Construction of 5-(Alkylamino)-6-aryl/alkylpyrazine-2,3-dicarbonitriles via the One-Pot Reaction of Alkyl Isocyanides with Aryl/Alkyl Carbonyl Chlorides and Diaminomaleonitrile: Fluorescence and Antimicrobial Activity Evaluation
Abstract
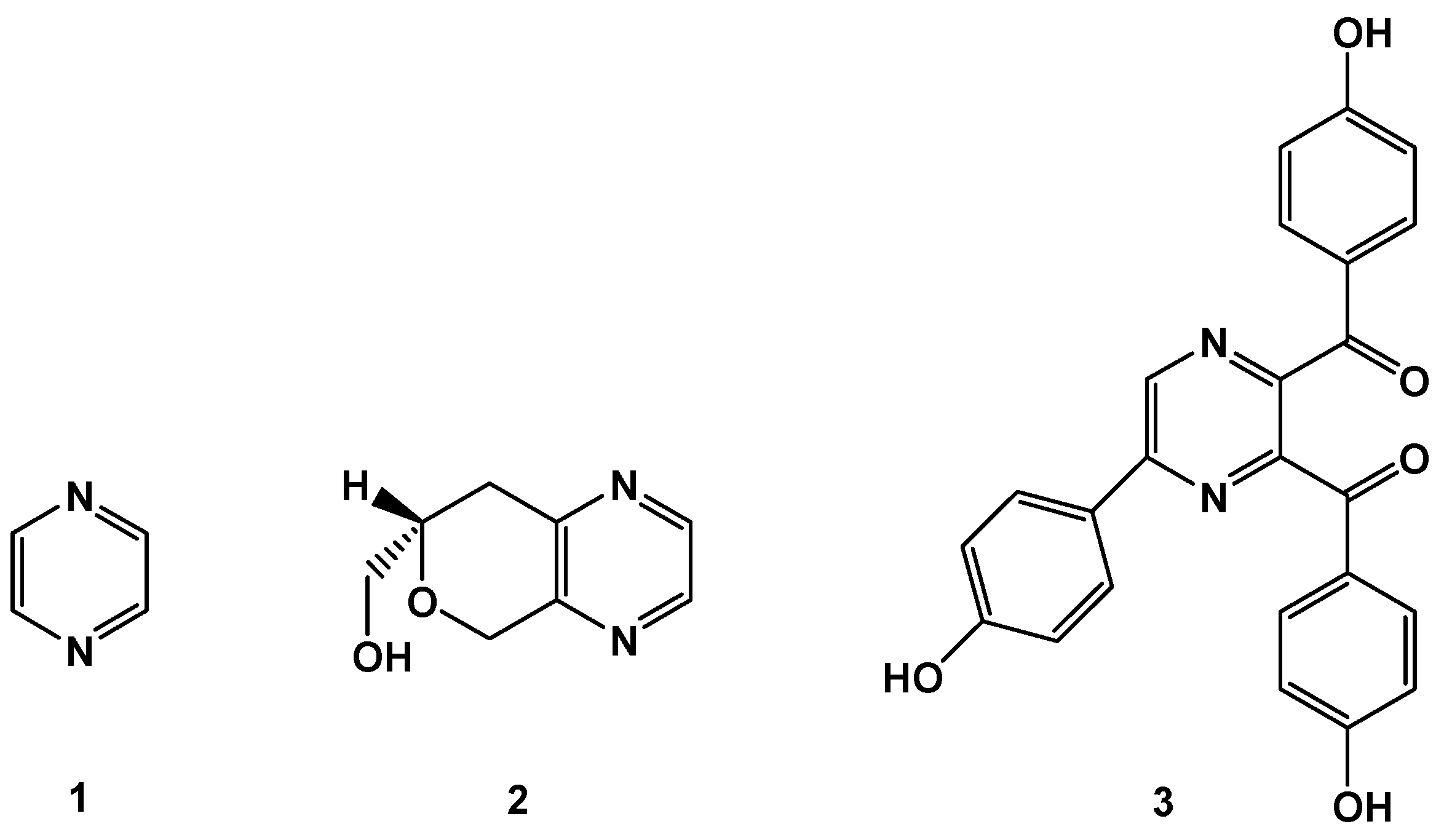
:1. Introduction
2. Results and Discussion
3. Fluorescence
4. Pharmacology
4.1. Methodology
4.2. Experimental Section
4.2.1. General
4.2.2. General Procedure for the Synthesis of Compounds 13a–k, 14c, j, k
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Al-Azmi, A.; Elassar, A.A.Z.; Booth, B.L. The chemistry of diaminomaleonitrile and its utility in heterocyclic synthesis. Tetrahedron 2003, 59, 2749–2763. [Google Scholar] [CrossRef]
- Al-Azmi, A. Recent developments in the chemistry of diaminomaleonitrile. Curr. Org. Synth. 2015, 12, 110–135. [Google Scholar] [CrossRef]
- Al-Azmi, A. Pyrazolo[1,5-a]pyrimidines: A close look into their synthesis and applications. Curr. Org. Synth. 2019, 23, 1–23. [Google Scholar] [CrossRef]
- Sato, N. Pyrazines, and their Benzo Derivatives. Compr. Heterocycl. Chem. III 2008, 8, 273–331. [Google Scholar]
- Watanabe, K.; Iguchi, K.; Fujimori, K. Clavulazine, a New Marine Pyrazine Congener from the Okinawan Soft Coral Clavularia viridis. Heterocycles 1998, 49, 269–274. [Google Scholar] [CrossRef]
- de Nino, A.; Maiuolo, L.; Costanzo, P.; Algieri, V.; Jiritano, A.; Olivito, F.; Tallarida, M.A. Recent Progress in Catalytic Synthesis of 1,2,3-Triazoles. Catalysts 2021, 11, 1120. [Google Scholar] [CrossRef]
- Maiuolo, L.; Algieri, V.; Olivito, F.; de Nino, A. Recent Developments on 1,3-Dipolar Cycloaddition Reactions by Catalysis in Green Solvents. Catalysts 2020, 10, 65. [Google Scholar] [CrossRef] [Green Version]
- Osborne, J.D.; Matthews, T.P.; McHardy, T.; Proisy, N.; Cheung, K.M.; Lainchbury, M.; Brown, N.; Walton, M.I.; Eve, P.D.; Boxall, K.J.; et al. Multiparameter Lead Optimization to Give an Oral Checkpoint Kinase 1 (CHK1) Inhibitor Clinical Candidate: (R)-5-((4-((Morpholin-2-ylmethyl)amino)-5-(trifluoromethyl)pyridin-2-yl)amino)pyrazine-2-carbonitrile (CCT245737). J. Med. Chem. 2016, 59, 5221–5237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iwai, K.; Yoshida, S.; Yoshimatsu, M.; Aoyama, K.; Kosugi, Y.; Kojima, T.; Morishita, N.; Dougan, D.R.; Snell, G.P.; Imamura, S.; et al. Design and Synthesis of Potent Inhibitor of Apoptosis (IAP) Proteins Antagonists Bearing an Octahydropyrrolo[1,2-a]pyrazine Scaffold as a Novel Proline Mimetic. J. Med. Chem. 2013, 56, 1228–1246. [Google Scholar]
- Lainchbury, M.; Matthews, T.P.; McHardy, T.; Boxall, K.J.; Walton, M.I.; Eve, P.D.; Hayes, A.; Valenti, M.R.; de Haven Brandon, A.K.; Box, G.; et al. Discovery of 3-alkoxyamino-5-(pyridin-2-ylamino)pyrazine-2-carbonitriles as selective, orally bioavailable CHK1 inhibitors. J. Med. Chem. 2012, 55, 10229–10240. [Google Scholar] [CrossRef] [PubMed]
- Buron, F.; Ple, N.; Turck, A.; Queguiner, G. Synthesis of Pyrazine Alcaloids from Botryllus leachi. Diazines 43. J. Org. Chem. 2005, 70, 2616. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.P.; Aithagani, S.K.; Yadav, M.; Singh, V.P.; Vishwakarma, R.A. Iron-catalyzed Cross-Coupling of Electron-Deficient Heterocycles and Quinone with Organoboron Species via Innate C–H Functionalization: Application in Total Synthesis of Pyrazine Alkaloid Botryllazine A. J. Org. Chem. 2013, 78, 2639–2648. [Google Scholar] [CrossRef] [PubMed]
- Chrovian, C.C.; Soyode-Johnson, A.; Ao, H.; Bacani, J.M.; Carruthers, N.I.; Lord, B.; Nguyen, L.; Rech, J.C.; Wang, Q.; Bhattacharya, A.; et al. Novel Phenyl-Substituted 5,6-Dihydro-[1,2,4]triazolo[4,3-a]pyrazine P2X7 Antagonists with Robust Target Engagement in Rat Brain. ACS Chem. Neurosci. 2016, 7, 490–497. [Google Scholar] [CrossRef] [PubMed]
- Chlupacova, M.K.; Kunes, J.; Buchta, V.; Vejsova, M.; Opletalova, V. Novel Pyrazine Analogs of Chalcones: Synthesis and Evaluation of Their Antifungal and Antimycobacterial Activity. Molecules 2015, 20, 1104–1117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jandourek, O.; Dolezal, M.; Paterova, P.; Kubicek, V.; Pesko, M.; Kunes, J.; Coffey, A.; Guo, J.; Kralova, K. N-Substituted 5-Amino-6-methylpyrazine-2,3-dicarbonitriles: Microwave-Assisted Synthesis and Biological Properties. Molecules 2014, 19, 651–671. [Google Scholar] [CrossRef] [PubMed]
- Miniyar, P.B.; Murumkar, P.R.; Patil, P.S.; Barmade, M.A.; Bothara, K.G. Unequivocal Role of Pyrazine Ring in Medicinally Important Compounds: A Review. Mini Rev. Med. Chem. 2013, 13, 1607–1625. [Google Scholar] [CrossRef] [PubMed]
- Üngören, S.H.; Dilekoğlu, E.; Koca, I. Synthesis of pyrazine-2,3-dicarbonitrile and 1,2,4-triazine-5(4H)-one derivatives from furan-2,3-diones. Chin. Chem. Lett. 2013, 24, 1130–1133. [Google Scholar] [CrossRef]
- Wang, F.; Wei, M.; Duan, X.; Liu, X.; Yao, S.; Wang, J.; Zhu, H.; Chen, C.; Gu, L.; Zhang, Y. Identification, synthesis and biological evaluation of pyrazine ring compounds from Talaromyces minioluteus (Penicillium minioluteum). Org. Chem. Front. 2020, 7, 3616–3624. [Google Scholar] [CrossRef]
- Zhang, Y.-B.; Wang, X.-L.; Liu, W.; Yang, Y.-S.; Tang, J.-F.; Zhu, H.-L. Design, synthesis and biological evaluation of heterocyclic azoles derivatives containing pyrazine moiety as potential telomerase inhibitors. Bioorganic Med. Chem. 2012, 20, 6356–6365. [Google Scholar] [CrossRef]
- Mortzfeld, F.; Hashem, C.; Vrankova, K.; Winkler, M.; Rudroff, F. Pyrazines: Synthesis and Industrial Application of these Valuable Flavor and Fragrance Compounds. Biotechnol. J. 2020, 15, 2000064. [Google Scholar] [CrossRef]
- Milić, J.V.; Schaack, C.; Hellou, N.; Isenrich, F.; Gershoni-Poranne, R.; Neshchadin, D.; Egloff, S.; Trapp, N.; Ruhlmann, L.; Boudon, C.; et al. Light-Responsive Pyrazine-Based Systems: Probing Aromatic Diarylethene Photocyclization. J. Phys. Chem. C 2018, 122, 19100–19109. [Google Scholar] [CrossRef]
- Zhang, C.Y.; Tour, J.M. Synthesis of Highly Functionalized Pyrazines by Ortho-Lithiation Reactions. Pyrazine Ladder Polymers. J. Am. Chem. Soc. 1999, 121, 8783–8790. [Google Scholar] [CrossRef]
- Lee, J.Y.; Aoyama, T.; Uchiyama, M.; Matsumoto, S. Synthesis and properties of liquid pyrazine dyes. Dyes Pigm. 2020, 174, 108030. [Google Scholar] [CrossRef]
- Kobak, R.Z.U.; Öztürk, E.S.; Koca, A.; Gül, A. The synthesis and cyclotetramerisation reactions of aryloxy-, arylalkyloxy-substituted pyrazine-2,3-dicarbonitriles and spectroelectrochemical properties of octakis(hexyloxy)-pyrazinoporphyrazine. Dyes Pigm. 2010, 86, 115–122. [Google Scholar] [CrossRef]
- Yavari, I.; Pashazadeh, R.; Hosseinpour, R. Synthesis of Functionalized Pyrazines and Quinoxalines from Nef-Isocyanide Adducts and 1,2-Diamines. Helvetica Chim. Acta. 2012, 95, 169–172. [Google Scholar] [CrossRef]
- Talebizadeh, M.; Abbasinejad, M.A.; Darehkordi, A. A Simple One-pot Three-component Synthesis of Dihydrobenzo[4,5]imidazo[2,1-b]thiazol-3-ols by Reaction of Acyl Chlorides, Isocyanides and 2-Mercaptobenzimidazoles. J. Heterocycl. Chem. 2018, 55, 2737–2743. [Google Scholar] [CrossRef]
- Shaabani, A.; Maleki, A.; Moghimi, J. A novel isocyanide-based three-component reaction: Synthesis of highly substituted 1,6-dihydropyrazine-2,3-dicarbonitrile derivatives. J. Org. Chem. 2007, 72, 6309–6311. [Google Scholar] [CrossRef] [PubMed]
- Novakova, V.; Hladík, P.; Filandrová, T.; Zajícová, I.; Krepsová, V.; Miletin, M.; Lenčo, J.; Zimcik, P. Structural factors influencing the intramolecular charge transfer and photoinduced electron transfer in tetrapyrazinoporphyrazines. Phys. Chem. Chem. Phys. 2014, 16, 5440–5446. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, M.; Perveen, Z.; Bortoluzzi, A.J.; Hameed, S.; Shah, M.R.; Tariq, M.; Din, G.; Muhammad, T.; Siddique, M.; Anwar, M. Synthesis, and characterization of novel iminobenzoates with terminal pyrazine moieties. Chem. Cent. J. 2018, 12, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Moloudi, M.; Kabirifard, H.; Piri, S.; Naghizadeh, E. Synthesis of 2,3-dicyanopyrazine and ethyl 5-amino-4,6-dicyanobiphenyl-3-carboxylate derivatives from ethyl aroylpyruvates. Heterocycl Comm. 2018, 24, 99–102. [Google Scholar] [CrossRef]
- Xu, L.; Zhao, Y.; Long, G.; Wang, Y.; Zhao, J.; Li, D.; Li, J.; Ganguly, R.; Li, Y.; Sun, H.; et al. Synthesis, structure, physical properties, and OLED application of pyrazine–triphenylamine fused conjugated compounds. RSC Adv. 2015, 5, 63080–63086. [Google Scholar] [CrossRef]

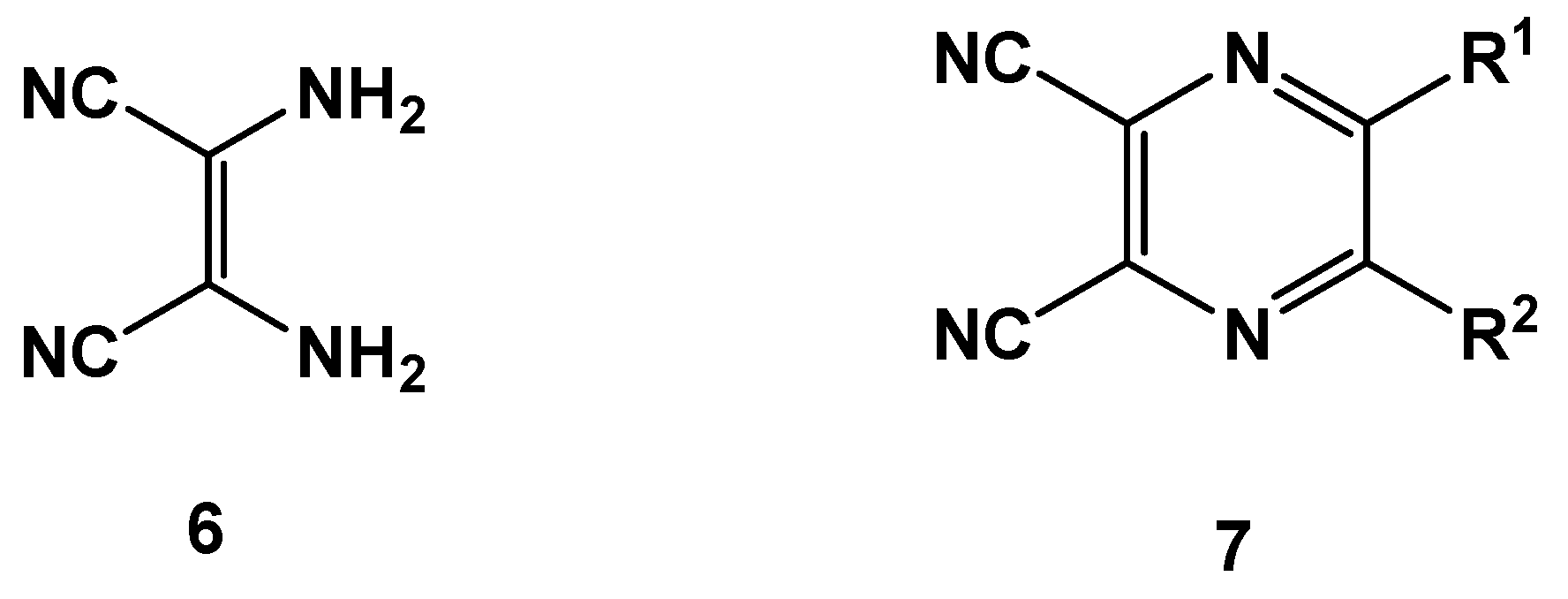

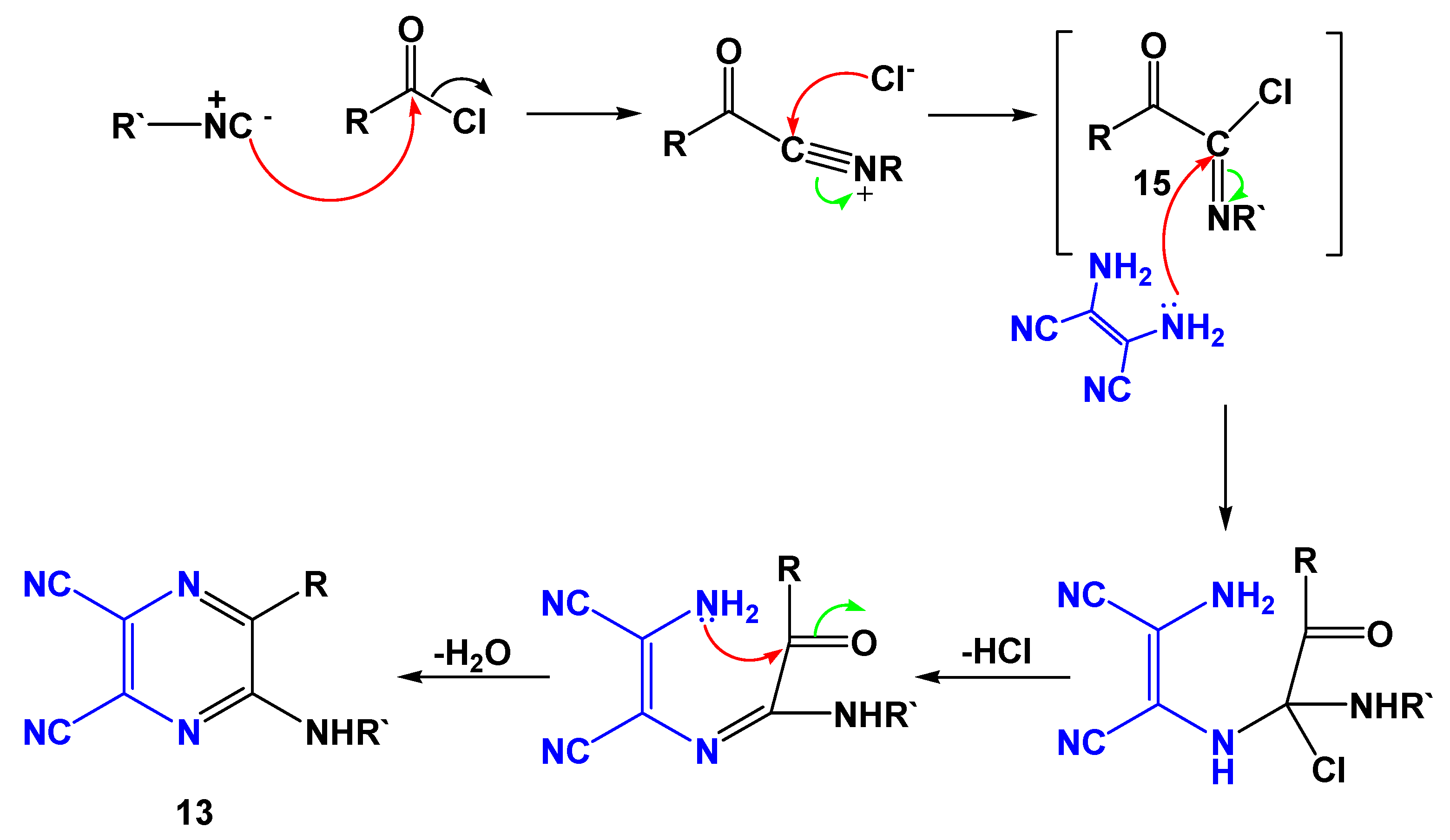
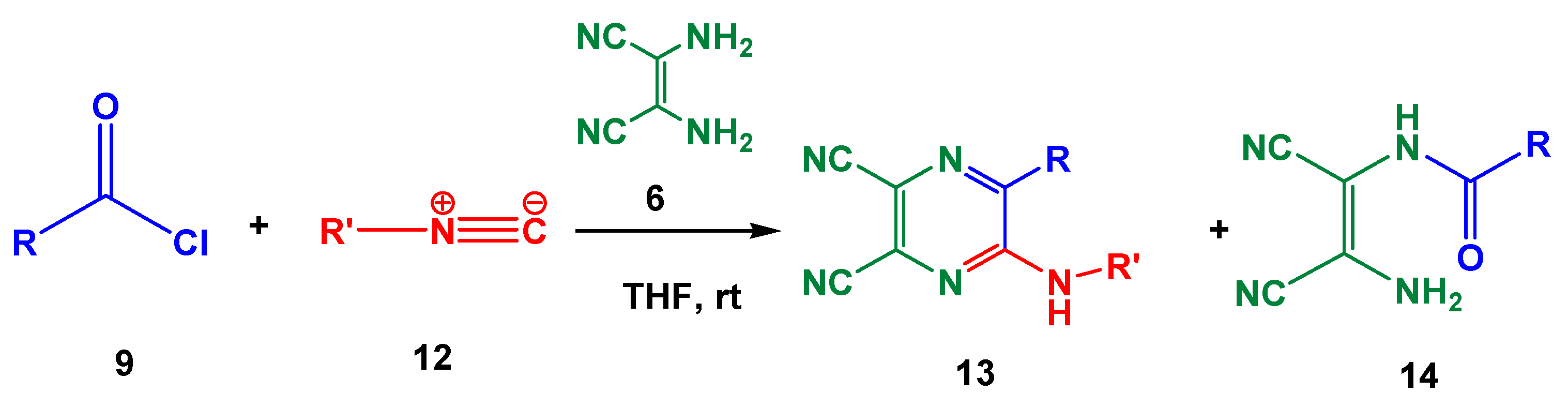
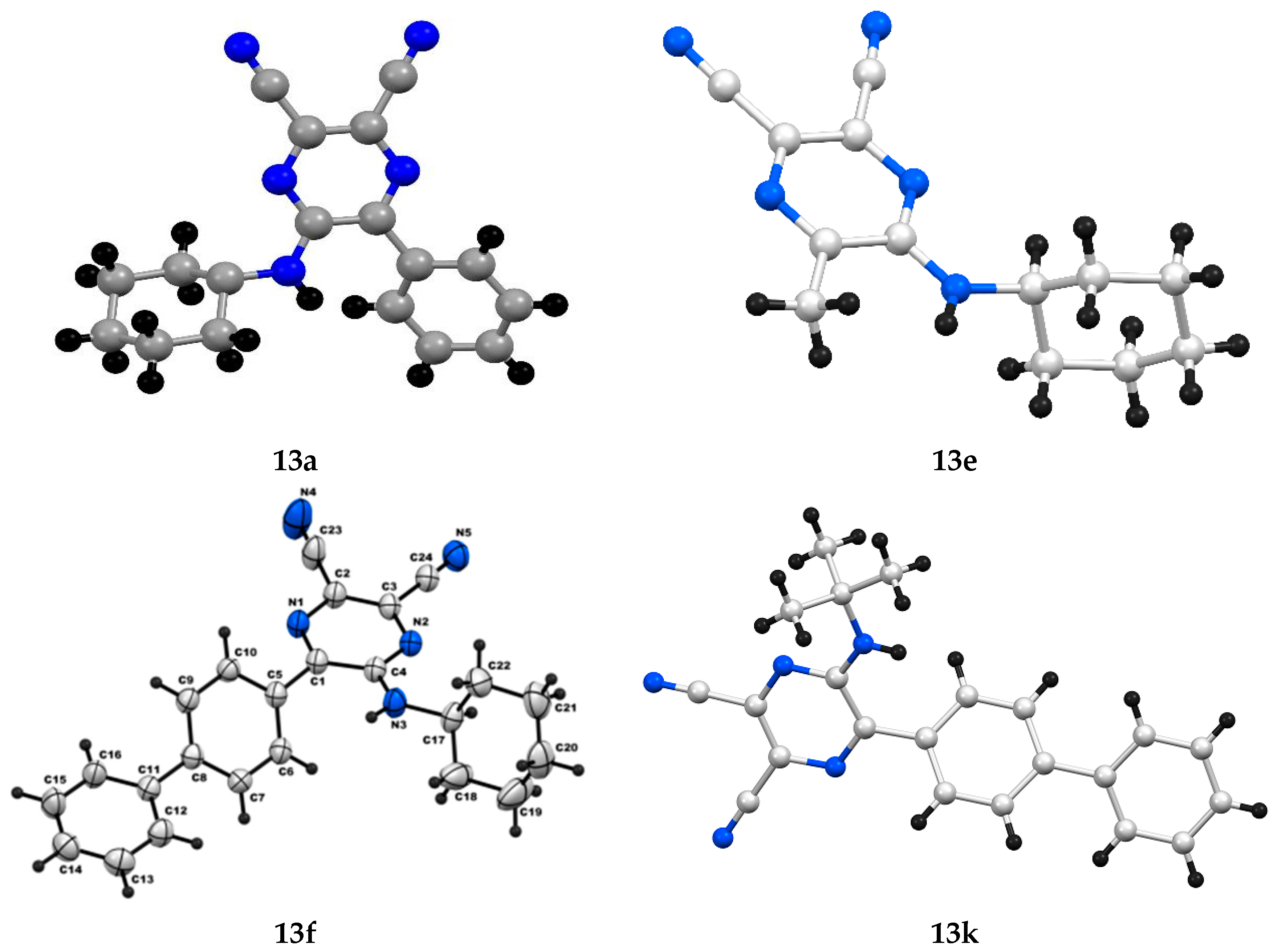
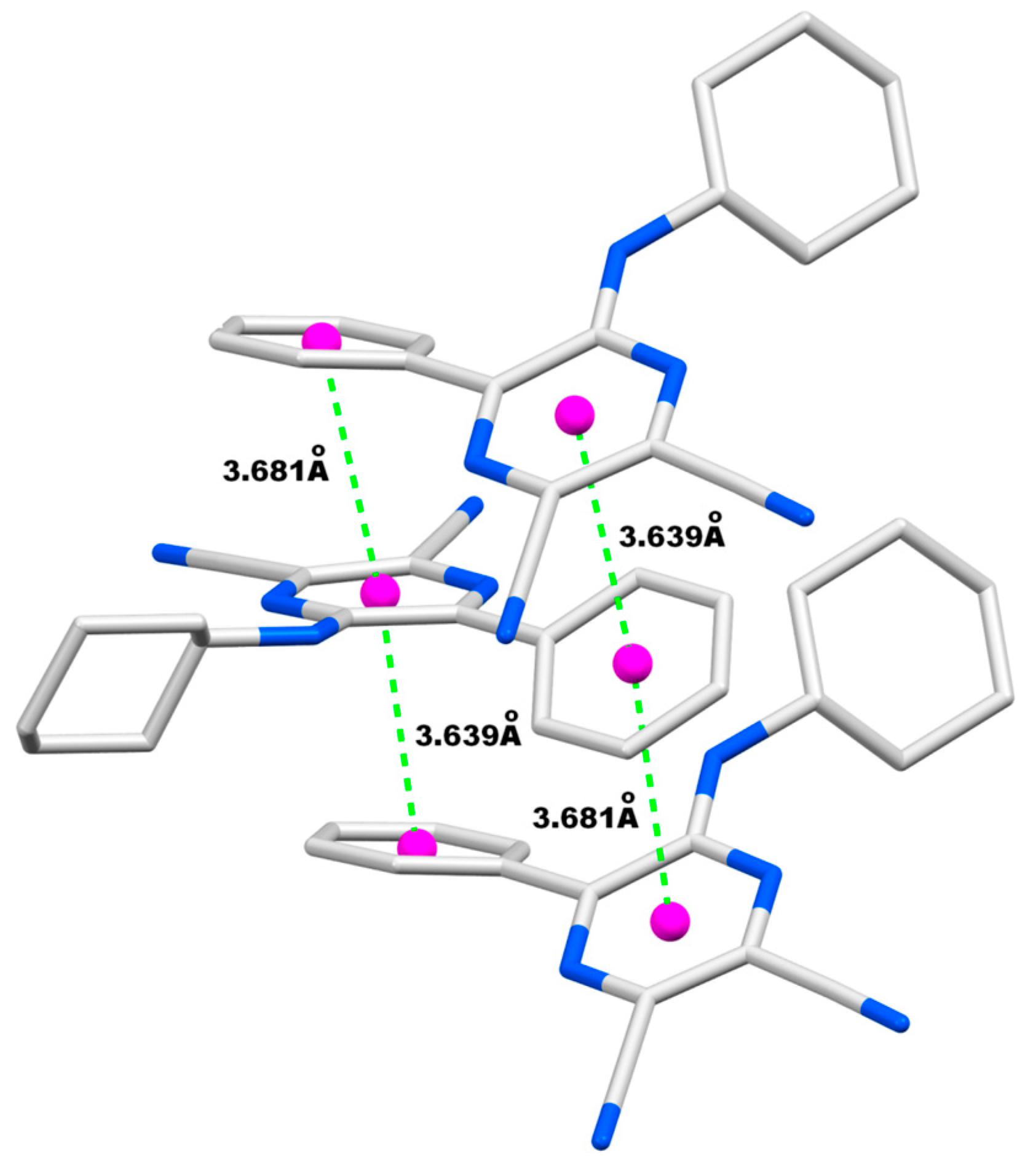
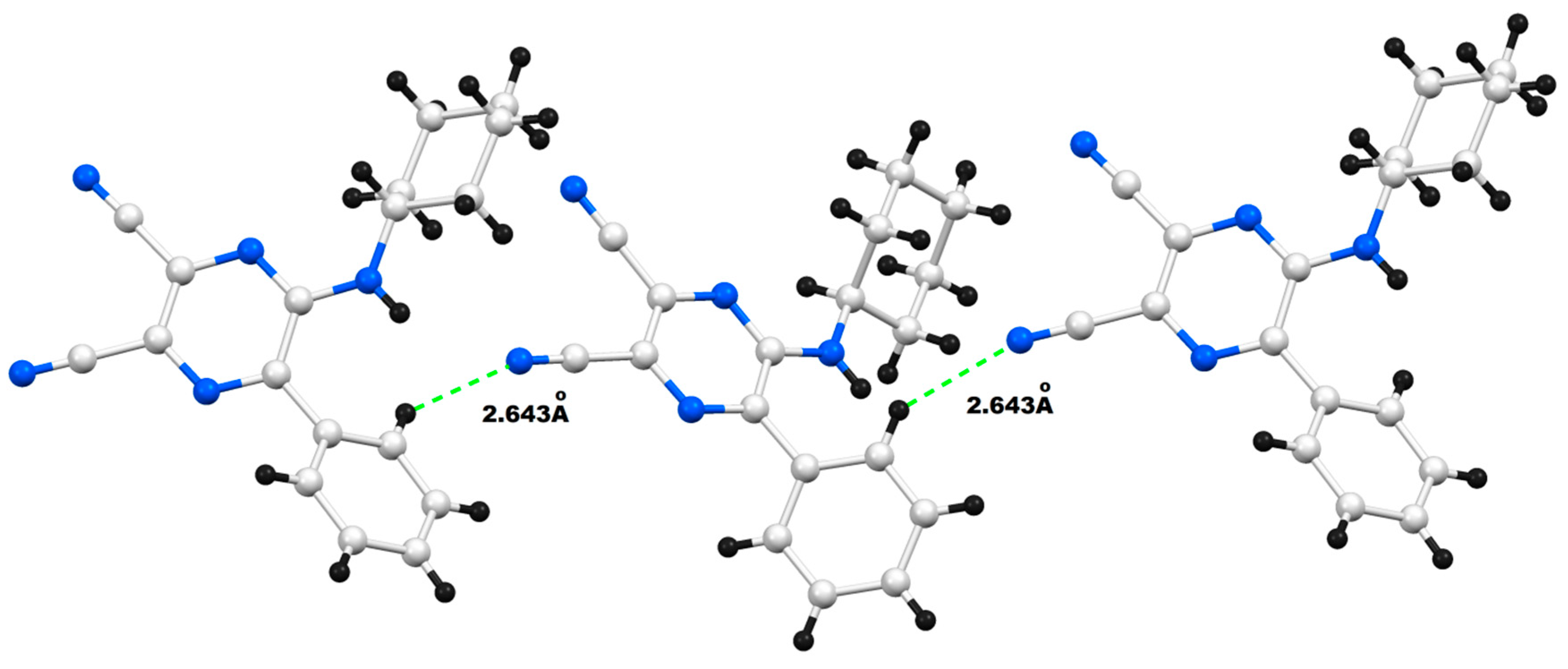
| Entry | R | R’ | 13 | Yield of 13% | 14 | Yield of 14% |
|---|---|---|---|---|---|---|
| 1 | C6H5 | Cyclohexyl | a | 88 | a | 8 |
| 2 | 4-CH3C6H4 | Cyclohexyl | b | 78 | - | |
| 3 | 4-ClC6H4 | Cyclohexyl | c | 83 | c | 10 |
| 4 | 4-CH3OC6H4 | Cyclohexyl | d | 80 | d | 10 |
| 5 | CH3 | Cyclohexyl | e | 72 | - | |
| 6 | 4-C6H5C6H4 | Cyclohexyl | f | 40 | f | 40 |
| 7 | CF3Py | Cyclohexyl | g | 87 | - | |
| 8 | CH3 | t-Bu | h | 37 | - | |
| 9 | C6H5 | t-Bu | i | 39 | i | 10 |
| 10 | 4-CH3OC6H4 | t-Bu | j | 41 | j | 15 |
| 11 | 4-C6H5C6H4 | t-Bu | k | 43 | k | 45 |
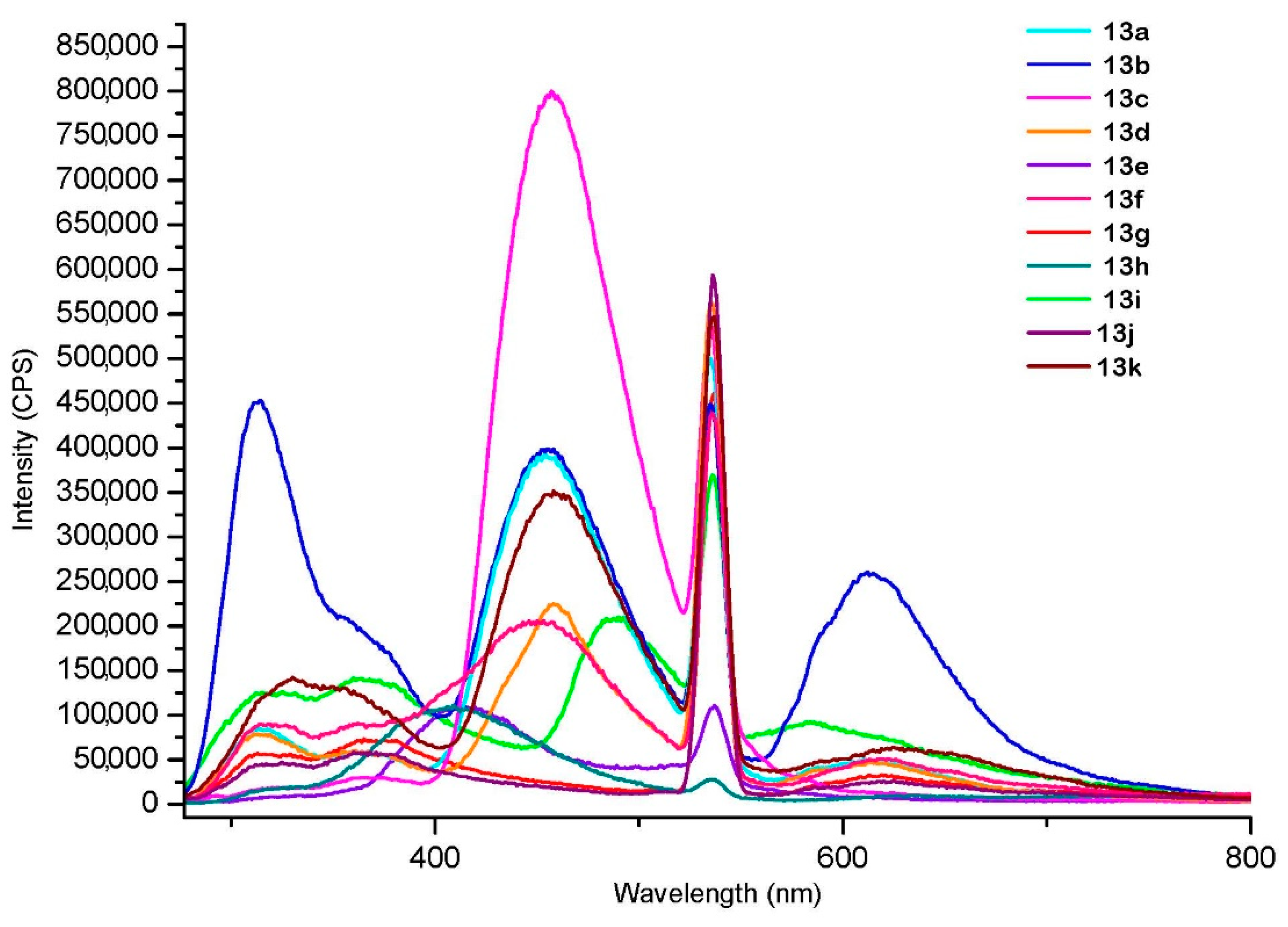
| Compound | Excitation at 267 nm λmax (Intensity in CPS) | Excitation at 303 nm λmax (Intensity in CPS) | Excitation at 373 nm λmax (Intensity in CPS) |
|---|---|---|---|
| 13a | 454 (4 × 105) | 454 (2.5 × 106) | 454 (3 × 106) |
| 13b | 455 (4 × 105) * | 455 (2.5 × 106) | 455 (3.7 × 106) |
| 13c | 457 (8 × 105) | 458 (4 × 106) | 457 (6 × 106) |
| 13d | 458 (2.5 × 105) | 459 (1.3 × 106) | 459 (2.5 × 106) |
| 13e | 414 (1.1 × 105) | 413 (2 × 106) | 421 (0.5 × 106) |
| 13f | 450 (2 × 105) | 456 (1.1 × 106) | 445 (2.3 × 106) |
| 13g | 358 (0.8 × 105) | 352 (0.25 × 106) | 421 (0.2 × 106) |
| 13h | 408 (1.1 × 105) | 406 (1.2 × 106) | 422 (0.4 × 106) |
| 13i | 490 (2.5× 105) | 486 (0.8 × 106) | 487 (0.97 × 106) |
| 13j | 368 (0.6 × 105) | 359 (0.17 × 106) | 420 (0.2 × 106) |
| 13k | 456 (3.5 × 105) | 461 (1.9× 106) | 458 (3.5 × 106) |
| Sample (1 mg/mL) | Inhibition Zone for Diameter (mm) for Bacteria, Mean (Min–Max) SD | |||
|---|---|---|---|---|
| E. coli | P. aeruginosa | S. aureus | B. subtilis | |
| 13a | 0 | 12.7 (12–15) 0.9 | 0 | 0 |
| 13b | 0 | 0 | 0 | 0 |
| 13c | 0 | 0 | 0 | 0 |
| 13d | 0 | 13.4 (12–18) 2.3 | 0 | 0 |
| 13e | 0 | 12.7 (11–13) 0.2 | 0 | 0 |
| 13f | 0 | 11.5 (11–12) 0.2 | 0 | 0 |
| 13g | 0 | 0 | 0 | 0 |
| 13h | 0 | 0 | 0 | 0 |
| 13i | 0 | 0 | 0 | 0 |
| 13j | 0 | 0 | 0 | 0 |
| 13k | 0 | 0 | 0 | 0 |
| (DMSO) | 0 | 0 | 0 | 0 |
| * Ampicillin | 34.33 (34–35) 0.5 | nil | 22.66 (22–23) 0.5 | 33.66 (33–34) 0.5 |
| * Kanamycin | 25.33 (25–26) 0.5 | 12.33 (12–13) 0.5 | 25.33 (25–26) 0.5 | 31 (31)0 |
| * Penicillin G | 23.66 (23–24) 0.5 | nil | 21.66 (21–23)1.1 | 33.66 (33–35) 1.1 |
| ** Cycloheximide | NA | NA | NA | NA |
| Sample (1 mg/mL) | Inhibition Zone for Diameter (mm) for Yeast, Mean (Min–Max) SD | |
|---|---|---|
| C. albicans | S. cerevisiae | |
| 13a | 0 | 0 |
| 13b | 9.5(9–10)0.5 | 0 |
| 13c | 0 | 0 |
| 13d | 0 | 0 |
| 13e | 0 | 0 |
| 13f | 8.6(8–10)0.6 | 0 |
| 13g | 0 | 0 |
| 13h | 0 | 0 |
| 13i | 0 | 0 |
| 13j | 0 | 0 |
| 13k | 0 | 0 |
| Negative control (DMSO) | 0 | 0 |
| * Ampicillin | NA | NA |
| * Kanamycin | NA | NA |
| * Penicillin G | NA | NA |
| ** Cycloheximide | 0 | 52.33 (51–53) 1.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Azmi, A.; John, E. Construction of 5-(Alkylamino)-6-aryl/alkylpyrazine-2,3-dicarbonitriles via the One-Pot Reaction of Alkyl Isocyanides with Aryl/Alkyl Carbonyl Chlorides and Diaminomaleonitrile: Fluorescence and Antimicrobial Activity Evaluation. Molecules 2022, 27, 8278. https://doi.org/10.3390/molecules27238278
Al-Azmi A, John E. Construction of 5-(Alkylamino)-6-aryl/alkylpyrazine-2,3-dicarbonitriles via the One-Pot Reaction of Alkyl Isocyanides with Aryl/Alkyl Carbonyl Chlorides and Diaminomaleonitrile: Fluorescence and Antimicrobial Activity Evaluation. Molecules. 2022; 27(23):8278. https://doi.org/10.3390/molecules27238278
Chicago/Turabian StyleAl-Azmi, Amal, and Elizabeth John. 2022. "Construction of 5-(Alkylamino)-6-aryl/alkylpyrazine-2,3-dicarbonitriles via the One-Pot Reaction of Alkyl Isocyanides with Aryl/Alkyl Carbonyl Chlorides and Diaminomaleonitrile: Fluorescence and Antimicrobial Activity Evaluation" Molecules 27, no. 23: 8278. https://doi.org/10.3390/molecules27238278
APA StyleAl-Azmi, A., & John, E. (2022). Construction of 5-(Alkylamino)-6-aryl/alkylpyrazine-2,3-dicarbonitriles via the One-Pot Reaction of Alkyl Isocyanides with Aryl/Alkyl Carbonyl Chlorides and Diaminomaleonitrile: Fluorescence and Antimicrobial Activity Evaluation. Molecules, 27(23), 8278. https://doi.org/10.3390/molecules27238278





