Plant Spices as a Source of Antimicrobial Synergic Molecules to Treat Bacterial and Viral Co-Infections
Abstract
1. Introduction
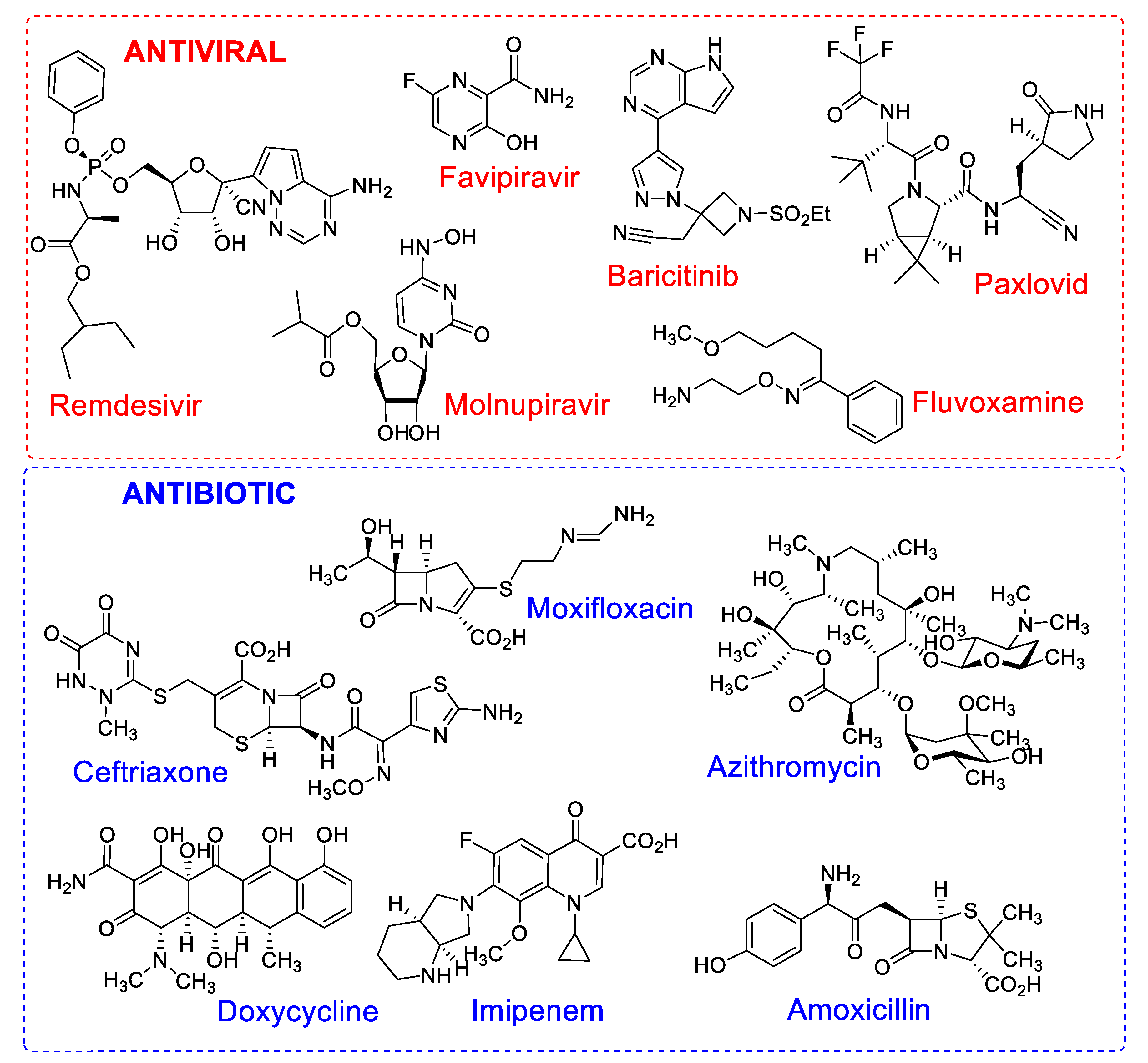
2. COVID 19: Context, Treatment and New Drugs Demand
2.1. COVID-19 Scenario
2.2. Combination Pharmacotherapy for Treatment of Patients with COVID-19
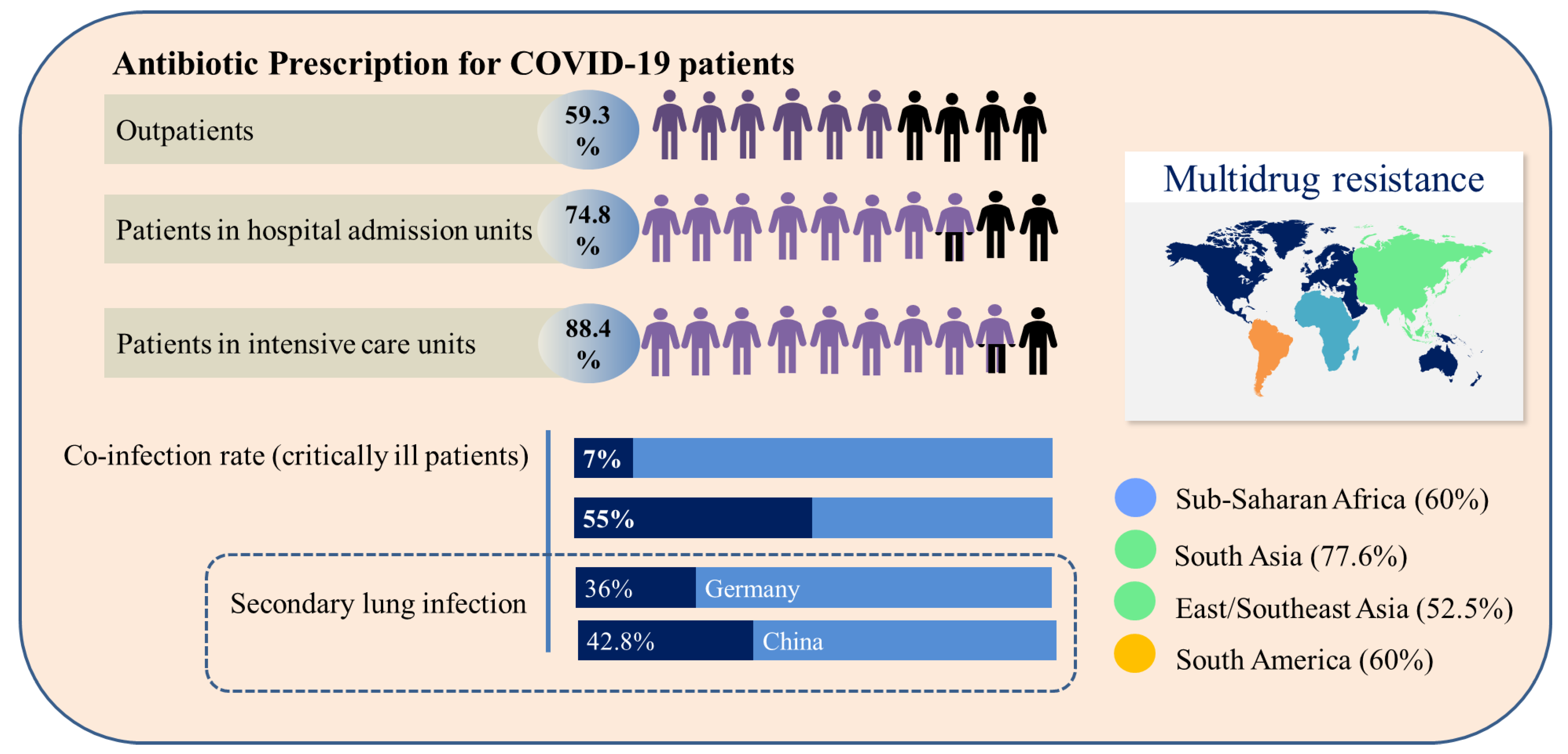
2.3. Prescription of Antibiotics for Patients with COVID-19 and Bacterial Resistance
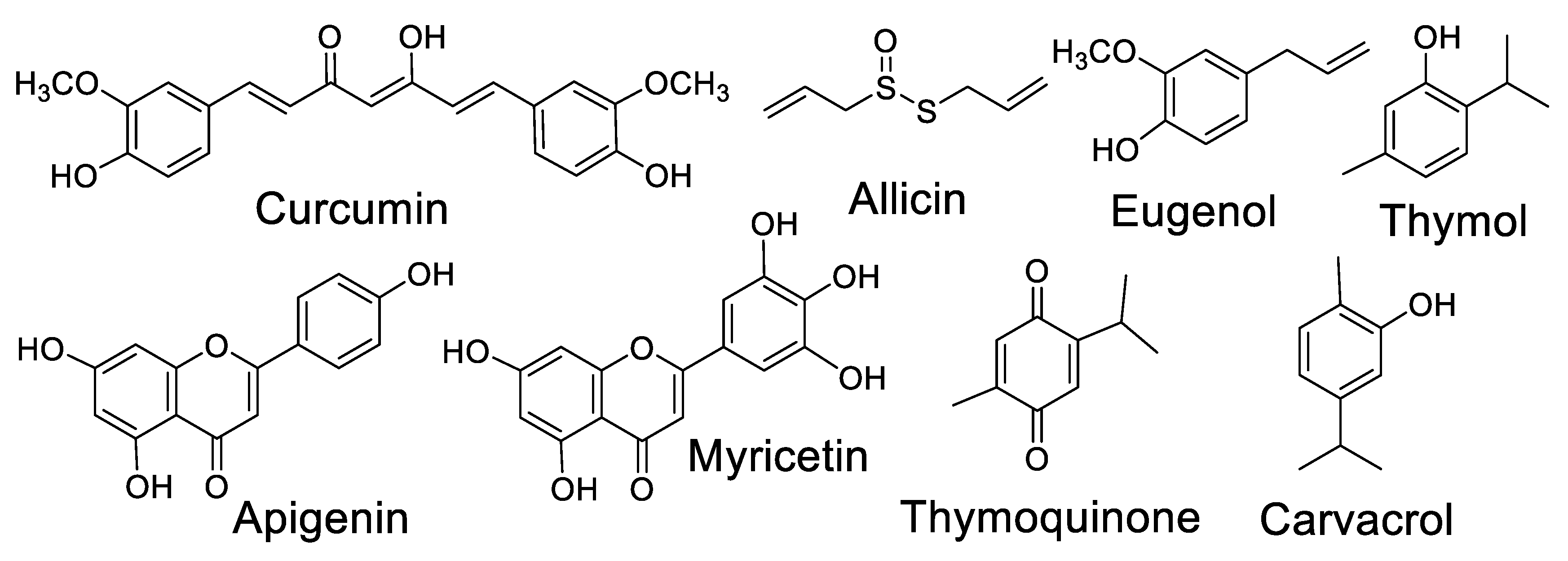
2.4. Medicinal and Spice Plants with Antibiotic Activity and Their Synergistic Effects with Industrialized Antibiotics
2.5. Potential of Plant Spices with Antibiotic Activity as Antiviral Agents
| Scientific Name [Popular Name] | Main Component | Antiviral Effects/COVID-19 Applications | Reference |
|---|---|---|---|
| C. longa [Turmeric] | Curcumin | Attenuation of poly(I:C)-induced immune and inflammatory responses by inhibiting the TLR3/TBK1/IFN-β cascade | [90] |
| Enhancement of oral drug delivery system (Labrasol® and tween 80 bicelles) | [91] | ||
| Molecular docking studies showed reliable ADME profile | [92] | ||
| Analogues as dual inhibitor of SARS-CoV-2 | [93] | ||
| Development of nanoformulations | [87,94] | ||
| Allium sativum [Garlic] | Allicin | Suppresses production and secretion of pro-inflammatory cytokines and stimulates of immune system cells (NK, lymphocytes, eosinophils and macrophages) | [95] |
| Suppression of pro-inflammatory cytokines TNF-α and CRP | [96] | ||
| Cinnamomum verum [Dalchini] | Eugenol | Inhibition of specific immune responses to allergens, reduces side effects of some anti-inflammatory drugs, antioxidant properties | [97] |
| Increases the bioavailability of antiviral drug saquinavir | [98] | ||
| Nigella sativa [Black cumin] | Thymoquinone | Inhibitory effects on viral spike protein with cellular angiotensin-converting enzyme 2 (ACE2) | [99] |
| Inhibition of RdRp of SARS-CoV-2, especially α-hederin; ongoing drug development strategy against SARS-CoV-2 | [99] | ||
| O. basilicum [Basil] | Apigenin | The phytoconstituents vicenin, sorientin and ursolic acid inhibit SARS-CoV-2 Mpro | [100] |
| Development of gellan gum hydrogel with basil oil nanoemulsion | [101] | ||
| O. vulgare [Oregano] | Carvacrol | Inhibition of viral replication and activity of SARS-CoV-2 3CLPRO | [102] |
| Potent inhibition of SARS-CoV-2 replication (modeling studies) | [103] | ||
| Thymus vulgaris [Thyme] | Thymol | Inhibits the viral spike protein, preventing SARS-CoV-2 entry | [103] |
| Essential oils induce cytopathogenic effect against SARS-CoV in Vero-E6 cells | [104] |
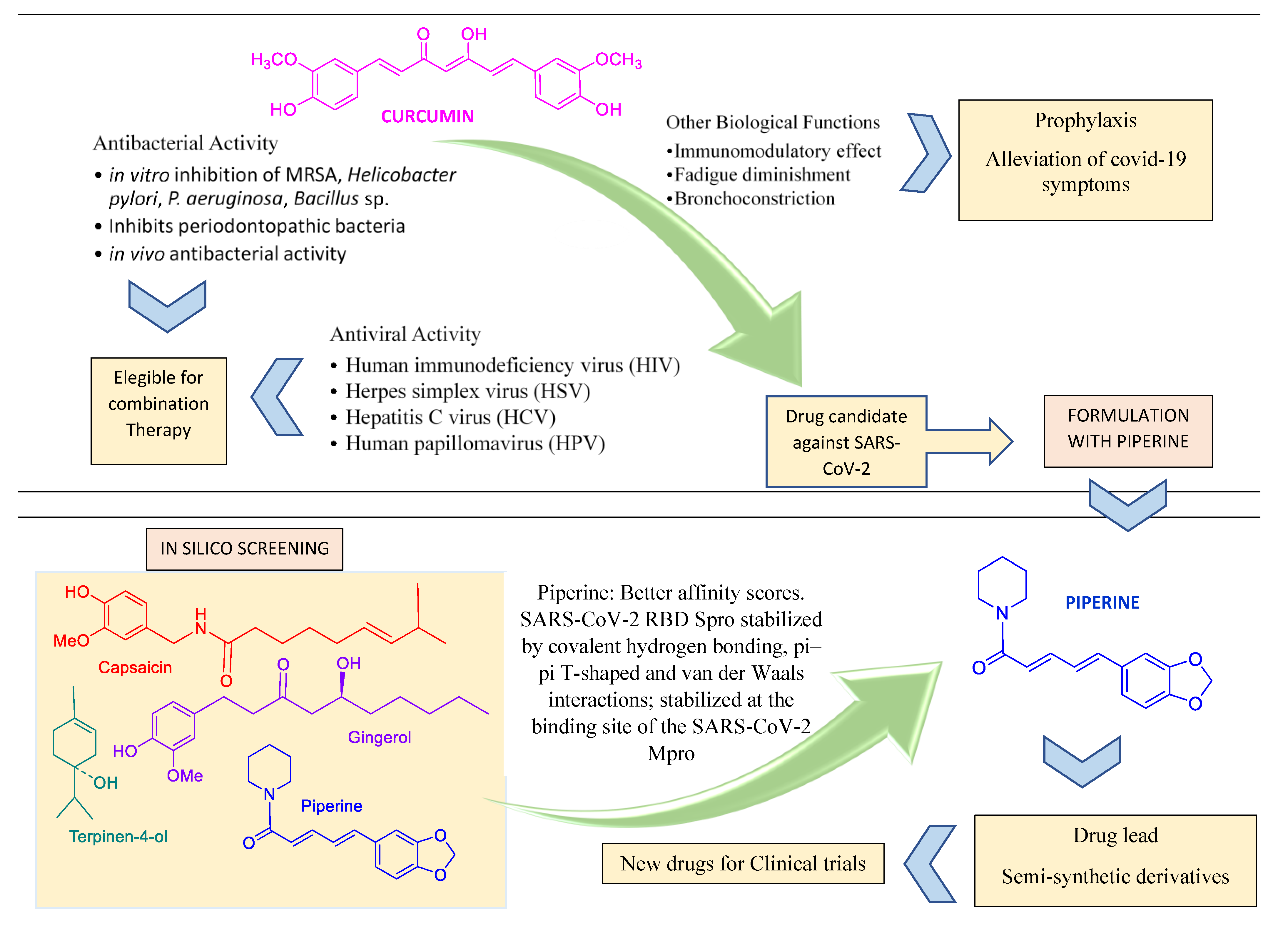
2.6. Recent Research in Spice-Derived Metabolites in COVID-19 Context
3. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Tompa, D.R.; Immanuel, A.; Srikanth, S.; Kadhirvel, S. Trends and strategies to combat viral infections: A review on FDA approved antiviral drugs. Int. J. Biol. Macromol. 2021, 172, 524–541. [Google Scholar] [CrossRef] [PubMed]
- Kausar, S.; Said, K.F.; Ishaq, M.; Akram, M.; Riaz, M.; Rasool, G.; Malik, A. A review: Mechanism of action of antiviral drugs. Int. J. Immunopathol. Pharmacol. 2021, 35, 20587384211002621. [Google Scholar] [CrossRef]
- Kwok, M.; Lee, C.; Li, H.S.; Deng, R.; Tsoi, C.; Ding, Q.; Poon, E.N. Remdesivir induces persistent mitochondrial and structural damage in human induced pluripotent stem cell-derived cardiomyocytes. Cardiovasc. Res. 2022, 118, 2652–2664. [Google Scholar] [CrossRef]
- Wen, W.; Chen, C.; Tang, J.; Wang, C.; Zhou, M.; Cheng, Y.; Mao, Q. Efficacy and safety of three new oral antiviral treatment (molnupiravir, fluvoxamine and Paxlovid) for COVID-19: A meta-analysis. Ann. Med. 2022, 54, 516–523. [Google Scholar] [CrossRef]
- Catalano, A.; Iacopetta, D.; Ceramella, J.; Scumaci, D.; Giuzio, F.; Saturnino, C.; Aquaro, S.; Rosano, C.; Sinicropi, M.S. Multidrug Resistance (MDR): A Widespread Phenomenon in Pharmacological Therapies. Molecules 2022, 27, 616. [Google Scholar] [CrossRef] [PubMed]
- Corrêa, R.C.G.; Heleno, A.S.; Alves, M.J.; Isabel, I.C.F.R. Bacterial resistance: Antibiotics of last generation used in clinical practice and the arise of natural products as new therapeutic alternatives. Curr. Pharm. Des. 2020, 26, 815–837. [Google Scholar] [CrossRef]
- Interagency Coordination Group on Antimicrobial Resistance (IACG). No Time to Wait: Securing the Future from Drug Resistant Infections. Available online: https://www.who.int/docs/default-source/documents/no-time-to-wait-securing-the-future-from-drug-resistant-infections-en.pdf (accessed on 21 October 2022).
- Ayaz, M.; Ullah, F.; Sadiq, A.; Ullah, F.; Ovais, M.; Ahmed, J.; Devkota, H.P. Synergistic interactions of phytochemicals with antimicrobial agents: Potential strategy to counteract drug resistance. Chem. Biol. Interact. 2019, 308, 294–303. [Google Scholar] [CrossRef]
- Huang, Y.; Li, H.; Peng, D.; Wang, Y.; Ren, Q.; Guo, Y. The production and exportation of artemisinin-derived drugs in China: Current status and existing challenges. Malaria J. 2016, 15, 365. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, S.; Dubey, N.K. Traditional medicinal plants as promising source of immunomodulator against COVID-19. J. Agric. Sci. 2020, 8, 126–138. [Google Scholar] [CrossRef]
- Akinbolade, S.; Coughlan, D.; Fairbairn, R.; McConkey, G.; Powell, H.; Ogunbayo, D.; Craig, D. Combination therapies for COVID-19: An overview of the clinical trials landscape. Br. J. Clin. Pharmacol. 2022, 88, 1590–1597. [Google Scholar] [CrossRef]
- Taher, M.; Tik, N.; Susanti, D. Drugs intervention study in COVID-19 management. Drug. Metab. Pers. Ther. 2021. [Google Scholar] [CrossRef]
- Berber, E.; Sumbria, D.; Kokkaya, S. A metabolic blueprint of COVID-19 and long-term vaccine efficacy. Drug Metab Pers Ther. 2022. [Google Scholar] [CrossRef]
- Dhama, K.; Karthik, K.; Khandia, R.; Munjal, A.; Tiwari, R.; Rana, R.; Khurana, S.K.; Sana, U.; Khan, R.; Alagawany, M.; et al. Medicinal and Therapeutic Potential of Herbs and Plant Metabolites/Extracts Countering Viral Pathogens—Current Knowledge and Future Prospects. Curr. Drug. Metab. 2018, 19, 236–263. [Google Scholar] [CrossRef]
- Takahashi, J.A.; Barbosa, B.V.R.; Lima, M.T.N.S.; Cardoso, P.G.; Contigli, C.; Pimenta, L.P.S. Antiviral fungal metabolites and some insights into their contribution to the current COVID-19 pandemic. Bioorg. Med. Chem. 2021, 46, 116366. [Google Scholar] [CrossRef] [PubMed]
- Weng, L.M.; Su, X.; Wang, X.Q. Pain Symptoms in Patients with Coronavirus Disease (COVID-19): A Literature Review. J. Pain Res. 2021, 14, 147–159. [Google Scholar] [CrossRef] [PubMed]
- Molla, M.A.; Yeasmin, M.; Slam, K.; Sharif, M.; Amin, R.; Nafisa, T.; Ghosh, A.K.; Parveen, M.; Arif, M.H.; Alam, J.A.J.; et al. Antibiotic Prescribing Patterns at COVID-19 Dedicated Wards in Bangladesh: Findings from a Single Center Study. Inf. Prev. Pract. 2021, 3, 100–134. [Google Scholar] [CrossRef]
- Mobaraki, P.D.; Zaidi, A.K. Consequences of Intubation in COVID-19 Patients: Are We Ready. IJOPL 2020, 10, 50–53. [Google Scholar] [CrossRef]
- Clancy, J.C.; Buehrle, J.D.; Nguyen, M.H. PRO: The COVID-19 pandemic will result in increased antimicrobial resistance rates. JAC Antimicrob. Resist. 2020, 2, dlaa049. [Google Scholar] [CrossRef] [PubMed]
- Falcone, M.; Tiseo, G.; Giordano, C.; Leonildi, A.; Menichini, M.; Vecchione, A.; Pistello, M.; Guarracino, F.; Ghiadoni, L.; Forfori, F.; et al. Predictors of hospital-acquired bacterial and fungal superinfections in COVID-19: A prospective observational study. J. Antimicrob. Chemother. 2020, 76, 1078–1084. [Google Scholar] [CrossRef]
- Hirschmann, R.; Bortolotto, C.C.; Silva, M.T.; Machado, A.K.F.; Xavier, M.O.; Fernandes, M.P.; Martins, R.C.; Bielemann, R.M.; Rodrigues, L.T.; Wehrmeister, F.C. Simultaneity of risk factors for chronic non-communicable diseases in a rural population of a Southern Brazilian city. Rev. Bras. Epidemiol. 2020, 23, E200066. [Google Scholar] [CrossRef]
- Ye, C.; Zhang, S.; Zhang, X.; Cai, H.; Gu, J.; Lian, J.; Lu, Y.; Jia, H.; Hu, J.; Jin, C.; et al. Impact of comorbidities on patients with COVID-19: A large retrospective study in Zhejiang, China. J. Med. Virol. 2020, 92, 2821–2829. [Google Scholar] [CrossRef] [PubMed]
- Coccia, M. Optimal levels of vaccination to reduce COVID-19 infected individuals and deaths: A global analysis. Environ. Res. 2022, 30, 10024. [Google Scholar] [CrossRef]
- Hoffmann, M.; Krüger, N.; Schulz, S.; Cossmann, A.; Rocha, C.; Kempf, A.; Pöhlmann, S. The Omicron variant is highly resistant against antibody-mediated neutralization: Implications for control of the COVID-19 pandemic. Cell 2022, 185, 447–456. [Google Scholar] [CrossRef] [PubMed]
- Choi, A.; Koch, M.; Wu, K.; Chu, L.; Ma, L.Z.; Hill, A.; Nunna, N.; Huang, W.; Oestreicher, J.; Colpitts, T.; et al. Safety and immunogenicity of SARS-CoV-2 variant mRNA vaccine boosters in healthy adults: An interim analysis. Nat. Med. 2021, 27, 2025–2031. [Google Scholar] [CrossRef]
- Javorac, D.; Grahovac, L.; Manic, L.; Srrojikovic, N.; Andelkovi, A.; Bulat, Z.; Dukic, D.; Curcic, M.; Djordjevic, A.B. An overview of the safety assessment of medicines currently used in the COVID-19 disease treatment. Food Chem. Toxicol. 2020, 144, 111639. [Google Scholar] [CrossRef]
- Salasc, F.; Lahlali, T.; Laurent, E.; Calatrava, M.R.; Pizzorno, A. Treatments for COVID-19: Lessons from 2020 and new therapeutic options. COPHAR 2022, 62, 43–49. [Google Scholar] [CrossRef]
- Shubhangi, K.; Darren, F. Repurposing drugs for treatment of SARS-CoV-2 infection: Computational design insights into mechanisms of action. J. Biomol. Struct. Dyn. 2022, 40, 1316–1330. [Google Scholar]
- Milas, S.; Poncelet, A.; Buttafuoco, F.; Pardo, A.; Lali, S.E.; Cherifi, S. Antibiotic use in patients with Coronavirus disease 2019 (COVID-19): Outcomes and associated factors. Acta Clin. Belg. 2022, 77, 579–587. [Google Scholar] [CrossRef] [PubMed]
- Bakowski, A.M.; Beutler, N.; Chen, E.; Trinh, T.; Nguyen, H.; Kirkpatrick, M.G.; Parren, M.; Yang, L.; Ricketts, J.; Gupta, A.K.; et al. Oral drug repositioning candidates and synergistic remdesivir combinations for the prophylaxis and treatment of COVID-19. bioRxiv 2020. [Google Scholar] [CrossRef]
- Khataniar, A.; Pathak, U.; Rajkhowa, S.; Jha, A.N. A Comprehensive Review of Drug Repurposing Strategies against Known Drug Targets of COVID-19. Covid 2022, 2, 148–167. [Google Scholar] [CrossRef]
- Langford, B.J.; So, M.; Raybardhan, S.; Leung, V.; Soucy, R.P.J.; Westwood, D.; Daneman, N.; Macfadden, D.R. Antibiotic prescribing in patients with COVID-19: Rapid review and meta-analysis. CMI 2021, 27, 520–531. [Google Scholar] [CrossRef]
- Miranda, C.; Silva, V.; Capita, R.; Calleja, C.A.; Igrejas, G.; Poeta, P. Implications of antibiotics use during the COVID-19 pandemic: Present and future. J. Antimicrob. Chemother. 2020, 75, 3413–3416. [Google Scholar] [CrossRef]
- Lucien, A.M.B.; Canarie, M.F.; Kilgore, P.E.; Denis, G.J.; Fenelon, N.; Pierre, M.; Cerpa, M.; Joseph, G.; Maki, G.; Zervos, M.J.; et al. Antibiotics and antimicrobial resistance in the COVID-19 era: Perspective from resource-limited settings. Int. J. Infect. Dis. 2021, 104, 250–254. [Google Scholar] [CrossRef]
- Su, L.; Tu, Y.; Kong, D.; Chen, D.; Zhang, C.; Zhang, W.; Zhuang, C.; Wang, Z. Drug repurposing of anti-infective clinical drugs: Discovery of two potential anti-cytokine storm agents. Biomed. Pharmacother. 2020, 131, 110643. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Liao, B.; Cheng, L.; Peng, X.; Xu, X.; Li, Y.; Hu, T.; Li, J.; Zhou, X.; Ren, B. The microbial coinfection in COVID-19. Appl. Microbiol. Biotechnol. 2020, 104, 7777–7785. [Google Scholar] [CrossRef]
- Chong, W.H.; Saha, B.K.; Ramani, A.; Chopra, A. State-of-the-art review of secondary pulmonary infections in patients with COVID-19 pneumonia. J. Infect. 2021, 49, 591–605. [Google Scholar] [CrossRef] [PubMed]
- Uchino, K.; Kolikonda, M.K.; Brown, D.; Kovi, S.; Collins, D.; Khawaja, Z.; Buletko, A.B.; Russman, A.N.; Hussain, M.S. Decline in Stroke Presentations During COVID-19 Surge. Stroke 2020, 51, 2544–2547. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.X.; Wang, C.Y.; Li, Y.Y.; Li, J.; Wan, Q.Q.; Chen, J.H.; Tay, F.R.; Niu, L.N. Considerations and Caveats in Combating ESKAPE Pathogens against Nosocomial Infections. Adv. Sci. 2020, 7, 1901872. [Google Scholar] [CrossRef] [PubMed]
- Nadeem, F.S.; Gohar, U.F.; Tahir, S.F.; Mukhtar, H.; Pornpukdeewattana, S.; Nukthamna, P.; Ali, A.M.M.; Bavisetty, S.C.B.; Massa, S. Antimicrobial resistance: More than 70 years of war between humans and bactéria. Crit. Rev. Microbiol. 2020, 5, 578–599. [Google Scholar] [CrossRef]
- Ilanko, A.; Cock, I.E. The interactive antimicrobial activity of conventional antibiotics and Petalostigma spp. extracts against bacterial triggers of some autoimmune inflammatory diseases. Pharmacogn. J. 2019, 11, 292–309. [Google Scholar] [CrossRef]
- Obakiro, S.B.; Kiyimba, K.; Paasi, G.; Napyo, A.; Anthierens, S.; Waako, P.; Royen, P.V.; Iramiot, J.S.; Goossens, H.; Kostyanev, T. Prevalence of antibiotic-resistant bacteria among patients in two tertiary hospitals in Eastern Uganda. J. Glob. Antimicrob. Resist. 2021, 25, 82–86. [Google Scholar] [CrossRef] [PubMed]
- Nelson, R.E.; Hatfield, K.M.; Wolford, H.; Samore, M.H.; Scott, R.D.; Reddy, S.C.; Paul, P.; Jernigan, J.A.; Baggs, L. National Estimates of Healthcare Costs Associated with Multidrug-Resistant Bacterial Infections Among patients hospitalized in the United States. Clin. Infect. Dis. 2021, 72, 17–26. [Google Scholar] [CrossRef]
- Abdulbari, A.; Ali, N.M.; Raghif, A.R.A.; Matloob, A.N. Comparison of Oral Isotretinoin vs Azithromycin in the Treatment of Acne Vulgaris. Indian J. Med. Forensic Med. Toxicol. 2021, 5, 1485–1489. [Google Scholar]
- Salmerón-Manzano, E.; Garrido-Cardenas, J.A.; Manzano-Agugliaro, F. Worldwide research trends on medicinal plants. Int. J. Environ. Res. Public Health 2020, 17, 3376. [Google Scholar] [CrossRef]
- Hao, D.C.; Xiao, P.G. Pharmaceutical resource discovery from traditional medicinal plants: Pharmacophylogeny and pharmacophylogenomics. Chin. Herb. Med. 2020, 12, 104–117. [Google Scholar] [CrossRef]
- Khan, T.; Sankhe, K.; Suvarna, V.; Sherje, A.; Patel, K.; Dravyakar, B. DNA gyrase inhibitors: Progress and synthesis of potent compounds as antibacterial agents. Biomed. Pharmacother. 2018, 103, 923–938. [Google Scholar] [CrossRef] [PubMed]
- Anand, U.; Jacobo-Herrera, N.; Altemimi, A.; Lakhssassi, N. A comprehensive review on medicinal plants as antimicrobial therapeutics: Potential avenues of biocompatible drug discovery. Metabolites 2019, 9, 258. [Google Scholar] [CrossRef]
- Baloyi, I.T.; Idowu, J.A.; Abdullahi, A.Y.; Sekelwa, C. Antibacterial, antiquorum sensing, antibiofilm activities and chemical profiling of selected South African medicinal plants against multi-drug resistant bacteria. J. Med. Plant Res. 2022, 16, 52–65. [Google Scholar]
- Nigussie, D.; Gail, D.; Beyene, T.T.; Malcolm, B.; Adefris, L.B.; Abebaw, F.; Eyasu, M. Antibacterial and Antifungal Activities of Ethiopian Medicinal Plants: A Systematic Review. Front. Pharmacol. 2021, 12, 1663–9812. [Google Scholar] [CrossRef]
- Manandhar, S.; Luitel, S.; Dahal, R.K. In Vitro Antimicrobial Activity of Some Medicinal Plants against Human Pathogenic Bacteria. J. Trop. Med. 2019, 2019, 1895340. [Google Scholar] [CrossRef]
- Choi, J.S.; Jung, H.C.; Baek, Y.J.; Kim, B.Y.; Lee, M.W.; Kim, H.D.; Kim, S.W. Antibacterial Activity of Green-Synthesized Silver Nanoparticles Using Areca catechu Extract against Antibiotic-Resistant Bacteria. Nanomaterials 2021, 11, 205. [Google Scholar] [CrossRef] [PubMed]
- Kebede, T.; Gadisa, E.; Tufa, A. Antimicrobial activities evaluation and phytochemical screening of some selected medicinal plants: A possible alternative in the treatment of multidrug-resistant microbes. PLoS ONE 2021, 16, e0249253. [Google Scholar] [CrossRef] [PubMed]
- Cech, N.B.; Junio, H.A.; Ackermann, L.W.; Kavanaugh, J.S.; Horswill, A.R. Quorum quenching and antimicrobial activity of goldenseal (Hydrastis canadensis) against methicillin-resistant Staphylococcus aureus (MRSA). Planta Med. 2012, 78, 1556–1561. [Google Scholar] [CrossRef] [PubMed]
- Richwagen, N.; Lyles, J.; Dale, B.; Quave, C. Antibacterial Activity of Kalanchoe mortagei and K. fedtschenkoi against ESKAPE Pathogens. Front. Pharmacol. 2019, 10, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Da Cruz, N.; Ferraz, A.C.; Moraes, T.D.F.S.; Lima, W.G.; Dos Santos, J.P.; Duarte, L.P.; de Magalhães, J.C. Pristimerin isolated from Salacia crassifolia (Mart. Ex. Schult.) G. Don. (Celastraceae) roots as a potential antibacterial agent against Staphylococcus aureus. J. Ethnopharmacol. 2021, 266, 113423. [Google Scholar]
- Mulat, M.; Khan, F.; Pandita, A. Chemical Composition and Antibacterial, Anti-biofilm and Anti-virulence Activities of Plant Extracts against Human Pathogenic Bacteria. Nat. Prod. J. 2022, 12, 54–68. [Google Scholar] [CrossRef]
- Schultz, F.; Anywar, G.; Tang, H. Targeting ESKAPE pathogens with anti-infective medicinal plants from the Greater Mpigi region in Uganda. Sci. Rep. 2020, 10, 11935. [Google Scholar] [CrossRef]
- Assis, F.V.; Ferreira, J.M.S.; Siqueira, F.L.; Gonçalves, I.E.; Lacerda, R.P.; Nascimento, R.A.; Araújo, S.G.; Trindade, J.T.; Herrera, K.M.S.; Lima, A.R.L.S. Antibacterial activity of Lamiaceae plant extracts in clinical isolates of multidrug-resistant bacteria. Anais da Academia Brasileira de Ciências 2018, 90, 1665–1670. [Google Scholar] [CrossRef]
- Al-sa’ady, A. Antibacterial screening for five local medicinal plants against nosocomial pathogens: Klebsiella pneumoniae and Staphylococcus epidermidis. Eurasia J. Biosci. 2020, 14, 553–559. [Google Scholar]
- Al-Bakri, A.G.; Othman, G.; Afif, F.U. Determination of the antibiofilm, antiadhesive, and anti-MRSA activities of seven Salvia species. Pharmacogn. Mag. 2010, 6, 264–270. [Google Scholar] [CrossRef]
- Tsai, C.C.; Lin, C.S.; Hsu, C.R. Using the Chinese herb Scutellaria barbata against extensively drug-resistant Acinetobacter baumannii infections: In vitro and in vivo studies. BMC Complement. Altern. Med. 2018, 18, 96. [Google Scholar] [CrossRef]
- Bouhrim, M.; Radi, F.Z.; Mechchate, H.; Al-zahrani, M.; Qurtam, A.A.; Aleissa, A.M.; Drioiche, A.; Handaq, N.; Zair, T. Phytochemical Analysis, Antimicrobial and Antioxidant Properties of Thymus zygis L. and Thymus willdenowii Boiss. Essential Oils. Plants 2022, 11, 15. [Google Scholar]
- Sun, L.; Song, F.; Shi, N.; Liu, F.; Li, S.; Li, P.; Zhang, W.; Jiang, X.; Zhang, Y.; Sun, L.; et al. Combination of four clinical indicators predicts the severe/critical symptom of patients infected COVID-19. J. Clin. Virol. 2020, 128, 104431. [Google Scholar] [CrossRef] [PubMed]
- Chanda, S.; Rakholiya, K. Combination therapy: Synergism between natural plant extracts and antibiotics against infectious diseases. Microbiol. Book Ser. 2011, 1, 520–529. [Google Scholar]
- Hemaiswarya, S.; Kruthiventi, A.K.; Doble, M. Synergism between natural products and antibiotics against infectious diseases. Phytomedicine 2008, 15, 639–652. [Google Scholar] [CrossRef]
- Ju, J.; Xie, Y.; Yu, H.; Guo, Y.; Cheng, Y.; Qian, H.; Yao, W. Synergistic interactions of plant essential oils with antimicrobial agents: A new antimicrobial therapy. Crit. Rev. Food Sci. Nutr. 2020, 62, 1740–1751. [Google Scholar] [CrossRef] [PubMed]
- Saquib, S.A.; AlQahtani, N.A.; Ahmad, I.; Kader, M.A.; Al Shahrani, S.S.; Asiri, E.A. Evaluation and Comparison of Antibacterial Efficacy of Herbal Extracts in Combination with Antibiotics on Periodontal pathobionts: An in vitro Microbiological Study. Antibiotics 2019, 8, 89. [Google Scholar] [CrossRef] [PubMed]
- Embaby, M.A.; El-Raey, M.A.; Zaineldain, M.; Almaghrabi, O.; Marrez, D.A. Synergistic effect and efflux pump inhibitory activity of Ficus nitida phenolic extract with tetracycline against some pathogenic bacteria. Toxin Rev. 2021, 40, 1187–1197. [Google Scholar] [CrossRef]
- Khleifat, K.M.; Matar, S.A.; Jaafreh, M.; Qaralleh, H.; Al-limoun, M.O.; Alsharafa, K.Y. Essential Oil of Centaurea damascena Aerial Parts, Antibacterial and Synergistic Effect. J. Essent. Oil-Bear. Plants 2019, 22, 356–367. [Google Scholar] [CrossRef]
- Dassanayake, M.K.; Khoo, T.J.; An, J. Antibiotic resistance modifying ability of phytoextracts in anthrax biological agent Bacillus anthracis and emerging superbugs: A review of synergistic mechanisms. Ann. Clin. Microbiol. Antimicrob. 2021, 20, 79. [Google Scholar] [CrossRef]
- Bezalwar, P.M.; Charde, V.N. Study on synergistic action of Coriandrum sativum seed extracts on antibiotics against multidrug resistant P. aeruginosa. Environ. Conserv. J. 2019, 20, 83–88. [Google Scholar] [CrossRef]
- Da Silva, J.B.; de Bessa, M.E.; Mayorga, O.A.S.; Andrade, V.T.; da Costa, Y.F.G.; de Freitas, M.R.; Alves, M.S. A promising antibiotic, synergistic and antibiofilm effects of Vernonia condensata Baker (Asteraceae) on Staphylococcus aureus. Microb. Pathog. 2018, 123, 385–392. [Google Scholar] [CrossRef]
- Maheshwari, M.; Althubiani, A.S.; Abulreesh, H.H.; Qais, F.A.; Khan, M.S.; Ahmad, I. Bioactive extracts of Carum copticum L. enhances efficacy of ciprofloxacin against MDR enteric bacteria. Saudi J. Biol. Sci. 2019, 26, 1848–1855. [Google Scholar] [CrossRef]
- Bahmani, M.; Taherikalani, M.; Khaksarian, M.; Rafieian-Kopaei, M.; Ashrafi, B.; Nazer, M.; Rashidipour, M. The synergistic effect of hydroalcoholic extracts of Origanum vulgare, Hypericum perforatum and their active components carvacrol and hypericin against Staphylococcus aureus. Future Sci. 2019, 5, FSO371. [Google Scholar] [CrossRef] [PubMed]
- Keawchai, K.; Chumkaew, P.; Permpoonpattana, P.; Srisawat, T. Synergistic effect of Hydnophytum formicarum tuber and Vatica diospyroides Symington cotyledon extracts with ampicillin on pathogenic bacteria. J. Appl. Biol. 2022, 10, 6–11. [Google Scholar]
- Silva, D.M.; Costa, P.A.; Ribon, A.B.; Purgato, G.A.; Gaspar, D.M.; Diaz, M.A. Plant Extracts Display Synergism with Different Classes of Antibiotics. Anais da Academia Brasileira de Ciências 2019, 91, e20180117. [Google Scholar] [CrossRef] [PubMed]
- Melguizo-Rodríguez, L.; García-Recio, E.; Ruiz, C.; De Luna-Bertos, E.; Illescas-Montes, R.; Costela-Ruiz, V.J. Biological properties and therapeutic applications of garlic and its components. Food Funct. 2022, 13, 2415–2426. [Google Scholar] [CrossRef] [PubMed]
- Rana, S.; Chauhan, P. Spices that heal: Review on untapped potential of lesser-known spices as immunity booster during COVID-19 pandemic. Ann. Phytomedicine 2022, 11, 7–11. [Google Scholar] [CrossRef]
- Mei, J.; Zhao, F.; Xu, R.; Huang, Y. A review on the application of spectroscopy to the condiments detection: From safety to authenticity. Crit. Rev. Food Sci. Nutr. 2021, 62, 6374–6389. [Google Scholar] [CrossRef] [PubMed]
- Long, Y.; Zhang, M.; Mujumdar, A.S.; Chen, J. Valorization of turmeric (Curcuma longa L.) rhizome: Effect of different drying methods on antioxidant capacity and physical properties. Dry. Technol. 2022, 40, 1609–1619. [Google Scholar] [CrossRef]
- Global Industry Analysts. Condiments Market—Growth, Trends, COVID-19 Impact and Forecasts (2022–2029). Available online: https://www.maximizemarketresearch.com/market-report/condiments-market/148426/#:~:text=Condiments%20Market%20Segment%20Analysis%3A&text=The%20Food%20Chain%20Service%20segment,dressing%20markets%20in%20the%20world (accessed on 25 October 2022).
- Hussain, Y.; Alam, W.; Ullah, H.; Dacrema, M.; Daglia, M.; Khan, H.; Arciola, C.R. Antimicrobial Potential of Curcumin: Therapeutic Potential and Challenges to Clinical Applications. Antibiotics 2022, 11, 322. [Google Scholar] [CrossRef]
- Thimmulappa, R.K.; Mudnakudu-Nagaraju, K.K.; Shivamallu, C.; Subramaniam, K.T.; Radhakrishnan, A.; Bhojraj, S.; Kuppusamy, G. Antiviral and immunomodulatory activity of curcumin: A case for prophylactic therapy for COVID-19. Heliyon 2021, 7, 6350. [Google Scholar] [CrossRef]
- Babaei, F.; Nassiri-asl, M.; Hosseinzadeh, H. Curcumin (a constituent of turmeric): New treatment option against COVID-19. Food Sci. Nutr. 2020, 8, 5215–5227. [Google Scholar] [CrossRef] [PubMed]
- Pawar, K.S.; Mastud, R.N.; Pawar, S.K.; Pawar, S.S.; Bhoite, R.R.; Bhoite, R.R.; Kulkarni, M.V.; Deshpande, A.R. Oral Curcumin with Piperine as Adjuvant Therapy for the Treatment of COVID-19: A Randomized Clinical Trial. Front. Pharmacol. 2021, 12, 669362. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, R.; Salari, S.; Sharifi, M.D.; Reihani, H.; Rostamiani, M.B.; Behmadi, M.; Elyasi, S. Oral nano-curcumin formulation efficacy in the management of mild to moderate outpatient COVID-19: A randomized triple-blind placebo-controlled clinical trial. Food Sci. Nutr. 2021, 9, 4068–4075. [Google Scholar] [CrossRef] [PubMed]
- Saber-Moghaddam, N.; Salari, S.; Hejazi, S.; Amini, M.; Taherzadeh, Z.; Eslami, S.; Elyasi, S. Oral nano-curcumin formulation efficacy in management of mild to moderate hospitalized coronavirus disease-19 patients: An open label nonrandomized clinical trial. Phytother Res. 2022, 35, 2616–2623. [Google Scholar] [CrossRef]
- Vahedian-Azimi, A.; Abbasifard, M.; Rahimi-Bashar, F.; Guest, P.C.; Majeed, M.; Mohammadi, A.; Sahebkar, A. Effectiveness of Curcumin on Outcomes of Hospitalized COVID-19 Patients: A Systematic Review of Clinical Trials. Nutrients 2022, 14, 256. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Chen, L.; Hu, C.; Fast, D.; Zhang, L.; Yang, B.; Kan, J.; Du, J. Curcumin attenuates poly(I:C)-induced immune and inflammatory responses in mouse macrophages by inhibiting TLR3/TBK1/IFNB cascade. J. Funct. Foods. 2022, 33, 104949. [Google Scholar] [CrossRef]
- Mahmoud, D.B.; Bakr, M.M.; Al-karmalawy, A.A. Scrutinizing the Feasibility of Nonionic Surfactants to Form Isotropic Bicelles of Curcumin: A Potential Antiviral Candidate Against COVID-19. AAPS PharmSciTech 2022, 23, 44. [Google Scholar] [CrossRef]
- Alici, H.; Tahtaci, H.; Demir, K. Design and various in silico studies of the novel curcumin derivatives as potential candidates against COVID-19—Associated main enzymes. Comput. Biol. Chem. 2022, 98, 107657. [Google Scholar] [CrossRef]
- Rampogu, S.; Lee, G.; Park, J.S.; Lee, K.W.; Kim, M.O. Molecular Docking and Molecular Dynamics Simulations Discover Curcumin Analogue as a Plausible Dual Inhibitor for SARS-CoV-2. Int. J. Mol. Sci. 2022, 23, 1771. [Google Scholar] [CrossRef] [PubMed]
- Asha, D.; Kushwaha, K.; Mishra, P.; Mrinalini, S.; Lilly, G.; Deepika, S. Nanocurcumin formulation: A possible therapeutic agent for post COVID inflammatory syndrome. Immunopharmacol. Immunotoxicol. 2022, 44, 141–146. [Google Scholar]
- Soleymani, S.; Naghizadeh, A.; Karimi, M. COVID-19: General strategies for herbal therapies. J. Evid.-Based Integr. Med. 2022, 27, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Kiraç, H.; Dalda, S.A.; Coskun, O.F. Morphological and molecular characterization of garlic (Allium sativum L.) genotypes sampled from Turkey. Genet. Resour. Crop. Evol. 2022, 69, 1833–1841. [Google Scholar] [CrossRef]
- Kiymaci, M.E.; Kaskatepe, B. Assessment of Cinnamon as an Antimicrobial Agent. In Promising Antimicrobials from Natural Products; Rai, M., Kosalec, I., Eds.; Springer: Cham, Switzerland, 2022; Volume 4, pp. 53–73. [Google Scholar]
- Gidwani, B.; Bhattacharya, R.; Shukla, S.S.; Pandey, R.K. Indian spices: Past, present and future challenges as the engine for bio-enhancement of drugs: Impact of COVID-19. J. Sci. Food Agric. 2022, 102, 3065–3077. [Google Scholar] [CrossRef] [PubMed]
- Mir, S.A.; Firoz, A.; Alaidarous, M.; Alshehri, B.; Dukhyil, A.B.; Banawas, S.; Alsagaby, A.S.; Alturaiki, W.; Bhat, A.G.; Kashoo, F.; et al. Identification of SARS-CoV-2 RNA-dependent RNA polymerase inhibitors from the major phytochemicals of Nigella sativa: An in silico approach. Saudi J. Biol. Sci. 2022, 29, 394–401. [Google Scholar] [CrossRef] [PubMed]
- Bharathi, M.; Sivamaruthi, B.S.; Kesika, P.; Thangaleela, S.; Chaiyasut, C. In Silico Screening of Potential Phytocompounds from Several Herbs against SARS-CoV-2 Indian Delta Variant B.1.617.2 to Inhibit the Spike Glycoprotein Trimer. Appl. Sci. 2022, 12, 665. [Google Scholar] [CrossRef]
- Chinnaiyan, S.K.; Pandiyan, R.; Natesan, S.; Chindam, S.; Gouti, A.K.; Sugumaran, A. Fabrication of basil oil Nanoemulsion loaded gellan gum hydrogel—Evaluation of its antibacterial and anti-biofilm potential. J. Drug Deliv. Sci. Technol. 2022, 68, 1773–2247. [Google Scholar] [CrossRef]
- Zrig, A. The Effect of Phytocompounds of Medicinal Plants on Coronavirus (2019-NCOV) Infection. Pharm. Chem. J. 2022, 55, 1080–1084. [Google Scholar] [CrossRef]
- Santos, S.; Barata, P.; Charmier, A. Cannabidiol and Terpene Formulation Reducing SARS-CoV-2 Infectivity Tackling a Therapeutic Strategy. Front. Immunol. 2022, 13, 841459. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.; Alam, M.; Khatoon, F.; Fatima, U.; Elasbali, A.M.; Adnan, M.; Islam, A.; Hassan, M.I.; Snoussi, M. Natural products can be used in therapeutic management of COVID-19: Probable mechanistic insights. Biomed. Pharmacother. 2022, 147, 112658. [Google Scholar] [CrossRef]
- Bhatwalkar, S.B.; Mondal, R.; Krishna, S.B.N.; Adam, J.K.; Govender, P.; Anupam, R. Antibacterial properties of organosulfur compounds of garlic (Allium sativum). Front. Microbiol. 2021, 12, 613077. [Google Scholar] [CrossRef]
- Torres, K.A.D.M.; Lima, S.M.R.R.; Torres, L.M.B.; Gamberini, M.T.; Silva, J.P.I.D. Garlic: An alternative treatment for group B Streptococcus. Microbiol. Spectr. 2021, 9, 121–170. [Google Scholar] [CrossRef]
- Rouf, R.; Uddin, S.J.; Sarker, D.K.; Islam, M.T.; Ali, E.S.; Shilpi, J.A.; Sarker, S.D. Antiviral potential of garlic (Allium sativum) and its organosulfur compounds: A systematic update of pre-clinical and clinical data. Trends Food Sci. Technol. 2020, 104, 219–234. [Google Scholar] [CrossRef]
- Thuy, B.T.P.; My, T.T.A.; Hai, N.T.T.; Hieu, L.T.; Hoa, T.T.; Thi, P.L.; Nhung, N.T.A. Investigation into SARS-CoV-2 resistance of compounds in garlic essential oil. ACS Omega 2020, 5, 8312–8320. [Google Scholar] [CrossRef]
- Garg, M.; Sharma, A.; Vats, S.; Tiwari, V.; Kumari, A.; Mishra, V.; Krishania, M. Vitamins in Cereals: A Critical Review of Content, Health Effects, Processing Losses, Bioaccessibility, Fortification, and Biofortification Strategies for Their Improvement. Front. Nutr. 2021, 8, 586815. [Google Scholar] [CrossRef]
- Zaer, A.M.; Norouzi, F.; Askari, V.R.; Khazdair, M.R.; Roshan, M.; Boskabady, M.; Hosseini, M.; Boskabady, M.H. The protective effect of Nigella sativa extract on lung inflammation and oxidative stress induced by lipopolysaccharide in rats. J. Ethnopharmacol. 2020, 253, 112653. [Google Scholar] [CrossRef]
- Koshak, A.E.; Koshak, E.A. Nigella sativa l. as a potential phytotherapy for COVID-19: A mini-review of in-silico studies. CTR 2020, 93, 100602. [Google Scholar]
- Islam, M.N.; Hossain, K.S.; Sarker, P.P.; Ferdous, J.; Hannan, M.A.; Rahman, M.M.; Uddin, M.J. Revisiting pharmacological potentials of Nigella sativa seed: A promising option for COVID-19 prevention and cure. Phytother. Res. 2021, 35, 1329–1344. [Google Scholar] [CrossRef]
- Prasanth, D.S.N.B.K.; Murahari, M.; Chandramohan, V.; Panda, S.P.; Atmakuri, L.R.; Guntupalli, C. In silico identification of potential inhibitors from Cinnamon against main protease and spike glycoprotein of SARS CoV-2. J. Biomol. Struct. Dyn. 2020, 39, 4618–4632. [Google Scholar] [CrossRef]
- D’aquila, P.; Paparazzo, E.; Crudo, M.; Bonacci, S.; Procopio, A.; Passarino, G.; Bellizzi, D. Antibacterial Activity and Epigenetic Remodeling of Essential Oils from Calabrian Aromatic Plants. Nutrients 2022, 14, 391. [Google Scholar] [CrossRef]
- Qureshi, W.; Saeed, F.; Ajaz, M.; Rasool, S.A. In vitro antimicrobial, antibiofilm and antiphage activity of thyme (Thymus vulgaris). Pak. J. Bot. 2022, 22, 660–666. [Google Scholar] [CrossRef]
- Kunnumakkara, A.B.; Rana, V.; Parama, D.; Banik, K.; Girisa, S.; Henamayee, S.; Aggarwal, B.B. COVID-19, cytokines, inflammation, and spices: How are they related? Life Sci. 2021, 284, 119201. [Google Scholar] [CrossRef]
- Nath, M.; Debnath, P. Therapeutic role of traditionally used Indian medicinal plants and spices in combating COVID-19 pandemic situation. J. Biomol. Struct. Dyn. 2022, 20, 1–20. [Google Scholar]
- Singh, N.A.; Kumar, P.; Kumar, N. Spices and herbs: Potential antiviral preventives and immunity boosters during COVID-19. Phytother. Res. 2021, 35, 2745–2757. [Google Scholar] [CrossRef]
- Kumar, B.; Zaidi, S.; Haque, S.; Dasgupta, N.; Hussain, A.; Pramodh, S.; Mishra, B.N. In silico studies reveal antiviral effects of traditional Indian spices on COVID-19. Curr. Pharm. Des. 2021, 27, 3462–3475. [Google Scholar] [CrossRef]
- Devan, A.R.; Nair, B.; Kumar, A.R.; Gorantla, J.N.; Nath, L.R. Unravelling the immune modulatory effect of Indian spices to impede the transmission of COVID-19: A promising approach. Curr. Pharm. Biotechnol. 2022, 23, 201–220. [Google Scholar] [CrossRef]
- Radhika, A.G.; Malik, H. Fight against COVID-19: Survey of Spices & Herbs Used in North India. Open J. Epidemiol. 2021, 11, 256–266. [Google Scholar]
- Sengupta, S.; Bhattacharyya, D.; Kasle, G.; Karmakar, S.; Sahu, O.; Ganguly, A.; Das Sarma, J. Potential Immunomodulatory Properties of Biologically Active Components of Spices Against SARS-CoV-2 and Pan β-Coronaviruses. Front. Cell. Infect. 2021, 11, 729622. [Google Scholar] [CrossRef] [PubMed]
- Khasamwala, R.H.; Ranjani, S.; Nivetha, S.S.; Hemalatha, S. COVID-19: An In Silico Analysis on Potential Therapeutic Uses of Trikadu as Immune System Boosters. Appl. Biochem. Biotechnol. 2022, 194, 291–301. [Google Scholar] [CrossRef]
- Rajamanickam, K.; Rathinavel, T.; Periyannan, V.; Ammashi, S.; Marimuthu, S.; Nasir Iqbal, M. Molecular insight of phytocompounds from Indian spices and its hyaluronic acid conjugates to block SARS-CoV-2 viral entry. J. Biomol. Struct. Dyn. 2022, 12, 1–20. [Google Scholar] [CrossRef]
- Rajan, M.; Gupta, P.; Kumar, A. Promising antiviral molecules from ayurvedic herbs and spices against COVID-19. Chin. J. Integr. Med. 2021, 27, 243–244. [Google Scholar]
- Ratnawatia, S.; Kumbara, A.; Pudentia, M.; Kusuma, W.; Soriente, A. The Indonesian Herbal Heritage Medicine during COVID-19 Pandemic. Rev. Int. Geogr. Educ. 2021, 11, 1611–1620. [Google Scholar]
- Mlozi, S.H. The role of natural products from medicinal plants against COVID-19: Traditional medicine practice in Tanzania. Heliyon 2022, 8, e09739. [Google Scholar] [CrossRef]
- Hajibeygi, R.; Mirghazanfari, S.M.; Pahlavani, N.; Jalil, A.T.; Alshahrani, S.H.; Rizaev, J.A.; Yekta, N.H. Effect of a diet based on Iranian traditional medicine on inflammatory markers and clinical outcomes in COVID-19 patients: A double-blind, randomized, controlled trial. Eur. J. Integr. Med. 2022, 55, 102179. [Google Scholar] [CrossRef]
- Bousquet, J.; Haahtela, T.; Blain, H.; Czarlewski, W.; Zuberbier, T.; Bedbrook, A.; Anto, J.M. Available and affordable complementary treatments for COVID-19: From hypothesis to pilot studies and the need for implementation. Clin. Transl. Allergy 2022, 12, e12127. [Google Scholar] [CrossRef]
- Johnson, T.S.; Narayana, D.A. Role of Spices in Offering Natural Immunity to Fight Various Diseases. Pharmacogn. Mag. 2021, 13, 600–613. [Google Scholar] [CrossRef]
- Farzana, M.; Shahriar, S.; Jeba, F.R.; Tabassum, T.; Araf, Y.; Ullah, M.; Hosen, M.J. Functional food: Complementary to fight against COVID-19. Be Beni Suef Univ. J. Basic Appl. Sci. 2022, 11, 33. [Google Scholar] [CrossRef]

| Plant Species [Botanical Family] | Active Against | Scope of Activity | Reference |
|---|---|---|---|
| Achyranthes aspera [Amaranthaceae] | MRSA ATCC 43300 | IZ 6.3 ± 0.6 mm; MIC 42.0 ± 14.4 mg/mL | [50] |
| MRPA ATCC 27853 | IZ 6.2 ± 0.3 mm; MIC 200.0 ± 0.0 mg/mL | ||
| MRKP ATCC 00603 | IZ 6.0 ± 0.0 mm; 50.0 ± 0.0 mg/mL | ||
| Acokathera oppositifolia [Apocynaceae] | MRKP ATCC 33495 | MIC 6.25 ± 0.0 mg/mL | [51] |
| Ageratina adenophora [Compositae] | MRSA ATCC 25923 | IZ 10 ± 0.0 mm; MIC 12.5 mg/mL | [52] |
| Areca catechu [Arecaceae] | MRPA CCARM 2092 | IZ 6.4 ± 0.5–16.3 ± 1.5 mm; MIC 5.6 µg/mL | [52] |
| MRAB CCARM 12005 | IZ 6.0 ± 0.0–17.7 ± 1.2 mm; MIC 5.6 µg/mL | ||
| Artemesia vulgaris [Compositae] | MRSA ATCC 25923 | IZ 10 ± 0.1 mm; MIC 12.5 mg/mL | [53] |
| Azadirachta indica [Meliaceae] | MRSA ATCC 43300 | IZ 6.2 ± 0.3 mm; MIC 33.3 ± 14.4 mg/mL | [50] |
| MRPA ATCC 27853 | IZ 6.4 ± 0.4 mm; MIC 50.0 ± 0.0 mg/mL | ||
| MRKP ATCC 00603 | IZ 6.1 ± 0.2 mm; MIC 41.7 ± 144 mg/mL | ||
| Cirsium englerianum [Asteraceae] | MRSA ATCC 25923 | IZ 28 ± 0.04 mm; MIC 16 μg/mL | [53] |
| Euphorbia depauperata [Euphorbiaceae] | MRSA ATCC 25923 | IZ 26 ± 0.02 mm; MIC 4 μg/mL | [53] |
| Hydrastis canadensis. [Ranunculaceae] | MRSA AH1677 | MIC 75 µg/mL | [54] |
| Kalanchoe fedtschenkoi [Crassulaceae] | MRAB CDC0033 | MIC 256 μg/mL | [55] |
| MREC CDC08 | MIC > 256 μg/mL | ||
| Lawsoniainermis [Lythracea] | MRSA ATCC 43300 | IZ 15.5 ± 0.5 mm; MIC 4.2 ± 2.0 mg/mL | [50] |
| MRPA ATCC 27853 | IZ 12.5 ± 0.5 mm; MIC 4.2 ± 1.8 mg/mL | ||
| MRKP ATCC 00603 | IZ 7.6 ± 0.5 mm; MIC 12.5 ± 0.0 mg/mL | ||
| Lippia adoensis [Verbenaceae] | MRSA ATCC 25923 | IZ 27 ± 0.56 mm; MIC 64 μg/mL | [49] |
| Lippia javanica [Verbenaceae] | MRPA ATCC 9721 | MIC 6.25 ± 3.2 mg/mL | [49] |
| Matricaria chamomilla [Asteraceae] | MRSA ATCC 43300 | IZ 30 ± 2 mm; MIC 0.781 mg/mL | [57] |
| MRPA ATCC 27853 | IZ 13.66 ± 1.52 mm; MIC 0.590 mg/mL | [57] | |
| Morella kandtiana [Myricaceae] | MRAB CDC 0033 | MIC > 256 μg/mL | [58] |
| MBKP CDC 0076 | MIC 256 μg/mL | ||
| Mentha sp [Lamiaceae] | MRAB CI | MIC > 2 mg/mL | [59] |
| MRKP CI | MIC >2 mg/mL | ||
| MRPA CI | MIC 2 mg/mL | ||
| Ocimun basilicum [Lamiaceae] | MRAB CI | MIC > 2 mg/mL | [59] |
| MRKP CI | MIC > 2 mg/mL | ||
| MRPA CI | MIC > 2 mg/mL | ||
| Oxalis corniculata [Oxalidaceae] | MRKP CDC 0076 | IZ 11 ± 0.0 mm; MIC 25 mg/mL | [53] |
| Plectranthus barbatus [Lamiaceae] | MRAB CI | MIC > 2 mg/mL | [59] |
| MRKP CI | MIC 1 mg/mL | ||
| MRPA CI | MIC 2 mg/mL | ||
| Punica granatum [Punicaceae] | MRKP CDC 0076 | IZ 19–45 ± 0.7 mm | [60] |
| Salvia triloba [Lamiaceae] | MRSA ATCC 6538 P | IZ 9.5 mm | [61] |
| Scutellaria barbata [Lamiaceae] | MRAB CDC 0033 | IZ 14–18 ± 0.0 mm; MIC 6.4 mg/mL | [62] |
| Thymus zygis L. [Lamiaceae] | MRSA ATCC 43300 | IZ 75 ± 00 mm; MIC 02 ± 0.0009 μL/mL | [63] |
| MRAB CDC 0033 | IZ 71.5 ± 0.1 mm; MIC 02 ± 0.001 μL/mL | ||
| Thymus willdenowii [Lamiaceae] | MRSA ATCC 43300 | IZ 33 ± 0.2 mm; MIC 04 ± 00 μL/mL | [63] |
| MRAB CDC 0033 | IZ 30 ± 00 mm; MIC 04 ± 0.001 μL/mL | ||
| Zanthoxylum chalybeum [Rutaceae] | MRSA ATCC 1677 | MIC 16 μg/mL | [58] |
| MREF ATCC 0044 | MIC 32 μg/mL |
| Target Pathogen | Plant Species | Synergy Effect | Reference |
|---|---|---|---|
| Aggregatibacter actinomycetemcomitans | Salvadora persica | More than doubled the activity combined with metronidazole | [68] |
| B. cereus, S. aureus, E. coli, and P. aeruginosa | Ficus nitida | Antibacterial activity was enhanced in the presence of tetracycline | [69] |
| E. coli and K. pneumoniae | Centaurea damascena | Synergetic effect combined with gentamicin (ineffective for E. coli), vancomycin, ampicillin and chloramphenicol (ineffective for K. pneumoniae) | [70] |
| MDRAB and MDRPsA | Pithecellobium clypearia | Synergistic effect with imipenem and tetracycline a | [71] |
| MDRPsA | Coriandrum sativum | Synergism in the presence of antibiotics including mezlocillin, cefoperazone, cefotaxime and levofloxacin | [72] |
| MRSA 1485279 | Vernonia condensata | High MIC reduction combined with ampicillin a | [73] |
| Multidrug-resistant enteric bacteria | Carum copticum | Reduced up to 64-fold MIC against E. coli with ciprofloxacin | [74] |
| P. mirabilis | Petalostigma spp. | Synergistic activity with penicillin-G, chloramphenicol and erythromycin | [41] |
| S. aureus ATCC 12600 | Origanum vulgare and Hypericum perforatum | Combined extracts (1:1) increased inhibition over 3 times more than the individual extracts | [75] |
| S. aureus ATCC 25923 and E. coli ATTC 25922 | Vatica diospyroides | Increased ampicillin efficacy; reduced the required antibiotic concentration by eight times | [76] |
| S. aureus strains 3993 and 4125 | Salvia officinalis, Senna macranthera, and Plectranthus ornatus | Up to 8-fold reductions in the MIC, especially associated to ampicillin, kanamycin and gentamicin | [77] |
| Treponema denticola | Cinnamomum zeylanicum | More than doubled the activity combined with amoxicillin | [68] |
| Scope | Reference |
|---|---|
| Indian Spices and Ayurvedic Herbs | |
| Spices with anti-inflammatory properties with suggested beneficial action in the prevention and treatment of COVID-19 associated cytokine storm. | [116] |
| Spices useful for future design of new protease inhibitors effective against SARS-CoV-2. | [117] |
| Antiviral activities of spices, herbs, and derivatives, mechanisms of action, and prospects for future studies. | [118] |
| Mechanism of action of spices regularly used for cooking purpose to enhance the taste of food in India. | [98] |
| In silico evaluation of Indian traditional spices with medicinal properties for their inhibitory activity against SARS-CoV-2 spike proteins (SP) and main proteases (Mpro). | [119] |
| Immune impact of various Indian spices, potential to tackle the novel coronavirus, safety and toxicity aspects. | [120] |
| Traditional herbs used for protection against COVID-19 in North India. | [121] |
| Modulation of host immune responses by spice-derived bioactive components with protective immunity in COVID-19. | [122] |
| Preventive effect of Trikadu (mixture of Zingiber officinale, Piper nigrum and Piper longum) by action in the immune system. | [123] |
| Docking of gingerol, thymol, thymohydroquinone, cyclocurcumin, hydrazinocurcumin, components of Indian medicinal plants (ginger, black cumin, turmeric) against initially deposited spike structural proteins (PDB ID 6WPT) and mutant variant D-614G (PDB ID 6XS6). | [124] |
| Quick screening of traditional herbs/spices phytoconstituents by in silico study in polyherbal/Ayurvedic formulations. | [125] |
| Indonesian herbal medicines | |
| Several healthy drinks related to the COVID-19 pandemic. | [126] |
| Tanzanian Traditional Medicine | |
| Phytochemical screening of medicinal plants used to combat COVID-19 in Tanzania. | [127] |
| Persian Traditional Medicine | |
| New traditional Persian medicine-based drug, efficacy and safety assessment in COVID-19 patients with major symptoms. | [128] |
| Other | |
| Available and affordable complementary treatments for COVID-19. | [129] |
| Scientific evidence on potential role of spices in offering innate and adaptive immunity to human body. | [130] |
| Role of functional foods through modulating the host immune system and promoting the synthesis of agents effective against the coronavirus. | [131] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duarte, N.B.A.; Takahashi, J.A. Plant Spices as a Source of Antimicrobial Synergic Molecules to Treat Bacterial and Viral Co-Infections. Molecules 2022, 27, 8210. https://doi.org/10.3390/molecules27238210
Duarte NBA, Takahashi JA. Plant Spices as a Source of Antimicrobial Synergic Molecules to Treat Bacterial and Viral Co-Infections. Molecules. 2022; 27(23):8210. https://doi.org/10.3390/molecules27238210
Chicago/Turabian StyleDuarte, Nathália Barroso Almeida, and Jacqueline Aparecida Takahashi. 2022. "Plant Spices as a Source of Antimicrobial Synergic Molecules to Treat Bacterial and Viral Co-Infections" Molecules 27, no. 23: 8210. https://doi.org/10.3390/molecules27238210
APA StyleDuarte, N. B. A., & Takahashi, J. A. (2022). Plant Spices as a Source of Antimicrobial Synergic Molecules to Treat Bacterial and Viral Co-Infections. Molecules, 27(23), 8210. https://doi.org/10.3390/molecules27238210







