Fast and Sensitive Determination of Cadmium and Selenium in Rice by Direct Sampling Electrothermal Vaporization Inductively Coupled Plasma Mass Spectrometry
Abstract
:1. Introduction
2. Results and Discussion
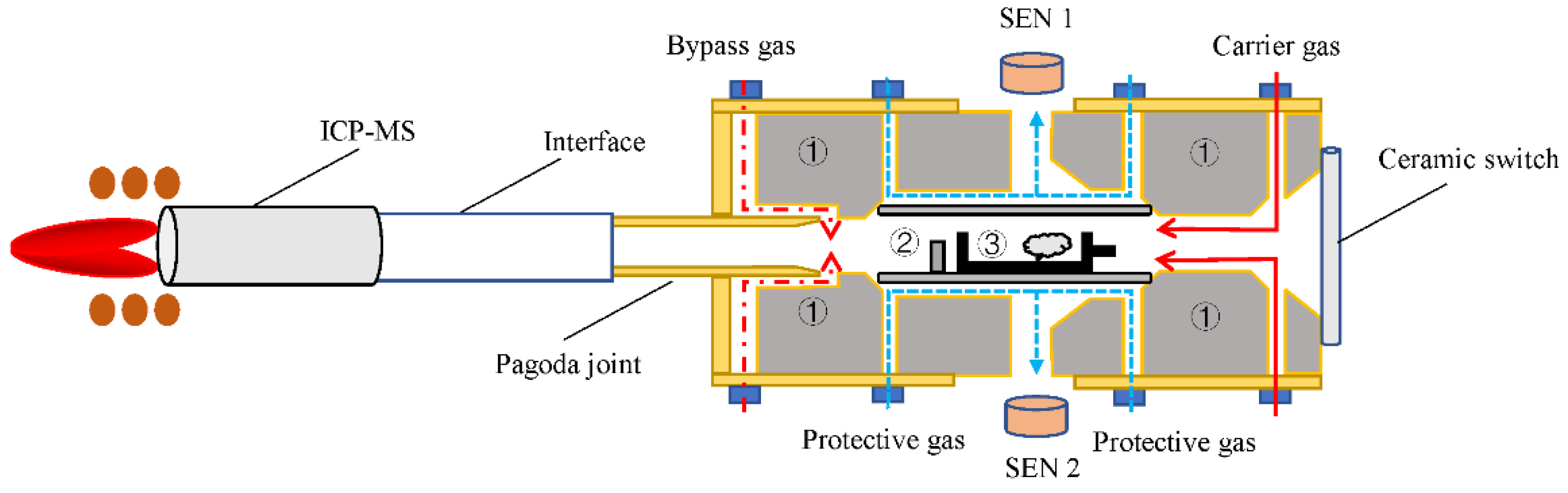
2.1. Design of Gas Line System
2.2. Sample Dehydration and Ashing
2.3. Vaporization of Cd and Se
2.4. Interference Study
2.5. Analytical Performances and Rice Sample Analysis
3. Materials and Methods
3.1. Instrumentation
3.2. Chemicals and Standards
3.3. Analytical Procedures of Direct Sampling ETV-ICP-MS
3.4. Analytical Procedures of Microwave Digestion ICP-MS
3.5. Sample Preparation
3.6. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Birringer, M.; Pilawa, S.; Flohé, L. Trends in selenium biochemistry. Nat. Prod. Rep. 2002, 19, 693–718. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.; Yin, C.; Wang, X.; Shao, S.; Chen, C.; Zhang, H. Selenium translocation in the soil-rice system in the Enshi seleniferous area, Central China. Sci. Total Environ. 2019, 669, 83–90. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Li, H.; Meng, F.; Sun, X.; Feng, X.; Chen, J. Methionine Sulfoxide Reductase B1 Regulates Hepatocellular Carcinoma Cell Proliferation and Invasion via the Mitogen-Activated Protein Kinase Pathway and Epithelial-Mesenchymal Transition. Oxid. Med. Cell Longev. 2018, 2018, 5287971. [Google Scholar] [CrossRef]
- Dinh, Q.T.; Cui, J.; Huang, J.; Tran, T.A.T.; Wang, D.; Yang, w. Selenium distribution in the Chinese environment and its relationship with human health: A review. Environ. Int. 2018, 112, 294–309. [Google Scholar] [CrossRef] [PubMed]
- Sun, G.X.; Meharg, A.A.; Li, G.; Chen, Z.; Yang, L.; Chen, S.C. Distribution of soil selenium in China is potentially controlled by deposition and volatilization? Sci. Rep. 2016, 6, 20953. [Google Scholar] [CrossRef] [Green Version]
- Swaminathan, S. Trace Elements, Toxic Metals, and Metalloids in Kidney Disease. In Nutritional Management of Renal Disease; Elsevier: Amsterdam, The Netherlands, 2013; pp. 339–349. [Google Scholar]
- Farooq, M.U.; Zhu, J. The paradox in accumulation behavior of cadmium and selenium at different planting times in rice. Environ. Sci. Pollut. Res. 2019, 26, 22421–22430. [Google Scholar] [CrossRef]
- Xing, P.; Li, X.; Li, F.; Mao, X. Novel solid sampling electrothermal vaporization atomic absorption spectrometry for fast detection of cadmium in grain samples. J. Anal. At. Spectrom. 2021, 36, 285–293. [Google Scholar] [CrossRef]
- Meharg, A.A.; Norton, G.; Deacon, C.; Williams, P.; Adomako, E.E.; Price, A. Variation in Rice Cadmium Related to Human Exposure. Environ. Sci. Technol. 2013, 47, 5613–5618. [Google Scholar] [CrossRef] [PubMed]
- Shao, Z.; Xue, M.; Liu, Q.; Li, P.; Fang, Y.; Hu, Q. Determination of cadmium in rice bran oils by ICP-MS with rapid ultrasound-assisted acid leaching extraction. J. Consum. Prot. Food Saf. 2020, 15, 193–198. [Google Scholar] [CrossRef]
- Kierulf, A.; Watts, M.; Koch, I.; Beauchemin, D. Continuous Online Leaching System Coupled with Inductively Coupled Plasma Mass Spectrometry for Assessment of Cr, As, Cd, Sb, and Pb in Soils. At. Spectrosc. 2022, 43, 107–116. [Google Scholar]
- Rodríguez Giraldo, Y.; Rodríguez Sánchez, E.; Torres, L.G.; Montenegro, A.C.; Pichimata, M.A. Development of validation methods to determine cadmium in cocoa almond from the beans by ICP-MS and ICP-OES. Talanta Open 2022, 5, 100078. [Google Scholar] [CrossRef]
- Schneider, M.; de Quadros, D.P.C.; Welz, B.; Carasek, E.; de Oliverira Bastos, D.; Rorig, L. A novel extraction-based procedure for the determination of cadmium in marine macro-algae using HR-CS GF AAS. Anal. Methods 2017, 9, 5400–5406. [Google Scholar] [CrossRef]
- Wang, B.; Feng, L.; Mao, X.; Liu, J.; Yu, C.; Ding, L. Direct determination of trace mercury and cadmium in food by sequential electrothermal vaporization atomic fluorescence spectrometry using tungsten and gold coil traps. J. Anal. At. Spectrom. 2018, 33, 1209–1216. [Google Scholar] [CrossRef]
- Hagiwara, K.; Koike, Y.; Aizawa, M.; Nakamura, T. On-site Determination of Arsenic, Selenium, and Chromium(VI) in Drinking Water Using a Solid-phase Extraction Disk/Handheld X-ray Fluorescence Spectrometer. Anal. Sci. 2018, 34, 1309–1315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Papaslioti, E.M.; Parviainen, A.; Román Alpiste, M.J.; Marchesi, C.; Garrido, C.J. Quantification of potentially toxic elements in food material by laser ablation-inductively coupled plasma-mass spectrometry (LA-ICP-MS) via pressed pellets. Food Chem. 2019, 274, 726–732. [Google Scholar] [CrossRef]
- Li, P.-C.; Jiang, S.J. Electrothermal vaporization inductively coupled plasma-mass spectrometry for the determination of Cr, Cu, Cd, Hg and Pb in rice flour. Anal. Chim. Acta 2003, 495, 143–150. [Google Scholar] [CrossRef]
- Mao, X.; Zhang, Y.; Liu, J.; Wang, M.; Qian, Y.; Zhang, Z. Simultaneous trapping of Zn and Cd by a tungsten coil and its application to grain analysis using electrothermal inductively coupled plasma mass spectrometry. RSC Adv. 2016, 6, 48699–48707. [Google Scholar] [CrossRef]
- Zhang, Y.; Mao, X.; Liu, J.; Wang, M.; Qian, Y.; Gao, C. Direct determination of cadmium in foods by solid sampling electrothermal vaporization inductively coupled plasma mass spectrometry using a tungsten coil trap. Spectrochim. Acta Part B At. Spectrosc. 2016, 118, 119–126. [Google Scholar] [CrossRef]
- Grindlay, G.; Mora, J.; Gras, L.; de Loos Vollebrege, M.T.C. Ultratrace determination of Pb, Se and As in wine samples by electrothermal vaporization inductively coupled plasma mass spectrometry. Anal. Chim. Acta 2009, 652, 154–160. [Google Scholar] [CrossRef] [Green Version]
- Hu, X.; Cao, Z.; Sun, W.; Yang, H.; Xu, P.; Zhu, Z. Accurate determination of arsenic and selenium in plant food samples by using ICP-MS/MS. Anal. Methods 2016, 8, 6150–6157. [Google Scholar] [CrossRef]
- de Souza, J.R.; da Silva, L.; da Rocha, M.S.; Saint’Pierre, T.D. Dynamic Reaction Cell-ICP-MS as a Powerful Tool for Quality Control of a Se-Enriched Dietary Supplement. Food Anal. Methods 2017, 10, 3088–3097. [Google Scholar] [CrossRef]
- Medvedev, N.S.; Volzhenin, A.V.; Saprykin, A.I. Multi-elemental Analysis of High-purity Molybdenum by Electrothermal Vaporization-Inductively Coupled Plasma Mass Spectrometry. At. Spectrom. 2021, 42, 71–78. [Google Scholar] [CrossRef]
- Sugiyama, N. Attenuation of doubly charged ion interferences on arsenic and selenium by ICP-MS under low kinetic energy collision cell conditions with hydrogen cell gas. J. Anal. At. Spectrom. 2021, 36, 294–302. [Google Scholar] [CrossRef]
- Patočka, J.; Černohorský, T.; Krejčová, A.; Slang, S. Carbon microparticles as a physical carrier for ETV-ICP-MS. Talanta 2019, 204, 555–560. [Google Scholar] [CrossRef] [PubMed]
- Ediger, R.D.; Beres, S.A. The role of chemical modifiers in analyte transport loss interferences with electrothermal vaporization ICP-mass spectrometry. Spectrochim. Acta Part B At. Spectrosc. 1992, 47, 907–922. [Google Scholar] [CrossRef]
- Araujo, R.G.O.; Oleszczuk, N.; Rampaszzo, R.T.; Costa, P.A.; Silva, M.M.; Vale, M.G.R. Comparison of direct solid sampling and slurry sampling for the determination of cadmium in wheat flour by electrothermal atomic absorption spectrometry. Talanta 2008, 77, 400–406. [Google Scholar] [CrossRef]
- Savio, M.; Cerutti, S.; Martinez, L.D.; Smichowski, P.; Gil, R.A. Study of matrix effects and spectral interferences in the determination of lead in sediments, sludges and soils by SR-ETAAS using slurry sampling. Talanta 2010, 82, 523–527. [Google Scholar] [CrossRef]
- Kántor, T. Electrothermal vaporization and laser ablation sample introduction for flame and plasma spectrometric analysis of solid and solution samples. Spectrochim. Acta Part B At. Spectrosc. 2001, 56, 1523–1563. [Google Scholar] [CrossRef]
- Peng, T.; Sheng, X.; Hu, B.; Jiang, Z. Direct analysis of silicon carbide by fluorination assisted electrothermal vaporization inductively coupled plasma atomic emission spectrometry using a slurry sampling technique. Analyst 2000, 125, 2089–2093. [Google Scholar] [CrossRef]
- Hu, B.; Jiang, Z.; Qin, Y.; Zeng, Y. Fluorination with polytetrafluoroethylene slurry in electrothermal vaporization-inductively coupled plasma-atomic emission spectrometry. Anal. Chim. Acta 1996, 319, 255–264. [Google Scholar] [CrossRef]
- Jiang, Z.; Hu, B.; Qin, Y.; Zeng, Y.e. Fluorination Assisted Electrothermal Vaporization Inductively Coupled Plasma Atomic Emission Spectrometry: An Effective Method for Direct Determination of Refractory Elements in Solid Biological Samples. Microchem. J. 1996, 53, 326–336. [Google Scholar] [CrossRef]
- Sadiq, N.; Beauchemin, D. Optimization of the operating conditions of solid sampling electrothermal vaporization coupled to inductively coupled plasma optical emission spectrometry for the sensitive direct analysis of powdered rice. Anal. Chim. Acta 2014, 851, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Rodney, W.F.; Nancy, J.M.-I. Influence of sample matrix components on the selection of calibration strategies in electrothermal vaporization inductively coupled plasma mass spectrometry. Spectrochim. Acta Part B At. Spectrosc. 1996, 51, 1591–1599. [Google Scholar]
- Feng, L.; Liu, J. An integrated quartz tube atom trap coupled with solid sampling electrothermal vapourization and its application to detect trace lead in food samples by atomic fluorescence spectrometry. J. Anal. At. Spectrom. 2016, 31, 2253–2260. [Google Scholar] [CrossRef]
- Wang, B.; Liu, T.; Liu, J.; Mao, X.; Na, X.; Chen, G.; Lv, Z.; Qia, Y. A Novel Strategy of Anti-interference for Solid Dilution in Elemental Analysis Using Spontaneous Surface Dispersion Theory. At. Spectrosc. 2020, 41, 119–126. [Google Scholar] [CrossRef]
- Turner, J.; Hill, S.J.; Evans, E.H.; Fairman, B.; Briche, C.S.J.W. Accurate analysis of selenium in water and serum using ETV-ICP-MS with isotope dilution. J. Anal. At. Spectrom. 2000, 15, 743–746. [Google Scholar] [CrossRef]
- Li, Y.T.; Jiang, S.J.; Sahayam, A.C. Electrothermal Vaporization Inductively Coupled Plasma Mass Spectrometry for the Determination of Cr, Cd, Hg, and Pb in Honeys. Food Anal. Methods 2017, 10, 434–441. [Google Scholar] [CrossRef]
- Szydlowski, F.J.; Peck, E.; Bax, B. Optimization of Pyrolytic Coating Procedure for Graphite Tubes Used in Atomic Absorption Spectrometry. Appl. Spectrosc. 1978, 32, 402–404. [Google Scholar] [CrossRef]
- Tong, R.; Guo, W. Slurry nebulisation ICP-MS direct determination of high field strength elements (Nb, Ta, Zr, and Hf) in silicate rocks. RSC Adv. 2019, 9, 32435–32440. [Google Scholar] [CrossRef] [Green Version]
- Cui, H.; Guo, W.; Jin, L.; Peng, Y.e.; Hu, S. Elemental screening of the plant-based food by slurry nebulization ICP–MS. J. Anal. At. Spectrom. 2020, 35, 592–599. [Google Scholar] [CrossRef]



| Substances | Added Cd a (ng) | Measured Cd by the Proposed Method (ng) | Recovery b (%) | Added Se a (ng) | Measured Se by the Proposed Method (ng) | Recovery b (%) |
|---|---|---|---|---|---|---|
| K2Cr2O7 | 0.2 | 0.176 ± 0.003 | 88 | 0.2 | 0.187 ± 0.003 | 93 |
| Urea | 0.2 | 0.239 ± 0.009 | 120 | 0.2 | 0.185 ± 0.004 | 92 |
| NH4H2PO4 | 0.2 | 0.197 ± 0.020 | 99 | 0.2 | 0.232 ± 0.016 | 116 |
| Fe | 0.2 | 0.204 ± 0.013 | 102 | 0.2 | 0.200 ± 0.001 | 100 |
| Mg | 0.2 | 0.179 ± 0.007 | 90 | 0.2 | 0.193 ± 0.002 | 97 |
| Na | 0.2 | 0.189 ± 0.010 | 95 | 0.2 | 0.192 ± 0.002 | 96 |
| Ca | 0.2 | 0.206 ± 0.011 | 103 | 0.2 | 0.198 ± 0.009 | 99 |
| As | 0.2 | 0.191 ± 0.009 | 95 | 0.2 | 0.198 ± 0.004 | 99 |
| Pb | 0.2 | 0.222 ± 0.009 | 111 | 0.2 | 0.214 ± 0.011 | 107 |
| Samples a | Cd | Se | ||||||
|---|---|---|---|---|---|---|---|---|
| ICP-MS or Certified (ng·g−1) | This Method (ng·g−1) | RSD (%) | Recovery b (%) | ICP-MS or Certified (ng·g−1) | This Method (ng·g−1) | RSD (%) | Recovery b (%) | |
| THX | 169 ± 2 | 174 ± 2 | 1.0 | 103 | 50 ± 4 | 53 ± 1 | 1.0 | 105 |
| JS | 149 ± 1 | 151 ± 2 | 1.6 | 101 | 126 ± 4 | 123 ± 2 | 1.7 | 98 |
| ZS | 19 ± 1 | 20 ± 1 | 4.0 | 106 | 36 ± 2 | 36 ± 2 | 4.7 | 100 |
| QY | 134 ± 1 | 132 ± 4 | 3.1 | 98 | 33 ± 3 | 31 ± 1 | 3.6 | 93 |
| HN-1 | 419 ± 2 | 421 ± 5 | 1.3 | 100 | 25 ± 3 | 26 ± 1 | 5.3 | 105 |
| HN-2 | 213 ± 1 | 223 ± 1 | 0.4 | 105 | 27 ± 2 | 26 ± 1 | 4.3 | 98 |
| FZ | 4 ± 1 | 4 ± 1 | 7.7 | 100 | 17 ± 2 | 17 ± 1 | 4.3 | 100 |
| GBW10010a | 53 ± 4 | 47 ± 2 | 4.9 | 89 | 36 ± 8 | 40 ± 2 | 5.2 | 111 |
| GBW10045a | 320 ± 40 | 309 ± 5 | 1.5 | 97 | 60 ± 1 | 67 ± 2 | 2.7 | 112 |
| Procedure | ETV Temperature (°C) | Heating Time (s) | Holding Time (s) | Carrier Gas Flow (mL min−1) | Bypass Gas Flow (mL min−1) | Signal Acquisition |
|---|---|---|---|---|---|---|
| Dehydration | 200 | 25 | 15 | 300 | 100 | |
| Ashing | 450 | 20 | 20 | 300 | 100 | |
| Vaporization | 1900 | 7 | 5 | 500 | 600 | Yes |
| Detection | ||||||
| Cleaning | 2100 | 5 | 5 | 800 | 500 |
| Instrument Parameters | Setting Value |
|---|---|
| RF power/(W) | 1400 |
| Auxiliary flow/(L min−1) | 1 |
| Cooling gas flow/(L min−1) | 14 |
| Sampling depth/(mm) | 2 |
| Sampling cone/interception cone | Ni/Cu |
| Scanning times | 1 |
| Scan mode | peak hopping scan |
| Dwell time/(ms) | 10 |
| Isotopes | 111Cd, 82Se |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lan, G.; Li, X.; Jia, H.; Yu, X.; Wang, Z.; Yao, J.; Mao, X. Fast and Sensitive Determination of Cadmium and Selenium in Rice by Direct Sampling Electrothermal Vaporization Inductively Coupled Plasma Mass Spectrometry. Molecules 2022, 27, 8176. https://doi.org/10.3390/molecules27238176
Lan G, Li X, Jia H, Yu X, Wang Z, Yao J, Mao X. Fast and Sensitive Determination of Cadmium and Selenium in Rice by Direct Sampling Electrothermal Vaporization Inductively Coupled Plasma Mass Spectrometry. Molecules. 2022; 27(23):8176. https://doi.org/10.3390/molecules27238176
Chicago/Turabian StyleLan, Guanyu, Xue Li, Hongyu Jia, Xiaofeng Yu, Zhaohui Wang, Jijun Yao, and Xuefei Mao. 2022. "Fast and Sensitive Determination of Cadmium and Selenium in Rice by Direct Sampling Electrothermal Vaporization Inductively Coupled Plasma Mass Spectrometry" Molecules 27, no. 23: 8176. https://doi.org/10.3390/molecules27238176
APA StyleLan, G., Li, X., Jia, H., Yu, X., Wang, Z., Yao, J., & Mao, X. (2022). Fast and Sensitive Determination of Cadmium and Selenium in Rice by Direct Sampling Electrothermal Vaporization Inductively Coupled Plasma Mass Spectrometry. Molecules, 27(23), 8176. https://doi.org/10.3390/molecules27238176





