J.; Qiu, Y.; Watt, R.K.; Pena, D.; Miller, J.E.; van Swol, F.; Shelnutt, J.A. Synthesis of peptide-nanotube platinum-nanoparticle composites. Chem. Commun. 2004, 9, 1044–1045. [Google Scholar] [CrossRef]



| Hydrogel | P5 | P21 | VIT 1 | Mechanical Properties | |
|---|---|---|---|---|---|
| (w/v) | (w/v) | G’ (Pa) | G” (Pa) | ||
| H0 | 0.5% | - | - | 11.2 ± 7.0 | 5.1 ± 8.4 |
| H1 | 0.71% | - | ✓ | 111.2 ± 27.5 | 17.0 ± 3.8 |
| H2 | 1.0% | - | ✓ | 914.8 ± 224.5 | 180.6 ± 66.8 |
| H3 | 2.0% | - | ✓ | 13,226.0 ± 2329.7 | 2483.2 ± 454.9 |
| H4 | 1.0% | 0.017% | ✓ | 875.6 ± 204.1 | 162.4 ± 45.5 |
| H5 | 1.0% | 0.034% | ✓ | 8133.2 ± 1638.0 | 1492.3 ± 178.0 |
| H6 | 1.0% | 0.084% | ✓ | 13,581.5 ± 1491.0 | 2163.2 ± 644.0 |
| H7 | 1.0% | 0.170% | ✓ | 14,387.0 ± 1495.2 | 1375.6 ± 318.4 |
| H8 | 2.0% | 0.034% | ✓ | 14,739.5 ± 1962.2 | 2226.0 ± 404.0 |
| H9 | 2.0% | 0.067% | ✓ | 38,718.0 ± 6066.5 | 6307.6 ± 1214.6 |
| H10 | 2.0% | 0.17% | ✓ | 39,566.7 ± 5048.0 | 6114.1 ± 1115.3 |
| H11 | 2.0% | 0.34% | ✓ | 38,072.5 ± 5119.1 | 5416.1 ± 1040.1 |
| Hydrogel | P5 | FF | VIT 1 | Mechanical Properties | |
|---|---|---|---|---|---|
| (w/v) | (w/v) | G’ (Pa) | G” (Pa) | ||
| HF1 | 1.0% | 0.025% | ✓ | 1869 ± 381 | 426 ± 18 |
| HF2 | 1.0% | 0.050% | ✓ | 1087 ± 198 | 250 ± 25 |
| HF3 | 1.0% | 0.125% | ✓ | 378 ± 77 | 75 ± 15 |
| HF4 | 1.0% | 0.25% | - | 26.0 ± 3.3 | 5.6 ± 0.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scelsi, A.; Bochicchio, B.; Smith, A.M.; Laezza, A.; Saiani, A.; Pepe, A. Hydrogels from the Assembly of SAA/Elastin-Inspired Peptides Reveal Non-Canonical Nanotopologies. Molecules 2022, 27, 7901. https://doi.org/10.3390/molecules27227901
Scelsi A, Bochicchio B, Smith AM, Laezza A, Saiani A, Pepe A. Hydrogels from the Assembly of SAA/Elastin-Inspired Peptides Reveal Non-Canonical Nanotopologies. Molecules. 2022; 27(22):7901. https://doi.org/10.3390/molecules27227901
Chicago/Turabian StyleScelsi, Alessandra, Brigida Bochicchio, Andrew M. Smith, Antonio Laezza, Alberto Saiani, and Antonietta Pepe. 2022. "Hydrogels from the Assembly of SAA/Elastin-Inspired Peptides Reveal Non-Canonical Nanotopologies" Molecules 27, no. 22: 7901. https://doi.org/10.3390/molecules27227901
APA StyleScelsi, A., Bochicchio, B., Smith, A. M., Laezza, A., Saiani, A., & Pepe, A. (2022). Hydrogels from the Assembly of SAA/Elastin-Inspired Peptides Reveal Non-Canonical Nanotopologies. Molecules, 27(22), 7901. https://doi.org/10.3390/molecules27227901








