Stabilizing Halogen-Bonded Complex between Metallic Anion and Iodide
Abstract
1. Introduction
2. Methods
2.1. Experimental Methods
2.2. Computational Methods
3. Results and Discussion
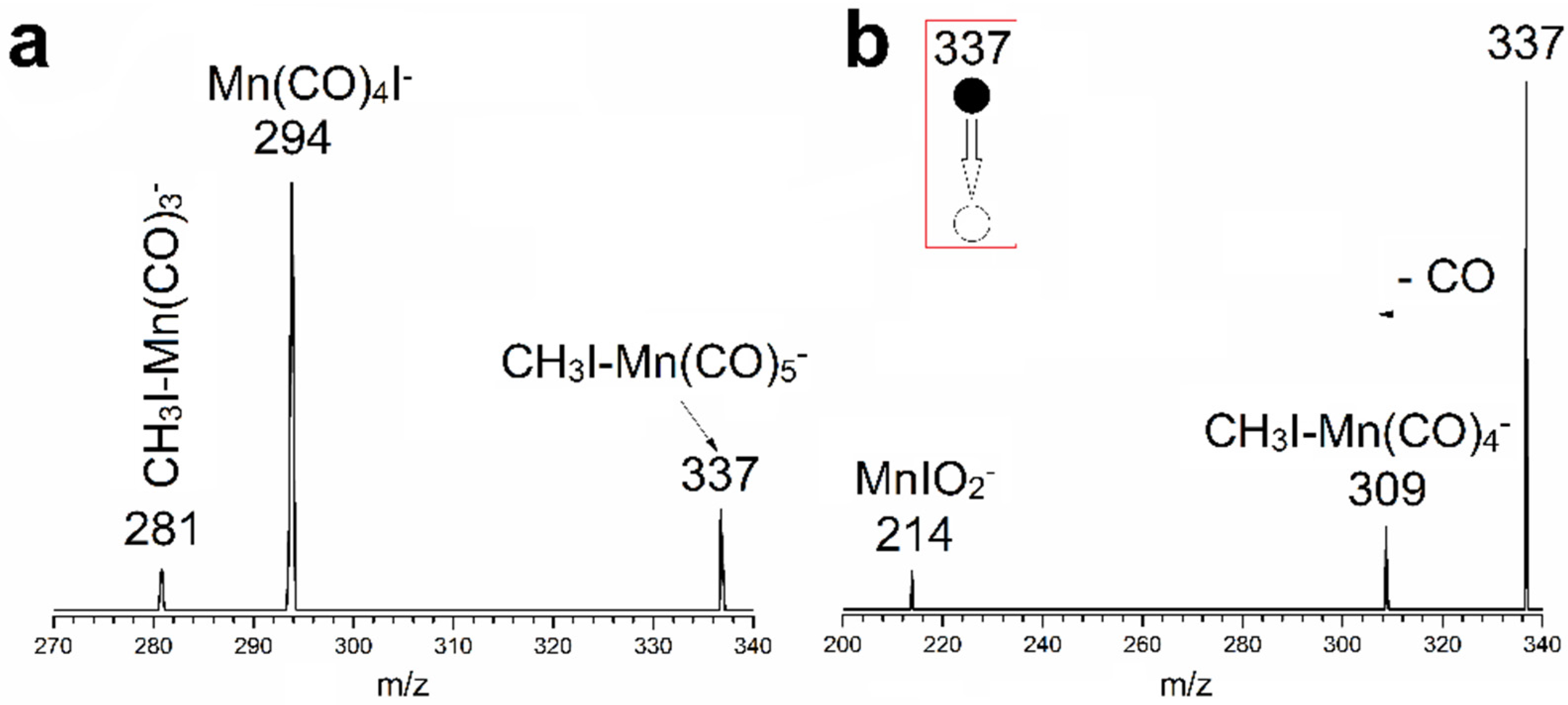
3.1. Mass Spectrometry
3.2. Density Functional Theory Calculation
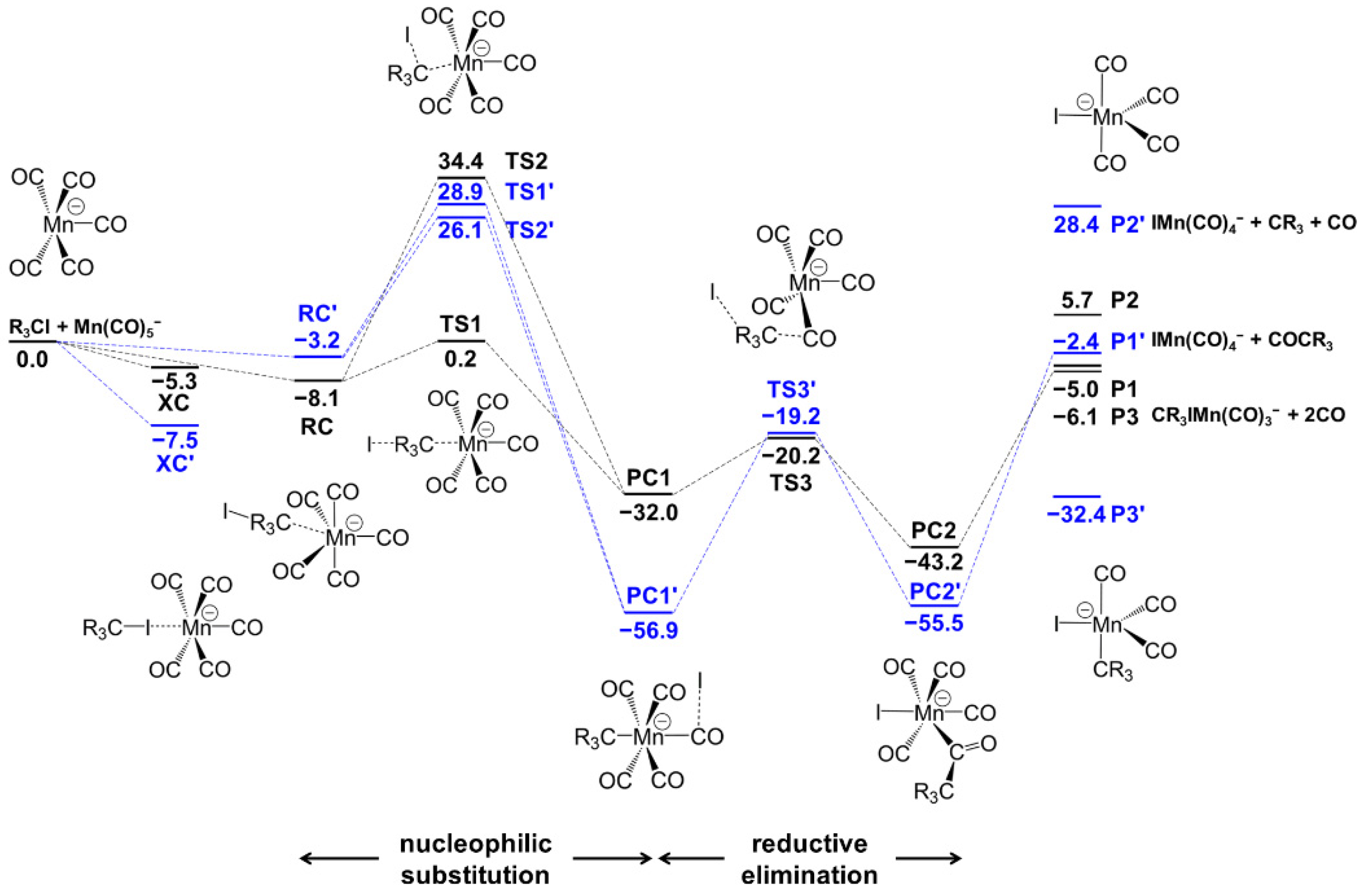
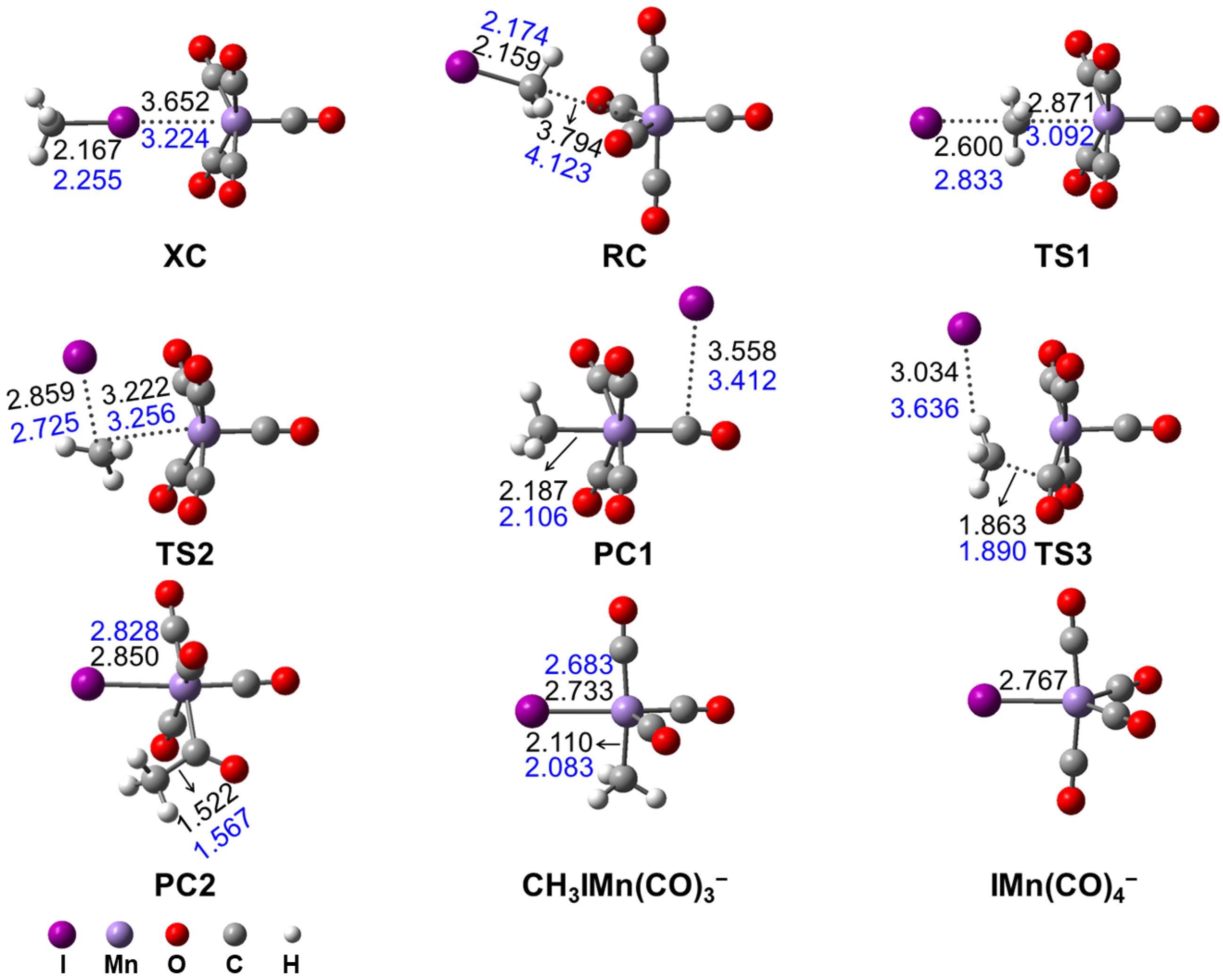
3.2.1. Mn(CO)5− + CH3I Reaction Mechanism
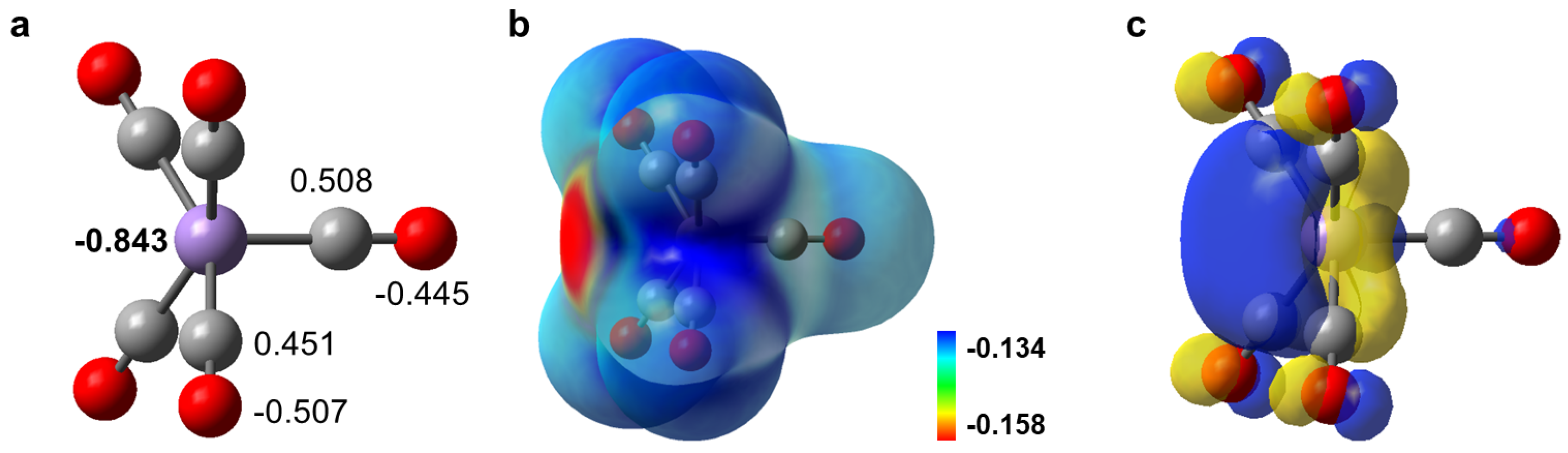
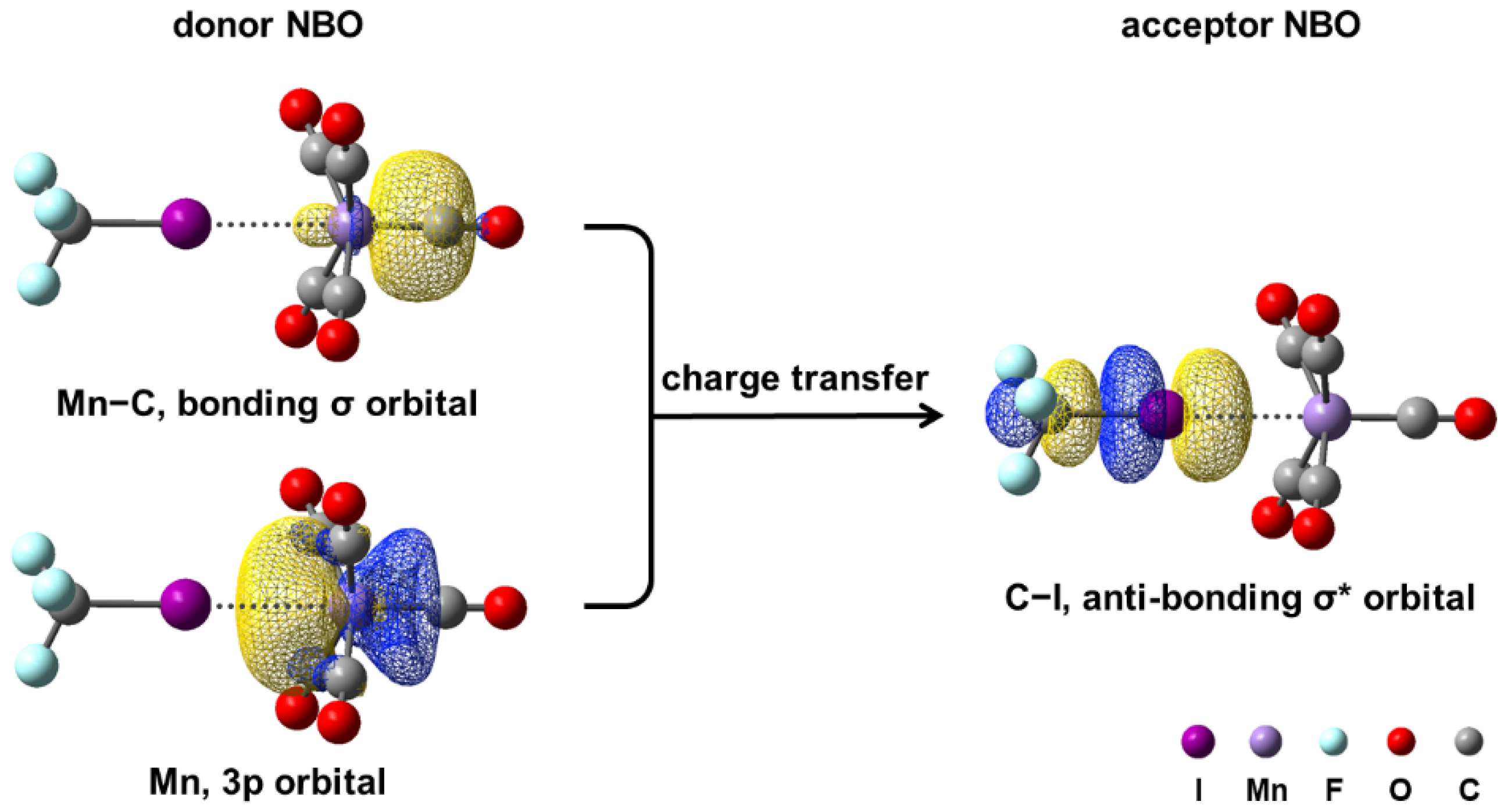
3.2.2. Stabilizing Halogen-Bonded Complex by CF3I
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cavallo, G.; Metrangolo, P.; Milani, R.; Pilati, T.; Priimagi, A.; Resnati, G.; Terraneo, G. The Halogen Bond. Chem. Rev. 2016, 116, 2478–2601. [Google Scholar] [CrossRef] [PubMed]
- Gou, Q.; Feng, G.; Evangelisti, L.; Vallejo-López, M.; Spada, L.; Lesarri, A.; Cocinero, E.J.; Caminati, W. Internal Dynamics in Halogen-Bonded Adducts: A Rotational Study of Chlorotrifluoromethane–Formaldehyde. Chem. Eur. J. 2015, 21, 4148–4152. [Google Scholar] [CrossRef] [PubMed]
- Inscoe, B.; Rathnayake, H.; Mo, Y. Role of Charge Transfer in Halogen Bonding. J. Phys. Chem. A 2021, 125, 2944–2953. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wang, J.; Zheng, Y.; Feng, G.; Gou, Q. Halogen Bond in the Water Adduct of Chloropentafluoroethane Revealed by Rotational Spectroscopy. J. Chem. Phys. 2018, 149, 154307. [Google Scholar] [CrossRef] [PubMed]
- Kolář, M.H.; Hobza, P. Computer Modeling of Halogen Bonds and Other σ-Hole Interactions. Chem. Rev. 2016, 116, 5155–5187. [Google Scholar] [CrossRef] [PubMed]
- Robertson, C.C.; Wright, J.S.; Carrington, E.J.; Perutz, R.N.; Hunter, C.A.; Brammer, L. Hydrogen Bonding vs. Halogen Bonding: The Solvent Decides. Chem. Sci. 2017, 8, 5392–5398. [Google Scholar] [CrossRef] [PubMed]
- Szabó, I.; Olasz, B.; Czakó, G. Deciphering Front-Side Complex Formation in SN2 Reactions via Dynamics Mapping. J. Phys. Chem. Lett. 2017, 8, 2917–2923. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, W.; Jin, W.J. σ-Hole Bond vs π-Hole Bond: A Comparison Based on Halogen Bond. Chem. Rev. 2016, 116, 5072–5104. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Herbers, S.; Gou, Q.; Caminati, W.; Grabow, J.U. Chlorine “Equatorial Belt” Activation of CF3Cl by CO2: The C···Cl Tetrel Bond Dominance in CF3Cl–CO2. J. Phys. Chem. Lett. 2021, 12, 3907–3913. [Google Scholar] [CrossRef] [PubMed]
- Desiraju, G.R.; Ho, P.S.; Kloo, L.; Legon, A.C.; Marquardt, R.; Metrangolo, P.; Politzer, P.; Resnati, G.; Rissanen, K. Definition of the Halogen Bond (IUPAC Recommendations 2013). Pure Appl. Chem. 2013, 85, 1711–1713. [Google Scholar] [CrossRef]
- Clark, T. σ-Holes. WIREs Comput. Mol. Sci. 2013, 3, 13–20. [Google Scholar] [CrossRef]
- Clark, T.; Hennemann, M.; Murray, J.S.; Politzer, P. Halogen Bonding: The σ-Hole. J. Mol. Model. 2007, 13, 291–296. [Google Scholar] [CrossRef]
- Auffinger, P.; Hays, F.A.; Westhof, E.; Ho, P.S. Halogen Bonds in Biological Molecules. Proc. Natl. Acad. Sci. USA 2004, 101, 16789–16794. [Google Scholar] [CrossRef] [PubMed]
- Gilday, L.C.; Robinson, S.W.; Barendt, T.A.; Langton, M.J.; Mullaney, B.R.; Beer, P.D. Halogen Bonding in Supramolecular Chemistry. Chem. Rev. 2015, 115, 7118–7195. [Google Scholar] [CrossRef]
- Mukherjee, A.; Tothadi, S.; Desiraju, G.R. Halogen Bonds in Crystal Engineering: Like Hydrogen Bonds yet Different. Acc. Chem. Res. 2014, 47, 2514–2524. [Google Scholar] [CrossRef]
- Priimagi, A.; Cavallo, G.; Metrangolo, P.; Resnati, G. The Halogen Bond in the Design of Functional Supramolecular Materials: Recent Advances. Acc. Chem. Res. 2013, 46, 2686–2695. [Google Scholar] [CrossRef]
- Sirimulla, S.; Bailey, J.B.; Vegesna, R.; Narayan, M. Halogen Interactions in Protein–Ligand Complexes: Implications of Halogen Bonding for Rational Drug Design. J. Chem. Inf. Model. 2013, 53, 2781–2791. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Liu, G.; Ciborowski, S.; Bowen, K. Stabilizing Otherwise Unstable Anions with Halogen Bonding. Angew. Chem. Int. Ed. 2017, 56, 9897–9900. [Google Scholar] [CrossRef] [PubMed]
- Muramatsu, S.; Koyasu, K.; Tsukuda, T. Formation of Grignard Reagent-like Complex CH3−M−I− via Oxidative Addition of CH3I on Coinage Metal Anions M− (M = Cu, Ag, Au) in the Gas Phase. Chem. Lett. 2017, 46, 676–679. [Google Scholar] [CrossRef]
- Muramatsu, S.; Koyasu, K.; Tsukuda, T. Oxidative Addition of CH3I to Au– in the Gas Phase. J. Phys. Chem. A 2016, 120, 957–963. [Google Scholar] [CrossRef]
- Wang, F.; Ji, X.; Ying, F.; Zhang, J.; Zhao, C.; Xie, J. Computational Studies of Coinage Metal Anion M− + CH3X (X = F, Cl, Br, I) Reactions in Gas Phase. Molecules 2022, 27, 307. [Google Scholar] [CrossRef]
- Zhang, X.; Bowen, K. Designer Metallic Acceptor-Containing Halogen Bonds: General Strategies. Chem. Eur. J. 2017, 23, 5439–5442. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Truhlar, D.G. The M06 Suite of Density Functionals for Main Group Thermochemistry, Thermochemical Kinetics, Noncovalent Interactions, Excited States, and Transition Elements: Two New Functionals and Systematic Testing of Four M06-Class Functionals and 12 Other Functionals. Theor. Chem. Acc. 2008, 120, 215–241. [Google Scholar] [CrossRef]
- Kendall, R.A.; Dunning, T.H., Jr.; Harrison, R.J. Electron Affinities of the First-Row Atoms Revisited. Systematic Basis Sets and Wave Functions. J. Chem. Phys. 1992, 96, 6796–6806. [Google Scholar] [CrossRef]
- Dunning, T.H., Jr. Gaussian Basis Sets for Use in Correlated Molecular Calculations. I. The Atoms Boron through Neon and Hydrogen. J. Chem. Phys. 1989, 90, 1007–1023. [Google Scholar] [CrossRef]
- Balabanov, N.B.; Peterson, K.A. Systematically Convergent Basis Sets for Transition Metals. I. All-Electron Correlation Consistent Basis Sets for the 3d Elements Sc–Zn. J. Chem. Phys. 2005, 123, 064107. [Google Scholar] [CrossRef] [PubMed]
- Mulliken, R.S. Electronic Population Analysis on LCAO–MO Molecular Wave Functions. I. J. Chem. Phys. 1955, 23, 1833–1840. [Google Scholar] [CrossRef]
- Peterson, K.A.; Figgen, D.; Goll, E.; Stoll, H.; Dolg, M. Systematically Convergent Basis Sets with Relativistic Pseudopotentials. II. Small-Core Pseudopotentials and Correlation Consistent Basis Sets for the Post-d Group 16–18 Elements. J. Chem. Phys. 2003, 119, 11113–11123. [Google Scholar] [CrossRef]
- Peterson, K.A.; Shepler, B.C.; Figgen, D.; Stoll, H. On the Spectroscopic and Thermochemical Properties of ClO, BrO, IO, and Their Anions. J. Phys. Chem. A 2006, 110, 13877–13883. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Revision A.03; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Mensa-Bonsu, G.; Tozer, D.J.; Verlet, J.R.R. Photoelectron Spectroscopic Study of I−·ICF3: A Frontside Attack SN2 Pre-Reaction Complex. Phys. Chem. Chem. Phys. 2019, 21, 13977–13985. [Google Scholar] [CrossRef]
- Reed, A.E.; Curtiss, L.A.; Weinhold, F. Intermolecular Interactions from a Natural Bond Orbital, Donor-Acceptor Viewpoint. Chem. Rev. 1988, 88, 899–926. [Google Scholar] [CrossRef]
- Reed, A.E.; Weinstock, R.B.; Weinhold, F. Natural Population Analysis. J. Chem. Phys. 1985, 83, 735–746. [Google Scholar] [CrossRef]
- Ji, X.; Zhao, C.; Xie, J. Investigating the Role of Halogen-Bonded Complexes in Microsolvated Y−(H2O)n + CH3I SN2 reactions. Phys. Chem. Chem. Phys. 2021, 23, 6349–6360. [Google Scholar] [CrossRef] [PubMed]
| kcal/mol | ∆Eelec | ∆(Eelec + ZPE) | ∆H298.15K | ∆G298.15K | ||||
|---|---|---|---|---|---|---|---|---|
| R | H | F | H | F | H | F | H | F |
| Mn(CO)5− + CR3I | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| XC | −5.4 | −17.5 | −5.3 | −17.5 | −3.9 | −16.5 | 1.4 | −7.0 |
| RC | −8.6 | −3.6 | −9.1 | −3.2 | −7.3 | −1.8 | −0.2 | 3.8 |
| TS1 | 0.4 | 29.1 | 0.2 | 28.9 | 1.2 | 30.3 | 8.6 | 37.3 |
| TS2 | 35.2 | 27.2 | 34.4 | 26.1 | 35.4 | 27.6 | 43.0 | 35.2 |
| PC1 | −31.7 | −56.6 | −32.0 | −56.9 | −31.1 | −55.1 | −22.4 | −47.6 |
| TS3 | −20.3 | −18.8 | −20.2 | −19.2 | −19.5 | −17.8 | −10.4 | −9.9 |
| PC3 | −44.4 | −56.7 | −43.2 | −55.5 | −42.4 | −54.9 | −33.3 | −43.5 |
| Mn(CO)4I− + COCR3 | −3.0 | −0.7 | −5.0 | −2.4 | −3.9 | −1.4 | −8.2 | −5.8 |
| Mn(CO)4I− + CO + CR3 | 13.1 | 33.4 | 5.7 | 28.4 | 8.4 | 30.2 | −4.7 | 16.3 |
| CR3I−Mn(CO)3− + 2CO | −1.6 | −28.3 | −6.1 | −32.4 | −4.4 | −29.7 | −15.5 | −43.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ying, F.; Yuan, X.; Zhang, X.; Xie, J. Stabilizing Halogen-Bonded Complex between Metallic Anion and Iodide. Molecules 2022, 27, 8069. https://doi.org/10.3390/molecules27228069
Ying F, Yuan X, Zhang X, Xie J. Stabilizing Halogen-Bonded Complex between Metallic Anion and Iodide. Molecules. 2022; 27(22):8069. https://doi.org/10.3390/molecules27228069
Chicago/Turabian StyleYing, Fei, Xu Yuan, Xinxing Zhang, and Jing Xie. 2022. "Stabilizing Halogen-Bonded Complex between Metallic Anion and Iodide" Molecules 27, no. 22: 8069. https://doi.org/10.3390/molecules27228069
APA StyleYing, F., Yuan, X., Zhang, X., & Xie, J. (2022). Stabilizing Halogen-Bonded Complex between Metallic Anion and Iodide. Molecules, 27(22), 8069. https://doi.org/10.3390/molecules27228069








