Development and Validation for Quantification of Cephapirin and Ceftiofur by Ultraperformance Liquid Chromatography with Triple Quadrupole Mass Spectrometry
Abstract
:1. Introduction
2. Results and Discussion
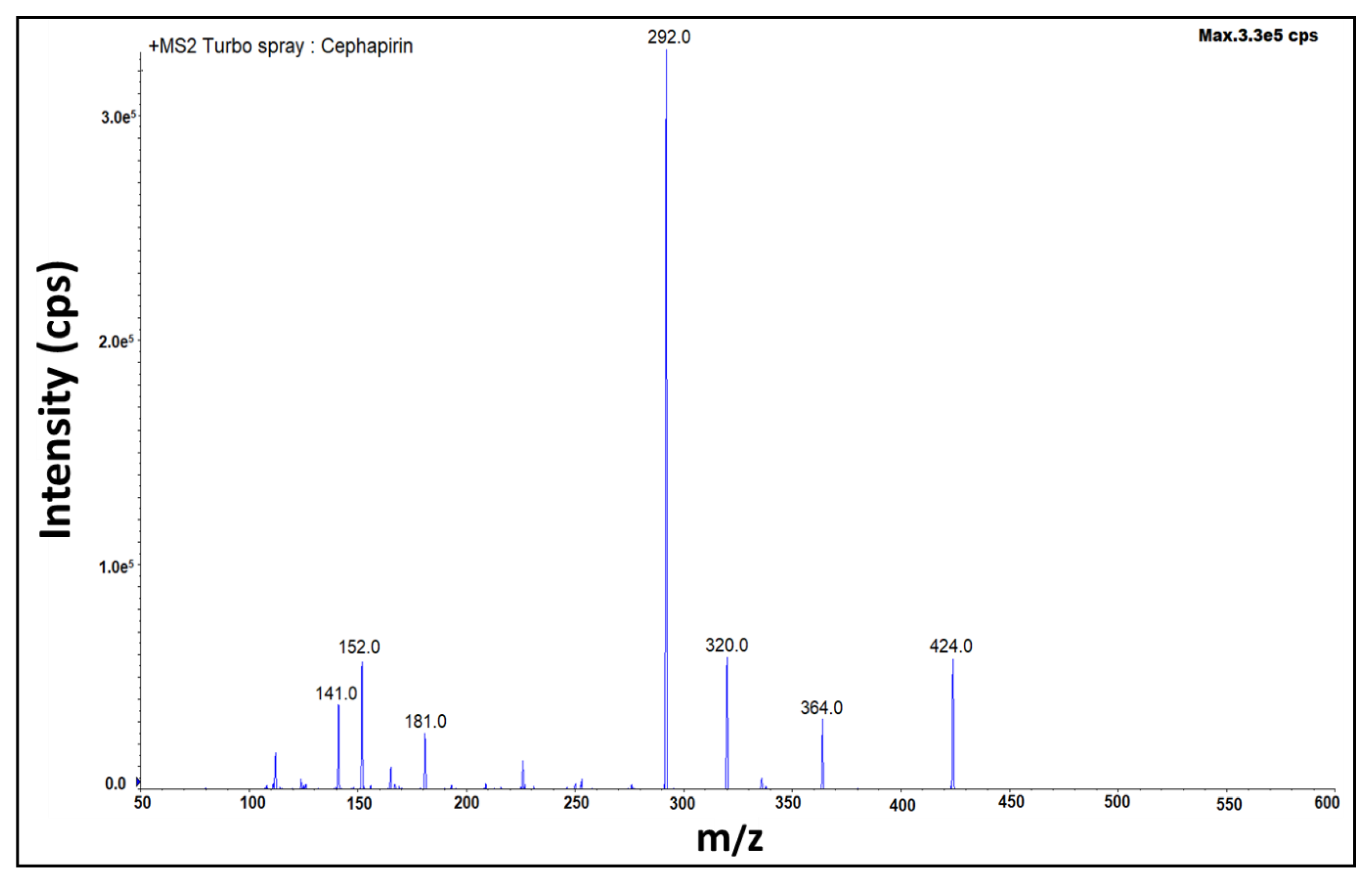
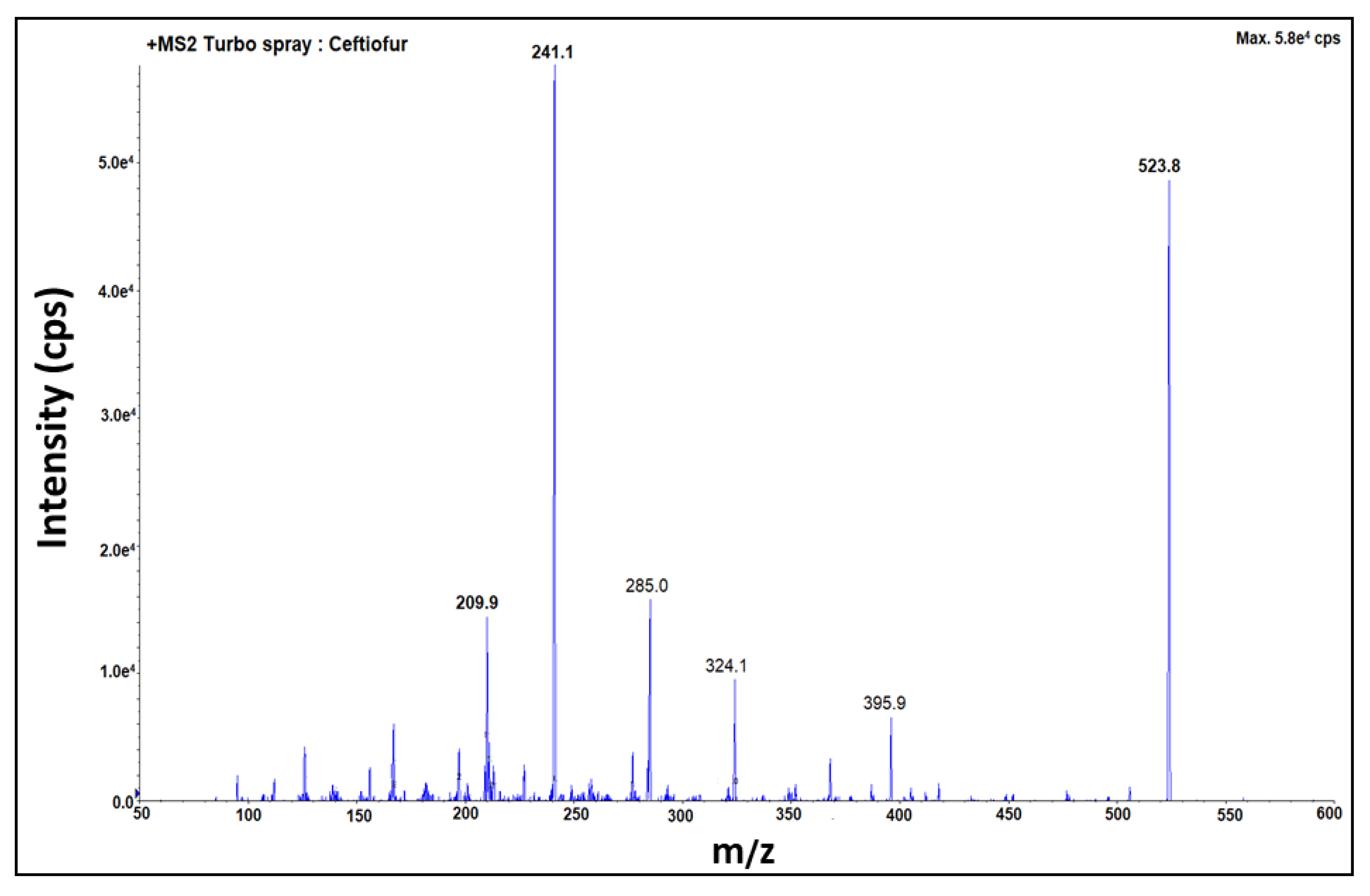
2.1. Optimization of Mass Spectrometric Parameters
2.2. Optimization of Chromatographic Conditions
2.3. Method Validation Study
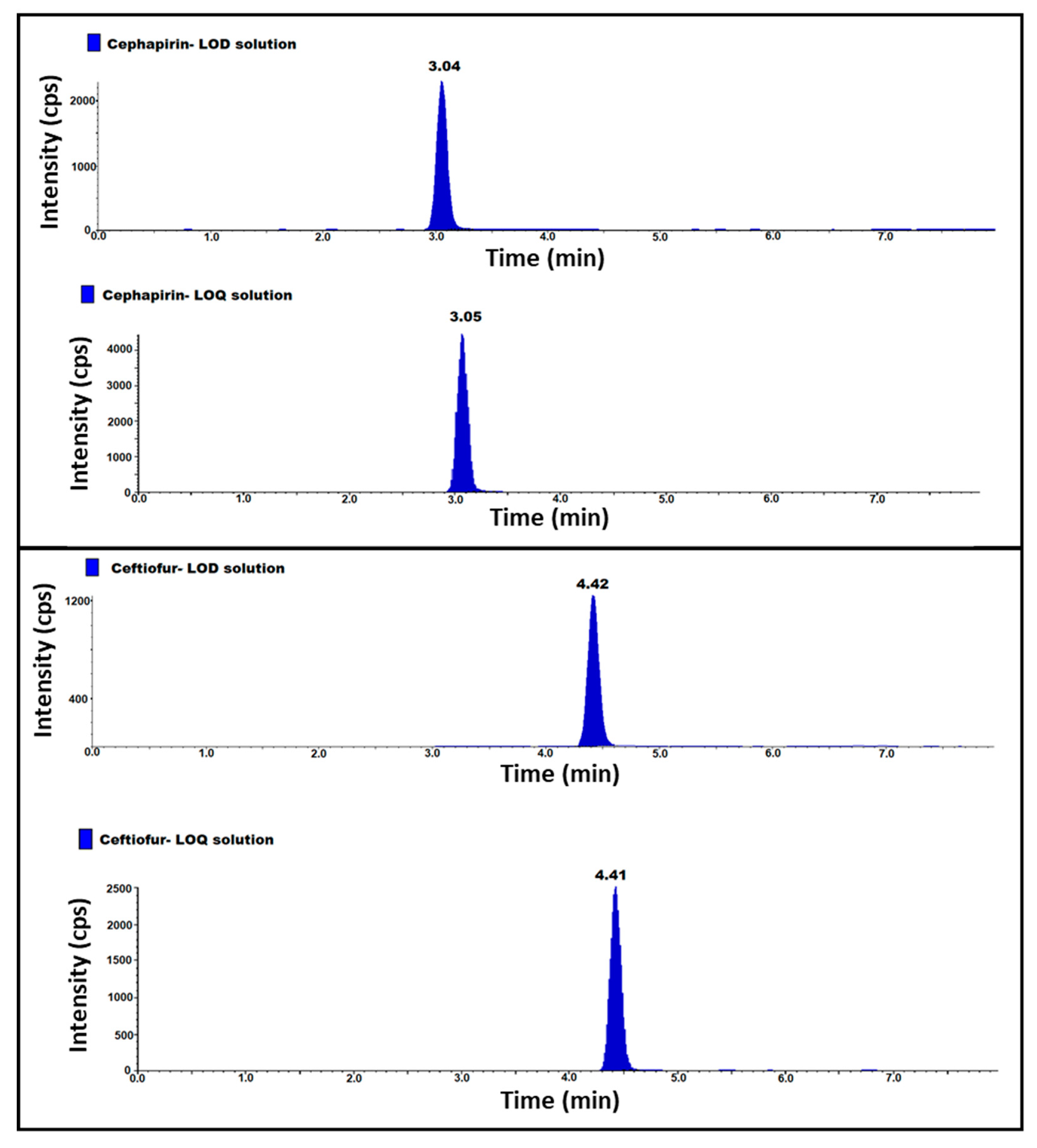
2.4. Specificity and System Suitability
2.5. LOD, LOQ and LOQ Precision
2.6. Linearity and Range
2.7. Method Precision
2.8. Intermediate Precision
2.9. Accuracy
2.10. Robustness
2.11. Solution Stability
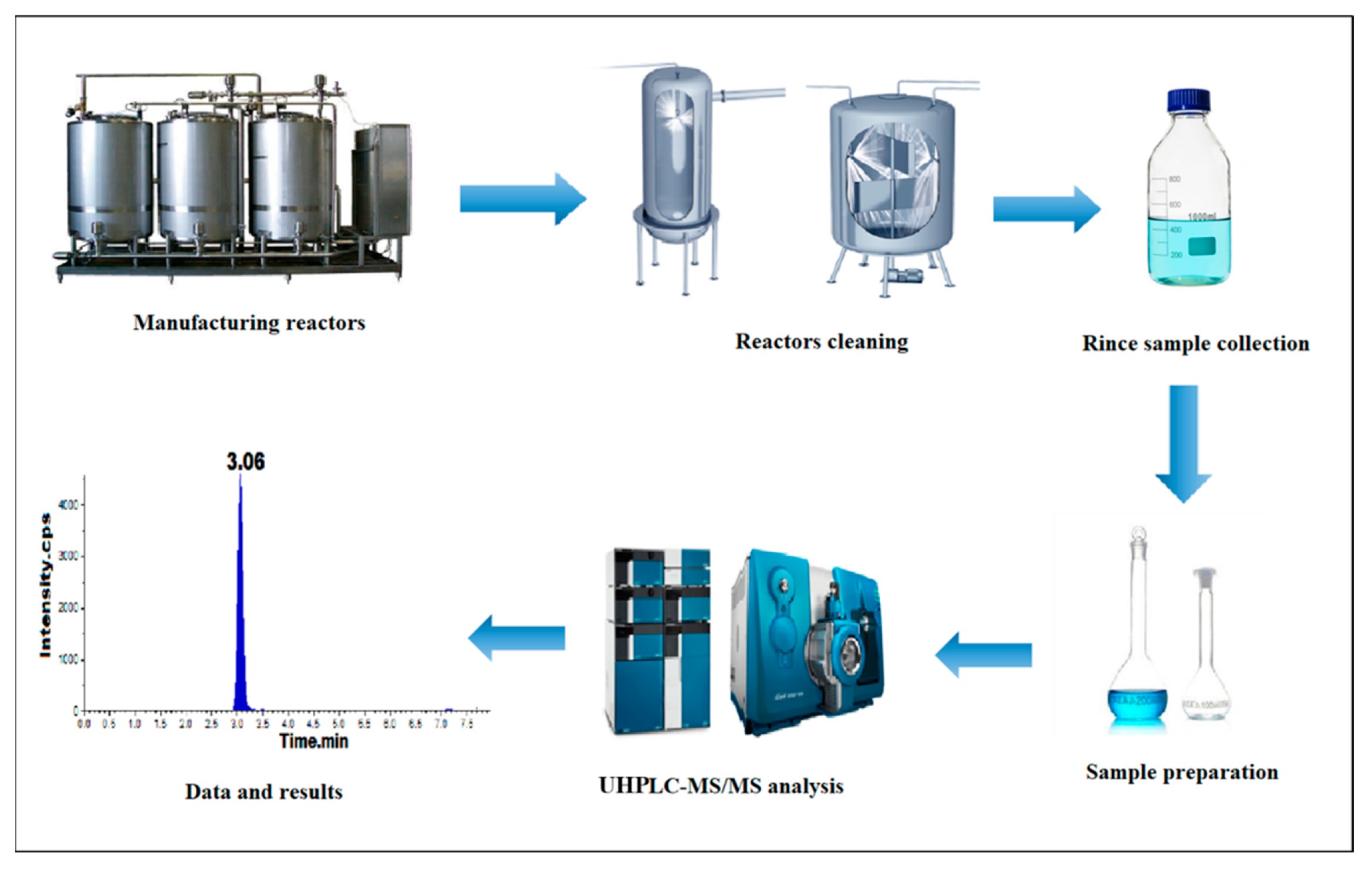
3. Material and Methods
3.1. Materials and Reagents
3.2. Equipment
3.3. Chromatographic Conditions
3.4. Mass Spectrometer Conditions
3.5. Preparation of Standard and Test Sample Solutions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Fabio, P.; Alessandro, C.; Stefania, S.; Iolanda, G.; Elena, M.; Aldo, C. The requirements for manufacturing highly active or sensitising drugs comparing Good Manufacturing Practices. Acta Biomed. Ateneo Parm. 2019, 90, 288. [Google Scholar]
- Bush, K.; Bradford, P.A. β-Lactams and β-lactamase inhibitors: An overview. Cold Spring Harb. Perspect. Med. 2016, 6, a025247. [Google Scholar] [CrossRef] [PubMed]
- Picard, M.; Robitaille, G.; Karam, F.; Daigle, J.-M.; Bédard, F.; Biron, É.; Tardif, M.R.; Lacombe-Barrios, J.; Bégin, P. Cross-reactivity to cephalosporins and carbapenems in penicillin-allergic patients: Two systematic reviews and meta-analyses. J. Allergy Clin. Immunol. Pract. 2019, 7, 2722–2738.e2725. [Google Scholar] [CrossRef] [PubMed]
- Macy, E.; Contreras, R. Health care use and serious infection prevalence associated with penicillin “allergy” in hospitalized patients: A cohort study. J. Allergy Clin. Immunol. 2014, 133, 790–796. [Google Scholar] [CrossRef]
- Pichichero, M.E.; Zagursky, R. Penicillin and cephalosporin allergy. Ann. Allergy Asthma Immunol. 2014, 112, 404–412. [Google Scholar] [CrossRef]
- Caubet, J.-C.; Kaiser, L.; Lemaître, B.; Fellay, B.; Gervaix, A.; Eigenmann, P.A. The role of penicillin in benign skin rashes in childhood: A prospective study based on drug rechallenge. J. Allergy Clin. Immunol. 2011, 127, 218–222. [Google Scholar] [CrossRef]
- Montañez, M.I.; Mayorga, C.; Bogas, G.; Barrionuevo, E.; Fernandez-Santamaria, R.; Martin-Serrano, A.; Laguna, J.J.; Torres, M.J.; Fernandez, T.D.; Doña, I. Epidemiology, mechanisms, and diagnosis of drug-induced anaphylaxis. Front. Immunol. 2017, 8, 614. [Google Scholar] [CrossRef] [Green Version]
- Gruchalla, R.S.; Pirmohamed, M. Antibiotic allergy. N. Engl. J. Med. 2006, 354, 601–609. [Google Scholar] [CrossRef]
- Song, E.; Yu, M.; Wang, Y.; Hu, W.; Cheng, D.; Swihart, M.T.; Song, Y. Multi-color quantum dot-based fluorescence immunoassay array for simultaneous visual detection of multiple antibiotic residues in milk. Biosens. Bioelectron. 2015, 72, 320–325. [Google Scholar] [CrossRef]
- Ray, P.; Knowlton, K.F.; Shang, C.; Xia, K. Development and validation of a UPLC-MS/MS method to monitor cephapirin excretion in dairy cows following intramammary infusion. PLoS ONE 2014, 9, e112343. [Google Scholar] [CrossRef]
- Llopis, B.; Funck-Brentano, C.; Tissot, N.; Bleibtreu, A.; Jaureguiberry, S.; Fourniols, E.; Aubry, A.; Zahr, N. Development and validation of a UPLC-MS/MS method for simultaneous quantification of levofloxacin, ciprofloxacin, moxifloxacin and rifampicin in human plasma: Application to the therapeutic drug monitoring in osteoarticular infections. J. Pharm. Biomed. Anal. 2020, 183, 113137. [Google Scholar] [CrossRef] [PubMed]
- Jyrkkanen, J. Antibiotic induced changes to mitochondria result in potential contributions to carcinogenesis, heart pathologies, other medical conditions and ecosystem risks. J. Cardiol. Cardiovasc. Med. 2020, 5, 163–171. [Google Scholar]
- Trejnowska, E.; Deptuła, A.; Tarczyńska-Słomian, M.; Knapik, P.; Jankowski, M.; Misiewska-Kaczur, A.; Tamowicz, B.; Śmiechowicz, J.; Antończyk, R.; Armatowicz, P. Surveillance of antibiotic prescribing in intensive care units in Poland. Can. J. Infect. Dis. Med. Microbiol. 2018, 2018, 5670238. [Google Scholar] [CrossRef] [PubMed]
- Chaudhry, S.B.; Veve, M.P.; Wagner, J.L. Cephalosporins: A focus on side chains and β-lactam cross-reactivity. Pharmacy 2019, 7, 103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zagursky, R.J.; Pichichero, M.E. Cross-reactivity in β-lactam allergy. J. Allergy Clin. Immunol. Pract. 2018, 6, 72–81.e71. [Google Scholar] [CrossRef] [PubMed]
- Terico, A.T.; Gallagher, J.C. Beta-lactam hypersensitivity and cross-reactivity. J. Pharm. Pract. 2014, 27, 530–544. [Google Scholar] [CrossRef] [PubMed]
- Torres, M.J.; Blanca, M. The complex clinical picture of β-lactam hypersensitivity: Penicillins, cephalosporins, monobactams, carbapenems, and clavams. Med. Clin. 2010, 94, 805–820. [Google Scholar] [CrossRef]
- Bozcal, E.; Dagdeviren, M. Toxicity of β-lactam antibiotics: Pathophysiology, molecular biology and possible recovery strategies. In Poisoning: From Specific Toxic Agents to Novel Rapid and Simplified Techniques for Analysis; InTechOpen: Rijeka, Croatia, 2017; pp. 87–105. [Google Scholar]
- Dickson, S.D.; Salazar, K.C. Diagnosis and management of immediate hypersensitivity reactions to cephalosporins. Clin. Rev. Allergy Immunol. 2013, 45, 131–142. [Google Scholar] [CrossRef]
- Chow, K.; Hui, A.; Szeto, C. Neurotoxicity induced by beta-lactam antibiotics: From bench to bedside. Eur. J. Clin. Microbiol. Infect. Dis. 2005, 24, 649–653. [Google Scholar] [CrossRef]
- Ana, K.M.S.; Madriaga, J.; Espino, M.P. β-Lactam antibiotics and antibiotic resistance in Asian lakes and rivers: An overview of contamination, sources and detection methods. Environ. Pollut. 2021, 275, 116624. [Google Scholar] [CrossRef]
- Gothwal, R.; Shashidhar, T. Antibiotic pollution in the environment: A review. Clean Soil Air Water 2015, 43, 479–489. [Google Scholar] [CrossRef]
- Nikolaou, A.; Meric, S.; Fatta, D. Occurrence patterns of pharmaceuticals in water and wastewater environments. Anal. Bioanal. Chem. 2007, 387, 1225–1234. [Google Scholar] [CrossRef] [PubMed]
- Olatoye, I.O.; Daniel, O.F.; Ishola, S.A. Screening of antibiotics and chemical analysis of penicillin residue in fresh milk and traditional dairy products in Oyo state, Nigeria. Vet. World 2016, 9, 948–954. [Google Scholar] [CrossRef] [Green Version]
- Food, U.; Administration, D. Guidance for Industry: Non-Penicillin Beta-Lactam Drugs: A CGMP Framework for Preventing Cross-Contamination; US Food and Drug Administration (FDA): Silver Spring, MD, USA, 2013.
- Carter, G.G. A Review of Procedures for the Detection of Residual Penicillins in Drugs; National Center for Antibiotics Analysis, Bureau of Drugs, FDA, BY-Lines: Silver Spring, MD, USA, 1977; Volume 8.
- Okerman, L.; De Wasch, K.; Van Hoof, J. Detection of antibiotics in muscle tissue with microbiological inhibition tests: Effects of the matrix. Analyst 1998, 123, 2361–2365. [Google Scholar] [CrossRef]
- Kricka, L.J. Selected strategies for improving sensitivity and reliability of immunoassays. Clin. Chem. 1994, 40, 347–357. [Google Scholar] [CrossRef] [PubMed]
- Hujer, A.M.; Page, M.G.; Helfand, M.S.; Yeiser, B.; Bonomo, R.A. Development of a sensitive and specific enzyme-linked immunosorbent assay for detecting and quantifying CMY-2 and SHV β-lactamases. J. Clin. Microbiol. 2002, 40, 1947–1957. [Google Scholar] [CrossRef] [Green Version]
- Petrişor, C.; Gherman-Ionică, N.; Bologa, R.; Sfichi, M.; Hagău, N. Basophil activation test versus radio-immunoassay in the diagnosis of β-lactam immediate-type hypersensitivity reactions. Rev. Romana Med. Lab. 2013, 21, 415–422. [Google Scholar] [CrossRef] [Green Version]
- Han, M.; Gong, L.; Wang, J.; Zhang, X.; Jin, Y.; Zhao, R.; Yang, C.; He, L.; Feng, X.; Chen, Y. An octuplex lateral flow immunoassay for rapid detection of antibiotic residues, aflatoxin M1 and melamine in milk. Sens. Actuators B Chem. 2019, 292, 94–104. [Google Scholar] [CrossRef]
- Kuhn, J.; Aylaz, G.; Sari, E.; Marco, M.; Yiu, H.H.; Duman, M. Selective binding of antibiotics using magnetic molecular imprint polymer (MMIP) networks prepared from vinyl-functionalized magnetic nanoparticles. J. Hazard. Mater. 2020, 387, 121709. [Google Scholar] [CrossRef]
- Ahmadian, E.; Eftekhari, A.; Kavetskyy, T.; Khosroushahi, A.Y.; Turksoy, V.A.; Khalilov, R. Effects of quercetin loaded nanostructured lipid carriers on the paraquat-induced toxicity in human lymphocytes. Pestic. Biochem. Physiol. 2020, 167, 104586. [Google Scholar] [CrossRef]
- Joshi, A.; Kim, K.-H. Recent advances in nanomaterial-based electrochemical detection of antibiotics: Challenges and future perspectives. Biosens. Bioelectron. 2020, 153, 112046. [Google Scholar] [CrossRef] [PubMed]
- Eftekhari, A.; Arjmand, A.; Asheghvatan, A.; Švajdlenková, H.; Šauša, O.; Abiyev, H.; Ahmadian, E.; Smutok, O.; Khalilov, R.; Kavetskyy, T. The potential application of magnetic nanoparticles for liver fibrosis theranostics. Front. Chem. 2021, 9, 674786. [Google Scholar] [CrossRef]
- Ahmadian, E.; Janas, D.; Eftekhari, A.; Zare, N. Application of carbon nanotubes in sensing/monitoring of pancreas and liver cancer. Chemosphere 2022, 302, 134826. [Google Scholar] [CrossRef] [PubMed]
- Verdier, M.-C.; Tribut, O.; Tattevin, P.; Le Tulzo, Y.; Michelet, C.; Bentué-Ferrer, D. Simultaneous determination of 12 β-lactam antibiotics in human plasma by high-performance liquid chromatography with UV detection: Application to therapeutic drug monitoring. Antimicrob. Agents Chemother. 2011, 55, 4873–4879. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohamed, G. Spectrophotometric determination of ampicillin, dicluxacillin, flucloxacillin and amoxicillin antibiotic drugs: Ion-pair formation with molybdenum and thiocyanate. J. Pharm. Biomed. Anal. 2001, 24, 561–567. [Google Scholar] [CrossRef]
- Salem, H.; Saleh, G.A. Selective spectrophotometric determination of phenolic β-lactam antibiotics. J. Pharm. Biomed. Anal. 2002, 28, 1205–1213. [Google Scholar] [CrossRef]
- Denooz, R.; Charlier, C. Simultaneous determination of five β-lactam antibiotics (cefepim, ceftazidim, cefuroxim, meropenem and piperacillin) in human plasma by high-performance liquid chromatography with ultraviolet detection. J. Chromatogr. B 2008, 864, 161–167. [Google Scholar] [CrossRef]
- Nemutlu, E.; Kır, S.; Katlan, D.; Beksac, M.S. Simultaneous multiresponse optimization of an HPLC method to separate seven cephalosporins in plasma and amniotic fluid: Application to validation and quantification of cefepime, cefixime and cefoperazone. Talanta 2009, 80, 117–126. [Google Scholar] [CrossRef]
- McWhinney, B.C.; Wallis, S.C.; Hillister, T.; Roberts, J.A.; Lipman, J.; Ungerer, J.P. Analysis of 12 beta-lactam antibiotics in human plasma by HPLC with ultraviolet detection. J. Chromatogr. B 2010, 878, 2039–2043. [Google Scholar] [CrossRef]
- Bekoe, S.O.; Bak, S.A.; Björklund, E.; Krogh, K.A.; Okine, N.N.; Adosraku, R.K.; Styrishave, B.; Hansen, M. Determination of thirteen antibiotics in drug products–A new LC-MS/MS tool for screening drug product quality. Anal. Methods 2014, 6, 5847–5855. [Google Scholar] [CrossRef]
- Qiu, C.; Zhu, H.; Ruzicka, C.; Keire, D.; Ye, H. A general LC-MS/MS method for monitoring potential β-lactam contamination in drugs and drug-manufacturing surfaces. AAPS J. 2018, 20, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Pan, X.-D.; Huang, B.-F.; Han, J.-L. Quantification of 16 β-lactams in chicken muscle by QuEChERS extraction and UPLC-Q-Orbitrap-MS with parallel reaction monitoring. J. Pharm. Biomed. Anal. 2017, 145, 525–530. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Yin, Y.; Zheng, G.; Liu, S.; Zhao, C.; Ma, L.; Shan, Q.; Dai, X.; Wei, L.; Lin, J. Determining β-lactam antibiotics in aquaculture products by modified QuECHERS combined with ultra-high performance liquid chromatography-tandem mass spectrometry (UHPLC-MS/MS). Arab. J. Chem. 2022, 15, 103912. [Google Scholar] [CrossRef]
- Guideline, I.H.T. Validation of Analytical Procedures: Text and Methodology. Q2 2005, 1, 05. [Google Scholar]
- Vaclavik, L.; Krynitsky, A.J.; Rader, J.I. Targeted analysis of multiple pharmaceuticals, plant toxins and other secondary metabolites in herbal dietary supplements by ultra-high performance liquid chromatography–quadrupole-orbital ion trap mass spectrometry. Anal. Chim. Acta 2014, 810, 45–60. [Google Scholar] [CrossRef] [PubMed]
- Jaderson, M.; Park, J.-H. Evaluation of matrix effects in quantifying microbial secondary metabolites in indoor dust using ultraperformance liquid chromatograph–tandem mass spectrometer. Saf. Health Work 2019, 10, 196–204. [Google Scholar] [CrossRef]


| Validation Parameters | Typical Acceptance Criteria | Results | |
|---|---|---|---|
| Cephapirin | Ceftiofur | ||
| System suitability | RSD (%) for peak area response (n = 6) should be ≤15.0. | 1.63 | 1.13 |
| Specificity | Retention time of analyte in all the solutions. | 3.0 | 4.4 |
| Interference from blank | No interference from blank | ||
| LOD | Concentration of LOD in ppb | 0.151 | 0.152 |
| S/N value should be ≥3 | 22 | 35 | |
| LOQ | Concentration of LOQ in ppb | 0.412 | 0.411 |
| s/n value should be ≥10 | 55 | 72 | |
| LOQ precision | RSD (%) for six replicate injections of LOQ solution should be ≤15.0% | 1.7 | 2.1 |
| Linearity | Range (ppb) | 0.412 to 1.511 | 0.411 to 1.542 |
| Square of Correlation coefficient (r2) should not be less than 0.99 | 0.9998 | 0.9994 | |
| Validation Parameters | Typical Acceptance Criteria | Results | |
|---|---|---|---|
| Cephapirin | Ceftiofur | ||
| Method precision | RSD (%) for six preparations (n = 6) of spiked sample at a specification level should be ≤15.0 | 1.0 | 0.6 |
| Intermediate precision | RSD (%) for six preparations (n = 6) of spiked sample at a specification level should be ≤15.0 | 1.1 | 1.0 |
| RSD (%) for preparations (n = 12) of MP and IP spiked sample at a specification level should be ≤20.0 | ≤20.0 | ≤20.0 | |
| Accuracy | LOQ average recovery (n = 3) should be between 70% to 130%. | 94.0 | 96.5 |
| 50% average recovery (n = 3) should be between 80% to 120%. | 95.2 | 94.5 | |
| 100% average recovery (n = 3) should be between 80% to 120%. | 96.6 | 93.5 | |
| 150% average recovery (n = 3) should be between 80% to 120%. | 92.8 | 90.7 | |
| Robustness | Plus (+) flow 0.7 mL/min: spiked sample concentration % difference and retention time | 1.1% 2.8 min | 1.8% 4.2 min |
| Minus (−) flow 0.5 mL/min: spiked sample concentration % difference and retention time | 1.4% 3.2 min | 1.6% 4.6 min | |
| Plus (+) oven 42 °C: spiked sample concentration % difference and retention time | 2.0% 2.9 min | 1.9% 4.3 min | |
| Minus (−) oven 38 °C: spiked sample concentration % difference and retention time | 1.8% 3.1 min | 1.7% 4.5 min | |
| Solution Stability | Standard and 100% spiked solution stored at ambient laboratory conditions (25 ± 5 °C) and refrigerated conditions (2–8 °C) were studied for 48 h | Solutions are stable for 48 h | |
| Parameter | Condition | |
|---|---|---|
| Liquid Chromatography Conditions | ||
| Mobile phase A | 0.15% formic acid in water | |
| Mobile phase B | acetonitrile | |
| Auto-sampler temperature | 8 °C | |
| Column temperature | 40 °C | |
| Flow rate | 0.6 mL/min | |
| Injection volume | 50 μL | |
| Gradient program (time in min/mobile phase A) | 0/80, 5/5, 5.5/80, 8/80 | |
| Diluent | Water | |
| Run time | 8 min | |
| Mass spectrometry conditions | ||
| Source and Ionization mode | ESI- Positive | |
| Detection mode | MRM | |
| MRM transitions (m/z) selected | Cephapirin | Ceftiofur |
| For qualification | 424.0 > 320.0 | 523.8 > 285.0 |
| For quantification | 424.0 > 292.0 | 523.8 > 241.1 |
| Collision energy (CE) | 22 | 25 |
| De-clustering potential (DP) | 40 | |
| Entrance potential (EP) | 10 | |
| MS temperature | 400 °C | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chittireddy, H.N.P.R.; Kumar, J.V.S.; Bhimireddy, A.; Shaik, M.R.; Shaik, A.H.; Alwarthan, A.; Shaik, B. Development and Validation for Quantification of Cephapirin and Ceftiofur by Ultraperformance Liquid Chromatography with Triple Quadrupole Mass Spectrometry. Molecules 2022, 27, 7920. https://doi.org/10.3390/molecules27227920
Chittireddy HNPR, Kumar JVS, Bhimireddy A, Shaik MR, Shaik AH, Alwarthan A, Shaik B. Development and Validation for Quantification of Cephapirin and Ceftiofur by Ultraperformance Liquid Chromatography with Triple Quadrupole Mass Spectrometry. Molecules. 2022; 27(22):7920. https://doi.org/10.3390/molecules27227920
Chicago/Turabian StyleChittireddy, Hari Naga Prasada Reddy, J. V. Shanmukha Kumar, Anuradha Bhimireddy, Mohammed Rafi Shaik, Althaf Hussain Shaik, Abdulrahman Alwarthan, and Baji Shaik. 2022. "Development and Validation for Quantification of Cephapirin and Ceftiofur by Ultraperformance Liquid Chromatography with Triple Quadrupole Mass Spectrometry" Molecules 27, no. 22: 7920. https://doi.org/10.3390/molecules27227920
APA StyleChittireddy, H. N. P. R., Kumar, J. V. S., Bhimireddy, A., Shaik, M. R., Shaik, A. H., Alwarthan, A., & Shaik, B. (2022). Development and Validation for Quantification of Cephapirin and Ceftiofur by Ultraperformance Liquid Chromatography with Triple Quadrupole Mass Spectrometry. Molecules, 27(22), 7920. https://doi.org/10.3390/molecules27227920







