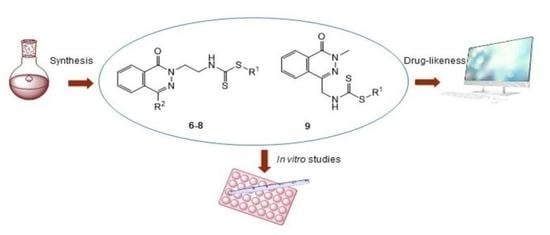
Novel Phthalazin-1(2H)-One Derivatives Displaying a Dithiocarbamate Moiety as Potential Anticancer Agents
Abstract
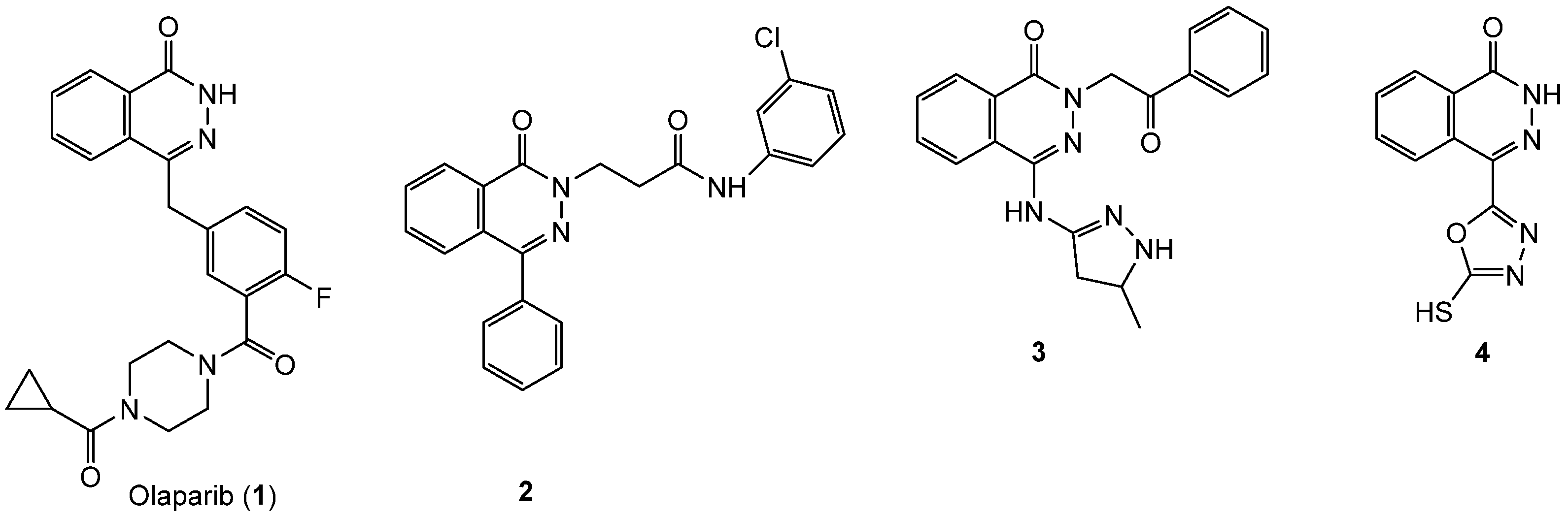
:1. Introduction
2. Results and Discussion
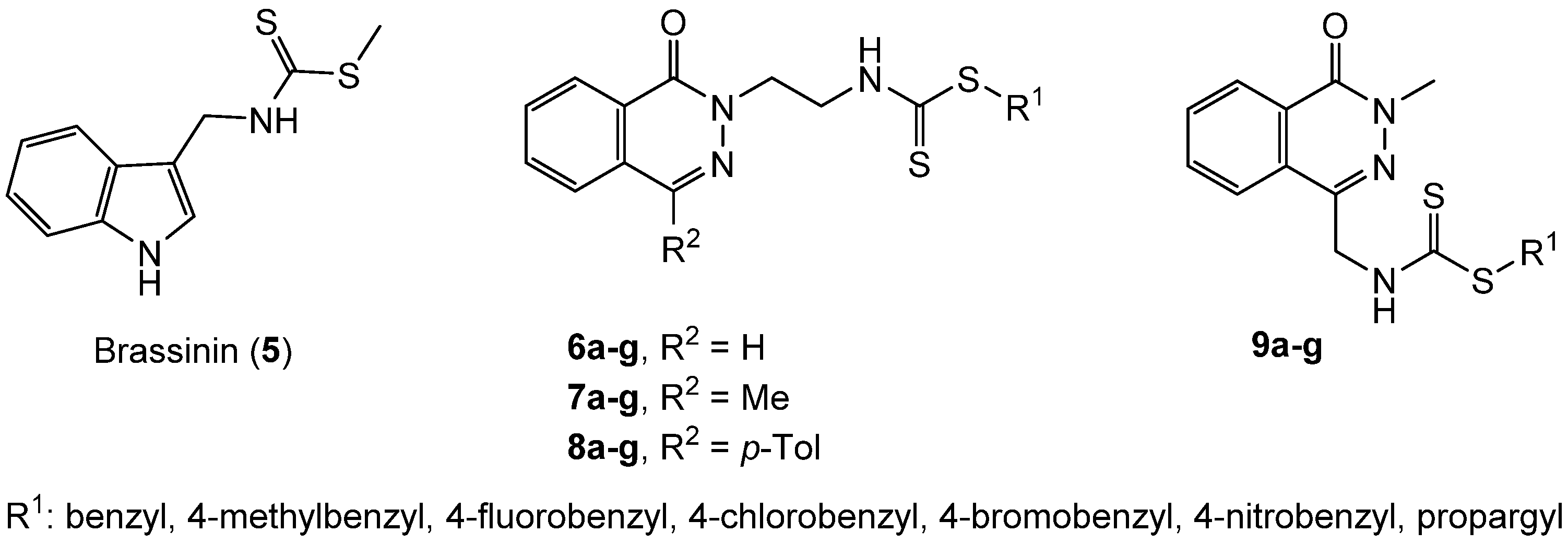
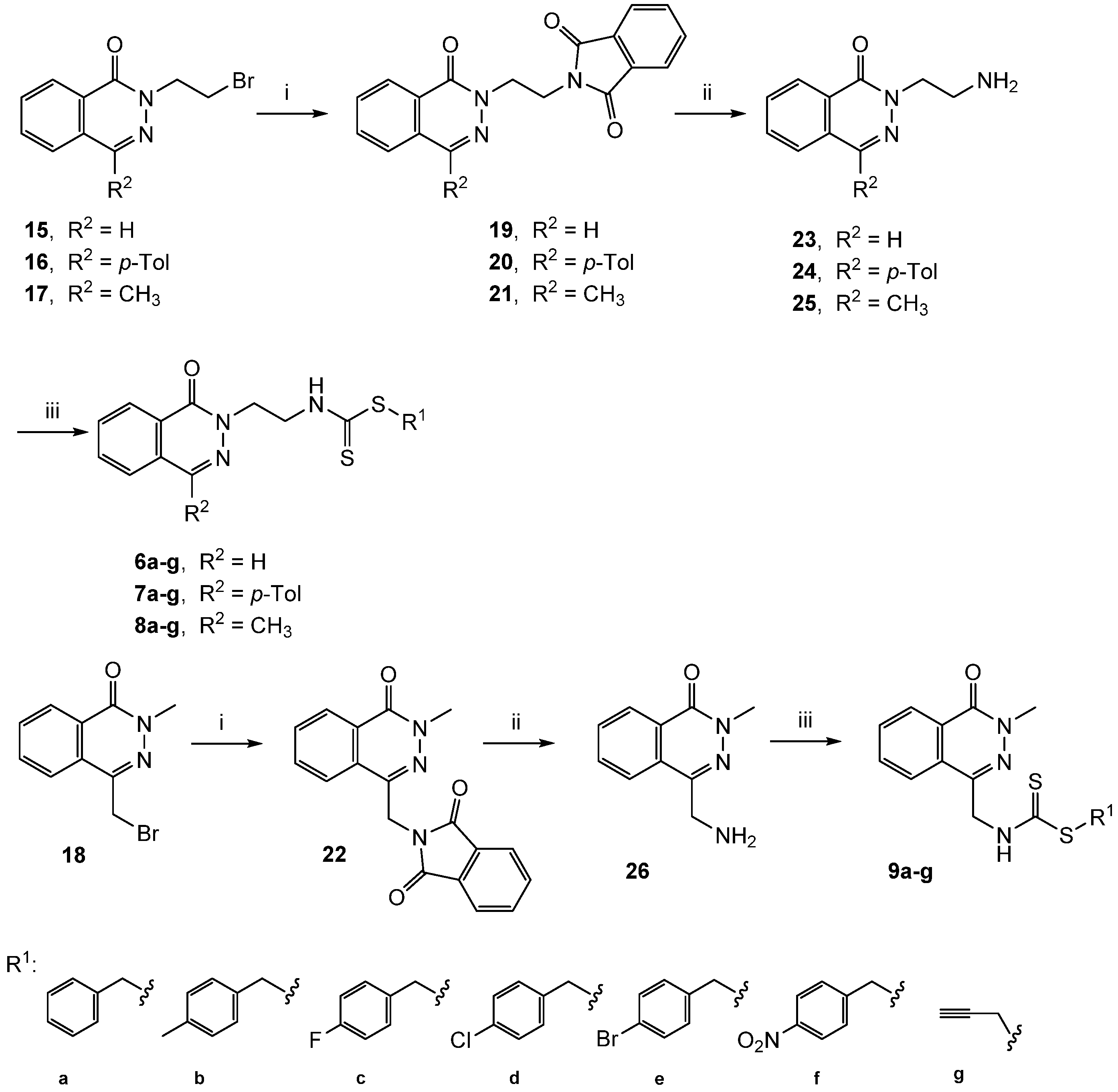
2.1. Chemistry
2.2. Pharmacology
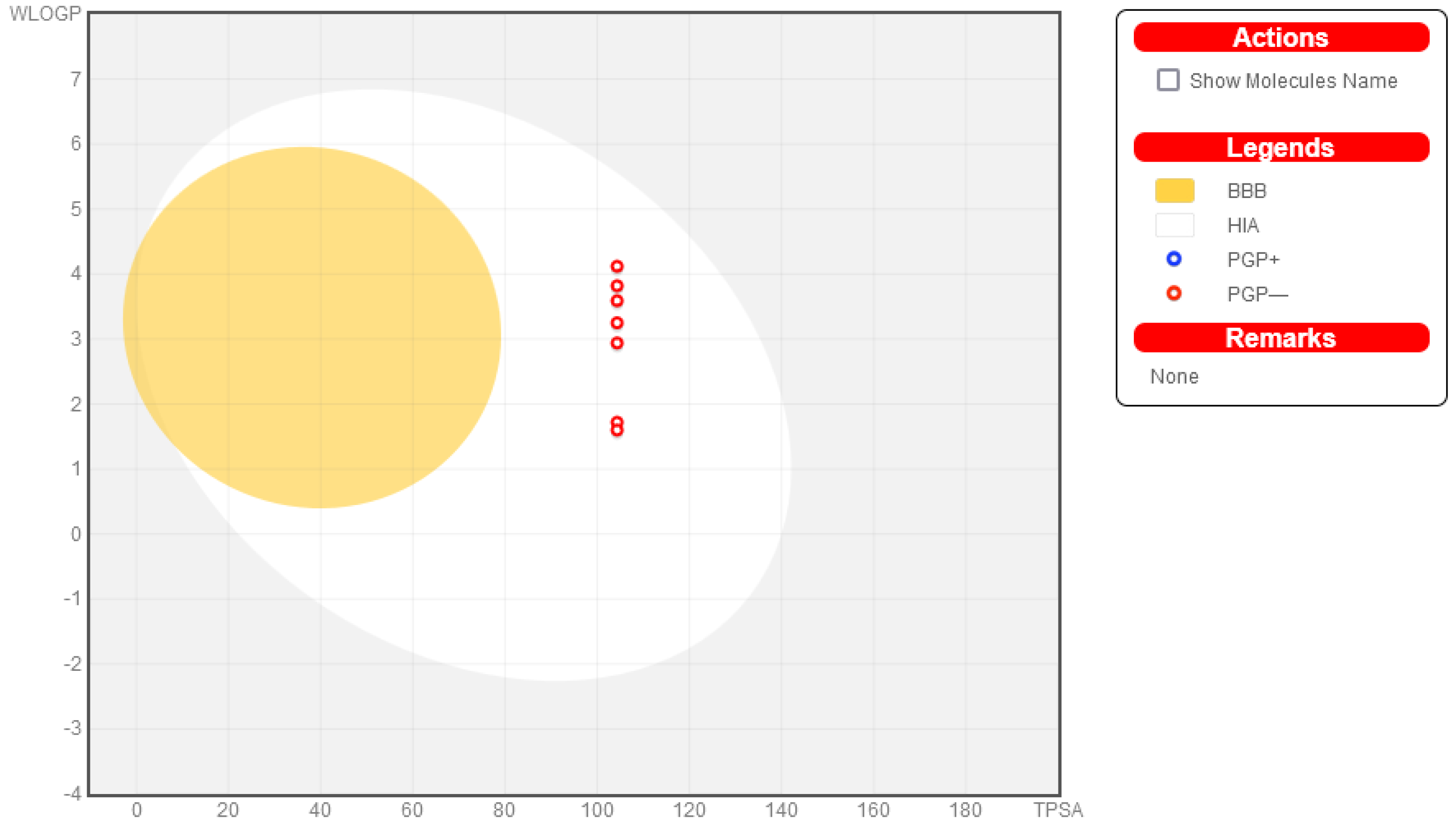
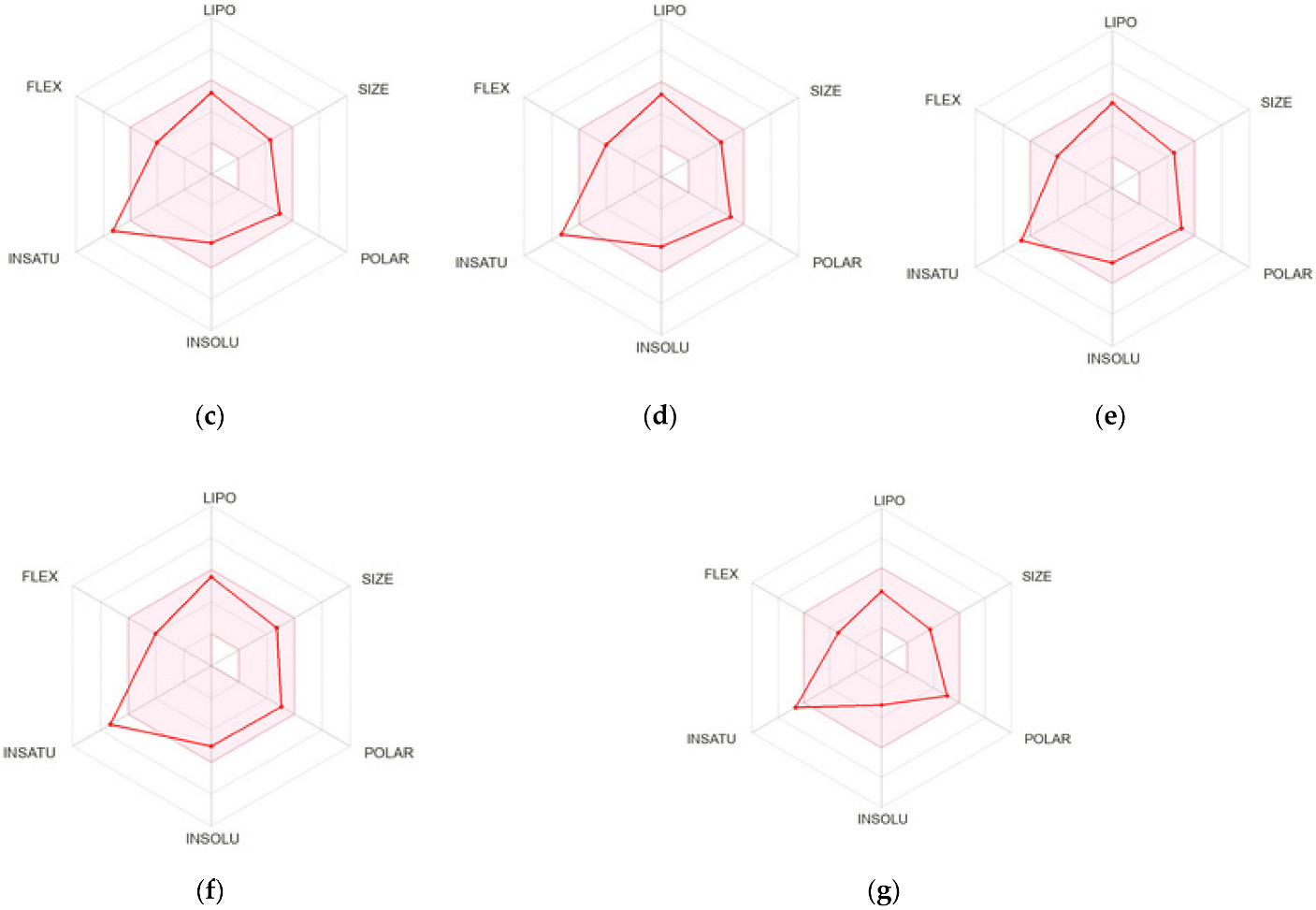
2.3. Drug-Like and Toxicity Properties Prediction
3. Materials and Methods
3.1. Materials and Instrumentation
3.2. Chemical Synthesis
3.2.1. General Procedure for the Preparation of 2-(Phthalazinylalkyl)isoindoline-1,3-Diones (19–22)
3.2.2. General Procedure for the Preparation of Aminoalkyl Phthalazin-1(2H)-Ones (23–26)
3.2.3. General Procedure for the Preparation of Phthalazinyle Alkyl Dithiocarbamates (6–9)
3.3. Antiproliferative Activity Studies
3.4. Drug-Like and Toxicity Properties Prediction
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Dagenais, G.R.; Leong, D.P.; Rangarajan, S.; Lanas, F.; Lopez-Jaramillo, P.; Gupta, R.; Diaz, R.; Avezum, A.; Oliveira, G.B.F.; Wielgosz, A.; et al. Variations in common diseases, hospital admissions, and deaths in middle-aged adults in 21 countries from five continents (PURE): A prospective cohort study. Lancet 2020, 395, 785–794. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Ward, R.A.; Fawell, S.; Floc’h, N.; Flemington, V.; McKerrecher, D.; Smith, P.D. Challenges and Opportunities in Cancer Drug Resistance. Chem. Rev. 2021, 121, 3297–3351. [Google Scholar] [CrossRef] [PubMed]
- Kamal, A.; Azeeza, S.; Bharathi, E.V.; Malik, M.S.; Shetti, R.V. Search for new and novel chemotherapeutics for the treatment of human malignancies. Mini Rev. Med. Chem. 2010, 10, 405–435. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira Pedrosa, M.; Duarte da Cruz, R.M.; de Oliveira Viana, J.; de Moura, R.O.; Ishiki, H.M.; Barbosa Filho, J.M.; Diniz, M.F.; Scotti, M.T.; Scotti, L.; Bezerra Mendonca, F.J. Hybrid compounds as direct multitarget ligands: A review. Curr. Top. Med. Chem. 2017, 17, 1044–1079. [Google Scholar] [CrossRef]
- Nepali, K.; Sharma, S.; Sharma, M.; Bedi, P.M.; Dhar, K.L. Rational approaches, design strategies, structure activity relationship and mechanistic insights for anticancer hybrids. Eur. J. Med. Chem. 2014, 77, 422–487. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.; Suryan, A.; Kumar, S.; Sharma, S. Phthalazinone scaffold: Emerging tool in the development of target based novel anticancer agents. Anticancer Agents Med. Chem. 2020, 20, 2228–2245. [Google Scholar] [CrossRef]
- Vila, N.; Besada, P.; Costas, T.; Costas-Lago, M.C.; Terán, C. Phthalazin-1(2H)-one as a remarkable scaffold in drug Discovery. Eur. J. Med. Chem. 2015, 97, 462–482. [Google Scholar] [CrossRef]
- Terán, C.; Besada, P.; Vila, N.; Costas-Lago, M.C. Recent advances in the synthesis of phthalazin-1(2H)-one core as a relevant pharmacophore in medicinal chemistry. Eur. J. Med. Chem. 2019, 161, 468–478. [Google Scholar] [CrossRef]
- Menear, K.A.; Adcock, C.; Boulter, R.; Cockcroft, X.L.; Copsey, L.; Cranston, A.; Dillon, K.J.; Drzewiecki, J.; Garman, S.; Gomez, S.; et al. 4-[3-(4-Cyclopropanecarbonylpiperazine-1-carbonyl)-4-fluorobenzyl]-2H-phthalazin-1-one: A Novel Bioavailable Inhibitor of Poly(ADP-ribose) Polymerase-1. J. Med. Chem. 2008, 51, 6581–6591. [Google Scholar] [CrossRef]
- Almahli, H.; Hadchity, E.; Jaballah, M.Y.; Daher, R.; Ghabbour, H.A.; Kabil, M.M.; Al-shakliah, N.S.; Eldehna, W.M. Development of novel synthesized phthalazinone-based PARP-1 inhibitors with apoptosis inducing mechanism in lung cancer. Bioorg. Chem. 2018, 77, 443–456. [Google Scholar] [CrossRef] [PubMed]
- Xin, M.; Sun, J.; Huang, W.; Tang, F.; Liu, Z.; Jin, Q.; Wang, J. Design and synthesis of novel phthalazinone derivatives as potent poly(ADP-ribose)polymerase 1 inhibitors. Future Med. Chem. 2020, 12, 1691–1707. [Google Scholar] [CrossRef] [PubMed]
- Prime, M.E.; Courtney, S.M.; Brookfield, F.A.; Marston, R.W.; Walker, V.; Warne, J.; Boyd, A.E.; Kairies, N.A.; von der Saal, W.; Limberg, A.; et al. Phthalazinone pyrazoles as potent, selective, and orally bioavailable inhibitors of Aurora-A kinase. J. Med. Chem. 2011, 54, 312–319. [Google Scholar] [CrossRef]
- Hekal, M.H.; El-Naggar, A.M.; Abu El-Azm, F.S.M.; El-Sayed, W.M. Synthesis of new oxadiazol-phthalazinone derivatives with anti-proliferative activity; molecular docking, pro-apoptotic, and enzyme inhibition profile. RSC Adv. 2020, 10, 3675–3688. [Google Scholar] [CrossRef] [Green Version]
- Kaul, L.; Süss, R.; Zannettino, A.; Richter, K. The revival of dithiocarbamates: From pesticides to innovative medical treatments. iScience 2021, 24, 102092. [Google Scholar] [CrossRef]
- Shinde, N.D.; Sakla, A.P.; Shankaraiah, M. An insight into medicinal attributes of dithiocarbamates: Bird’s eye view. Bioorg. Chem. 2020, 105, 104347. [Google Scholar] [CrossRef]
- Gaspari, P.; Banerjee, T.; Malachowski, W.P.; Muller, A.J.; Prendergast, G.C.; DuHadaway, J.; Bennett, S.; Donovan, A.M. Structure-activity study of brassinin derivatives as indoleamine 2,3-dioxygenase inhibitors. J. Med. Chem. 2006, 49, 684–692. [Google Scholar] [CrossRef] [Green Version]
- Banerjee, T.; Duhadaway, J.B.; Gaspari, P.; Sutanto-Ward, E.; Munn, D.H.; Mellor, A.L.; Malachowski, W.P.; Prendergast, G.C.; Muller, A.J. A key in vivo antitumor mechanism of action of natural product-based brassinins is inhibition of indoleamine 2,3-dioxygenase. Oncogene 2008, 27, 2851–2857. [Google Scholar] [CrossRef] [Green Version]
- Omar, A.M.E.; AboulWafa, O.M.; El-Shoukrofy, M.S.; Amr, M.E. Benzoxazole derivatives as new generation of anti-breast cancer agents. Bioorg. Chem. 2020, 96, 103593. [Google Scholar] [CrossRef]
- Zheng, Y.C.; Duan, Y.C.; Ma, J.L.; Xu, R.M.; Zi, X.; Lv, W.L.; Wang, M.M.; Ye, X.W.; Zhu, S.; Mobley, D.; et al. Triazole-dithiocarbamate based selective lysine specific demethylase 1 (LSD1) inactivators inhibit gastric cancer cell growth, invasion, and migration. J. Med. Chem. 2013, 56, 8543–8560. [Google Scholar] [CrossRef]
- Su, Y.; Li, R.; Ning, X.; Lin, Z.; Zhao, X.; Zhou, J.; Liu, J.; Jin, Y.; Yin, Y. Discovery of 2,4-diarylaminopyrimidine derivatives bearing dithiocarbamate moiety as novel FAK inhibitors with antitumor and anti-angiogenesis activities. Eur. J. Med. Chem. 2019, 177, 32–46. [Google Scholar] [CrossRef]
- Ding, P.P.; Gao, M.; Mao, B.B.; Cao, S.L.; Liu, C.H.; Yang, C.R.; Li, Z.F.; Liao, J.; Zhao, H.; Li, Z.; et al. Synthesis and biological evaluation of quinazolin-4(3H)-one derivatives bearing dithiocarbamate side chain at C2-position as potential antitumor agents. Eur. J. Med. Chem. 2016, 108, 364–373. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.L.; Wang, Y.; Zhu, L.; Liao, J.; Guo, Y.W.; Chen, L.L.; Liu, H.Q. Synthesis and cytotoxic activity of N-((2-methyl-4(3H)-quinazolinon-6-yl)methyl)dithiocarbamates. Eur. J. Med. Chem. 2010, 45, 3850–3857. [Google Scholar] [CrossRef] [PubMed]
- Fu, D.J.; Zhang, S.Y.; Liu, Y.C.; Zhang, L.; Liu, J.J.; Song, J.; Zhao, R.H.; Li, F.; Sun, H.H.; Liu, H.M.; et al. Design, synthesis and antiproliferative activity studies of novel dithiocarbamate-chalcone derivatives. Bioorg. Med. Chem. Lett. 2016, 26, 3918–3922. [Google Scholar] [CrossRef] [PubMed]
- Terán, C.; Besada, P.; Costas, T.; Vila, N.; Costas-Lago, M.C. Compuestos de Estructura Híbrida Piridaziona Ditiocarbamato Con Actividad Antineoplásica. Spanish Patent ES2469990B1, 27 January 2015. [Google Scholar]
- Abo-elmagd, N.E.; George, R.F.; Ezzat, M.A.; Arafa, R.K. New 1-phthalazinone scaffold based compounds: Design, cytotoxicity and protein kinase inhibition activity. Mini Rev. Med. Chem. 2018, 18, 1759–1774. [Google Scholar] [CrossRef] [PubMed]
- Costas-Lago, M.C.; Vila, N.; Rahman, A.; Besada, P.; Rozas, I.; Brea, P.; Loza, M.I.; González-Romero, E.; Terán, C. Novel pyridazin-3(2H)-one-based guanidine derivatives as potential DNA minor groove binders with anticancer activity. ACS Med. Chem. Lett. 2022, 13, 463–469. [Google Scholar] [CrossRef]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef] [Green Version]
- Daina, A.; Zoete, V. A BOILED-Egg to predict gastrointestinal absorption and brain penetration of small molecules. ChemMedChem 2016, 11, 1117–1121. [Google Scholar] [CrossRef] [Green Version]
- Montarani, F.; Ecker, G.F. Prediction of drug-ABC-transporter interaction-recent advances and future challenges. Adv. Drug Deliv. Rev. 2015, 86, 17–26. [Google Scholar] [CrossRef] [Green Version]
- Sharom, F.J. ABC multidrug transporters: Structure, function and role in chemoresistence. Pharmacohenomics 2008, 9, 105–127. [Google Scholar] [CrossRef]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feney, P.J. Experimental and computational approaches to estimate solubility and premeabilty in drug discoveryand development settings. Adv. Drug. Deliv. Rev. 2001, 46, 3–26. [Google Scholar] [CrossRef]
- Ghose, A.K.; Viswanadhan, V.N.; Wendoloski, J.J. A knowledge-based approach in designing combinatorial or medicinal chemistry libraries for drug discovery. 1. A qualitative and quantitative characterization of known drug databases. J Comb. Chem. 1999, 1, 55–68. [Google Scholar] [CrossRef]
- Veber, D.F.; Johnson, S.R.; Cheng, H.Y.; Smith, B.R.; Ward, K.W.; Kopple, K.D. Molecular properties that influence the oral bioavailability of drug candidates. J. Med. Chem. 2002, 45, 2615–2623. [Google Scholar] [CrossRef]
- Egan, W.J.; Merz, K.M.; Baldwin, J.J. Prediction of drug absorption using multivariate statistics. J. Med. Chem. 2000, 43, 3867–3877. [Google Scholar] [CrossRef]
- Muegge, I.; Heald, S.L.; Brittelli, D. Simple selection criteria for drug-like chemical matter. J. Med. Chem. 2001, 44, 1841–1846. [Google Scholar] [CrossRef]
- Banerjee, P.; Eckert, A.O.; Schrey, A.K.; Preissner, R. ProTox-II: A webserver for the prediction of toxicity of chemicals. Nucleic Acids Res. 2018, 46, W257–W263. [Google Scholar] [CrossRef] [Green Version]
- Vila, N.; Besada, P.; Viña, D.; Sturlese, M.; Moro, S.; Terán, C. Synthesis, biological evaluation and molecular modeling studies of phthalazin-1(2H)-one derivatives as novel cholinesterase inhibitors. RSC Adv. 2016, 6, 46170–46185. [Google Scholar] [CrossRef]
- Besada, P.; Viña, D.; Costas, T.; Costas-Lago, M.C.; Vila, N.; Torres-Terán, I.; Sturlese, M.; Moro, S.; Terán, C. Pyridazinones containing dithiocarbamoyl moieties as a new class of selective MAO-B inhibitors. Bioorg. Chem. 2021, 115, 105203. [Google Scholar] [CrossRef]
- Descôteaux, C.; Provencher-Mandeville, J.; Mathieu, I.; Perron, V.; Mandal, S.K.; Asselin, E.; Berube, G. Synthesis of 17-beta-estradiol platinum(II) complexes: Biological evaluation on breast cancer cell lines. Bioorg. Med. Chem. Lett. 2003, 13, 3927–3931. [Google Scholar] [CrossRef]
- Reithofer, M.R.; Valiahdi, S.M.; Jakupec, M.A.; Arion, V.B.; Egger, A.; Galanski, M.; Keppler, B.K. Novel di- and tetracarboxylatoplatinum(IV) complexes. synthesis, characterization, cytotoxic activity, and DNA platination. J. Med. Chem. 2007, 50, 6692–6699. [Google Scholar] [CrossRef]
- Martinez, A.; Lorenzo, J.; Prieto, M.J.; Llorens, R.; Font-Bardia, M.; Solans, X.; Avilés, F.X.; Moreno, V. Synthesis, characterization and biological activity of trans-platinum(II) and trans-platinum(IV) complexes with 4-hydroxymethylpyridine. ChemBioChem 2005, 6, 2068–2077. [Google Scholar] [CrossRef] [PubMed]
- SwissADME. Available online: http://www.swissadme.ch (accessed on 26 September 2022).
- ProTox-II. Available online: https://tox-new.charite.de/protox_II/ (accessed on 27 September 2022).


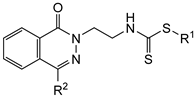 | IC50 a (µM) | ||||
|---|---|---|---|---|---|
| Compound | R2 | R1 | A-2780 | NCI-H460 | MCF-7 |
| 6a | H |  | 88 ± 15 | >100 | 75 ± 1 |
| 7a | p-Tol | 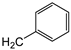 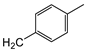 | 10 ± 1 | >100 | 33 ± 1 |
| 8a | Me | 29 ± 3 | >100 | >100 | |
| 6b | H | 78 ± 6 | >100 | 22 ± 2 | |
| 7b | p-Tol |  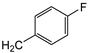 | 17 ± 1 | >100 | 26 ± 1 |
| 8b | Me | 27 ± 1 | >100 | 37 ± 3 | |
| 6c | H | 61 ± 6 | >100 | 36 ± 6 | |
| 7c | p-Tol | 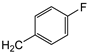 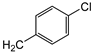 | 15 ± 1 | >100 | 51 ± 1 |
| 8c | Me | 44 ± 7 | >100 | >100 | |
| 6d | H | 15 ± 1 | >100 | 46 ± 1 | |
| 7d | p-Tol | 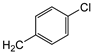  | 20 ± 1 | >100 | >100 |
| 8d | Me | 19 ± 1 | >100 | 24 ± 1 | |
| 6e | H | 5.53 ± 0.09 | >100 | 20 ± 1 | |
| 7e | p-Tol | 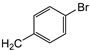 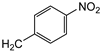 | 17 ± 1 | >100 | >100 |
| 8e | Me | 7.51 ± 0.13 | >100 | >100 | |
| 6f | H | 55 ± 10 | >100 | 25 ± 1 | |
| 7f | p-Tol | 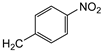 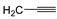 | 10 ± 1 | >100 | 40 ± 1 |
| 8f | Me | 11 ± 1 | >100 | 14 ± 1 | |
| 6g | H | 5.20 ± 0.13 | >100 | 7.64 ± 0.5 | |
| 7g | p-Tol | 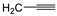 - | 24 ± 1 | 43 ± 1 | 28 ± 1 |
| 8g | Me | 23 ± 1 | >100 | ND | |
| Cisplatin | - | 0.54 ± 0.01 | 5.54 ± 0.23 | 13 ± 1 |
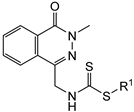 | IC50 a (µM) | |||
|---|---|---|---|---|
| Compound | R1 | A-2780 | NCI-H460 | MCF-7 |
| 9a |  | 12 ± 1 | 7.36 ± 0.08 | 12 ± 1 |
| 9b |  | 32 ± 3 | 8.49 ± 0.25 | 10 ± 1 |
| 9c | 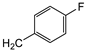 | 43 ± 4 | 12 ± 1 | 84 ± 15 |
| 9d | 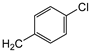 | 34 ± 5 | 7.77 ± 0.17 | 39 ± 4 |
| 9e | 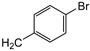 | 24 ± 1 | 12 ± 1 | 33 ± 2 |
| 9f | 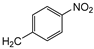 | 12 ± 1 | 25 ± 1 | 62 ± 2 |
| 9g | 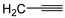 | 6.75 ± 0.12 | 34 ± 1 | 29 ± 2 |
| Cisplatin | - | 0.54 ± 0.01 | 5.54 ± 0.23 | 13 ± 1 |
| Compound | Molecular Weight (g/mol) | Heavy Atoms | Arom. Heavy Atoms | Fraction Csp3 | Rotable Bonds | H-Bond Aceptors | H-Bond Donors | Molar Refractivity | TPSA (Å2) | Log Po/w |
|---|---|---|---|---|---|---|---|---|---|---|
| 6e | 434.37 | 25 | 16 | 0.17 | 7 | 2 | 1 | 112.06 | 104.31 | 3.92 |
| 8e | 448.40 | 26 | 16 | 0.21 | 7 | 2 | 1 | 117.02 | 104.31 | 4.24 |
| 6g | 303.40 | 20 | 10 | 0.21 | 6 | 2 | 1 | 87.65 | 104.31 | 2.28 |
| 9a | 355.48 | 24 | 16 | 0.17 | 6 | 2 | 1 | 104.52 | 104.31 | 3.36 |
| 9b | 369.50 | 25 | 16 | 0.21 | 6 | 2 | 1 | 109.48 | 104.31 | 3.73 |
| 9d | 389.92 | 25 | 16 | 0.17 | 6 | 2 | 1 | 109.53 | 104.31 | 3.85 |
| 9g | 288.39 | 19 | 10 | 0.31 | 4 | 2 | 0 | 85.00 | 92.28 | 2.94 |
| Compound | DL50 (mg/kg) | Toxicity Class | Average Similarity (%) | Prediction Accuracy (%) |
|---|---|---|---|---|
| 6e | 350 | 4 | 47.19 | 54.26 |
| 8e | 350 | 4 | 53.95 | 67.38 |
| 6g | 350 | 4 | 46.36 | 54.26 |
| 9a | 500 | 4 | 47.02 | 54.26 |
| 9b | 500 | 4 | 47.63 | 54.26 |
| 9d | 500 | 4 | 49.72 | 54.26 |
| 9g | 500 | 4 | 47.18 | 54.26 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vila, N.; Besada, P.; Brea, J.; Loza, M.I.; Terán, C. Novel Phthalazin-1(2H)-One Derivatives Displaying a Dithiocarbamate Moiety as Potential Anticancer Agents. Molecules 2022, 27, 8115. https://doi.org/10.3390/molecules27238115
Vila N, Besada P, Brea J, Loza MI, Terán C. Novel Phthalazin-1(2H)-One Derivatives Displaying a Dithiocarbamate Moiety as Potential Anticancer Agents. Molecules. 2022; 27(23):8115. https://doi.org/10.3390/molecules27238115
Chicago/Turabian StyleVila, Noemí, Pedro Besada, José Brea, María Isabel Loza, and Carmen Terán. 2022. "Novel Phthalazin-1(2H)-One Derivatives Displaying a Dithiocarbamate Moiety as Potential Anticancer Agents" Molecules 27, no. 23: 8115. https://doi.org/10.3390/molecules27238115
APA StyleVila, N., Besada, P., Brea, J., Loza, M. I., & Terán, C. (2022). Novel Phthalazin-1(2H)-One Derivatives Displaying a Dithiocarbamate Moiety as Potential Anticancer Agents. Molecules, 27(23), 8115. https://doi.org/10.3390/molecules27238115