Macrocyclic Ionic Liquids with Amino Acid Residues: Synthesis and Influence of Thiacalix[4]arene Conformation on Thermal Stability
Abstract
:1. Introduction
2. Results and Discussion
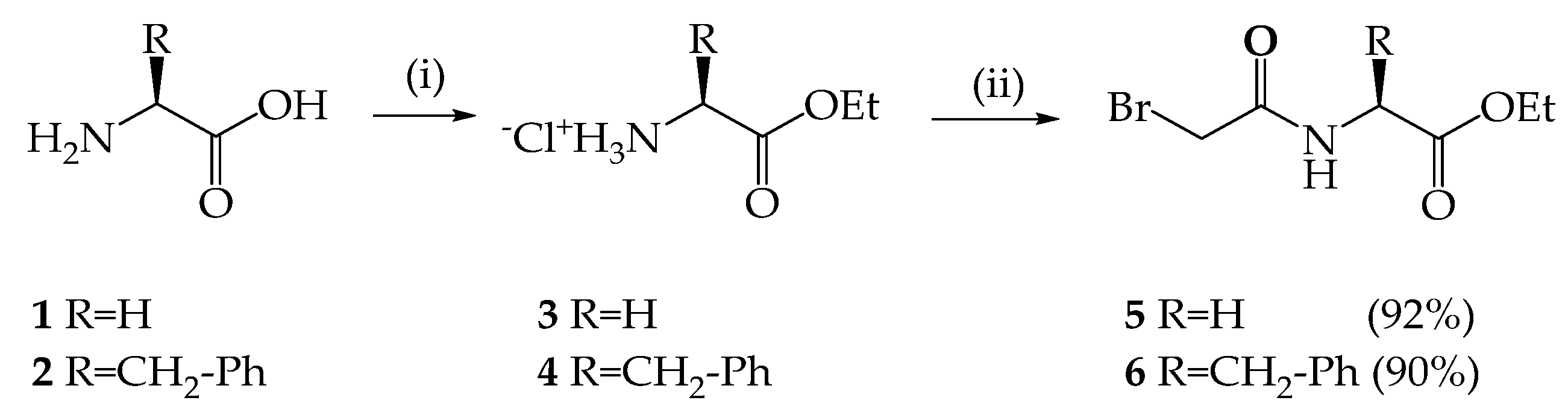
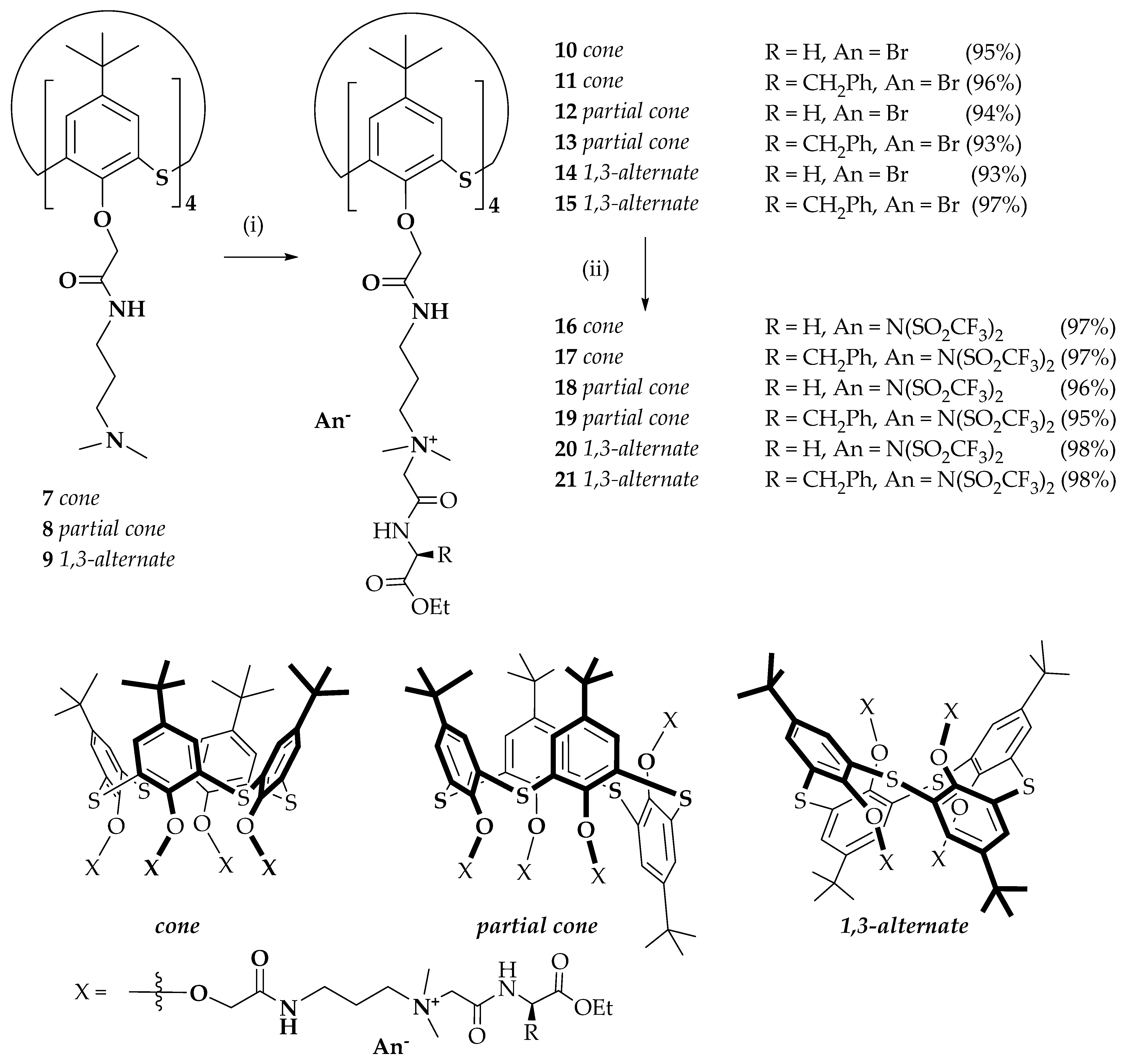
2.1. Synthesis of p-tert-butylthiacalix[4]arenes Containing Quaternary Ammonium Groups and Fragments of Glycine and L-phenylalanine
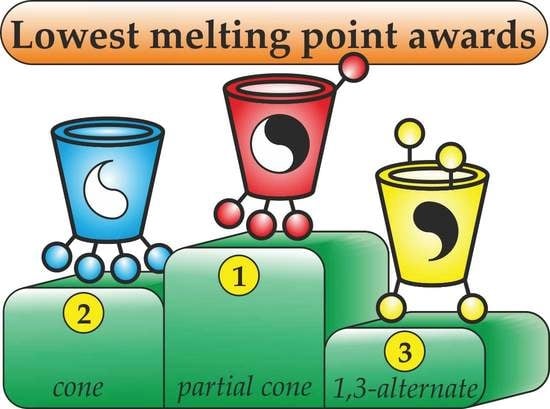
2.2. The Study of Thermal Stability of the Obtained Thiacalix[4]arene Based ILs
3. Materials and Methods
3.1. General
3.2. Procedure for the Synthesis of the Compound 4
L–Phenylalanine Ethyl Ester Hydrochloride (4) [66]
3.3. Procedure for the Synthesis of the Compound 6
N-Bromoacetyl-L-Phenylalanine Ethyl Ester (6) [66]
3.4. General Procedure for the Synthesis of the Compounds 10–15
3.4.1. 5,11,17,23-Tetra-tert-butyl-25,26,27,28-tetrakis{N-[3′-(dimethyl{[(S)-ethoxycarbonylbenzylmethyl]aminocarbonylmethyl}ammonio)propyl]aminocarbonylmethoxy}-2,8,14,20-thiacalix[4]arene Tetrabromide in cone Conformation (11)
3.4.2. 5,11,17,23-Tetra-tert-butyl-25,26,27,28-tetrakis{N-[3′-(dimethyl{[ethoxycarbonylmethyl]aminocarbonylmethyl}ammonio)propyl]aminocarbonylmethoxy}-2,8,14,20-tetrathiacalix[4]arene Tetrabromide in partial cone Conformation (12)
3.4.3. 5,11,17,23-Tetra-tert-butyl-25,26,27,28-tetrakis{N-[3′-(dimethyl{[(S)-ethoxycarbonylbenzylmethyl]aminocarbonylmethyl}ammonio)propyl]aminocarbonylmethoxy}-2,8,14,20-thiacalix[4]arene Tetrabromide in partial cone Conformation (13)
3.4.4. 5,11,17,23-Tetra-tert-butyl-25,26,27,28-tetrakis{N-[3′-(dimethyl{[(S)-ethoxycarbonylbenzylmethyl]aminocarbonylmethyl}ammonio)propyl]aminocarbonylmethoxy}-2,8,14,20-thiacalix[4]arene tetrabromide in 1,3-alternate Conformation (15)
3.5. General Procedure for the Synthesis of the Compounds 16–21
3.5.1. 5,11,17,23-Tetra-tert-butyl-25,26,27,28-tetrakis{N-[3′-(dimethyl{[(S)-ethoxycarbonylbenzylmethyl]aminocarbonylmethyl}ammonio)propyl]aminocarbonylmethoxy}-2,8,14,20-thiacalix[4]arene tetra[bis(trifluoromethylsulfonyl)imide] in cone Conformation (17)
3.5.2. 5,11,17,23-Tetra-tert-butyl-25,26,27,28-tetrakis{N-[3′-(dimethyl{[ethoxycarbonylmethyl]aminocarbonylmethyl}ammonio)propyl]aminocarbonylmethoxy}-2,8,14,20-tetrathiacalix[4]arene tetra[bis(trifluoromethylsulfonyl)imide] in partial cone Conformation (18)
3.5.3. 5,11,17,23-Tetra-tert-butyl-25,26,27,28-tetrakis{N-[3′-(dimethyl{[(S)-ethoxycarbonylbenzylmethyl]aminocarbonylmethyl}ammonio)propyl]aminocarbonylmethoxy}-2,8,14,20-thiacalix[4]arene tetra[bis(trifluoromethylsulfonyl)imide] in partial cone Conformation (19)
3.5.4. 5,11,17,23-Tetra-tert-butyl-25,26,27,28-tetrakis{N-[3′-(dimethyl{[(S)-ethoxycarbonylbenzylmethyl]aminocarbonylmethyl}ammonio)propyl]aminocarbonylmethoxy}-2,8,14,20-thiacalix [4]arene tetra[bis(trifluoromethylsulfonyl)imide] in 1,3-alternate Conformation (21)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Patil, K.R.; Surwade, A.D.; Rajput, P.J.; Shaikh, V.R. Investigations of Solute–Solvent Interactions in Aqueous Solutions of Amino Acids Ionic Liquids Having the Common Nitrate as Anion at Different Temperatures. J. Mol. Liq. 2021, 329, 115546. [Google Scholar] [CrossRef]
- Fabre, E.; Murshed, S.M.S. A Review of the Thermophysical Properties and Potential of Ionic Liquids for Thermal Applications. J. Mater. Chem. A 2021, 9, 15861–15879. [Google Scholar] [CrossRef]
- Nikfarjam, N.; Ghomi, M.; Agarwal, T.; Hassanpour, M.; Sharifi, E.; Khorsandi, D.; Ali Khan, M.; Rossi, F.; Rossetti, A.; Nazarzadeh Zare, E.; et al. Antimicrobial Ionic Liquid-Based Materials for Biomedical Applications. Adv. Funct. Mater. 2021, 31, 2104148. [Google Scholar] [CrossRef]
- Xu, C.; Yang, G.; Wu, D.; Yao, M.; Xing, C.; Zhang, J.; Zhang, H.; Li, F.; Feng, Y.; Qi, S.; et al. Roadmap on Ionic Liquid Electrolytes for Energy Storage Devices. Chem. Asian J. 2021, 16, 549–562. [Google Scholar] [CrossRef] [PubMed]
- Minea, A.A.; Sohel Murshed, S.M. Ionic Liquids-Based Nanocolloids—A Review of Progress and Prospects in Convective Heat Transfer Applications. Nanomaterials 2021, 11, 1039. [Google Scholar] [CrossRef]
- Buettner, C.S.; Cognigni, A.; Schröder, C.; Bica-Schröder, K. Surface-Active Ionic Liquids: A Review. J. Mol. Liq. 2022, 347, 118160. [Google Scholar] [CrossRef]
- Correia, D.M.; Fernandes, L.C.; Fernandes, M.M.; Hermenegildo, B.; Meira, R.M.; Ribeiro, C.; Ribeiro, S.; Reguera, J.; Lanceros-Méndez, S. Ionic Liquid-Based Materials for Biomedical Applications. Nanomaterials 2021, 11, 2401. [Google Scholar] [CrossRef]
- Maculewicz, J.; Świacka, K.; Stepnowski, P.; Dołżonek, J.; Białk-Bielińska, A. Ionic Liquids as Potentially Hazardous Pollutants: Evidences of Their Presence in the Environment and Recent Analytical Developments. J. Hazard. Mater. 2022, 437, 129353. [Google Scholar] [CrossRef]
- Zhuang, W.; Hachem, K.; Bokov, D.; Javed Ansari, M.; Taghvaie Nakhjiri, A. Ionic Liquids in Pharmaceutical Industry: A Systematic Review on Applications and Future Perspectives. J. Mol. Liq. 2022, 349, 118145. [Google Scholar] [CrossRef]
- Niu, H.; Wang, L.; Guan, P.; Zhang, N.; Yan, C.; Ding, M.; Guo, X.; Huang, T.; Hu, X. Recent Advances in Application of Ionic Liquids in Electrolyte of Lithium Ion Batteries. J. Energy Storage 2021, 40, 102659. [Google Scholar] [CrossRef]
- Rauber, D.; Hofmann, A.; Philippi, F.; Kay, C.W.M.; Zinkevich, T.; Hanemann, T.; Hempelmann, R. Structure-Property Relation of Trimethyl Ammonium Ionic Liquids for Battery Applications. Appl. Sci. 2021, 11, 5679. [Google Scholar] [CrossRef]
- Cho, C.-W.; Pham, T.P.T.; Zhao, Y.; Stolte, S.; Yun, Y.-S. Review of the Toxic Effects of Ionic Liquids. Sci. Total Environ. 2021, 786, 147309. [Google Scholar] [CrossRef] [PubMed]
- El Seoud, O.A.; Keppeler, N.; Malek, N.I.; Galgano, P.D. Ionic Liquid-Based Surfactants: Recent Advances in Their Syntheses, Solution Properties, and Applications. Polymers 2021, 13, 1100. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, A.R.P.; Paredes, X.; Cristino, A.F.; Santos, F.J.V.; Queirós, C.S.G.P. Ionic Liquids—A Review of Their Toxicity to Living Organisms. Int. J. Mol. Sci. 2021, 22, 5612. [Google Scholar] [CrossRef] [PubMed]
- Himani; Pratap Singh Raman, A.; Babu Singh, M.; Jain, P.; Chaudhary, P.; Bahadur, I.; Lal, K.; Kumar, V.; Singh, P. An Update on Synthesis, Properties, Applications and Toxicity of the ILs. J. Mol. Liq. 2022, 364, 119989. [Google Scholar] [CrossRef]
- Chen, Y.; Han, X.; Liu, Z.; Li, Y.; Sun, H.; Wang, H.; Wang, J. Thermal Decomposition and Volatility of Ionic Liquids: Factors, Evaluation and Strategies. J. Mol. Liq. 2022, 366, 120336. [Google Scholar] [CrossRef]
- Holbrey, J.D.; Seddon, K.R. The Phase Behaviour of 1-Alkyl-3-Methylimidazolium Tetrafluoroborates; Ionic Liquids and Ionic Liquid Crystals. J. Chem. Soc., Dalton Trans. 1999, 13, 2133–2140. [Google Scholar] [CrossRef]
- Shmukler, L.E.; Fedorova, I.V.; Fadeeva, Y.A.; Safonova, L.P. The Physicochemical Properties and Structure of Alkylammonium Protic Ionic Liquids of RnH4-nNX (n = 1–3) Family. A Mini–Review. J. Mol. Liq. 2021, 321, 114350. [Google Scholar] [CrossRef]
- Vereshchagin, A.N.; Frolov, N.A.; Egorova, K.S.; Seitkalieva, M.M.; Ananikov, V.P. Quaternary Ammonium Compounds (QACs) and Ionic Liquids (ILs) as Biocides: From Simple Antiseptics to Tunable Antimicrobials. Int. J. Mol. Sci. 2021, 22, 6793. [Google Scholar] [CrossRef]
- Zhuravlev, O.E.; Voronchikhina, L.I.; Gorbunova, D.V. Comparative Characteristics of Thermal Stability of Quaternary Ammonium and Pyridinium Tetrachloroferrates. Russ. J. Gen. Chem. 2022, 92, 348–354. [Google Scholar] [CrossRef]
- Egorova, K.S.; Gordeev, E.G.; Ananikov, V.P. Biological Activity of Ionic Liquids and Their Application in Pharmaceutics and Medicine. Chem. Rev. 2017, 117, 7132–7189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khachatrian, A.A.; Mukhametzyanov, T.A.; Yakhvarov, D.G.; Sinyashin, O.G.; Garifullin, B.F.; Rakipov, I.T.; Mironova, D.A.; Burilov, V.A.; Solomonov, B.N. Intermolecular Interactions between Imidazolium- and Cholinium-Based Ionic Liquids and Lysozyme: Regularities and Peculiarities. J. Mol. Liq. 2022, 348, 118426. [Google Scholar] [CrossRef]
- Melo, C.I.; Bogel-Łukasik, R.; Nunes da Ponte, M.; Bogel-Łukasik, E. Ammonium Ionic Liquids as Green Solvents for Drugs. Fluid Phase Equilib. 2013, 338, 209–216. [Google Scholar] [CrossRef] [Green Version]
- Bisht, M.; Jha, I.; Venkatesu, P. Comprehensive Evaluation of Biomolecular Interactions between Protein and Amino Acid Based-Ionic Liquids: A Comparable Study between [Bmim][Br] and [Bmim][Gly] Ionic Liquids. ChemistrySelect 2016, 1, 3510–3519. [Google Scholar] [CrossRef]
- Schindl, A.; Hagen, M.L.; Muzammal, S.; Gunasekera, H.A.D.; Croft, A.K. Proteins in Ionic Liquids: Reactions, Applications, and Futures. Front. Chem. 2019, 7, 347. [Google Scholar] [CrossRef] [Green Version]
- Ossowicz, P.; Klebeko, J.; Roman, B.; Janus, E.; Rozwadowski, Z. The Relationship between the Structure and Properties of Amino Acid Ionic Liquids. Molecules 2019, 24, 3252. [Google Scholar] [CrossRef] [Green Version]
- Miao, S.; Atkin, R.; Warr, G. Design and Applications of Biocompatible Choline Amino Acid Ionic Liquids. Green Chem. 2022, 24, 7281–7304. [Google Scholar] [CrossRef]
- Shukla, S.K.; Mikkola, J.-P. Use of Ionic Liquids in Protein and DNA Chemistry. Front. Chem. 2020, 8, 598662. [Google Scholar] [CrossRef]
- Egorova, K.S.; Posvyatenko, A.V.; Larin, S.S.; Ananikov, V.P. Ionic Liquids: Prospects for Nucleic Acid Handling and Delivery. Nucleic Acids Res. 2021, 49, 1201–1234. [Google Scholar] [CrossRef]
- Patel, R.; Kumari, M.; Khan, A.B. Recent Advances in the Applications of Ionic Liquids in Protein Stability and Activity: A Review. Appl. Biochem. Biotechnol. 2014, 172, 3701–3720. [Google Scholar] [CrossRef]
- Egorova, K.S.; Seitkalieva, M.M.; Posvyatenko, A.V.; Ananikov, V.P. An Unexpected Increase of Toxicity of Amino Acid-Containing Ionic Liquids. Toxicol. Res. 2015, 4, 152–159. [Google Scholar] [CrossRef]
- Yadav, R.; Kahlon, N.K.; Kumar, S.; Devunuri, N.; Venkatesu, P. Biophysical Study on the Phase Transition Behaviour of Biocompatible Thermoresponsive Polymer Influenced by Tryptophan-Based Amino Acid Ionic Liquids. Polymer 2021, 228, 123871. [Google Scholar] [CrossRef]
- Sahoo, D.K.; Jena, S.; Tulsiyan, K.D.; Dutta, J.; Chakrabarty, S.; Biswal, H.S. Amino-Acid-Based Ionic Liquids for the Improvement in Stability and Activity of Cytochrome c: A Combined Experimental and Molecular Dynamics Study. J. Phys. Chem. B 2019, 123, 10100–10109. [Google Scholar] [CrossRef]
- Durga, G.; Kalra, P.; Kumar Verma, V.; Wangdi, K.; Mishra, A. Ionic Liquids: From a Solvent for Polymeric Reactions to the Monomers for Poly(Ionic Liquids). J. Mol. Liq. 2021, 335, 116540. [Google Scholar] [CrossRef]
- Yang, B.; Yang, G.; Zhang, Y.-M.; Zhang, S.X.-A. Recent Advances in Poly(Ionic Liquid)s for Electrochromic Devices. J. Mater. Chem. C 2021, 9, 4730–4741. [Google Scholar] [CrossRef]
- Barrulas, R.V.; Zanatta, M.; Casimiro, T.; Corvo, M.C. Advanced Porous Materials from Poly(Ionic Liquid)s: Challenges, Applications and Opportunities. Chem. Eng. J. 2021, 411, 128528. [Google Scholar] [CrossRef]
- Thapaliya, B.P.; Puskar, N.G.; Slaymaker, S.; Feider, N.O.; Do-Thanh, C.-L.; Schott, J.A.; Jiang, D.; Teague, C.M.; Mahurin, S.M.; Dai, S. Synthesis and Characterization of Macrocyclic Ionic Liquids for CO2 Separation. Ind. Eng. Chem. Res. 2021, 60, 8218–8226. [Google Scholar] [CrossRef]
- Ogoshi, T.; Ueshima, N.; Yamagishi, T.; Toyota, Y.; Matsumi, N. Ionic Liquid Pillar[5]Arene: Its Ionic Conductivity and Solvent-Free Complexation with a Guest. Chem. Commun. 2012, 48, 3536. [Google Scholar] [CrossRef]
- Zhou, J.; Rao, L.; Yu, G.; Cook, T.R.; Chen, X.; Huang, F. Supramolecular Cancer Nanotheranostics. Chem. Soc. Rev. 2021, 50, 2839–2891. [Google Scholar] [CrossRef]
- Wang, M.; Fang, S.; Yang, S.; Li, Q.; Khashab, N.M.; Zhou, J.; Huang, F. Separation of Ethyltoluene Isomers by Nonporous Adaptive Crystals of Perethylated and Perbromoethylated Pillararenes. Mater. Today Chem. 2022, 24, 100919. [Google Scholar] [CrossRef]
- Selivanova, N.; Gubaidullin, A.; Padnya, P.; Stoikov, I.; Galyametdinov, Y. Phase Behaviour, Structural Properties and Intermolecular Interactions of Systems Based on Substituted Thiacalix[4]Arene and Nonionic Surfactants. Liq. Cryst. 2018, 46, 415–421. [Google Scholar] [CrossRef]
- Selivanova, N.M.; Zimina, M.V.; Padnya, P.L.; Stoikov, I.I.; Gubaidullin, A.T.; Galyametdinov, Y.G. Development of Efficient Luminescent Soft Media by Incorporation of a Hetero-Ligand Macrocyclic Terbium Complex into a Lyomesophase. Russ. Chem. Bull. 2020, 69, 1763–1770. [Google Scholar] [CrossRef]
- Shurpik, D.N.; Padnya, P.L.; Stoikov, I.I.; Cragg, P.J. Antimicrobial Activity of Calixarenes and Related Macrocycles. Molecules 2020, 25, 5145. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Guo, H.; Jiao, Z.; Li, C.; Ye, J. Calixarene Ionic Liquids: Excellent Phase Transfer Catalysts for Nucleophilic Substitution Reaction in Water. J. Iran. Chem. Soc. 2012, 9, 327–332. [Google Scholar] [CrossRef]
- Padnya, P.L.; Porfireva, A.V.; Evtugyn, G.A.; Stoikov, I.I. Solid Contact Potentiometric Sensors Based on a New Class of Ionic Liquids on Thiacalixarene Platform. Front. Chem. 2018, 6, 594. [Google Scholar] [CrossRef]
- Padnya, P.L.; Terenteva, O.S.; Akhmedov, A.A.; Iksanova, A.G.; Shtyrlin, N.V.; Nikitina, E.V.; Krylova, E.S.; Shtyrlin, Y.G.; Stoikov, I.I. Thiacalixarene Based Quaternary Ammonium Salts as Promising Antibacterial Agents. Bioorg. Med. Chem. 2021, 29, 115905. [Google Scholar] [CrossRef]
- Podyachev, S.N.; Zairov, R.R.; Mustafina, A.R. 1,3-Diketone Calix[4]Arene Derivatives—A New Type of Versatile Ligands for Metal Complexes and Nanoparticles. Molecules 2021, 26, 1214. [Google Scholar] [CrossRef]
- Iampolska, A.D.; Kharchenko, S.G.; Voitenko, Z.V.; Shishkina, S.V.; Ryabitskii, A.B.; Kalchenko, V.I. Synthesis of Thiacalix[4]Arene Task-Specific Ionic Liquids. Phosphorus Sulfur Silicon Relat. Elem. 2016, 191, 174–179. [Google Scholar] [CrossRef]
- Alishahi, N.; Mohammadpoor-Baltork, I.; Tangestaninejad, S.; Mirkhani, V.; Moghadam, M.; Kia, R. Calixarene Based Ionic Liquid as an Efficient and Reusable Catalyst for One-Pot Multicomponent Synthesis of Polysubstituted Pyridines and Bis-pyridines. ChemistrySelect 2019, 4, 5903–5910. [Google Scholar] [CrossRef]
- Padnya, P.L.; Andreyko, E.A.; Gorbatova, P.A.; Parfenov, V.V.; Rizvanov, I.K.; Stoikov, I.I. Towards Macrocyclic Ionic Liquids: Novel Ammonium Salts Based on Tetrasubstituted p-Tert-Butylthiacalix[4]Arenes. RSC Adv. 2017, 7, 1671–1686. [Google Scholar] [CrossRef]
- Zhang, S.; Sun, N.; He, X.; Lu, X.; Zhang, X. Physical Properties of Ionic Liquids: Database and Evaluation. J. Phys. Chem. Ref. Data 2006, 35, 1475–1517. [Google Scholar] [CrossRef]
- Xu, C.; Cheng, Z. Thermal Stability of Ionic Liquids: Current Status and Prospects for Future Development. Processes 2021, 9, 337. [Google Scholar] [CrossRef]
- Villanueva, M.; Coronas, A.; García, J.; Salgado, J. Thermal Stability of Ionic Liquids for Their Application as New Absorbents. Ind. Eng. Chem. Res. 2013, 52, 15718–15727. [Google Scholar] [CrossRef]
- Moldoveanu, S.C. Pyrolysis of Amino Acids and Small Peptides. In Pyrolysis of Organic Molecules, 2nd ed.; Moldoveanu, S.C., Ed.; Elsevier: London, UK, 2019; pp. 555–633. [Google Scholar] [CrossRef]
- Efimova, A.; Pfützner, L.; Schmidt, P. Thermal Stability and Decomposition Mechanism of 1-Ethyl-3-Methylimidazolium Halides. Thermochim. Acta 2015, 604, 129–136. [Google Scholar] [CrossRef]
- Lorenzo, M.; Vilas, M.; Verdía, P.; Villanueva, M.; Salgado, J.; Tojo, E. Long-Term Thermal Stabilities of Ammonium Ionic Liquids Designed as Potential Absorbents of Ammonia. RSC Adv. 2015, 5, 41278–41284. [Google Scholar] [CrossRef]
- Kurnia, K.A.; Wilfred, C.D.; Murugesan, T. Thermophysical Properties of Hydroxyl Ammonium Ionic Liquids. J. Chem. Thermodyn. 2009, 41, 517–521. [Google Scholar] [CrossRef]
- Pucci, F.; Rooman, M. Improved Insights into Protein Thermal Stability: From the Molecular to the Structurome Scale. Phil. Trans. R. Soc. A. 2016, 374, 20160141. [Google Scholar] [CrossRef] [Green Version]
- Wingreen, N.S.; Li, H.; Tang, C. Designability and Thermal Stability of Protein Structures. Polymer 2004, 45, 699–705. [Google Scholar] [CrossRef] [Green Version]
- Maheshwari, A.S.; Archunan, G. Distribution of Amino Acids in Functional Sites of Proteins with High Melting Temperature. Bioinformation 2012, 8, 1176–1181. [Google Scholar] [CrossRef]
- Jelesarov, I.; Karshikoff, A. Defining the Role of Salt Bridges in Protein Stability. Methods Mol. Biol. 2008, 490, 227–260. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.; Tsai, C.-J.; Nussinov, R. Factors Enhancing Protein Thermostability. Protein Eng. Des. Sel. 2000, 13, 179–191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Padnya, P.L.; Andreyko, E.A.; Mostovaya, O.A.; Rizvanov, I.K.; Stoikov, I.I. The Synthesis of New Amphiphilic P-Tert-Butylthiacalix[4]Arenes Containing Peptide Fragments and Their Interaction with DNA. Org. Biomol. Chem. 2015, 13, 5894–5904. [Google Scholar] [CrossRef] [PubMed]
- Andreyko, E.A.; Padnya, P.L.; Stoikov, I.I. Supramolecular Self-Assembly of Water-Soluble Nanoparticles Based on Amphiphilic p-Tert-Butylthiacalix[4]Arenes with Silver Nitrate and Fluorescein. Colloids Surf. A 2014, 454, 74–83. [Google Scholar] [CrossRef]
- Andreyko, E.A.; Padnya, P.L.; Daminova, R.R.; Stoikov, I.I. Supramolecular “Containers”: Self-Assembly and Functionalization of Thiacalix[4]Arenes for Recognition of Amino- and Dicarboxylic Acids. RSC Adv. 2014, 4, 3556–3565. [Google Scholar] [CrossRef]
- Nazarova, A.; Shurpik, D.; Padnya, P.; Mukhametzyanov, T.; Cragg, P.; Stoikov, I. Self-Assembly of Supramolecular Architectures by the Effect of Amino Acid Residues of Quaternary Ammonium Pillar[5]Arenes. Int. J. Mol. Sci. 2020, 21, 7206. [Google Scholar] [CrossRef]

| Compounds | Amino Acid Fragments/Anion | tBu | OCH2 | ArH | NHCH2CH2CH2N+ |
|---|---|---|---|---|---|
| 10 (cone) * | Gly/Br− | 1.12 | 5.03 | 7.36 | 8.68 |
| 11 (cone) | L-Phe/Br− | 1.07 | 4.81 | 7.39 | 8.50 |
| 12 (partial cone) | Gly/Br− | 1.00, 1.17, 1.27 | 4.40 (2JHH = 13.6 Hz), 4.49, 4.51, 4.80 (2JHH = 13.6 Hz) | 7.67, 7.01 (4JHH = 2.4 Hz), 7.65 (4JHH = 2.4 Hz), 7.75 | 8.36, 8.45, 8.50 |
| 13 (partial cone) | L-Phe/Br− | 1.00, 1.10, 1.27 | 4.42 (2JHH = 13.5 Hz), 4.48, 4.51, 4.79 (2JHH = 13.5 Hz) | 7.67, 7.01 (4JHH = 2.4 Hz), 7.65 (4JHH = 2.4 Hz), 7.75 | 8.31, 8.41, 8.48 |
| 14 (1,3-alternate) * | Gly/Br− | 1.20 | 3.99 | 7.60 | 8.04 |
| 15 (1,3-alternate) | L-Phe/Br− | 1.19 | 4.00 | 7.59 | 8.02 |
| 16 (cone) * | Gly/NTf2− | 1.11 | 4.89 | 7.35 | 8.48 |
| 17 (cone) | L-Phe/NTf2− | 1.06 | 4.79 | 7.38 | 8.48 |
| 18 (partial cone) | Gly/NTf2− | 1.00, 1.27, 1.30 | 4.39 (2JHH = 13.6 Hz), 4.49, 4.51, 4.78 (2JHH = 13.6 Hz) | 7.67, 7.01 (4JHH = 2.4 Hz), 7.65 (4JHH = 2.4 Hz), 7.75 | 8.31, 8.41, 8.50 |
| 19 (partial cone) | L-Phe/NTf2− | 1.00, 1.27, 1.28 | 4.40 (2JHH = 13.5 Hz), 4.49, 4.52, 4.79 (2JHH = 13.5 Hz) | 7.67, 7.02 (4JHH = 2.4 Hz), 7.65 (4JHH = 2.4 Hz), 7.75 | 8.28, 8.40, 8.48 |
| 20 (1,3-alternate) * | Gly/NTf2− | 1.20 | 3.99 | 7.59 | 8.03 |
| 21 (1,3-alternate) | L-Phe/NTf2− | 1.19 | 3.99 | 7.59 | 8.00 |
| Amino Acid Fragments | Br− | N(SO2CF3)2− | ||||
|---|---|---|---|---|---|---|
| Cone | Partial Cone | 1,3-alternate | Cone | Partial Cone | 1,3-alternate | |
| Gly | 114 * (10) | 105 (12) | 112 * (14) | 63 * (16) | 50 (18) | 73 * (20) |
| L-Phe | 118 (11) | 110 (13) | 123 (15) | 64 (17) | 55 (19) | 75 (21) |
| Compounds | Amino Acid Fragments | Tonset | T10% | T50% |
|---|---|---|---|---|
| 16(cone) | Gly | 320 | 320 | 405 |
| 18 (partial cone) | 324 | 321 | 365 | |
| 20 (1,3-alternate) | 327 | 329 | 391 | |
| 17 (cone) | L-Phe | 317 | 314 | 389 |
| 19 (partial cone) | 305 | 305 | 414 | |
| 21 (1,3-alternate) | 323 | 321 | 382 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Terenteva, O.; Bikmukhametov, A.; Gerasimov, A.; Padnya, P.; Stoikov, I. Macrocyclic Ionic Liquids with Amino Acid Residues: Synthesis and Influence of Thiacalix[4]arene Conformation on Thermal Stability. Molecules 2022, 27, 8006. https://doi.org/10.3390/molecules27228006
Terenteva O, Bikmukhametov A, Gerasimov A, Padnya P, Stoikov I. Macrocyclic Ionic Liquids with Amino Acid Residues: Synthesis and Influence of Thiacalix[4]arene Conformation on Thermal Stability. Molecules. 2022; 27(22):8006. https://doi.org/10.3390/molecules27228006
Chicago/Turabian StyleTerenteva, Olga, Azamat Bikmukhametov, Alexander Gerasimov, Pavel Padnya, and Ivan Stoikov. 2022. "Macrocyclic Ionic Liquids with Amino Acid Residues: Synthesis and Influence of Thiacalix[4]arene Conformation on Thermal Stability" Molecules 27, no. 22: 8006. https://doi.org/10.3390/molecules27228006
APA StyleTerenteva, O., Bikmukhametov, A., Gerasimov, A., Padnya, P., & Stoikov, I. (2022). Macrocyclic Ionic Liquids with Amino Acid Residues: Synthesis and Influence of Thiacalix[4]arene Conformation on Thermal Stability. Molecules, 27(22), 8006. https://doi.org/10.3390/molecules27228006