Effect of Solvothermal Temperature on Morphology and Supercapacitor Performance of Ni-MOF
Abstract
1. Introduction
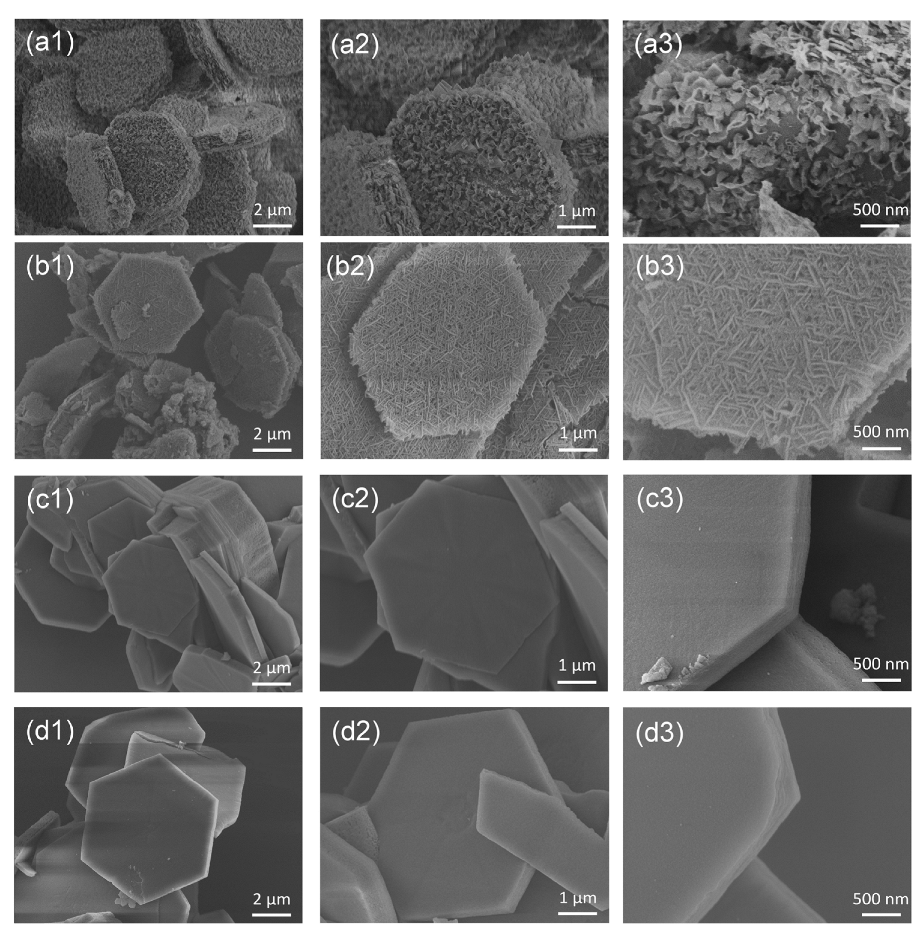
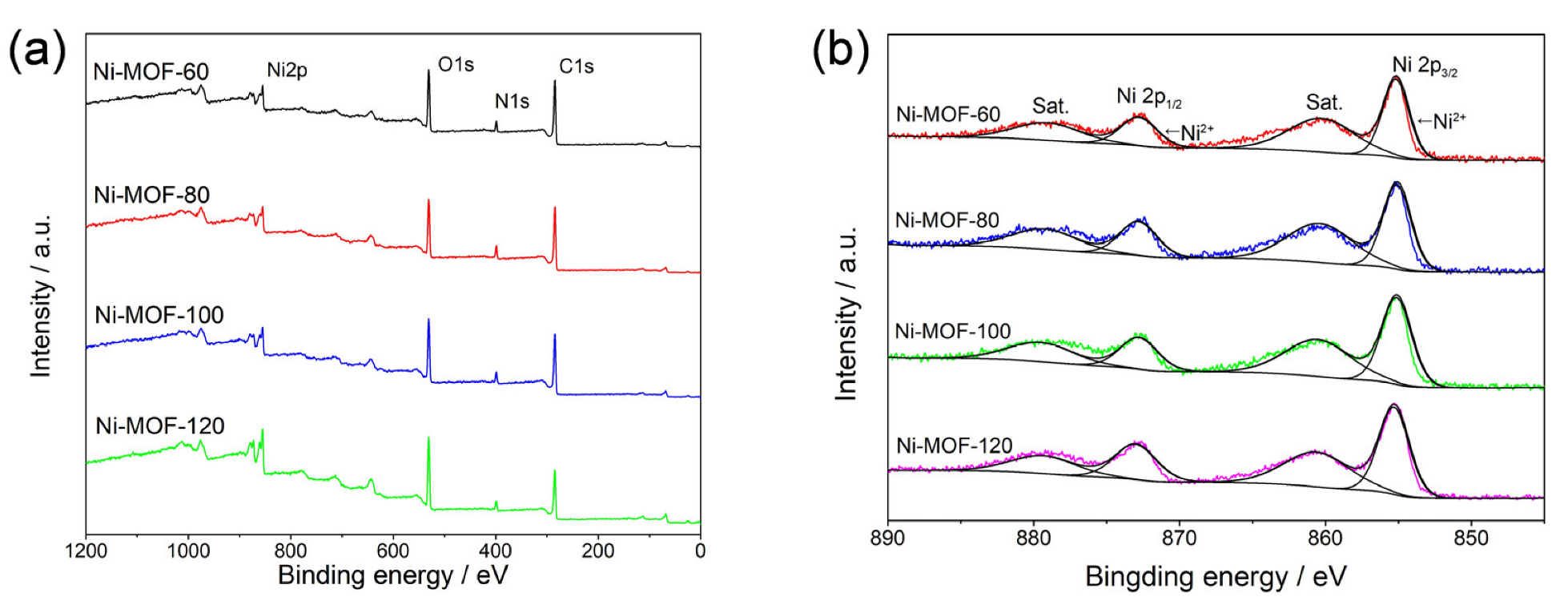
2. Results and Discussion
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Gui, Q.; Ba, D.; Li, L.; Liu, W.; Li, Y.; Liu, J. Recent advances in materials and device technologies for aqueous hybrid supercapacitors. Sci. China Mater. 2022, 65, 10–31. [Google Scholar] [CrossRef]
- Tan, C.L.; Cao, X.H.; Wu, X.J.; He, Q.Y.; Yang, J.; Zhang, X.; Chen, J.Z.; Zhao, W.; Han, S.K.; Nam, G.H.; et al. Recent Advances in Ultrathin Two-Dimensional Nanomaterials. Chem. Rev. 2017, 117, 6225–6331. [Google Scholar] [CrossRef]
- Liu, W.; Yin, R.; Xu, X.; Zhang, L.; Shi, W.; Cao, X. Structural Engineering of Low-Dimensional Metal-Organic Frameworks: Synthesis, Properties, and Applications. Adv. Sci. 2019, 6, 1802373. [Google Scholar] [CrossRef]
- Li, Y.; Xu, Y.; Liu, Y.; Pang, H. Exposing {001} Crystal Plane on Hexagonal Ni-MOF with Surface-Grown Cross-Linked Mesh-Structures for Electrochemical Energy Storage. Small 2019, 15, 1902463. [Google Scholar] [CrossRef]
- Xiao, X.; Zheng, S.S.; Li, X.R.; Zhang, G.X.; Guo, X.T.; Xue, H.G.; Pang, H. Facile synthesis of ultrathin Ni-MOF nanobelts for high-efficiency determination of glucose in human serum. J. Mater. Chem. B. 2017, 5, 5234–5239. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.S.; Xue, H.G.; Pang, H. Supercapacitors based on metal coordination materials. Coord. Chem. Rev. 2018, 373, 2–21. [Google Scholar] [CrossRef]
- Zheng, S.S.; Li, X.R.; Yan, B.Y.; Hu, Q.; Xu, Y.X.; Xiao, X.; Xue, H.G.; Pang, H. Transition-Metal (Fe, Co, Ni) Based Metal-Organic Frameworks for Electrochemical Energy Storage. Adv. Energy Mater. 2017, 7, 1602733. [Google Scholar] [CrossRef]
- Zhou, D.; Ni, J.; Li, L. Self-supported multicomponent CPO-27 MOF nanoarrays as high-performance anode for lithium storage. Nano Energy 2019, 57, 711–717. [Google Scholar] [CrossRef]
- Zuo, W.; Xie, C.; Xu, P.; Li, Y.; Liu, J. A Novel Phase-Transformation Activation Process toward Ni-Mn-O Nanoprism Arrays for 2.4 V Ultrahigh-Voltage Aqueous Supercapacitors. Adv. Mater. 2017, 29, 1703463. [Google Scholar] [CrossRef]
- Zuo, W.; Li, R.; Zhou, C.; Li, Y.; Xia, J.; Liu, J. Battery-Supercapacitor Hybrid Devices: Recent Progress and Future Prospects. Adv. Sci. 2017, 4, 1600539. [Google Scholar] [CrossRef]
- Sun, S.; Zhai, T.; Liang, C.; Savilov, S.V.; Xia, H. Boosted crystalline/amorphous Fe2O3-δ core/shell heterostructure for flexible solid-state pseudocapacitors in large scale. Nano Energy 2018, 45, 390–397. [Google Scholar] [CrossRef]
- Chhetri, K.; Dahal, B.; Mukhiya, T.; Tiwari, A.P.; Muthurasu, A.; Kim, T.; Kim, H.; Kim, H.Y. Integrated hybrid of graphitic carbon-encapsulated CuxO on multilayered mesoporous carbon from copper MOFs and polyaniline for asymmetric supercapacitor and oxygen reduction reactions. Carbon 2021, 179, 89–99. [Google Scholar] [CrossRef]
- Ni, J.; Li, Y. Carbon Nanomaterials in Different Dimensions for Electrochemical Energy Storage. Adv. Energy Mater. 2016, 6, 1600278. [Google Scholar] [CrossRef]
- Jiang, Y.; Liu, J. Definitions of Pseudocapacitive Materials: A Brief Review. Energy Environ. Mater. 2019, 2, 30–37. [Google Scholar] [CrossRef]
- Chen, M.; Xia, X.; Yin, J.; Chen, Q. Construction of Co3O4 nanotubes as high-performance anode material for lithium-ion batteries. Electrochim. Acta 2015, 160, 15–21. [Google Scholar] [CrossRef]
- Han, Y.; Lu, Y.Z.; Shen, S.H.; Zhong, Y.; Liu, S.; Xia, X.H. Enhancing the Capacitive Storage Performance of Carbon Fiber Textile by Surface and Structural Modulation for Advanced Flexible Asymmetric Supercapacitors. Adv. Funct. Mater. 2019, 29, 1806329. [Google Scholar] [CrossRef]
- Li, R.; Ren, X.; Zhang, F.; Du, C.; Liu, J. Synthesis of Fe3O4@SnO2 core–shell nanorod film and its application as a thin-film supercapacitor electrode. Chem. Commun. 2012, 48, 5010. [Google Scholar] [CrossRef]
- Xia, H.; Hong, C.Y.; Li, B.; Zhao, B.; Lin, Z.X.; Zheng, M.B.; Savilov, S.; Aldoshin, S. Facile Synthesis of Hematite Quantum-Dot/Functionalized Graphene-Sheet Composites as Advanced Anode Materials for Asymmetric Supercapacitors. Adv. Funct. Mater. 2015, 25, 627–635. [Google Scholar] [CrossRef]
- Li, Y.; Li, Y.; Xu, J.; Feng, T.; Yao, Q.; Xie, J.; Xia, H. Fe2O3 Nanoneedles on Ultrafine Nickel Nanotube Arrays as Efficient Anode for High-Performance Asymmetric Supercapacitors. Adv. Funct. Mater. 2017, 27, 1606728. [Google Scholar] [CrossRef]
- Jabeen, N.; Hussain, A.; Xia, Q.; Sun, S.; Zhu, J.; Xia., H. High-Performance 2.6 V Aqueous Asymmetric Supercapacitors based on In Situ Formed Na0.5MnO2 Nanosheet Assembled Nanowall Arrays. Adv. Mater. 2017, 29, 1700804. [Google Scholar] [CrossRef]
- Xia, X.H.; Tu, J.P.; Zhang, Y.Q.; Wang, X.L.; Gu, C.D.; Zhao, X.B.; Fan, H.J. High-Quality Metal Oxide Core/Shell Nanowire Arrays on Conductive Substrates for Electrochemical Energy Storage. ACS Nano 2012, 6, 5531–5538. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.T.; Li, W.T.; Zhang, Q.Y.; Liu, Y.Y.; Yuan, G.Q.; Braunstein, P.; Pang, H. Ultrasmall metal (Fe, Co, Ni) nanoparticles strengthen silicon oxide embedded nitrogen-doped carbon superstructures for long-cycle-life Li-ion-battery anodes. Chem. Eng. J. 2022, 432, 134413. [Google Scholar] [CrossRef]
- Xie, D.; Xia, X.H.; Wang, Y.D.; Wang, D.H.; Zhong, Y.; Tang, W.J.; Wang, X.L.; Tu, J.P. Nitrogen-Doped Carbon Embedded MoS2 Microspheres as Advanced Anodes for Lithium- and Sodium-Ion Batteries. Chem. A Eur. J. 2016, 22, 11617–11623. [Google Scholar] [CrossRef]
- Zhai, T.; Wan, L.M.; Sun, S.; Chen, Q.; Sun, J.; Xia, Q.Y.; Xia, H. Phosphate Ion Functionalized Co3O4 Ultrathin Nanosheets with Greatly Improved Surface Reactivity for High Performance Pseudocapacitors. Adv. Mater. 2017, 29, 1604167. [Google Scholar] [CrossRef]
- Cao, X.H.; Zheng, B.; Shi, W.H.; Yang, J.; Fan, Z.X.; Luo, Z.M.; Tui, X.H.; Chen, B.; Yan, Q.Y.; Zhang, H. Reduced Graphene Oxide-Wrapped MoO3 Composites Prepared by Using Metal-Organic Frameworks as Precursor for All-Solid-State Flexible Supercapacitors. Adv. Mater. 2015, 27, 4695–4701. [Google Scholar] [CrossRef]
- Ni, J.F.; Zhu, X.C.; Yuan, Y.F.; Wang, Z.Z.; Li, Y.B.; Ma, L.; Dai, A.; Li, M.; Wu, T.P.; Shanhbazian, R.; et al. Rooting binder-free tin nanoarrays into copper substrate via tin-copper alloying for robust energy storage. Nat. Commun. 2020, 11, 1212. [Google Scholar] [CrossRef] [PubMed]
- Hähnel, T.; Kalies, G.; Krishna, R.; Möllmer, J.; Hofmann, J.; Kobalz, M.; Krautscheid, H. Adsorptive separation of C2/C3/C4-hydrocarbons on a flexible Cu-MOF: The influence of temperature, chain length and bonding character. Microporous Mesoporous Mater. 2016, 224, 392–399. [Google Scholar] [CrossRef]
- Sheberla, D.; Bachman, J.C.; Elias, J.S.; Sun, C.-J.; Shao-Horn, Y.; Dincă, M. Conductive MOF electrodes for stable supercapacitors with high areal capacitance. Nat. Mater. 2017, 16, 220–224. [Google Scholar] [CrossRef]
- Azadfalah, M.; Sedghi, A.; Hosseini, H.; Mirhosseini, S.S. Synergic effect of physically-mixed metal organic framework based electrodes as a high efficient material for supercapacitors. J. Energy Storage 2021, 44, 103248. [Google Scholar] [CrossRef]
- Salunkhe, A.D.; Pagare, P.K.; Torane, A.P. Review on Recent Modifications in Nickel Metal-Organic Framework Derived Electrode (Ni-MOF) Materials for Supercapacitors. J. Inorg. Organomet. Polym. Mater. 2022, 32, 10904–10935. [Google Scholar] [CrossRef]
- Feng, C.; Lv, C.P.; Li, Z.Q.; Zhao, H.; Huang, H.H. A porous 2D Ni-MOF material with a high supercapacitive performance. J. Solid State Chem. 2018, 265, 244–247. [Google Scholar] [CrossRef]
- Wang, C.H.; Zhang, D.W.; Liu, S.; Yamauchi, Y.; Zhang, F.B.; Kaneti, Y.V. Ultrathin nanosheet-assembled nickel-based metal–organic framework microflowers for supercapacitor applications. Chem. Commun. 2022, 58, 1009–1012. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Y.; Pei, J.; Chen, D.H.; Yan, C.S.; Hu, Y.Y.; Zhang, Q.; Chen, G. Mixed-metallic MOF based electrode materials for high performance hybrid supercapacitors. J. Mater. Chem. A 2017, 5, 1094–1102. [Google Scholar] [CrossRef]
- Pan, Y.; Han, Y.H.; Chen, Y.J.; Li, D.; Tian, Z.; Guo, L.; Wang, Y.Z. Benzoic acid-modified 2D Ni-MOF for high-performance supercapacitors. Electrochim. Acta 2022, 403, 139679. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, S.X.; Lu, W.; Lei, D.; Tian, Y.H.; Guo, M.G.; Mi, P.P.; Qu, N.; Zhao, Y.Y. MXenes induced formation of Ni-MOF microbelts for high-performance supercapacitors. J. Colloid Interface Sci. 2021, 592, 95–102. [Google Scholar] [CrossRef]
- Gao, S.; Sui, Y.; Wei, F.; Qi, J.; Meng, Q.; He, Y. Facile synthesis of cuboid Ni-MOF for high-performance supercapacitors. J. Mater. Sci. 2018, 53, 6807–6818. [Google Scholar] [CrossRef]
- Kale, A.M.; Manikandan, R.; Justin Raj, C.; Dennyson Savariraj, A.; Voz, C.; Kim, B.C. Protonated nickel 2-methylimidazole framework as an advanced electrode material for high-performance hybrid supercapacitor. Mater. Today Energy 2021, 21, 100736. [Google Scholar] [CrossRef]
- Yan, Y.; Gu, P.; Zheng, S.S.; Zheng, M.B.; Pang, H.; Xue, H.G. Facile synthesis of an accordion-like Ni-MOF superstructure for high-performance flexible supercapacitors. J. Mater. Chem. A 2016, 4, 19078–19085. [Google Scholar] [CrossRef]
- Wang, J.W.; Ma, Y.X.; Kang, X.Y.; Yang, H.J.; Liu, B.L.; Li, S.S.; Zhang, X.D.; Ran, F. A novel moss-like 3D Ni-MOF for high performance supercapacitor electrode material. J. Solid State Chem. 2022, 309, 122994. [Google Scholar] [CrossRef]
- Du, P.; Dong, Y.; Liu, C.; Wei, W.; Liu, D.; Liu, P. Fabrication of hierarchical porous nickel based metal-organic framework (Ni-MOF) constructed with nanosheets as novel pseudo-capacitive material for asymmetric supercapacitor. J. Colloid Interface Sci. 2018, 518, 57–68. [Google Scholar] [CrossRef]
- Manikandan, M.R.; Cai, K.P.; Hu, Y.D.; Li, C.L.; Zhang, J.T.; Zheng, Y.P.; Liang, Y.F.; Song, H.R.; Shang, M.Y.; Shi, X.N.; et al. Influence of hydrothermal reaction time on the supercapacitor performance of Ni-MOF nanostructures. Appl. Phys. A Mater. Sci. Process. 2021, 127, 421–430. [Google Scholar] [CrossRef]
- Xuan, W.; Ramachandran, R.; Zhao, C.; Wang, F. Influence of synthesis temperature on cobalt metal-organic framework (Co-MOF) formation and its electrochemical performance towards supercapacitor electrodes. J. Solid State Electrochem. 2018, 22, 3873–3881. [Google Scholar] [CrossRef]
- Sun, Y.X.; Sun, W.Y. Influence of temperature on metal-organic frameworks. Chin. Chem. Lett. 2014, 25, 823–828. [Google Scholar] [CrossRef]
- Li, C.; Li, X.J.; Zhao, Z.Y.; Li, F.L.; Xue, J.Y.; Niu, Z.; Gu, H.W.; Braunstein, P.; Lang, J.P. Iron-doped NiCo-MOF hollow nanospheres for enhanced electrocatalytic oxygen evolution. Nanoscale 2020, 12, 14004–14010. [Google Scholar] [CrossRef] [PubMed]
- Ko, S.; Tang, X.; Gao, F.; Wang, C.; Liu, H.; Liu, Y. Selective catalytic reduction of NOx with NH3 on Mn, Co-BTC-derived catalysts: Influence of thermal treatment temperature. J. Solid State Chem. 2022, 307, 122843. [Google Scholar] [CrossRef]
- Bhattarai, R.M.; Chhetri, K.; Saud, S.; Teke, S.; Kim, S.J.; Mok, Y.S. Eco-Friendly Synthesis of Cobalt Molybdenum Hydroxide 3D Nanostructures on Carbon Fabric Coupled with Cherry Flower Waste-Derived Activated Carbon for Quasi-Solid-State Flexible Asymmetric Supercapacitors. ACS Appl. Nano Mater. 2022, 5, 160–175. [Google Scholar] [CrossRef]
- Otun, K.O. Temperature-controlled activation and characterization of iron-based metal-organic frameworks. Inorg. Chim. Acta 2020, 507, 119563. [Google Scholar] [CrossRef]
- Zhang, X.; Xiang, S.; Du, Q.; Bi, F.; Xie, K.; Wang, L. Effect of calcination temperature on the structure and performance of rod-like MnCeOx derived from MOFs catalysts. Mol. Catal. 2022, 522, 112226. [Google Scholar] [CrossRef]
- Seetharaj, R.; Vandana, P.V.; Arya, P.; Mathew, S. Dependence of solvents, pH, molar ratio and temperature in tuning metal organic framework architecture. Arab. J. Chem. 2019, 12, 295–315. [Google Scholar] [CrossRef]
- Wu, Y.Q.; Xie, L.H.; Qin, X.; Sun, Y.X.; Xie, Y.B.; Li, J.R. Continuous Crystalline Membranes of a Ni(II)-Based Pillared-Layer Metal-Organic Framework In Situ Grown on Nickel Foam with Two Orientations. Crystals 2018, 8, 383. [Google Scholar] [CrossRef]
- Guo, X.; Li, W.; Geng, P.; Zhang, Q.; Pang, H.; Xu, Q. Construction of SiOx/nitrogen-doped carbon superstructures derived from rice husks for boosted lithium storage. J. Colloid Interface Sci. 2022, 606, 784–792. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.; Zhou, H.; Xue, H.; Braunstein, P.; Pang, H. Pillared-layer Ni-MOF nanosheets anchored on Ti3C2 MXene for enhanced electrochemical energy storage. J. Colloid Interface Sci. 2022, 614, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Chhetri, K.; Dahal, B.; Tiwari, A.P.; Mukhiya, T. Controlled Selenium Infiltration of Cobalt Phosphide Nanostructure Arrays from a Two-Dimensional Cobalt Metal-Organic Framework: A Self-Supported Electrode for Flexible Quasi-Solid-State Asymmetric Supercapacitors. ACS Appl. Energy Mater. 2021, 4, 404–415. [Google Scholar] [CrossRef]
- Gautam, K.P.; Acharya, D.; Bhatta, I.; Subedi, V.; Das, M.; Neupane, S.; Kunwar, J.; Chhetri, K.; Yadav, A.P. Nickel Oxide-Incorporated Polyaniline Nanocomposites as an Efficient Electrode Material for Supercapacitor Application. Inorganics 2022, 10, 86. [Google Scholar] [CrossRef]
- Wang, H.; Zhu, C.F.; Wu, M.Z.; Zheng, F.C.; Gao, Y.H.; Niu, H.L. Synthesis of a novel double-ligand nickel conductive metal-organic framework material and its electrochemical characterization for supercapacitors. J. Mater. Sci. 2021, 56, 2517–2527. [Google Scholar] [CrossRef]
- Xiao, Y.; Wei, W.; Zhang, M.J.; Jiao, S.; Shi, Y.C.; Ding, S.J. Facile Surface Properties Engineering of High-Quality Graphene: Toward Advanced Ni-MOF Heterostructures for High-Performance Supercapacitor Electrode. ACS Appl. Energy Mater. 2019, 2, 2169–2177. [Google Scholar] [CrossRef]
- Li, Y.; Song, L.L.; Han, Y.H.; Wang, G.Y. Electrochemical Performance of Ni-MOFs for Supercapacitors. IOP Conf. Ser. Mater. Sci. Eng. 2018, 317, 1757–1760. [Google Scholar] [CrossRef]
- Hang, X.X.; Xue, Y.D.; Chen, Y.; Du, M.; Du, L.T.; Pang, H. From Co-MOF to CoNi-MOF to Ni-MOF: A Facile Synthesis of 1D Micro-/Nanomaterials. Inorg. Chem. 2021, 60, 13168–13176. [Google Scholar] [CrossRef]
- Wang, Z.K.; Chen, J.Q.; Bi, R.; Dou, W.; Wang, K.B.; Mao, F.F.; Wu, H.; Wang, S.S. Supercapacitor and oxygen evolution reaction performances based on morphology-dependent Co-MOFs. J. Solid State Chem. 2020, 283, 121128. [Google Scholar] [CrossRef]
- Ramachandran, R.; Zhao, C.H.; Luo, D.; Wang, K.; Wang, F. Synthesis of copper benzene-1, 3, 5-tricarboxylate metal organic frameworks with mixed phases as the electrode material for supercapacitor applications. Appl. Surf. Sci. 2018, 460, 33–39. [Google Scholar] [CrossRef]
- Zhang, Y.D.; Lin, B.P.; Sun, Y.; Zhang, X.Q.; Yang, H.; Wang, J.C. Carbon nanotubes@metal–organic frameworks as Mn-based symmetrical supercapacitor electrodes for enhanced charge storage. RSC Adv. 2015, 5, 58100–58106. [Google Scholar] [CrossRef]
- Xu, J.; Yang, Y.H.; Wang, Y.; Cao, J.Y.; Chen, Z.D. Enhanced electrochemical properties of manganese-based metal organic framework materials for supercapacitors. J. Appl. Electrochem. 2019, 49, 1091–1102. [Google Scholar] [CrossRef]
- Otun, K.O.; Zong, S.; Hildebrandt, D.; Liu, X.Y. Self-assembled Zn-functionalized Ni-MOF as an efficient electrode for electrochemical energy storage. J. Phys. Chem. Solids 2022, 167, 110779. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, W.; Guo, X.; Pang, H. Effect of Solvothermal Temperature on Morphology and Supercapacitor Performance of Ni-MOF. Molecules 2022, 27, 8226. https://doi.org/10.3390/molecules27238226
Shen W, Guo X, Pang H. Effect of Solvothermal Temperature on Morphology and Supercapacitor Performance of Ni-MOF. Molecules. 2022; 27(23):8226. https://doi.org/10.3390/molecules27238226
Chicago/Turabian StyleShen, Wanxin, Xiaotian Guo, and Huan Pang. 2022. "Effect of Solvothermal Temperature on Morphology and Supercapacitor Performance of Ni-MOF" Molecules 27, no. 23: 8226. https://doi.org/10.3390/molecules27238226
APA StyleShen, W., Guo, X., & Pang, H. (2022). Effect of Solvothermal Temperature on Morphology and Supercapacitor Performance of Ni-MOF. Molecules, 27(23), 8226. https://doi.org/10.3390/molecules27238226




