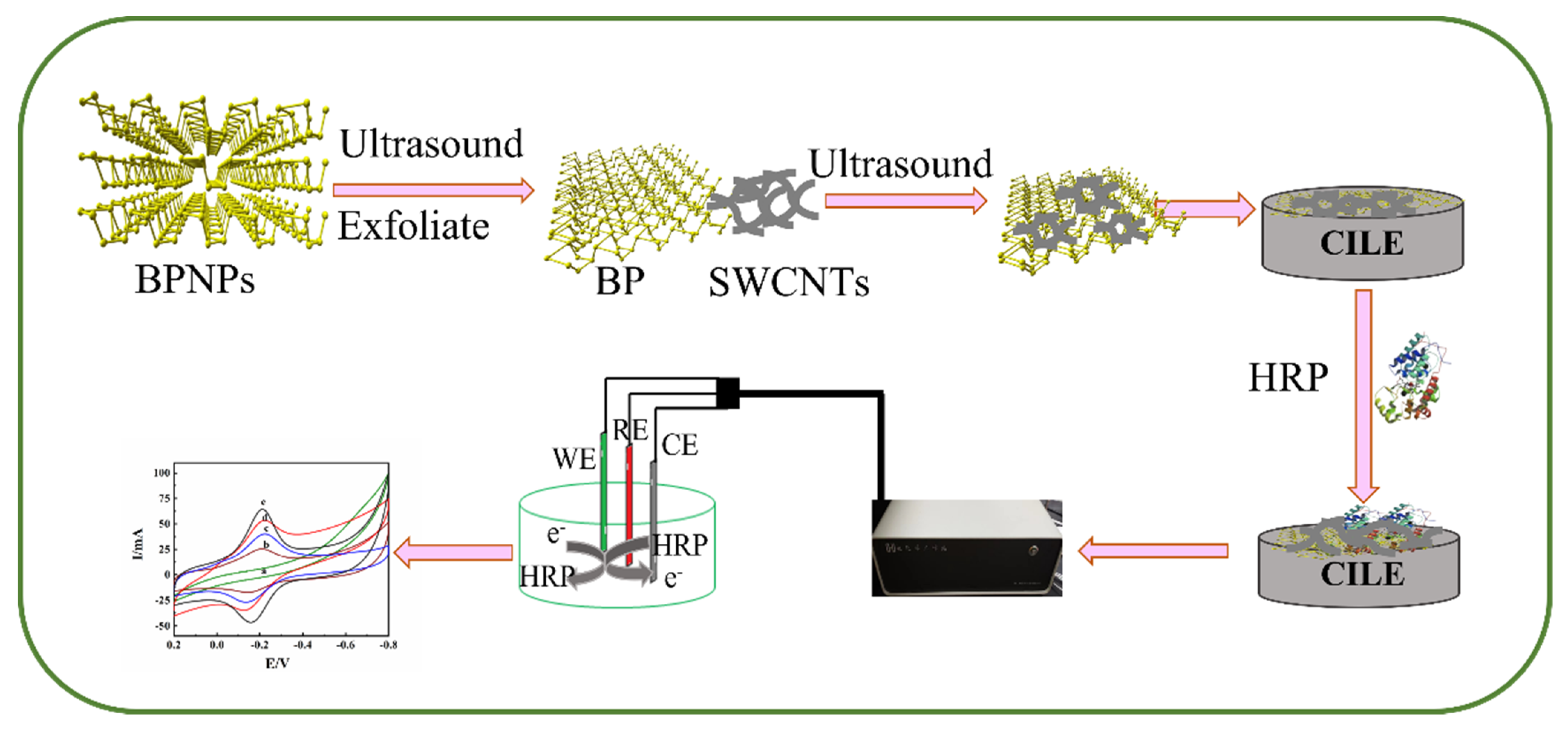
Preparation and Application of Electrochemical Horseradish Peroxidase Sensor Based on a Black Phosphorene and Single-Walled Carbon Nanotubes Nanocomposite
Abstract
:1. Introduction
2. Results and Discussion
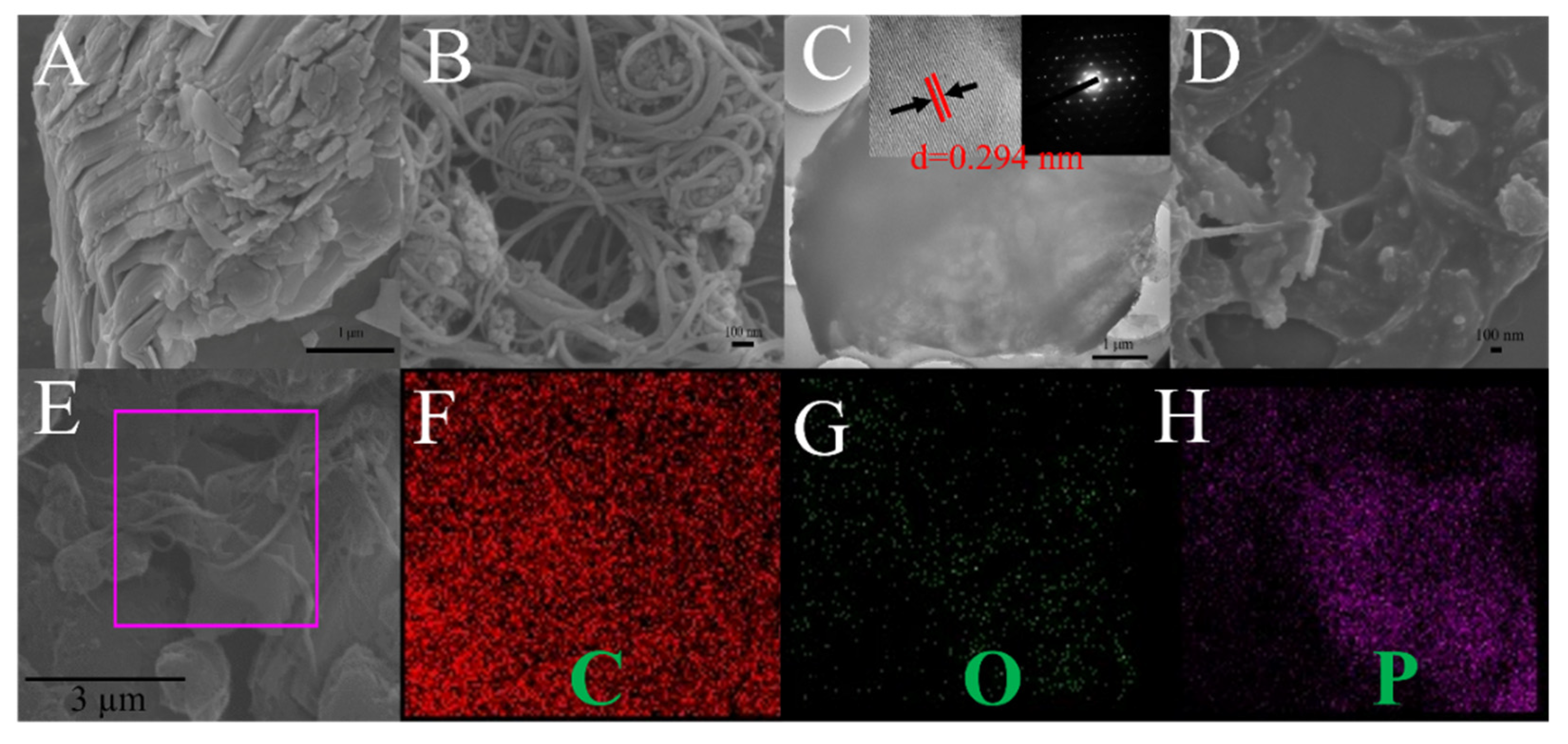
2.1. Characterization of SWCNTs-BP Nanocomposite
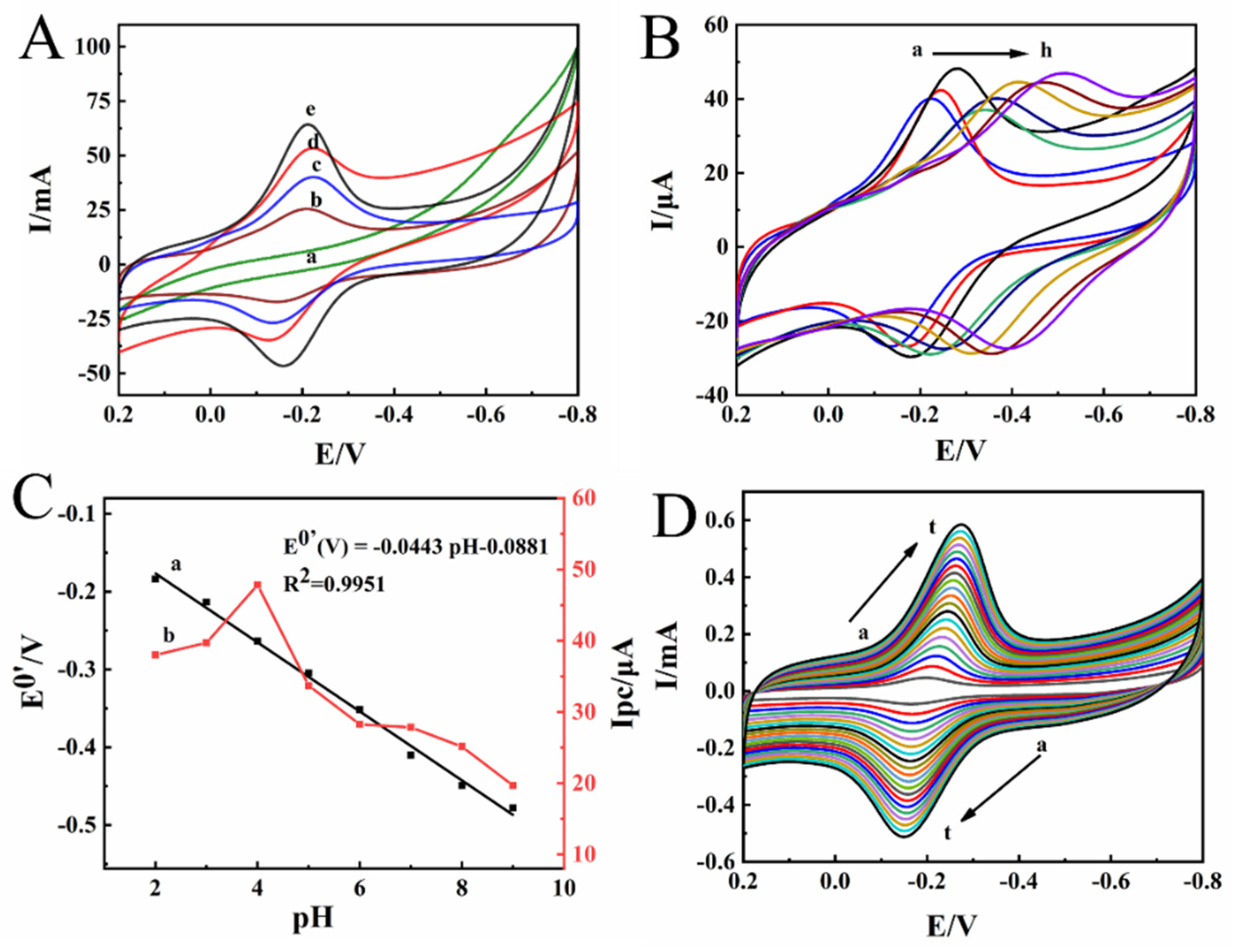
2.2. Electrochemical Properties of the Modified Electrodes
2.3. Direct Electrochemical Behavior of HRP
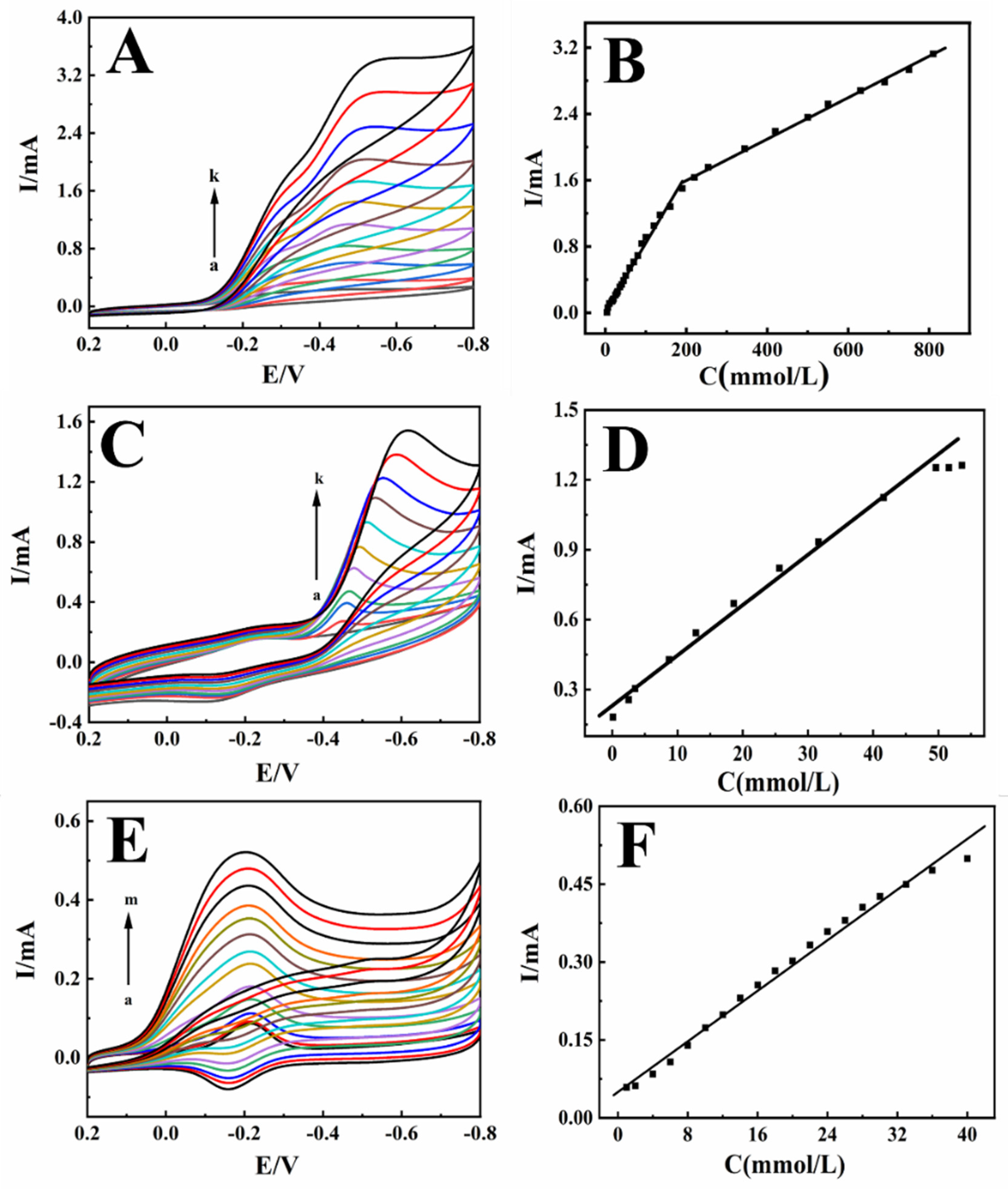
2.4. Electrocatalytic Activity
2.5. Analytical Applications
2.6. Stability and Reproducibility
3. Materials and Methods
3.1. Reagents and Chemicals
3.2. Instruments
3.3. Electrochemical Measurements
3.4. Preparation of SWCNTs-BP Nanocomposite
3.5. Preparation of Nafion/HRP/SWCNTs-BP/CILE
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Xu, J.W.; Ma, T.M.; Zhang, M.; Zeng, H. Electro-catalysis on H2O2 reduction of electrode capped by nanotubes-polypyrrole composite with hemoglobin integration. J. Mater. Sci. Mater. Electron. 2021, 32, 6064–6079. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, Y.H.; Ma, T.M.; Zeng, H. Comparison in electro-catalytic function to reduction of hydrogen peroxide for two nano-structure electrodes with myoglobin immobilization. Chem. Phy. Lett. 2020, 738, 136904. [Google Scholar] [CrossRef]
- Zhang, S.W.; Ma, T.M.; Zeng, H.; Wang, F. Direct electro-chemistry and electro-catalytic reduction on hydrogen peroxide of horseradish peroxidase based electrode on the basis of graphene oxide-magnetic nano-particle composite. J. Inorg. Organomet. Polym. Mater. 2021, 31, 741–755. [Google Scholar] [CrossRef]
- Niu, X.L.; Xie, H.; Luo, G.L.; Men, Y.L.; Zhang, W.L.; Sun, W. Platinum-3D graphene oxide areogel nanocomposite for direct electrochemistry and electrocatalysis of horseradish peroxidase. J. Electrochem. Soc. 2018, 165, B713–B719. [Google Scholar] [CrossRef]
- Liu, H.; Guo, K.; Lv, J.; Gao, Y.; Duan, C.Y.; Deng, L.; Zhu, Z.F. A novel nitrite biosensor based on the direct electrochemistry of horseradish peroxidase immobilized on porous Co3O4 nanosheets and reduced graphene oxide composite modified electrode. Sens. Actuators B 2017, 238, 249–256. [Google Scholar] [CrossRef] [Green Version]
- Horst, C.; Silwana, B.; Iwuoha, E.; Somerset, V. Electrocatalytic evaluation of a horseradish peroxidase biosensor based on a novel Bi-Ag bimetallic nanocomposite. Procedia Technol. 2017, 27, 179–182. [Google Scholar] [CrossRef]
- Choi, J.R.; Yong, K.W.; Choi, J.Y.; Nilghaz, A.; Lin, Y.; Xu, J.; Lu, X.N. Black phosphorus and its biomedical applications. Theranostics 2018, 8, 1005–1026. [Google Scholar] [CrossRef]
- Zhu, D.Q.; Zhang, M.L.; Pu, L.; Gai, P.P.; Li, F. Nitrogen-enriched conjugated polymer enabled metal-free carbon nanozymes with efficient oxidase-like activity. Small 2022, 18, 2104993. [Google Scholar] [CrossRef]
- Yu, L.; Chang, J.F.; Zhuang, X.Y.; Li, H.Y.; Hou, T.; Li, F. Two-dimensional cobalt-doped Ti3C2 MXene nanozyme-nediated homogeneous electrochemical strategy for pesticides assay based on in situ generation of electroactive substances. Anal. Chem. 2022, 94, 3669–3676. [Google Scholar] [CrossRef]
- Wu, J.H.; Lv, W.X.; Yang, Q.T.; Li, H.Y.; Li, F. Label-free homogeneous electrochemical detection of microRNA based on target-induced anti-shielding against the catalytic activity of two-dimension nanozyme. Biosens. Bioelectron. 2021, 171, 112707. [Google Scholar] [CrossRef]
- Huang, Y.H.; Yan, L.J.; Wang, B.; Zhu, L.; Shao, B.; Niu, Y.Y.; Zhang, X.P.; Yin, P.; Ge, Y.Q.; Sun, W.; et al. Recent applications of black phosphorus and its related composites in electrochemistry and bioelectrochemistry: A mini review. Electrochem. Commun. 2021, 129, 107095. [Google Scholar] [CrossRef]
- Ge, X.X.; Xia, Z.H.; Guo, S.J. Recent advances on black phosphorus for biomedicine and biosensing. Adv. Funct. Mater. 2019, 29, 1900318. [Google Scholar] [CrossRef]
- Li, K.X.; Qiao, X.J.; Zhao, H.Y.; He, Y.P.; Sheng, Q.L.; Yue, T.L. Ultrasensitive and label-free electrochemical aptasensor based on carbon dots-black phosphorus nanohybrid for the detection of ochratoxins A. Microchem. J. 2021, 168, 106378. [Google Scholar] [CrossRef]
- Yi, J.Q.; Chen, X.P.; Weng, Q.H.; Zhou, Y.; Han, Z.Z.; Chen, J.H.; Li, C.Y. A simple electrochemical pH sensor based on black phosphorus nanosheets. Electrochem. Commun. 2020, 118, 106796. [Google Scholar] [CrossRef]
- Liu, H.W.; Hu, K.; Yan, D.; Chen, R.; Zou, Y.Q.; Liu, H.B.; Wang, S.Y. Recent advances on black phosphorus for energy storage, catalysis, and sensor applications. Adv. Mater. 2018, 30, 1800295. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Wang, H.D.; Li, H.N.; Guo, Z.N.; Tian, B.N.; Cui, Y.X.; Li, Z.F.; Li, G.H.; Zhang, H.; Wu, Y.C. Recent advances in black phosphorus/carbon hybrid composites: From improved stability to applications. J. Mater. Chem. 2020, 8, 4647–4676. [Google Scholar] [CrossRef]
- Qiao, H.; Liu, H.T.; Huang, Z.Y.; Ma, Q.; Luo, S.W.; Li, J.; Liu, Y.D.; Zhong, J.X.; Qi, X. Black phosphorus nanosheets modified with Au nanoparticles as high conductivity and high activity electrocatalyst for oxygen evolution reaction. Adv. Energy Mater. 2020, 10, 2002424. [Google Scholar] [CrossRef]
- Wu, T.Y.; Ma, Z.Y.; He, Y.Y.; Wu, X.J.; Tang, B.; Yu, Z.Y.; Wu, G.; Chen, S.; Bao, N.Z. A covalent black phosphorus/metal-organic framework hetero-nanostructure for high-performance flexible supercapacitors. Angew. Chem. Int. Ed. 2021, 60, 10366–10374. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhang, Y.H.; Zhuge, Z.; Tang, Y.H.; Tao, J.W.; Chen, Y. Synthesis of a poly-l-Lysine/black phosphorus hybrid for biosensors. Anal. Chem. 2018, 90, 3149–3155. [Google Scholar] [CrossRef]
- Zhuge, Z.; Tang, Y.H.; Tao, J.W.; Zhao, Y. Functionalized black phosphorus nanocomposite for biosensing. ChemElectroChem 2019, 6, 1129–1133. [Google Scholar] [CrossRef]
- Shi, F.; Wang, B.L.; Yan, L.J.; Wang, B.; Niu, Y.Y.; Wang, L.S.; Sun, W. In-situ growth of nitrogen-doped carbonized polymer dots on black phosphorus for electrochemical DNA biosensor of Escherichia coli O157: H7. Bioelectrochemistry 2022, 148, 108226. [Google Scholar] [CrossRef] [PubMed]
- Beitollahi, H.; Movahedifar, F.; Tajik, S.; Jahani, S. A review on the effects of introducing CNTs in the modification process of electrochemical sensors. Electroanalysis 2018, 30, 1195–1203. [Google Scholar] [CrossRef]
- Sun, W.; Li, X.Q.; Wang, Y.; Zhao, R.J.; Jiao, K. Electrochemistry and electrocatalysis of hemoglobin on multi-walled carbon nanotubes modified carbon ionic liquid electrode with hydrophilic EMIMBF4 as modifier. Electrochim. Acta 2009, 54, 4141–4148. [Google Scholar] [CrossRef]
- Sun, W.; Li, X.Q.; Zhai, Z.Q.; Jiao, K. Direct electrochemistry of hemoglobin on single-walled carbon nanotubes modified carbon ionic liquid electrode and its electrocatalysis. Electroanalysis 2008, 24, 2649–2654. [Google Scholar] [CrossRef]
- Deshmuk, M.A.; Celiesiute, R.; Ramanaviciene, A.; Shirsat, M.D.; Ramanavicius, A. EDTA_PANI/SWCNTs nanocomposite modified electrode for electrochemical determination of copper (II), lead (II) and mercury (II) ions. Electrochim. Acta 2018, 259, 930–938. [Google Scholar] [CrossRef]
- Zhao, L.Y.; Liu, H.Y.; Hu, N.F. Assembly of layer-by-layer films of heme proteins and single-walled carbon nanotubes: Electrochemistry and electrocatalysis. Anal. Bioanal. Chem. 2006, 384, 414–422. [Google Scholar] [CrossRef]
- Sun, W.; Sun, Z.L.; Zhang, L.Q.; Qi, X.W.; Li, G.J.; Wu, J.; Wang, M. Application of Fe3O4 mesoporous sphere modified carbon ionic liquid electrode as electrochemical hemoglobin biosensor. Colloids Sur. B 2013, 101, 177–182. [Google Scholar] [CrossRef]
- Shiddiky, M.J.A.; Torriero, A.A.J. Application of ionic liquids in electrochemical sensing systems. Biosens. Bioelectron. 2011, 26, 1775–1787. [Google Scholar] [CrossRef]
- Opallo, M.; Lesniewski, A. A review on electrodes modified with ionic liquids. J. Electroanal. Chem. 2011, 656, 2–16. [Google Scholar] [CrossRef]
- Liu, J.; Cheng, H.; Xie, H.; Luo, G.L.; Niu, Y.Y.; Zhang, S.Y.; Li, G.J.; Sun, W. Platinum nanoparticles decorating a biomass porous carbon nanocomposite-modified electrode for the electrocatalytic sensing of luteolin and application. RSV Adv. 2019, 9, 33607. [Google Scholar] [CrossRef]
- Ma, Y.H.; Zhan, G.Q.; Ma, M.; Wang, X.; Li, C.Y. Direct electron transfer of hemoglobin in a biocompatible electrochemical system based on zirconium dioxide nanotubes and ionic liquid. Bioelectrochemistry 2012, 84, 6–10. [Google Scholar] [CrossRef]
- Machabaphala, M.K.; Hlekelele, L.; Dlamini, L.N. A heterostructure of black phosphorus and zirconium-based MOF as a photocatalyst for photocatalytic applications. Mater. Lett. 2020, 281, 128660. [Google Scholar] [CrossRef]
- Reddy, D.A.; Kim, E.H.; Gopannagari, M.; Kim, Y.; Kumar, D.P.; Kim, T.K. Few layered black phosphorus/MoS2 nanohybrid: A promising co-catalyst for solar driven hydrogen evolution. Appl. Catal. B 2019, 241, 491–498. [Google Scholar] [CrossRef]
- Wang, P.F.; Liu, Y.; Jiang, N.; Jing, R.S.; Li, S.T.; Zhang, Q.; Liu, H.Q.; Xiu, J.S.; Li, Z.; Liu, Y.Y. Double s-scheme AgBr heterojunction co-modified with g-C3N4 and black phosphorus nanosheets greatly improves the photocatalytic activity and stability. J. Mol. Liq. 2021, 329, 115540. [Google Scholar] [CrossRef]
- Li, Q.; Cen, Y.; Huang, J.Y.; Li, X.J.; Zhang, H.; Geng, Y.F.; Yakobson, B.I.; Du, Y.; Tian, X.Q. Zinc oxide-black phosphorus composites for ultrasensitive nitrogen dioxide sensing. Nanoscale Horiz. 2018, 3, 525–531. [Google Scholar] [CrossRef] [PubMed]
- Barman, S.C.; Sharifuzzaman, M.; Zahed, M.A.; Park, C.; Yoon, S.H.; Zhang, S.P.; Kim, H.; Yoon, H.; Park, J.Y. A highly selective and stable cationic polyelectrolyte encapsulated black phosphorene based impedimetric immunosensor for interleukin-6 biomarker detection. Biosens. Bioelectron. 2021, 186, 113287. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H.; Zhao, M.; Zhang, J.J.; Ma, X.F.; Zhang, J.; Hua, T.J.; Tang, T.; Jia, J.F.; Wu, H.S. Electrocheical cathode exfoliation of bulky black phosphorus into fer-layer phosphorene nanosheets. Electrochem. Commun. 2018, 89, 10–13. [Google Scholar] [CrossRef]
- He, L.D.; Lu, Q.J.; Yang, Y.; Liu, Y.Q.; Zhu, Y.Z.; Mei, Y. Facile synthesis of holey phosphorene via low temperature electrochemical exfoliation for electrocatalytic nitrogen reduction. ChemistrySelect 2021, 6, 5021–5026. [Google Scholar] [CrossRef]
- He, L.D.; Wu, J.; Zhu, Y.Z.; Wang, Y.M.; Mei, Y. Covalent immobilization of black phosphorus quantum dots on Mxene for enhanced electrocatalytic nitrogen reduction. Ind. Eng. Chem. Res. 2021, 60, 5443–5450. [Google Scholar] [CrossRef]
- Niu, X.L.; Wen, Z.R.; Li, X.B.; Zhao, W.S.; Li, X.Y.; Huang, Y.Q.; Li, Q.T.; Li, G.J.; Sun, W. Fabrication of graphene and gold nanoparticle modified acupuncture needle electrode and its application in rutin analysis. Sens. Actuators B 2018, 255, 471–477. [Google Scholar] [CrossRef]
- Bond, A.M. Modern Polarographic Methods in Analytical Chemistry; Marcel Dekker: New York, NY, USA, 1980. [Google Scholar]
- Laviron, E. The use of linear potential sweep voltammetry and of a.c. voltammetry for the study of the surface electrochemical reaction of strongly adsorbed systems and of redox modified electrodes. J. Electroanal. Chem. 1979, 100, 263–270. [Google Scholar] [CrossRef]
- Laviron, E. General expression of the linear potential sweep voltammogram in the case of diffusionless electrochemical systems. J. Electroanal. Chem. 1979, 101, 19–28. [Google Scholar] [CrossRef]
- Niu, Y.Y.; Zou, R.Y.; Yones, H.A.; Li, X.B.; Li, X.Y.; Niu, X.L.; Chen, Y.; Li, P.; Sun, W. Electrochemical behavior of horseradish peroxidase on WS2 nanosheet-modified electrode and electrocatalytic investigation. J. Chin. Chem. Soc. 2018, 65, 1127–1135. [Google Scholar] [CrossRef]
- Chen, W.; Weng, W.J.; Yin, C.X.; Niu, X.L.; Li, G.J.; Xie, H.; Liu, J.; Sun, W. Fabrication of an electrochemical biosensor based on Nafion/horseradish peroxidase/Co3O4 NP/CILE and its electrocatalysis. Int. J. Electrochem. Sci. 2018, 13, 4741–4752. [Google Scholar] [CrossRef]
- Niu, Y.Y.; Xie, H.; Luo, G.L.; Weng, W.J.; Ruan, C.X.; Li, G.J.; Sun, W. Electrochemical performance of myoglobin based on TiO2-doped carbon nanofiber decorated electrode and its applications in biosensing. RSC Adv. 2019, 9, 4480–4487. [Google Scholar] [CrossRef] [Green Version]
- Zhan, T.R.; Wang, X.J.; Zhang, Y.M.; Song, Y.; Liu, X.L.; Xu, J.; Hou, W.G. Direct electrochemistry and electrocatalysis of hemoglobin immobilized in layered double hydroxides modified with amino functionalized ionic liquid through coprecipitation technique. Sens. Actuators B 2015, 220, 1232–1240. [Google Scholar] [CrossRef]
- Jiang, J.J.; Fan, W.J.; Du, X.Z. Nitrite electrochemical biosensing based on coupled graphend and gold nanoparticles. Biosens. Bioelectron. 2014, 51, 343–348. [Google Scholar] [CrossRef]
- Lijinsky, W.; Epstein, S.S. N-Nitrosamines as Environmental Carcinogens. Nature 1970, 225, 21–23. [Google Scholar] [CrossRef]
- Wang, R.K.; Peng, L.Z.; Zhao, J.Q.; Zhang, L.T.; Guo, C.P.; Zheng, W.H.; Chen, H.R. Gardenamide A protects RGC-5 cells from H2O2-induced oxidative stress insults by activating PI3K/Akt/eNOS signaling pathway. Int. J. Mol. Sci. 2015, 16, 22350–22367. [Google Scholar] [CrossRef] [Green Version]
- Ochoa, C.D.; Wu, R.F.; Terada, L.S. ROS signaling and ER stress in cardiovascular disease. Mol. Asp. Med. 2018, 63, 18–29. [Google Scholar] [CrossRef]
- Xie, H.; Li, X.Y.; Luo, G.L.; Niu, Y.Y.; Zou, R.Y.; Yin, C.X.; Huang, S.M.; Sun, W.; Li, G.J. Nano-diamond modified electrode for the investigation on direct electrochemistry and electrocatalytic behavior of myoglobin. Diam. Relat. Mater. 2019, 97, 107453. [Google Scholar] [CrossRef]
- Shan, W.J.; He, P.L.; Hu, N.F. Electrocatalytic reduction of nitric oxide and other substrates on hydrogel triblock copolymer pluronic films containing hemoglobin or myoglobin based on protein direct electrochemistry. Electrochim. Acta 2005, 51, 432–440. [Google Scholar] [CrossRef]
- Gomes, F.O.; Maia, L.B.; Cordas, C.; Delerue-Matos, C.; Moura, I.; Moura, J.J.G.; Morais, S. Nitric oxide detection using electrochemical third-generation biosensors-based on heme proteins and porphyrins. Electroanalysis 2018, 30, 2485–2503. [Google Scholar] [CrossRef] [Green Version]
- Li, X.Y.; Niu, X.L.; Zhao, W.S.; Chen, W.; Yin, C.X.; Men, Y.L.; Li, G.J.; Sun, W. Black phosphorene and PEDOT: PSS-modified electrode for electrochemistry of hemoglobin. Electrochem. Commun. 2018, 86, 68–71. [Google Scholar] [CrossRef]
- Kamin, R.A.; Wilson, G.S. Rotating ring-disk enzyme electrode for biocatalysis kinetic studies and characterization of the immobilized enzyme layer. Anal. Chem. 1980, 52, 1198–1205. [Google Scholar] [CrossRef]
- Li, X.Y.; Luo, G.L.; Xie, H.; Niu, Y.Y.; Li, X.B.; Zou, R.Y.; Xi, Y.R.; Xiong, Y.; Sun, W.; Li, G.J. Voltammetric sensing performances of a carbon ionic liquid electrode modified with black phosphorene and hemin. Microchim. Acta 2019, 186, 304–312. [Google Scholar] [CrossRef]
- Zheng, W.; Li, G.J.; Liu, L.H.; Chen, W.; Weng, W.J.; Sun, W. Electrochemical behaviors of horseradish peroxidase on MoS2 nanosheets modified electrode. Int. J. Electrochem. Sci. 2016, 11, 7584–7593. [Google Scholar] [CrossRef] [Green Version]
- Zheng, W.; Chen, W.; Weng, W.J.; Liu, L.H.; Li, G.J.; Wang, J.W.; Sun, W. Direct electron transfer of horseradish peroxidase at Co3O4–graphene nanocomposite modified electrode and electrocatalysis. J. Iran. Chem. Soc. 2017, 14, 925–932. [Google Scholar] [CrossRef]
- Zhu, Z.H.; Li, X.; Wang, Y.; Zeng, Y.; Sun, W.; Huang, X.T. Direct electrochemistry and electrocatalysis of horseradish peroxidase with hyaluronic acid-ionic liquid-cadmium sulfide nanorod composite material. Anal. Chim. Acta 2010, 670, 51–56. [Google Scholar] [CrossRef]
- Chen, G.Y.; Sun, H.; Hou, S.F. Electrochemistry and electrocatalysis of myoglobin immobilized in sulfonated graphene oxide and Nafion films. Anal Biochem. 2016, 502, 43–49. [Google Scholar] [CrossRef]
- Chen, W.; Weng, W.J.; Niu, X.L.; Li, X.Y.; Men, Y.L.; Sun, W.; Li, G.J.; Dong, L.F. Boron-doped graphene quantum dots modified electrode for electrochemistry and electrocatalysis of hemoglobin. J. Electroanal. Chem. 2018, 823, 137–145. [Google Scholar] [CrossRef]
- Zhan, T.R.; Wang, X.J.; Li, X.J.; Song, Y.; Hou, W.G. Hemoglobin immobilized in exfoliated Co2Al LDH-graphene nanocomposite film: Direct electrochemistry and electrocatalysis toward trichloroacetic acid. Sens. Actuators B 2016, 228, 101–108. [Google Scholar] [CrossRef]
- Feng, X.W.; Han, G.D.; Cai, J.H.; Wang, X.Y. Au@Carbon quantum dots-MXene nanocomposite as an electrochemical sensor for sensitive detection of nitrite. J. Colloid Interface Sci. 2022, 607, 1313–1322. [Google Scholar] [CrossRef] [PubMed]
- Rozada, T.D.C.; Pessoa, C.A.; Estrada, R.A.; Fiorin, B.C.; Fujiwara, S. Sol gel synthesis of 3-n-propyl(4-aminomethyl)pyridinium silsesquioxane chloride and the enhanced electrocatalytic activity of LbL films. J. Sol-Gel Sci. Technol. 2018, 87, 216–229. [Google Scholar]
- Mariyappan, V.; Chen, S.M.; Murugan, K.; Jeyapragasam, T.; Ramachandran, R. Electrochemical sensor based on cobalt ruthenium sulfide nanoparticles embedded on boron nitrogen co-doped reduced graphene oxide for the determination of nitrite. Colloids Surf. A 2022, 637, 128271. [Google Scholar] [CrossRef]
- Shen, J.; Liu, L.B.; Huang, W.S.; Wu, K.B. Polyvinylpyrrolidone-assisted solvent exfoliation of black phosphorus nanosheets and electrochemical sensing of p-nitrophenol. Anal. Chim. Acta 2021, 1167, 338594. [Google Scholar] [CrossRef]
- Sun, W.; Li, Y.Z.; Duan, Y.Y.; Jiao, K. Direct electrocatalytic oxidation of adenine and guanine on carbon ionic liquid electrode and the simultaneous determination. Biosens. Bioelectron. 2008, 24, 988–993. [Google Scholar] [CrossRef]

| Electrode | Ipc (μA) | Ipa (μA) | Epc (V) | Epa (V) | ΔE/mV | Ipc/Ipa | A (cm2) |
|---|---|---|---|---|---|---|---|
| CILE | 38.00 | 34.39 | 0.182 | 0.249 | 67 | 1.10 | 0.167 |
| Nafion/SWCNTs/CILE | 44.43 | 40.84 | 0.191 | 0.257 | 66 | 1.09 | 0.197 |
| Nafion/BP/CILE | 49.17 | 46.65 | 0.191 | 0.251 | 60 | 1.05 | 0.220 |
| Nafion/SWCNTs-BP/CILE | 71.31 | 62.77 | 0.202 | 0.254 | 52 | 1.14 | 0.309 |
| Modified Electrodes | Methods | Analyte | Linear Range (mmol/L) | Detection Limit (mmol/L) | Refs |
|---|---|---|---|---|---|
| Nafion/Mb/TiO2@CNF/CILE | CV | TCA | 5.0–105.0 | 1.6 | [46] |
| NaNO2 | 1.0–70.0 | 0.3 | |||
| H2O2 | 2.8–32.0 | 1.0 | |||
| BPE-PEDOT:PSS-hemin/CILE | CV | TCA | 2.0–180.0 | 0.67 | [57] |
| NaNO2 | 1.0–10.5 | 0.33 | |||
| H2O2 | 4.0–35.0 | 1.33 | |||
| Nafion/HRP/MoS2/CILE | CV | TCA | 10.0–63.0 | 0.67 | [58] |
| Nafion/HRP/Co3O4-GR/CILE | CV | TCA | 1.0–53.0 | 0.33 | [59] |
| HA-HRP-CdS-IL/CILE | CV | TCA | 1.6–18.0 | 0.53 | [60] |
| Nafion-Mb-SGO-GCE | CV | NaNO2 | 2.0–24.5 | 1.5 | [61] |
| Nafion/Hb/B-GQDs/CILE | CV | TCA | 7.0–420.0 | 2.3 | [62] |
| CV | NaNO2 | 1.0–80.0 | 0.3 | ||
| DPV | H2O2 | 4.0–24.0 | 1.3 | ||
| CTS/ELDH-GR-Hb/CILE | CV | TCA | 5.0–360 | 1.506 | [63] |
| Au@CQDs-Mxene/GCE | DPV | NaNO2 | 0.001–3.2 | 7.8 × 10−4 | [64] |
| (Si4ampy+Cl−/NiTsPc)11/ITO | DPV | NaNO2 | 0.113–0.860 | 0.026 | [65] |
| CRS/BN-RGO/GCE | DPV | NaNO2 | 1 × 10−6–1.29 | 1.59 × 10−5 | [66] |
| Nafion/HRP/SWCNTs-BP/CILE | CV | TCA | 1.0–810.0 | 0.33 | This work |
| NaNO2 | 0.8–49.6 | 0.27 | |||
| H2O2 | 1.0–40.0 | 0.33 |
| Sample | Detected (mmol/L) | Added (mmol/L) | Total (mmol/L) | Recovery (%) | RSD (%) |
|---|---|---|---|---|---|
| Medical facial peel | 9.21 | 10.00 | 18.63 | 94.2 | 1.23 |
| 20.00 | 28.65 | 97.2 | 2.45 | ||
| 30.00 | 40.50 | 104.3 | 2.61 | ||
| Soak water of pickled vegetables | 1.25 | 1.00 | 2.27 | 102.0 | 1.67 |
| 2.00 | 3.19 | 97.0 | 2.18 | ||
| 3.00 | 4.36 | 103.7 | 1.72 | ||
| 3% H2O2 disinfectant | 0.76 | 1.00 | 1.74 | 98.0 | 0.97 |
| 2.00 | 2.82 | 103.0 | 1.35 | ||
| 3.00 | 3.69 | 97.7 | 1.98 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Wang, L.; Wang, B.; Zhang, S.; Jiang, M.; Fu, W.; Sun, W. Preparation and Application of Electrochemical Horseradish Peroxidase Sensor Based on a Black Phosphorene and Single-Walled Carbon Nanotubes Nanocomposite. Molecules 2022, 27, 8064. https://doi.org/10.3390/molecules27228064
Li X, Wang L, Wang B, Zhang S, Jiang M, Fu W, Sun W. Preparation and Application of Electrochemical Horseradish Peroxidase Sensor Based on a Black Phosphorene and Single-Walled Carbon Nanotubes Nanocomposite. Molecules. 2022; 27(22):8064. https://doi.org/10.3390/molecules27228064
Chicago/Turabian StyleLi, Xiaoqing, Lisi Wang, Baoli Wang, Siyue Zhang, Meng Jiang, Wanting Fu, and Wei Sun. 2022. "Preparation and Application of Electrochemical Horseradish Peroxidase Sensor Based on a Black Phosphorene and Single-Walled Carbon Nanotubes Nanocomposite" Molecules 27, no. 22: 8064. https://doi.org/10.3390/molecules27228064






