Surfactant-Mediated Ultrasonic-Assisted Extraction and Purification of Antioxidants from Chaenomeles speciosa (Sweet) Nakai for Chemical- and Cell-Based Antioxidant Capacity Evaluation
Abstract
:1. Introduction
2. Results and Discussion
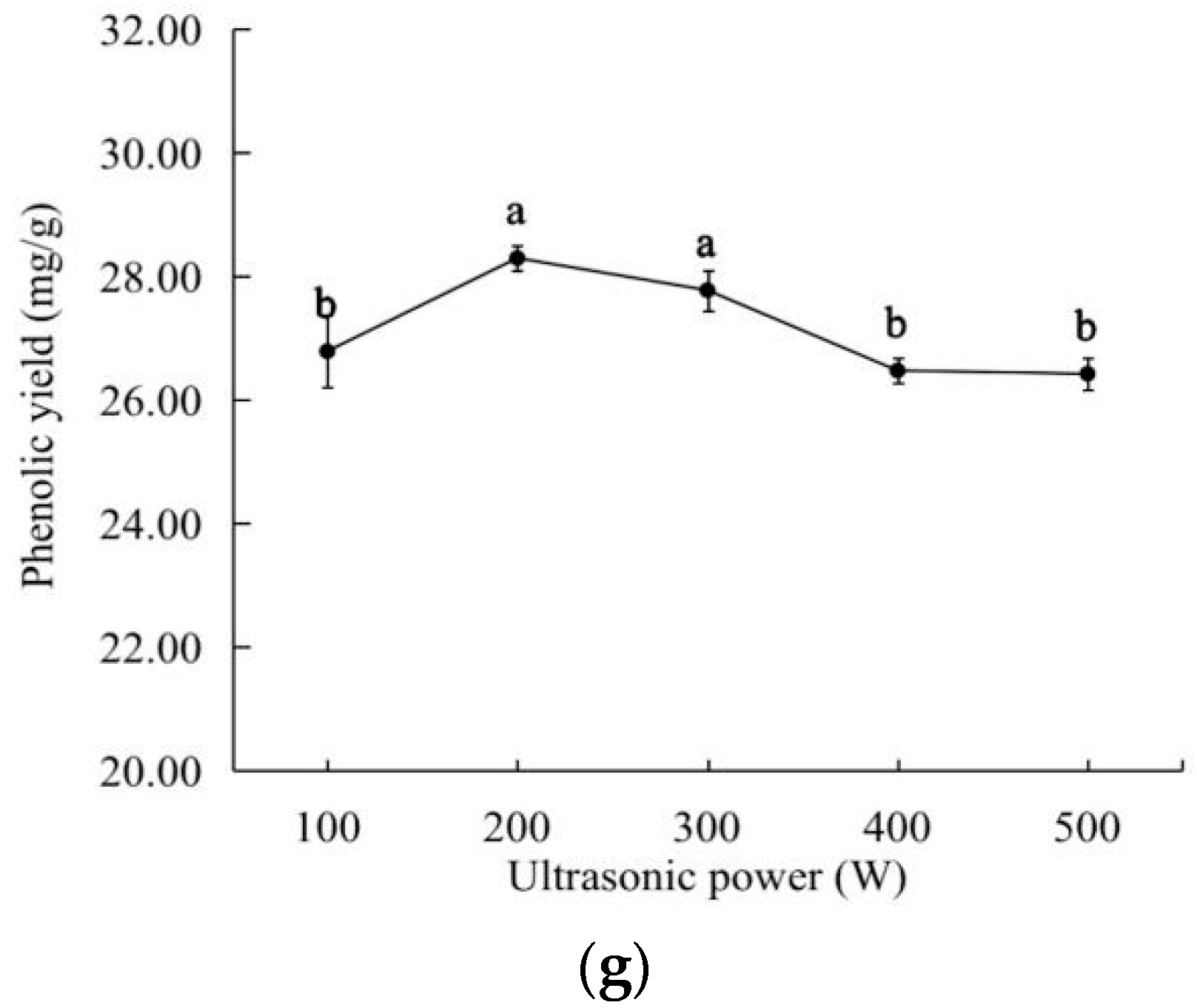
2.1. Effects of the Different Factors on the Extraction Yield of the Total Phenolics
2.2. Optimization for the Extraction of the Phenolics
2.2.1. Plackett–Burman Experiment Analysis
2.2.2. Response Model Establishment and the Variance Analysis
2.2.3. Response Surface Analysis
2.2.4. Optimal Conditions and the Model Validation
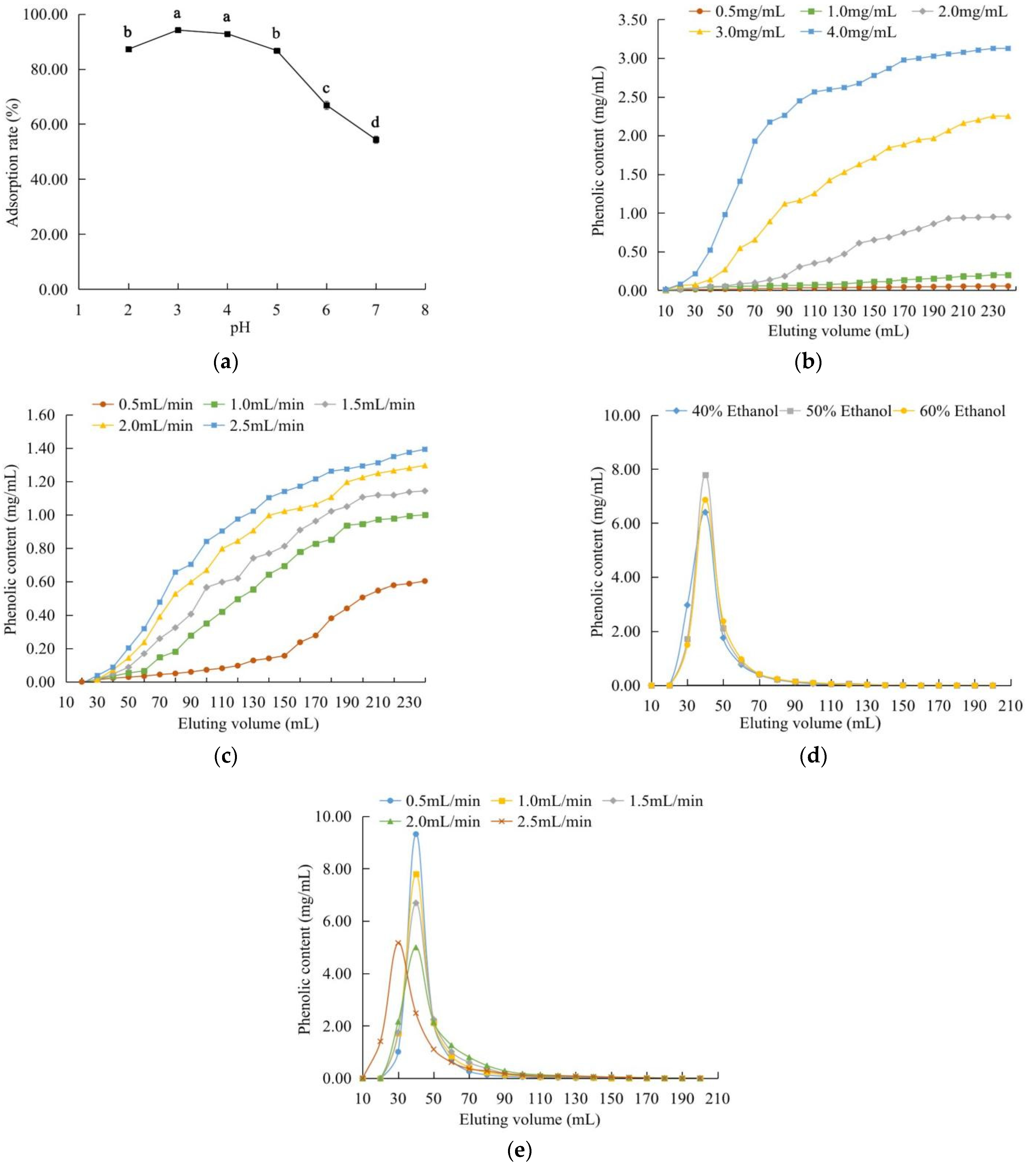
2.3. Effect of the Different Factors on the Purification of the Phenolic Extract
2.3.1. Static Adsorption and Desorption
2.3.2. Dynamic Adsorption and Desorption
2.4. Antioxidant Activities of the C. speciosa Antioxidant-Enriched Extract
2.4.1. Antioxidant Activity Assay
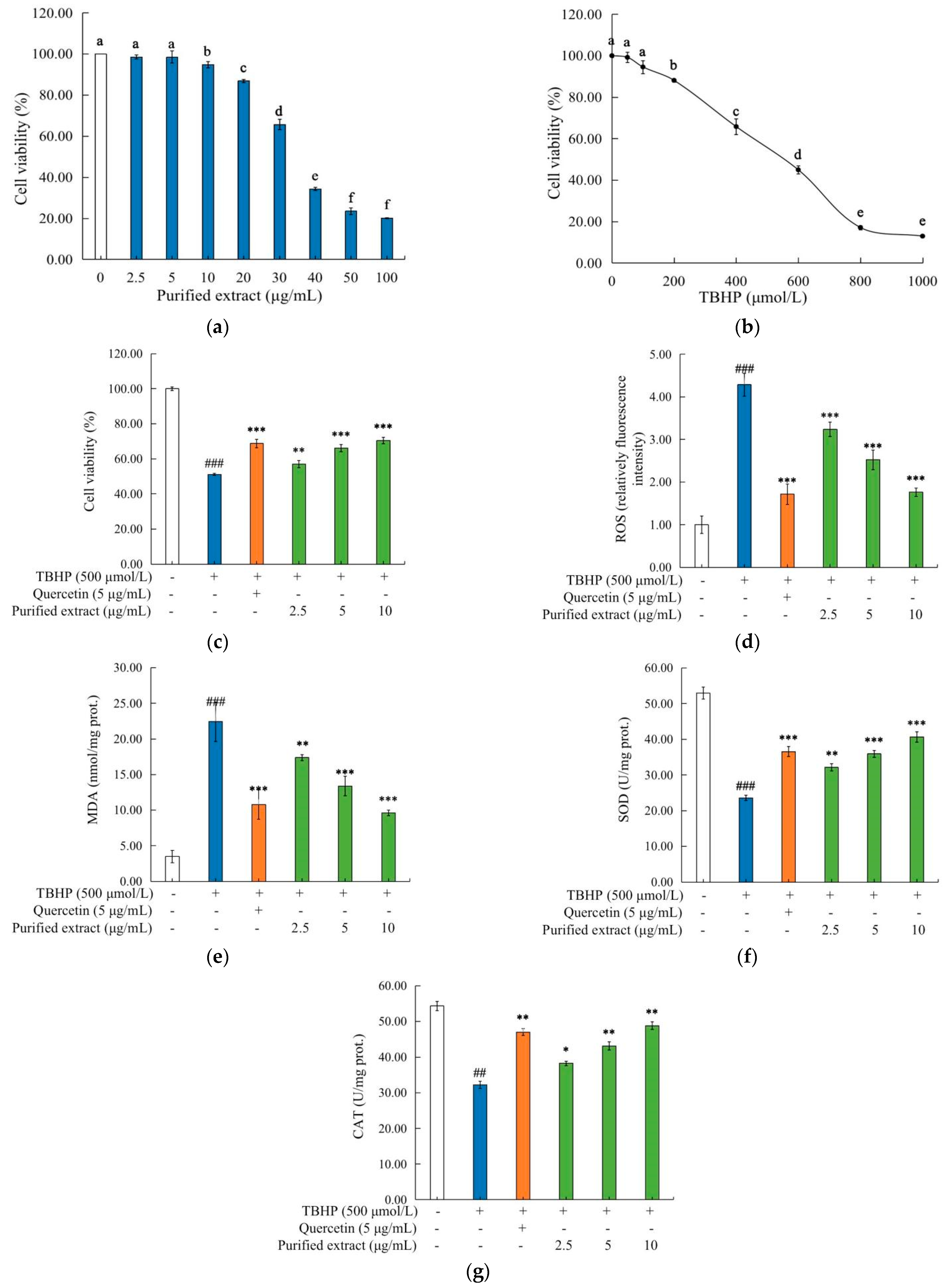
2.4.2. Effects of the Purified C. speciosa Antioxidant-Enriched Extract on the Viability of HepG2 Cells
2.4.3. Effects of the Purified C. speciosa Antioxidant-Enriched Extract on the Oxidative Stress Biomarkers
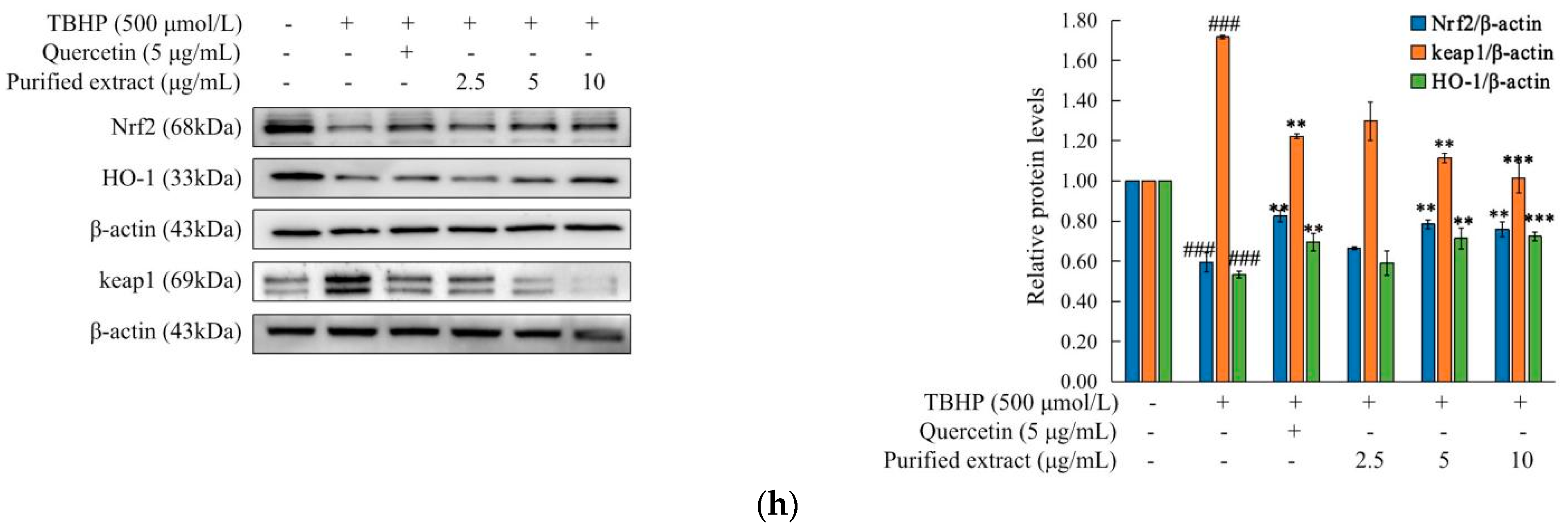
2.4.4. Effects of the Purified C. speciosa Antioxidant-Enriched Extract on the Nrf2/Keap1 Pathways in TBHP-Induced HepG2 Cells
3. Materials and Methods
3.1. Reagents and Materials
3.2. Extraction of the Antioxidants from C. speciosa Fruits
3.2.1. Single Factor Experiments
3.2.2. Plackett–Burman Design
3.2.3. Box–Behnken Design (BBD)
3.3. Purification of the Antioxidant-Enriched Extract Using the Macroporous Resins
3.3.1. Pretreatments of the Crude Antioxidant-Enriched Extract and the Macroporous Resins
3.3.2. Static Adsorption and Desorption Tests
Desorption rate (%) = [m2/(m0 − m1)] × 100
Recovery rate (%) = (m2/m0) × 100
3.3.3. Dynamic Adsorption and Desorption Tests
3.4. Chemical- and Cell-Based Antioxidant Capacity Evaluations
3.4.1. Total Reducing Power Assay
3.4.2. DPPH Assay
3.4.3. ABTS Assay
3.4.4. Cell Culture
3.4.5. Cell Viability Assay
3.4.6. Construction of the Oxidative Damage Cell Model
3.4.7. Determination of the Biomarkers Associated with the Oxidative Stress and Antioxidant Actions
3.4.8. Western Blot Analysis
3.5. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Kim, S.Y.; Park, C.; Jang, H.J.; Kim, B.; Bae, H.W.; Chung, I.Y.; Kim, E.S.; Cho, Y.H. Antibacterial strategies inspired by the oxidative stress and response networks. J. Microbiol. 2019, 57, 203–212. [Google Scholar] [CrossRef]
- Chen, H.H.; Li, G.F.; Luo, Z.S. Optimization of Extraction Process and Antioxidant Activity of Total Saponins from Panax notoginseng Flower with Surfactant and Ultrasonic Enzymatic Method. Food Ferment. Ind. 2020, 46, 178–186. [Google Scholar]
- Kansanen, E.; Kuosmanen, S.M.; Leinonen, H.; Levonen, A.L. The Keap1-Nrf2 pathway: Mechanisms of activation and dysregulation in cancer. Redox Biol. 2013, 1, 45–59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Devrim-Lanpir, A.; Bilgic, P.; Kocahan, T.; Deliceoğlu, G.; Rosemann, T.; Knechtle, B. Total Dietary Antioxidant Intake Including Polyphenol Content: Is it Capable to Fight against Increased Oxidants within the Body of Ultra-Endurance Athletes? Nutrients 2020, 12, 1877. [Google Scholar] [CrossRef] [PubMed]
- Setyaningsih, W.; Saputro, I.E.; Palma, M.; Barroso, C.G. Optimisation and validation of the microwave-assisted extraction of phenolic compounds from rice grains. Food Chem. 2015, 169, 141–149. [Google Scholar] [CrossRef]
- Solana, M.; Mirofci, S.; Bertucco, A. Production of phenolic and glucosinolate extracts from rocket salad by supercritical fluid extraction: Process design and cost benefits analysis. J. Food Eng. 2016, 168, 35–41. [Google Scholar] [CrossRef]
- Grunovaité, L.; Pukalskiené, M.; Pukalskas, A.; Venskutonis, P.R. Fractionation of black chokeberry pomace into functional ingredients using high pressure extraction methods and evaluation of their antioxidant capacity and chemical composition. J. Funct. Foods 2016, 24, 85–96. [Google Scholar] [CrossRef]
- Heleno, S.A.; Diz, P.; Prieto, M.A.; Barros, L.; Rodrigues, A.; Barreiro, M.F.; Ferreira, I.C.F.R. Optimization of ultrasound-assisted extraction to obtain mycosterols from Agaricus bisporus L. by response surface methodology and comparison with conventional Soxhlet extraction. Food Chem. 2016, 197, 1054–1063. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.F.; Liu, Z.L. Optimization and comparison of ultrasound/microwave assisted extraction (UMAE) and ultrasonic assisted extraction (UAE) of lycopene from tomatoes. Ultrason. Sonochem. 2008, 15, 731–737. [Google Scholar]
- Sharma, S.; Kori, S.; Parmar, A. Surfactant mediated extraction of total phenolic contents (TPC) and antioxidants from fruits juices. Food Chem. 2015, 185, 284–288. [Google Scholar] [CrossRef]
- He, S.; Shi, J.; Walid, E.; Ma, Y.; Xue, S.J. Extraction and purification of a lectin from small black kidney bean (Phaseolus vulgaris) using a reversed micellar system. Process Biochem. 2013, 20, 746–752. [Google Scholar] [CrossRef]
- Li, Y.Z.; Jiang, H.B. Research progress on chemical constituents and pharmacological effects of Wrinkled papaya. Chem. Technol. Dev. 2018, 7, 35–38. [Google Scholar]
- Zhang, S.Y.; Han, L.Y.; Zhang, H.; Xin, H.L. Chaenomeles speciosa: A review of chemistry and pharmacology. Biomed. Rep. 2014, 2, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Tang, K.; Yan, J. Research on the process of microwave-surfactant synergistic extraction of Momordica charantia saponins. Food Sci. Technol. 2015, 40, 181–184. [Google Scholar]
- Wang, X.L.; Wang, W.J.; Zhong, F.L.; Liu, Y. Study on Microwave Assisted Extraction of Total Flavonoids from Platycodon grandiflorum Stem with Surfactant and Its Antioxidant Activity. J. Henan Univ. Technol. Nat. Sci. Ed. 2016, 37, 86–91. [Google Scholar]
- Guo, M.; Shi, H.L.; Yang, H.; Gao, L.X. Optimization of the extraction process and antioxidant activity of total polyphenols in the constellation cabbage. Food Oils 2021, 34, 113–117. [Google Scholar]
- Zhu, X.; Guo, H.Y.; Tan, X.H.; Yu, X.J.; Zhang, Y.; Wang, F.; Deng, J.H. Study on the extraction process of total flavonoids from Maoyanberry by ultrasonic-anionic/cationic surfactants. Food Ind. Sci. Technol. 2016, 37, 213–218. [Google Scholar]
- Guo, H.Y.; Li, D.; Lei, X.; Wang, X.J.; Gang, Y.; Li, W.H. Response surface optimization and kinetic analysis of Zanthoxylum bungeanum polyphenols extraction process. Food Sci. 2018, 39, 247–253. [Google Scholar]
- Li, R.; Lu, X.X.; Zhang, D.W.; Huang, S.Y. Study on extraction technology of total phenolic acids and total triterpenoids from Imperata cylindrica by comprehensive evaluation method. Food Res. Dev. 2013, 34, 77–80. [Google Scholar]
- Lin, X.Q.; Ma, Z.H.; He, Z.M.; Chen, Y.; Peng, Y.R.; Lin, S.Z. Optimization of polyphenol extraction from Chinese fir seeds. J. For. Environ. 2017, 37, 47–53. [Google Scholar]
- Dadi, D.W.; Emire, S.A.; Hagos, A.D.; Bang, J. Effect of ultrasound-assisted extraction of moringa stenopetala leaves on bioactive compounds and their antioxidant activity. Food Technol. Biotechnol. 2019, 57, 77–86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shuai, L.; Liao, L.Y.; Duan, Z.H.; Xie, Y.H.; Liu, Y.F. Optimization of extraction technology of polysaccharides from passion fruit peel and its antioxidant activity. Sci. Technol. Food Ind. 2020, 41, 150–156. [Google Scholar]
- Sun, X.; Liu, X.; Zhao, G.R. Separation of salidroside from the fermentation broth of engineered Escherichia coli using macroporous adsorbent resins. Chin. J. Chem. Eng. 2021, 38, 2833–2840. [Google Scholar] [CrossRef]
- Wang, Y.H.; Zhou, M.; Dai, K.W.; Yang, Y.J. Purification technology of total triterpenoids in Orostachys fimbriatus (Turcz) Berger. with macroporous resin. Mol. Plant Breed. 2017, 15, 4654–4658. [Google Scholar]
- Jin, W.F.; Li, B.; Lu, S.C.; Bu, J.; Wang, Z.; Yu, Y.; Sun, Y.P.; Wang, G.; Wang, G.K. Purification process of total triterpenes from Kalimeris indica using macroporous resin. J. Anhui Univ. Tradit. Chin. Med. 2017, 36, 92–94. [Google Scholar]
- Hu, F.X.; Liu, C.; Wang, F.Q.; Zhou, C.X.; Zhu, M.T.; Sun-Waterhouse, D.X.; Wang, Z.S. Phenolic compounds from Chaenomeles speciosa alleviate inflammation in lipopolysaccharide-treated RAW264.7 macrophages via the NF-κB and MAPK pathways. Food Sci. Hum. Wellness 2022, Accepted, in press. [Google Scholar]
- Chen, N.; Xiang, J.; Liu, Y.; Li, H.; Yang, B. Preparation and characterization of antioxidant flavonoid-enriched extract from saffron by-product: A combination of ultrasound-assisted extraction and macroporous resin purification. Chem. Pap. 2021, 75, 2749–2763. [Google Scholar] [CrossRef]
- Mahindrakar, K.V.; Rathod, V.K. Ultrasonic assisted aqueous extraction of catechin and gallic acid from Syzygium cumini seed kernel and evaluation of total phenolic, flavonoid contents and antioxidant activity-sciencedirect. Chem. Eng. Process. Process Intensif. 2020, 149, 1–7. [Google Scholar] [CrossRef]
- Fan, Z.L.; Li, L.; Bai, X.L.; Zhang, H.; Liu, Q.R.; Zhang, H.; Fu, Y.J.; Moyo, R. Extraction optimization, antioxidant activity, and tyrosinase inhibitory capacity of polyphenols from Lonicera japonica. Food Sci. Nutr. 2019, 7, 1786–1794. [Google Scholar] [CrossRef] [Green Version]
- Du, H.; Wu, J.; Li, H.; Zhong, P.X.; Xu, P.X.; Li, C.H.; Ji, K.X.; Wang, L.S. Polyphenols and triterpenes from Chaenomeles fruits: Chemical analysis and antioxidant activities assessment. Food Chem. 2013, 141, 4260–4268. [Google Scholar] [CrossRef]
- Niedzielska, E.; Smaga, I.; Gawlik, M.; Moniczewski, A.; Stankowicz, P.; Pera, J.; Filip, M. Oxidative stress in neurodegenerative diseases. Mol. Neuro Biol. 2016, 53, 4094–4125. [Google Scholar] [CrossRef] [Green Version]
- Olsvik, P.A.; Kristensen, T.; Waagbo, R.; Rosseland, B.O.; Tollefsen, K.E.; Baeverfjord, G.; Berntssen, M.H.G. mRNA expression of antioxidant enzymes (SOD, CAT and GSH-Px) and lipid peroxidative stress in liver of Atlantic salmon (Salmo salar) exposed to hyperoxic water during smoltification. Comp. Biochem. Physiol. Toxicol. Pharmacol. 2005, 141, 314–323. [Google Scholar] [CrossRef]
- Nurhanani, R.; Sarni, M.J.; Azlina, A.A. Polyphenols from the extract and fraction of T. indica seeds protected HepG2 cells against oxidative stress. Bmc Complement. Altern. Med. 2015, 15, 438–457. [Google Scholar]
- Cores, Á.; Piquero, M.; Villacampa, M.; León, R.; Menéndez, J.C. NRF2 Regulation Processes as a Source of Potential Drug Targets against Neurodegenerative Diseases. BioMolecules 2020, 10, 904. [Google Scholar] [CrossRef]
- Lee, K.M.; Kwon, T.Y.; Kang, U.; Seo, E.K.; Kim, Y.S. Tussilagonone-induced Nrf2 pathway activation protects HepG2 cells from oxidative injury. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2017, 108, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Miao, J.; Zhao, C.; Li, X.; Chen, X.T.; Mao, X.H.; Huang, H.H.; Wang, T.T.; Gao, W.Y. Chemical Composition and Bioactivities of Two Common Chaenomeles Fruits in China: Chaenomeles speciosa and Chaenomeles sinensis. J. Food Sci. 2016, 81, 2049–2058. [Google Scholar] [CrossRef]
- Yao, Y.; Wang, H.; Xu, F.; Zhang, Y.; Wang, L. Insoluble-bound polyphenols of adlay seed ameliorate h2o2-induced oxidative stress in hepg2 cells via nrf2 signalling. Food Chem. 2020, 325, 126865. [Google Scholar] [CrossRef]
- Chen, K.; Jia, Y.; Yong, T.; Zhou, Y.; Peng, L.; Dan, Z.; Zhou, Q.; Zhang, T.; Zhu, J.; Mi, M. Supplementation of superfine powder prepared from chaenomeles speciosa fruit increases endurance capacity in rats via antioxidant and nrf2/are signaling pathway. Evid. Based Complement. Altern. Med. 2014, 2014, 976438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, R.; Zhang, Y.Y.; Lan, J.N.; Meng, Q.T. Ischemic Postconditioning Alleviates Intestinal Ischemia-Reperfusion Injury by Enhancing Autophagy and Suppressing Oxidative Stress through the Akt/GSK-3β/Nrf2 Pathway in Mice. Oxidative Med. Cell Longev. 2020, 2020, 6954764. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cui, L.; Zhang, Z.; Li, H.; Li, N.; Li, X.; Chen, T. Optimization of ultrasound assisted extraction of phenolic compounds and anthocyanins from perilla leaves using response surface methodology. Food Sci. Technol. Res. 2017, 23, 535–543. [Google Scholar] [CrossRef] [Green Version]
- Yan, J.L.; Fan, Y.; Qin, C.; Ou, X.; Chen, S.J.; Ao, X.L. Purification of total flavonoids from bamboo shoots of Pleioblastus amarus with macroporous resin and its anti-inflammation activity. Food Ferment. Ind. 2020, 46, 184–192. [Google Scholar]
- Cheng, H.; Liu, X.H.; Wang, C.; Wu, Z.; Wang, Y. Optimization of purification conditions for areca seeds using microporous resins. J. Food Meas. Charact. 2021, 15, 2440–2447. [Google Scholar] [CrossRef]
- Chang, H.W.; Tan, T.B.; Tan, P.Y.; Abas, F.; Lai, O.M.; Wang, Y.; Nehdi, N.; Tan, C.P. Microencapsulation of fish oil using thiol-modified β-lactoglobulin fibrils/chitosan complex: A study on the storage stability and invitro release. Food Hydrocoll. 2018, 80, 186–194. [Google Scholar] [CrossRef]
- Qiu, D.; Guo, J.; Yu, H.; Yan, J.; Yang, S.; Li, X.; Qin, J.C.; Zhang, Y.; Sun, J.; Cong, J.; et al. Antioxidant phenolic compounds isolated from wild Pyrus ussuriensis Maxim. fruit peels and leaves. Food Chem. 2018, 241, 182–187. [Google Scholar] [CrossRef] [PubMed]



| Run | Coded Variable Levels | Phenolic Yield (mg/g) | |||||
|---|---|---|---|---|---|---|---|
| X1 | X2 | X3 | X4 | X5 | X6 | ||
| 1 | −1 (0.2) | 1 (70) | 1 (1:35) | −1 (40) | 1 (40) | 1 (300) | 28.82 ± 0.21 |
| 2 | −1 (0.2) | 1 (70) | −1 (1:25) | 1 (60) | 1 (40) | −1 (100) | 27.82 ± 0.26 |
| 3 | −1 (0.2) | 1 (70) | 1 (1:35) | 1 (60) | −1 (20) | −1 (100) | 28.79 ± 0.30 |
| 4 | 1 (0.6) | 1 (70) | 1 (1:35) | −1 (40) | −1 (20) | −1 (100) | 28.09 ± 0.27 |
| 5 | 1 (0.6) | 1 (70) | −1 (1:25) | 1 (60) | 1 (40) | 1 (300) | 27.14 ± 0.37 |
| 6 | 1 (0.6) | −1 (50) | 1 (1:35) | 1 (60) | −1 (20) | 1 (300) | 32.48 ± 0.38 |
| 7 | 1 (0.6) | −1 (50) | 1 (1:35) | 1 (60) | 1 (40) | −1 (100) | 31.62 ± 1.29 |
| 8 | −1 (0.2) | −1 (50) | −1 (1:25) | 1 (60) | −1 (20) | 1 (300) | 30.02 ± 0.43 |
| 9 | −1 (0.2) | −1 (50) | 1 (1:35) | −1 (40) | 1 (40) | 1 (300) | 31.25 ± 0.80 |
| 10 | −1 (0.2) | −1 (50) | −1 (1:25) | −1 (40) | −1 (20) | −1 (100) | 29.04 ± 0.34 |
| 11 | 1 (0.6) | 1 (70) | −1 (1:25) | −1 (40) | −1 (20) | 1 (300) | 25.66 ± 0.38 |
| 12 | 1 (0.6) | −1 (50) | −1 (1:25) | −1 (40) | 1 (40) | −1 (100) | 28.04 ± 0.16 |
| Source | Sum of Squares | df | Mean Square | F Value | p-Value Probe > F |
|---|---|---|---|---|---|
| Model | 0.48 | 6 | 0.080 | 14.44 | 0.0051 |
| X1-X1 | 2.437 × 10−3 | 1 | 2.437 × 10−3 | 0.44 | 0.5360 |
| X2-X2 | 0.24 | 1 | 0.24 | 44.27 | 0.0012 ** |
| X3-X3 | 0.17 | 1 | 0.17 | 30.98 | 0.0026 ** |
| X4-X4 | 0.053 | 1 | 0.053 | 9.58 | 0.0270 * |
| X5-X5 | 1.268 × 10−4 | 1 | 1.268 × 10−4 | 0.023 | 0.8855 |
| X6-X6 | 7.351 × 10−3 | 1 | 7.351 × 10−3 | 1.33 | 0.3008 |
| Residual | 0.028 | 5 | 5.523 × 10−3 | ||
| Cor Total | 0.51 | 11 |
| Number | Coded Variable Levels | Phenolic Yield (mg/g) | ||
|---|---|---|---|---|
| X2 | X3 | X4 | ||
| 1 | 0 (60) | 0 (1:30) | 0 (50) | 32.23 ± 0.58 |
| 2 | −1 (50) | −1 (1:25) | 0 (50) | 30.21 ± 0.88 |
| 3 | −1 (50) | 0 (1:30) | 1 (60) | 30.41 ± 0.68 |
| 4 | −1 (50) | 1 (1:35) | 0 (50) | 31.54 ± 0.80 |
| 5 | 0 (60) | −1 (1:25) | 1 (60) | 30.05 ± 0.34 |
| 6 | 1 (70) | 0 (1:30) | 1 (60) | 28.42 ± 0.36 |
| 7 | 1 (70) | 0 (1:30) | −1 (40) | 27.58 ± 0.37 |
| 8 | 0 (60) | 1 (1:35) | −1 (40) | 30.35 ± 0.80 |
| 9 | 0 (60) | 1 (1:35) | 1 (60) | 30.78 ± 0.59 |
| 10 | 0 (60) | −1 (1:25) | −1 (40) | 29.45 ± 0.89 |
| 11 | 0 (60) | 0 (1:30) | 0 (50) | 32.46 ± 0.45 |
| 12 | 1 (70) | −1 (1:25) | 0 (50) | 29.15 ± 0.32 |
| 13 | −1 (50) | 0 (1:30) | −1 (40) | 28.81 ± 0.99 |
| 14 | 0 (60) | 0 (1:30) | 0 (50) | 32.55 ± 0.32 |
| 15 | 1 (70) | 1 (1:35) | 0 (50) | 29.98 ± 0.17 |
| 16 | 0 (60) | 0 (1:30) | 0 (50) | 32.05 ± 0.58 |
| 17 | 0 (60) | 0 (1:30) | 0 (50) | 32.47 ± 0.45 |
| Source | Sum of Squares | df | Mean Square | F Value | p-Value Probe > F |
|---|---|---|---|---|---|
| Model | 0.38 | 9 | 0.043 | 47.48 | <0.0001 *** |
| X2 | 0.043 | 1 | 0.043 | 47.92 | 0.0002 *** |
| X3 | 0.017 | 1 | 0.017 | 19.13 | 0.0033 ** |
| X4 | 0.016 | 1 | 0.016 | 17.91 | 0.0039 ** |
| X2X3 | 6.250 × 10−4 | 1 | 6.250 × 10−4 | 0.70 | 0.4296 |
| X2X4 | 1.444 × 10−3 | 1 | 1.444 × 10−3 | 1.62 | 0.8433 |
| X3X4 | 1.822 × 10−4 | 1 | 1.822 × 10−4 | 0.20 | 0.6645 |
| X22 | 0.13 | 1 | 0.13 | 150.74 | <0.0001 *** |
| X32 | 6.983 × 10−3 | 1 | 6.983 × 10−3 | 7.85 | 0.0265 * |
| X42 | 0.14 | 1 | 0.14 | 157.14 | <0.0001 *** |
| Residual | 6.228 × 10−3 | 7 | 8.897 × 10−4 | ||
| Lack of Fit | 3.091 × 10−3 | 3 | 1.030 × 10−3 | 1.31 | 0.3861 |
| Fure Error | 3.137 × 10−3 | 4 | 7.842 × 10−4 | ||
| Cor Total | 0.39 | 16 | |||
| R2 = 0.9840 | R2adj = 0.9634 | C.V. = 0.98 |
| Resin Type | Adsorption Capacity (mg/g) | Adsorption Rate (%) | Desorption Rate (%) | Recovery Rate (%) |
|---|---|---|---|---|
| AB-8 | 21.50 ± 0.47 c | 78.83 ± 1.05 c | 89.51 ± 3.24 a | 70.57 ± 3.03 b |
| D101 | 22.94 ± 0.63 b | 82.73 ± 1.27 c | 84.07 ± 2.88 b | 69.56 ± 2.93 b |
| LX-8 | 23.85 ± 0.96 ab | 87.64 ± 2.05 b | 29.37 ± 2.67 d | 25.71 ± 2.01 d |
| S-8 | 24.89 ± 0.67 a | 88.70 ± 1.50 b | 1.60 ± 1.63 e | 1.50 ± 1.45 e |
| LSA-900C | 25.19 ± 0.28 a | 90.72 ± 0.81 a | 84.84 ± 4.22 b | 76.95 ± 3.13 a |
| LSA-900E | 25.15 ± 0.33 a | 90.20 ± 0.47 ab | 71.48 ± 0.06 c | 64.44 ± 0.28 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, F.; Li, F.; Zheng, Z.; Sun-Waterhouse, D.; Wang, Z. Surfactant-Mediated Ultrasonic-Assisted Extraction and Purification of Antioxidants from Chaenomeles speciosa (Sweet) Nakai for Chemical- and Cell-Based Antioxidant Capacity Evaluation. Molecules 2022, 27, 7970. https://doi.org/10.3390/molecules27227970
Hu F, Li F, Zheng Z, Sun-Waterhouse D, Wang Z. Surfactant-Mediated Ultrasonic-Assisted Extraction and Purification of Antioxidants from Chaenomeles speciosa (Sweet) Nakai for Chemical- and Cell-Based Antioxidant Capacity Evaluation. Molecules. 2022; 27(22):7970. https://doi.org/10.3390/molecules27227970
Chicago/Turabian StyleHu, Fuxia, Feng Li, Zhenjia Zheng, Dongxiao Sun-Waterhouse, and Zhaosheng Wang. 2022. "Surfactant-Mediated Ultrasonic-Assisted Extraction and Purification of Antioxidants from Chaenomeles speciosa (Sweet) Nakai for Chemical- and Cell-Based Antioxidant Capacity Evaluation" Molecules 27, no. 22: 7970. https://doi.org/10.3390/molecules27227970





