Abstract
Bioprospecting natural products to find prominent agents for medical application is an area of scientific endeavor that has produced many clinically used bioactive compounds, including anticancer agents. These compounds come from plants, microorganisms, and marine life. They are so-called secondary metabolites that are important for a species to survive in the hostile environment of its respective ecosystem. The kingdom of Plantae has been an important source of traditional medicine in the past and is also enormously used today as an exquisite reservoir for detecting novel bioactive compounds that are potent against hard-to-treat maladies such as cancer. Cancer therapies, especially chemotherapies, are fraught with many factors that are difficult to manage, such as drug resistance, adverse side effects, less selectivity, complexity, etc. Here, we report the results of an exploration of the databases of PubMed, Science Direct, and Google Scholar for bioactive anticancer phytochemicals published between 2010 and 2020. Our report is restricted to new compounds with strong-to-moderate bioactivity potential for which mass spectroscopic structural data are available. Each of the phytochemicals reported in this review was assigned to chemical classes with peculiar anticancer properties. In our survey, we found anticancer phytochemicals that are reported to have selective toxicity against cancer cells, to sensitize MDR cancer cells, and to have multitarget effects in several signaling pathways. Surprisingly, many of these compounds have limited follow-up studies. Detailed investigations into the synthesis of more functional derivatives, chemical genetics, and the clinical relevance of these compounds are required to achieve safer chemotherapy.
1. Introduction
The prediction that global cancer incidence by 2040 would rise by 27.5 million new cases compared to the previously recorded 17 million in 2018 (Cancer Research, London, UK) is not overemphasized. Continuous ageing in the population is associated with this rise in the number of new cases. Current measures to deal with this predicament remain largely unsatisfactory due to the setbacks in various treatment methods [1]. Treatments available according to the National Cancer Institute include surgery, radiation therapy, chemotherapy, immunotherapy, hormone therapy, stem cell transplant, targeted therapy, and precision medicine. A recent approach in cancer treatment is photothermal therapy [2]. The choice of treatment depends on the type of cancer developed in the body. The goal of cancer-related research is to detect therapeutics that will have no or minimal side effects and reduce the complexity of chemotherapy.
For the context of the discussion here, lead compounds that have recently been identified from plants are examined. Many drugs available in the market as anticancer agents are secondary metabolites from microbes, marine life, and plants [3]. Natural products such as vinblastine [4], vincristine [5], etoposide [6], teniposide [7], taxol [8], navelbine [9], Taxotere [10], camptothecin [11], topotecan [12], and irinotecan [13] have been approved as chemotherapy agents; all are plant-derived [3,14]. However, the desire for a cancer cure is not really fulfilled, as many of the drugs available have severe side effects [14]. In this regard, it is appropriate to critically review efforts to date in bioprospecting and chemical genetics reports on new plant-derived compounds with increased activity and better therapeutic prospects. We reviewed the efforts in the bioprospecting of natural products from plants against cancers in the last decade.
2. Phytochemicals in Bioprospecting Research
The search for bioactive secondary metabolites in plants as anticancer agents is gaining increasing interest from researchers as it offers a more promising future in detecting novel compounds rather than the synthetic approach [15]. Natural products, especially those from plants, have a unique structural diversity, enabling new possibilities in drug research [16]. Traditional medicine has been a reliable source for obtaining useful information about plants with medicinal properties [17]. Some compounds were discovered by accident, i.e., by random screening of plants, microorganisms, and marine organisms or by bioactivity-guided fractionation [18]. Many drug lead structures were discovered through these methods; nevertheless, the precious gifts of nature to humankind still must be used satisfactorily. It is worth noting that among the sources of natural products, plants remain a huge vase of abundant chemical compounds with unprecedented biological activities and mechanistic action. Conversely, only a small portion of the world’s flora has been tested for their potential bioactive compounds [19,20] and surprisingly, drugs derived from plants are particularly low in number, unlike those from other natural product sources [21].
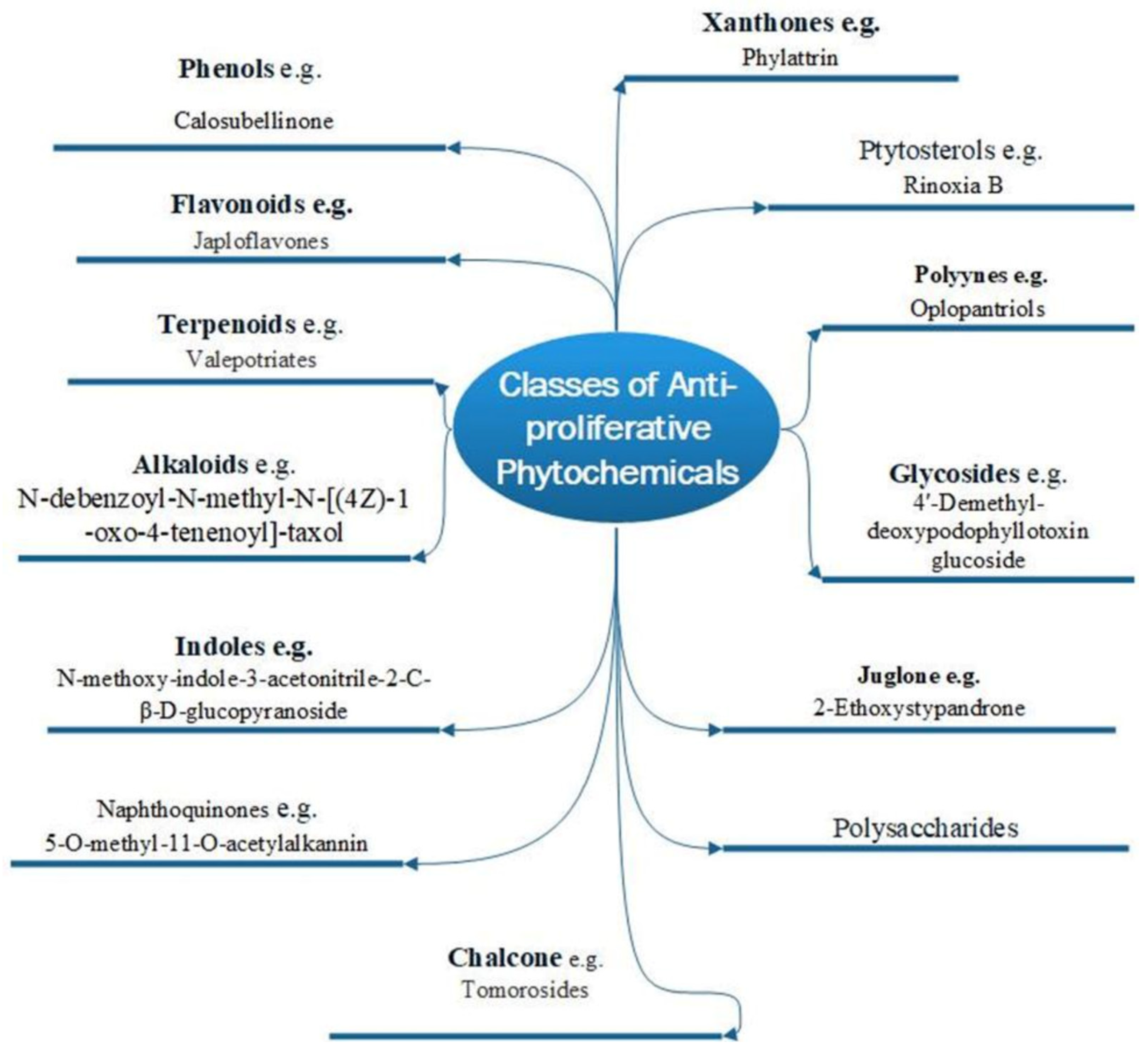
There are two basic types of phytochemicals: primary and secondary metabolites. The former class contains the components used for the basic physiological processes in plants, while the latter class is not necessarily used by plants’ physiology but ensures survival in an ecological context. These compounds are known as secondary metabolites, and some have been identified to promote human health and treatment of diseases [22]. Figure 1 shows different classes of plant bioactive secondary metabolites that are of clinical and pharmacological importance, with examples of newly purified compounds that have been studied for their anticancer potentials [23].
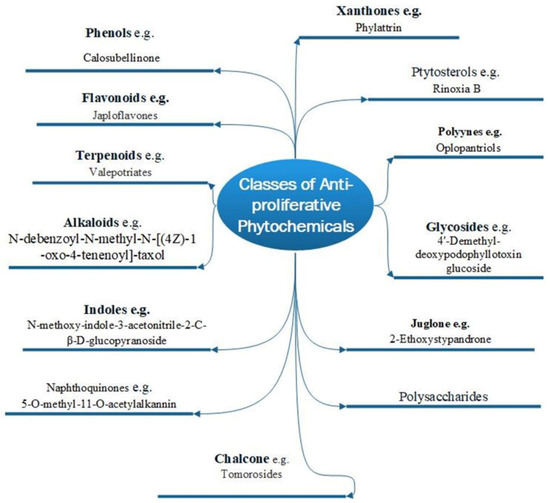
Figure 1.
Various phytochemical classes with examples of new members.
3. Carcinogenesis and Phytochemicals: Mechanisms of Action and Cellular Targets
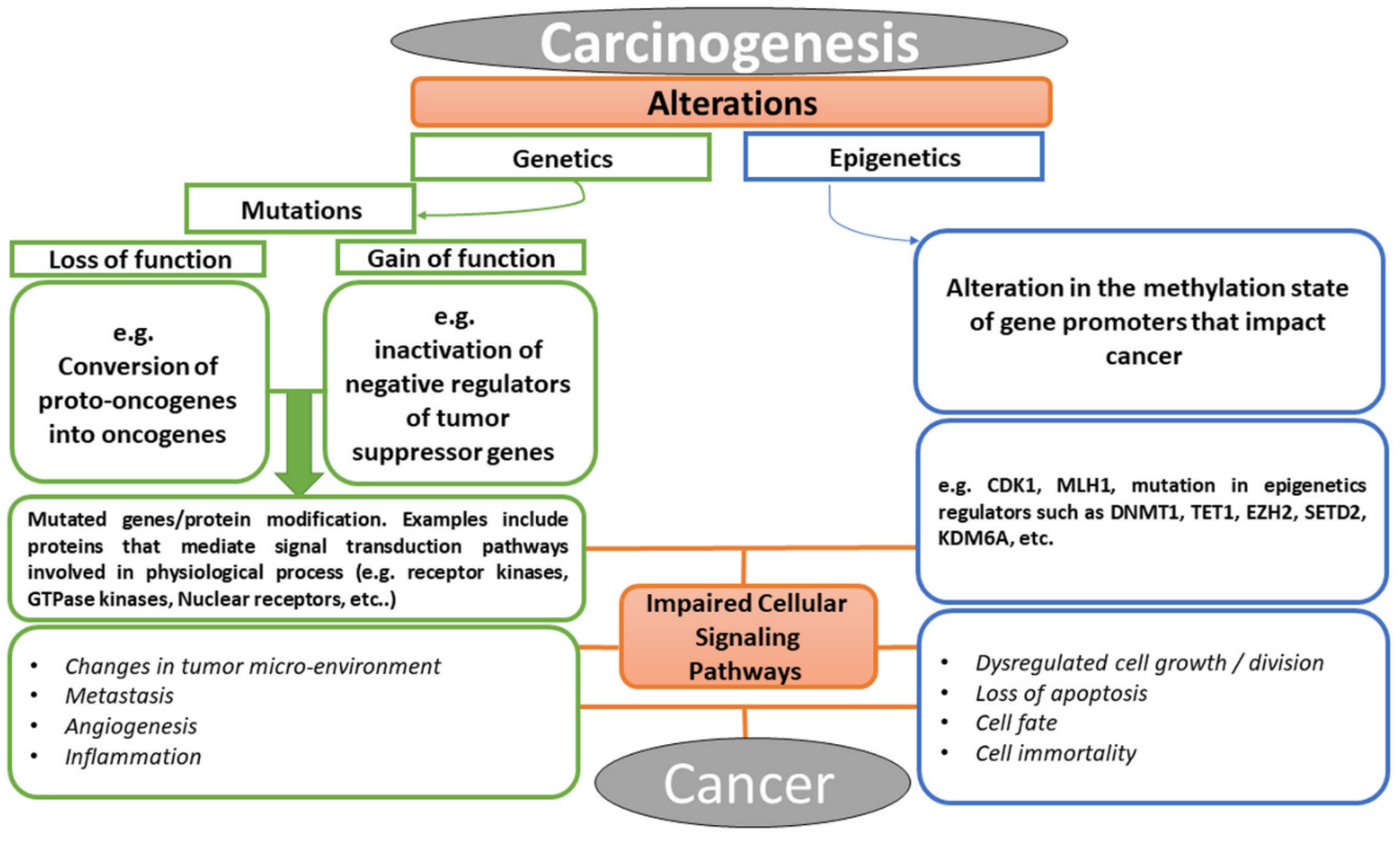
Cancer is a multifactorial disease with diverse etiological factors. The events that lead to cancer arise from the alterations in the genetic constituents of an individual, which may be in the form of mutation, epigenetic alteration, or perhaps the crosstalk between the two processes [24]. As illustrated in Figure 2, the conversion of cellular proto-oncogenes into oncogenes or inactivation of tumor suppressor genes (i.e., loss or gain of function mutations) result in carcinogenesis. Similarly, alteration in methylation or acetylation of promoters of certain genes could consequently result in aberrant proteins or lead to over-expression of some facilitating components of cell signaling pathways. Examples of these facilitators include growth factors and their receptors, such as RTKs, small GTPases, kinases, nuclear receptors, developmental signaling pathway components (e.g., Wnt [25], Hedgehog [26], Notch [27], Hippo [28]), nuclear targets of various signaling pathways (e.g., transcription factors, chromatin remodeler, tumor suppressor genes, cell cycle regulators) [29,30,31,32,33], and lots more [34,35]. These molecular facilitators have been the targets of bioactive compounds used in chemotherapy in the treatment of cancer. Today, the advancement in molecular biology has revolutionized all aspects of research in biology. Efforts in elucidating drug–target interaction have helped in revealing functions of some cellular proteins and in understanding the mechanism of action involved in drug phenotypes [36,37]. Targets of bioactive compounds (e.g., phytochemicals) range from genes to proteins responsible for several cellular processes. Among the notable cellular processes that small molecules target for their anticancer effects are cell death mechanisms (apoptosis and autophagy), metabolic pathways, cell cycle regulation, mitogenic signal transduction, angiogenesis, metastasis, replication, transcription, and translation machinery [38]. Meanwhile, the identification of a therapeutic window for cancer has been a major challenge in chemotherapy in that both the proliferating normal and cancer cells require the same metabolic needs [39]. The selectivity of anticancer drugs is proportional to their ability to have cellular targets that are peculiar to cancer in treatment. Unfortunately, most of the available anticancer drugs in the market lack this pedestal.
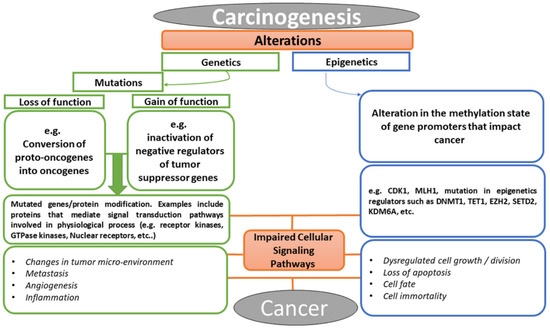
Figure 2.
Chronological events that lead to cancer, explaining the relationship between abnormal cellular signaling pathway and cancer formation.
Some of the available anticancer drugs are nonselective to cancer cells, targeting housekeeping proteins and genes indiscriminately in both highly proliferating transformed and normal cells [40]. However, most of the metabolic alterations in cancer often result in supporting proliferation (e.g., loss of function mutation in tumor suppressor gene p53). The wide merging in proliferation between transformed and nontransformed cells has been a major drawback to find a therapeutic window in metabolism-based chemotherapy [41].
In recognition of the relentless efforts in bioprospecting plants, reports from many studies have established promising and potent anticancer agents. Many of these phytochemicals are selective in targeting signal transduction pathways that are known to be adulterated in cancer cells [42]. Some of these agents modulate immune systems, control epigenetics and mutations, and leverage the expression of enzymatic products and the blockage of intracellular signal transduction cascades that may lead to the manifestation of cancer. Some other phytochemicals have also been reported to work in synergy with known anticancer drugs, thereby increasing their potency. Some can reverse the multiple drug resistance (MDR) phenotype.
The phytochemicals discussed below are new and have been established in various studies to possess anticancer/antiproliferative properties. These phytochemicals are assigned to their various established phytochemical groups, and the information on whether their mechanism of action and cellular targets is available in the literature was recorded.
4. Novel Glycosides
Glycosides include all phytochemicals that have saccharide moieties, i.e., glycosides can occur in any phytochemical class. Prominent among the glycosides with therapeutic values are flavonoid glycosides, anthraquinone glycosides, coumarin glycosides, cardiac glycosides, cyanogenic glucosides, indole glycosides, etc. Efforts in recent times have elucidated novel cellular targets specific to this class of chemicals. Two novel cellular targets, YYI/p65/p300 complex and VRKI/p53BPI, were recently reported to be the target of hyperoside (a flavonol glycoside) and ginsenoside Rg3, respectively [43,44]. The anticancer potency of cardiac glycosides is linked to their inhibition of sodium potassium ATPase (NKA), Ca2+ apoptosis induction, sequential activation of autophagy and apoptosis, modulations of topoisomerase II, fibroblast growth factors (FGF-2), and nuclear factor kappa B (NF-ƙB) inhibitions [45]. Nur77 (an orphan nuclear receptor) pathway has also been established as a target of some cardiac glycosides. Nur77 protein is expressed in cardiac glycoside-treated cells, which results in an apoptosis induction. This is due to an alteration in the mitochondria occurring because of the translocation of Nur77 from the nucleus to the cytoplasm [46].
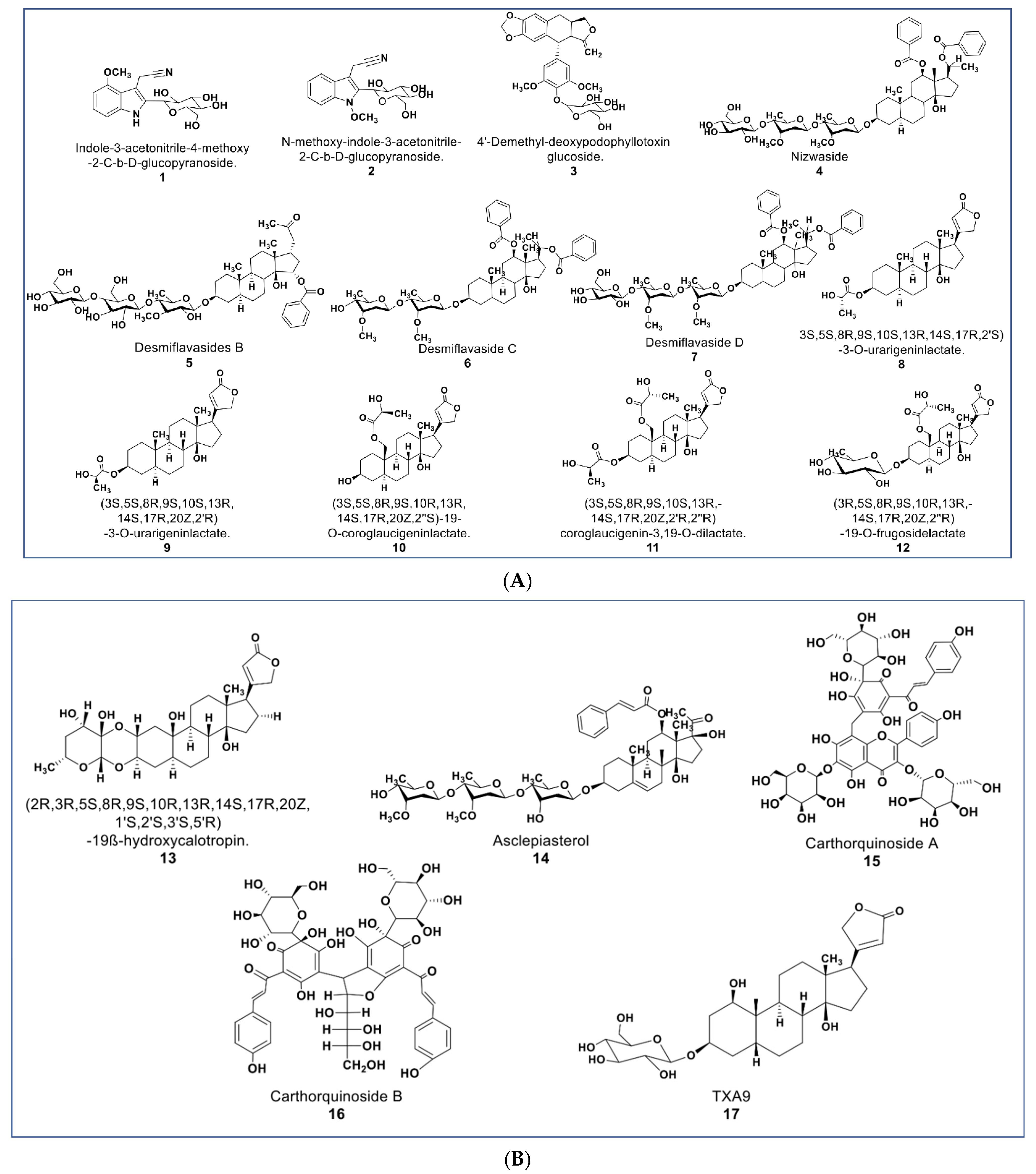
The chemical structures presented in Figure 3 contain some newly reported glycosides with promising antiproliferative activities, as well as some cardenolide lactates. Some new glucosides (compounds 1 and 2, Figure 3A) were reported to be cytotoxic against HL-60 and HepG2 (IC50 values 1.3 and 2.1 µM; 5.1 and 12.1 µM, respectively). No report was found as to the mechanisms involved in their antiproliferative activities [47]. Another glycoside (compound 3) reported by Zilla et. al. from Podophyllum hexandrum shows cytotoxicity against a panel of cancer cell lines (see Supplementary Table S1) with an IC50 value range of 0.208–0.291 µM [48].
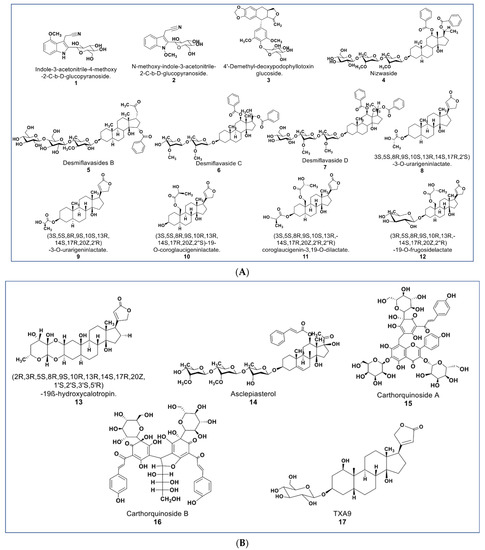
Figure 3.
(A): Representative members of novel glycosides from various plant sources (compounds 1–7 and 12). Compounds 8–11 are cardenolide lactates isolated from Asclepias curassavica. (B): Representative members of novel glycosides from various plant sources (compounds 13 to 17).
As described by Raees et al., some novel pregnane glycosides from Desmidorchis flava possess antiproliferative effects against breast and ovarian cancer cell lines [49,50]. Compounds 4–7 (Figure 3A) showed cytotoxicity on MDA-MB 231 and SKOV-3 cancer cells [51]. Additionally, compound 4 (nizwaside) showed a stronger antiproliferative effect in MDA-MB-231 cell lines than the known antitumor drug doxorubicin [49]. In contrast, compounds 6 and 7 showed no cytotoxicity on normal breast epithelial cell line MCF-10-2A [51].
A group of potent-to-moderate antiproliferative compounds was isolated from Asclepias curassavica [52]. These include four new cardenolide lactates plus one glucoside lactate (compounds 8–11, Figure 3A) and a new double-linked cardenolide glycoside (compound 13, Figure 3B). While compound 13 showed a strong cytotoxic effect on DU145 cells (IC50 0.29 µM), the cardenolide lactates showed moderate cytotoxicity (IC50 value range 1.66–16.96 µM) [52]. Other previously known compounds from this plant were identified to be cytotoxic against DU145 prostate cancer cells. These were six normal cardenolides (IC50 values: 0.33–0.92 µM), four double-linked cardenolide glycosides (IC50 Values: 0.03–0.28 µM), and some C-21 steroidal glycosides [53,54,55]. Compound 14 (asclepiasterol), a C-21 steroidal glycoside from A. curassavica, was reported to modulate the MDR phenotype in MCF-7/ADR and HepG-2/ADM cells at low concentrations (2.5–5.0 µM) and enhancing the cytotoxicity of anticancer drugs [56].
Two new quinochalcone C-glycosides (carthorquinosides A and B, Figure 3B) were isolated from Carthamus tinctorius. Both compounds inhibit inflammation in lipopolysaccharide (LPS)-stimulated HUVEC cells at low micromolar concentrations [57]. A novel cardiac glycoside purified from Streptocaulon juventas (compound 17) showed more potent antitumor activities against NSCLCs (IC50 values ranging from 0.006–0.44 µM) than Taxol [58].
5. Novel Polyphenolic Compounds
Polyphenolic compounds are generally characterized by the presence of an aromatic ring with hydroxyl groups attached. This phytochemical class is of medical importance to humans as antioxidants, antivirals, and anticarcinogens [59,60]. Polyphenols are subdivided into phenols and flavonoids and can occur as flavonoid glycosides [61]. Several studies have established phenolic compounds as modulators of multiple inflammatory components [62]. Polyphenolic anticancer activity is linked to the modulation of proteins that are involved in procancer signaling pathways, e.g., survival kinases, transcription factors, and growth factors [63]. Reports have demonstrated the role of polyphenols in targeting the PI3K/Akt/mTOR and Wnt/β-catenin signaling pathways and the downregulation of cell cycle regulatory proteins such as cyclins and its kinases. Polyphenols are also known as modulators of epigenetic regulators such as HDAC1 and HDAC2; tumor suppressor proteins p53, PTEN, p21, and NF-kβ, NRF2 and STATs p27 [64,65,66]. Flavonoids share some targets such as MAPK, PI3K/AKT, NF-κB, and NRF2 signaling pathways with phenols [67]. Reports from studies established some flavonoids as antimitotic and microtubule-targeting agents (destabilizers and stabilizers of microtubules) and inhibitors of polo-like kinase 1 (PLK1) [68,69,70,71].
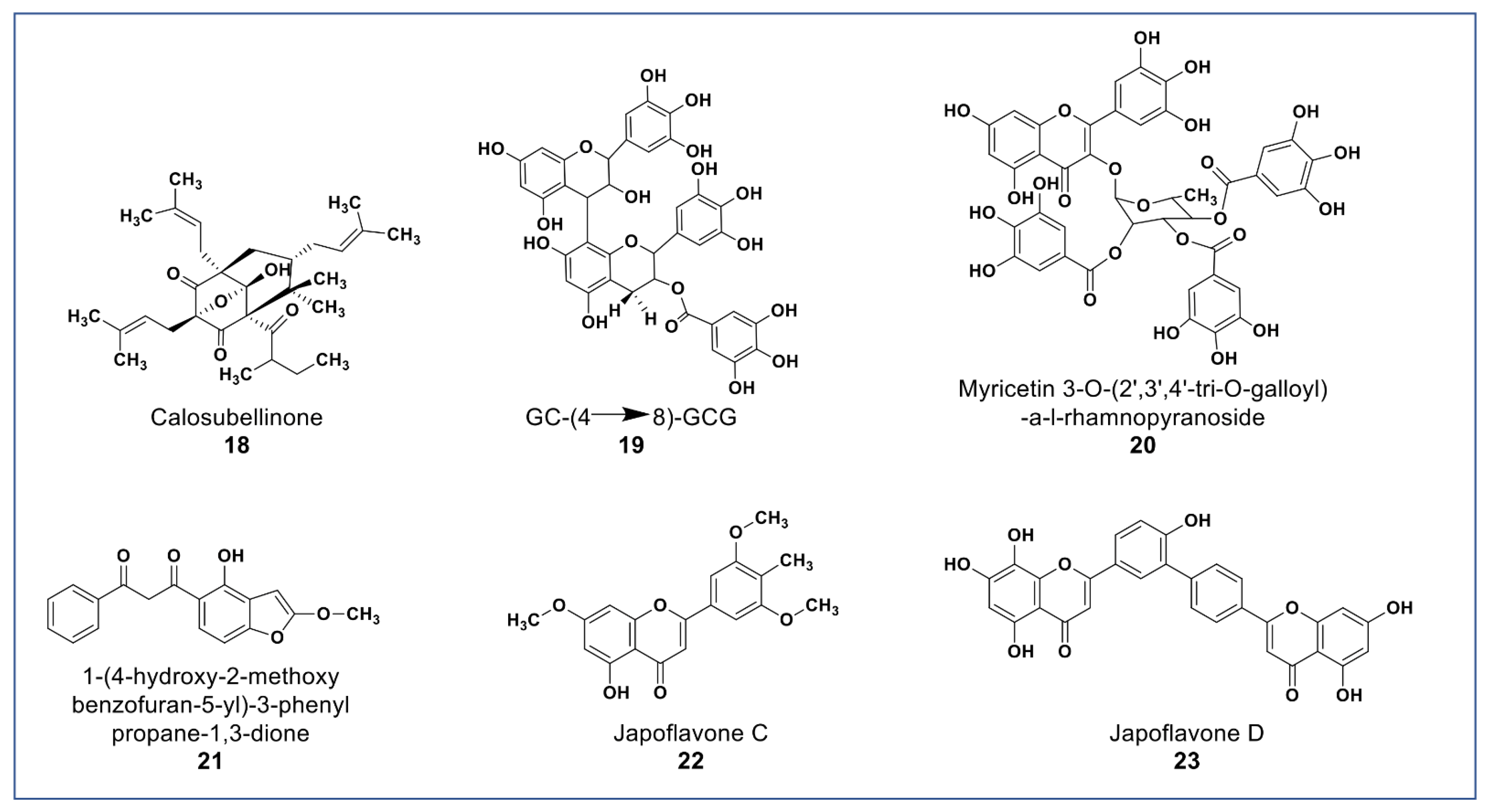
As shown in Figure 4, novel polyphenols from Calophyllum soulattri (compound 18) showed cytotoxicity against the MDA-MB-231 breast cancer cell line. Compound 18 was almost as active as cisplatin (IC50: 19.3 µM) on MDA-MB 231cells. It was found not to be cytotoxic against normal HEK293 cells [72].
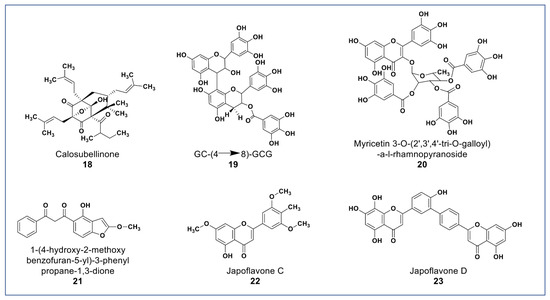
Figure 4.
Representative members of new polyphenolic phytochemicals from various plant sources (compounds 18 to 23).
A novel proanthocyanidin (compound 19, Figure 4) purified from Camellia ptilophylla proved to be antiangiogenic [73]. This compound suppressed microtubule formation in endothelial cells and cell migration in HMEC-1 cells. It inhibited intersegmental vessels formation (ISV) in Zebrafish and inhibited the phosphorylation of ERK, p38, and Akt in HMEC cells. MAPK and ERK signaling pathways play an important role in angiogenesis [73].
Compounds 20 and 21 (Figure 4) isolated from Leucaena leucocephala and Celosia argentea were reported to have potent antioxidant scavenging and reducing power, and to be cytotoxic against some cancer cells [74,75]. Compound 20 was reported to have IC50 value 2.41 µg/mL against HepG2 [74] while compound 21 (Figure 4A) was reported to have significant antioxidant and cytotoxic activities against Siha, MCF-7, HCT, and HT-29, and no cytotoxicity against Vero cells [74]. In addition to the novel polyphenols, two new flavones (compounds 22 and 23) isolated from Lonicera japonica suggested having hepatoprotective and antihepatoma properties due to activities in SMCC-7721 and HepG2 cells [76].
6. Novel Xanthones
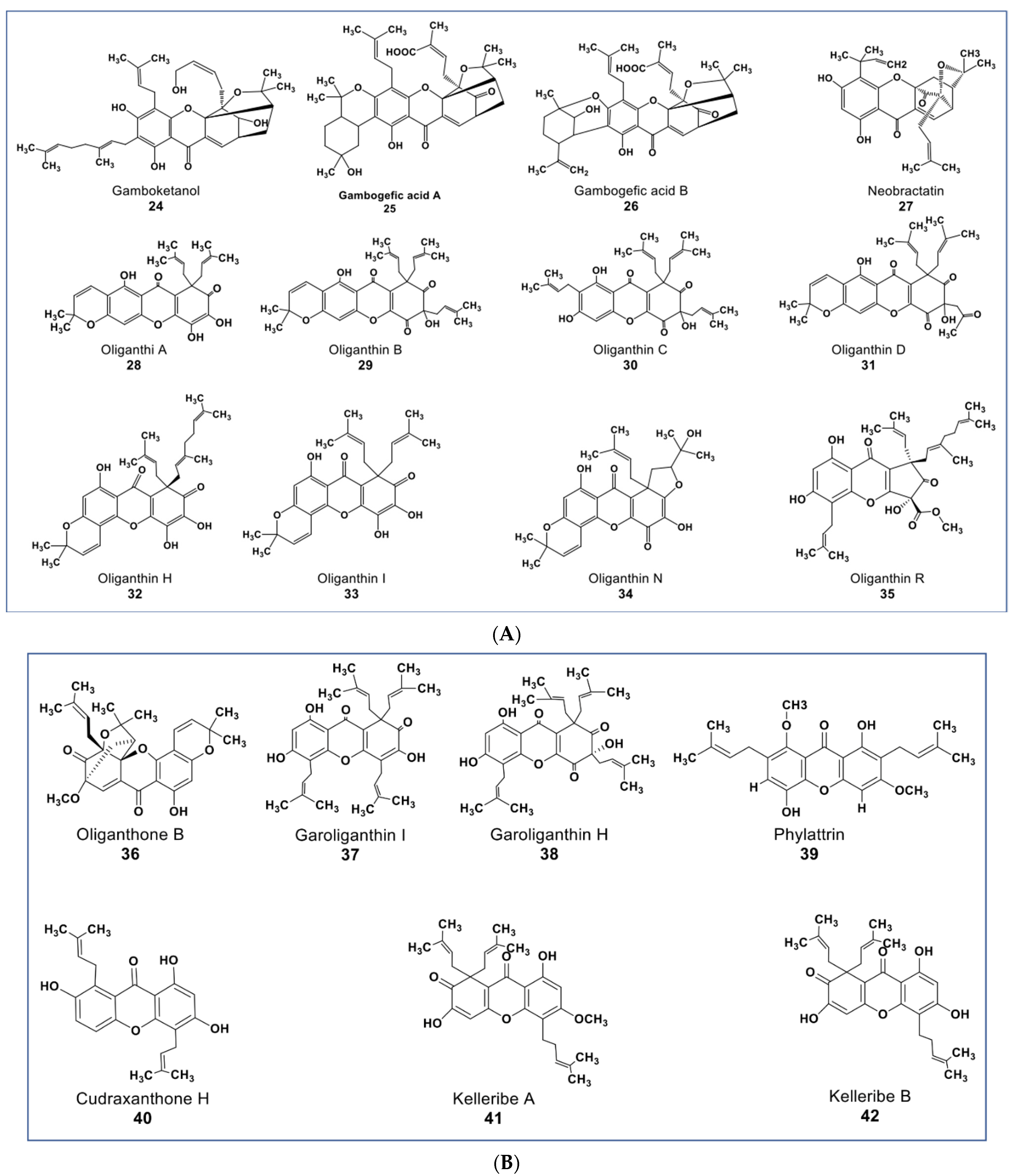
Xanthones are polyphenols that belong to the flavone subgroup. Many potent anticancer agents have been reported from this group (as shown in Figure 5A,B). The genus Garciniaea produces many anticancer xanthones [77,78,79,80]. Compounds 24, 25, and 26 (Figure 5A) are new xanthones that have not received due attention, though they have potent anticancer activities. These compounds were purified from Garcinia hanburyi and were reported to show cytotoxicity on cervical carcinoma cells with IC50 values of 3.82, 2.11, and 1.73 µM, respectively [79]. A caged-prenylxanthone (compound 27) isolated from Garcinia bracteata showed cytotoxic effects against a panel of cancer cell lines including A549, MCF7, SMMC-7721, SW480, and HL-60 cells at IC50 values ranging between 2.02–3.25 µM [81]. Compound 27 has been shown to induce growth inhibition via apoptosis and inhibited autophagy flux in A549 and HeLa cells [80]. Compound 27 also inhibited the growth of A549 and HeLa xenograft in mice a little bit higher than the taxol-positive control via the upregulation of muscleblind-like 2 (MBNL2) and CELF 6 RNA-binding proteins [82,83].
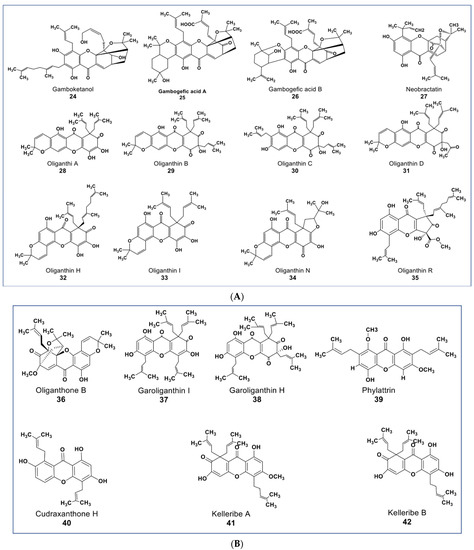
Figure 5.
(A): Representative members of new xanthone phytochemicals from various plant sources (compounds 24 to 35). (B): Representative members of new xanthone phytochemicals from various plant sources (compounds 36 to 42).
Some new xanthones from Garcinia oligantha were reported to be cytotoxic against many cancer cell lines [84,85,86,87]. Compounds 28–38 (Figure 5A,B) had IC50 values ranging from 1.52–<20 µM against A549, HepG2, HT-29, PC, HL-7702, and HeLa cells [86]. Compound 35 (Figure 5A) showed cytotoxic activities against quiescent prostate cancer cells (LNCaP) that are insensitive to Taxol at 20 µM [84]. Xanthones from other plants source include a novel diprenylated xanthone. Compound 39 (Figure 5B) from Calophyllum soulattri was reported to have moderately higher antiproliferative effects across nine different cancer cell lines than the positive controls quercetin and kaempferol (IC50 values from 9.2–22.10 µM, see Supplementary Table S1) [88]. Compound 40 from Cudrania tricuspidata showed antiproliferative effects on oral squamous cell carcinoma by targeting NF-κB and PIN1 signaling pathways [89]. Among five new compounds reported from Hypericum kelleri, compounds 41 and 42 (Figure 5B) showed potent cytotoxicity against HeLa cells with an IC50 value of 2.5 and 5.9 µM, respectively [90].
7. Novel Terpenoids
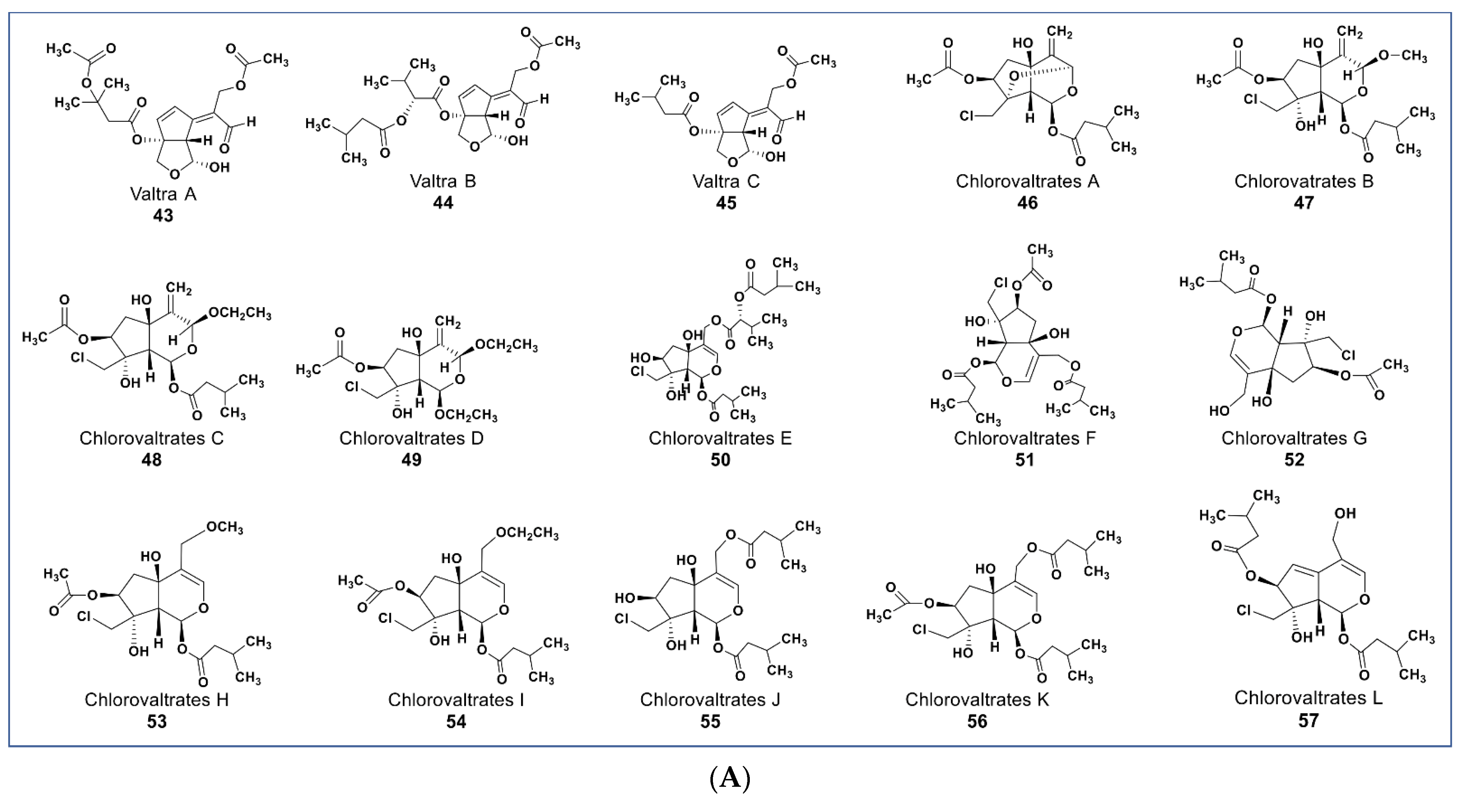
Valepotriates, which are triesters of monoterpene alcohol, are known for their excellent anticancer properties [91]. Novel compounds belonging to this chemical class include compounds 43–45 (Figure 6A) isolated from Valeriana jatamansi. These compounds obtained as degradation products of valepotriates show selective cytotoxicity against PC-3M and HCT-8 cancer cells with IC50 values between 2.1 and 6.5 µM [92]. Some new chlorinated valepotriates (compounds 46–60, Figure 6A,B) isolated from Valeriana jatamansi were reported to be cytotoxic on several cancer cell lines [93]. Some of these compounds (56–59) had potent activity on A-549, PC-3, HCT-8, and Bel-7402 cancer cells with IC50 values ranging between 1.06 and 10 µM.
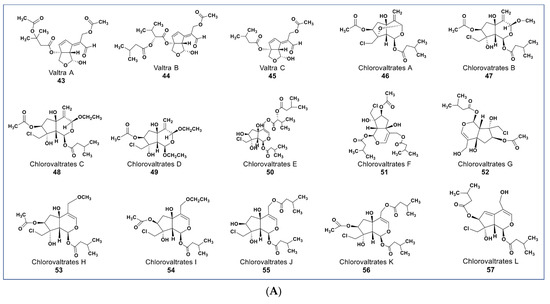
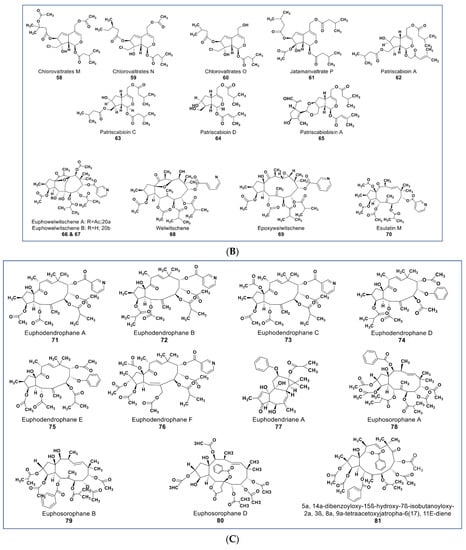
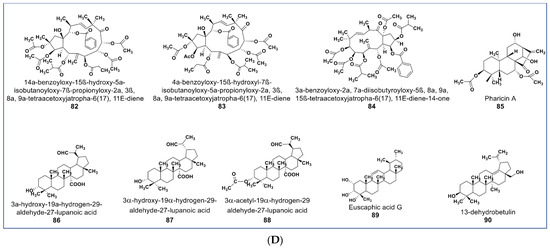
Figure 6.
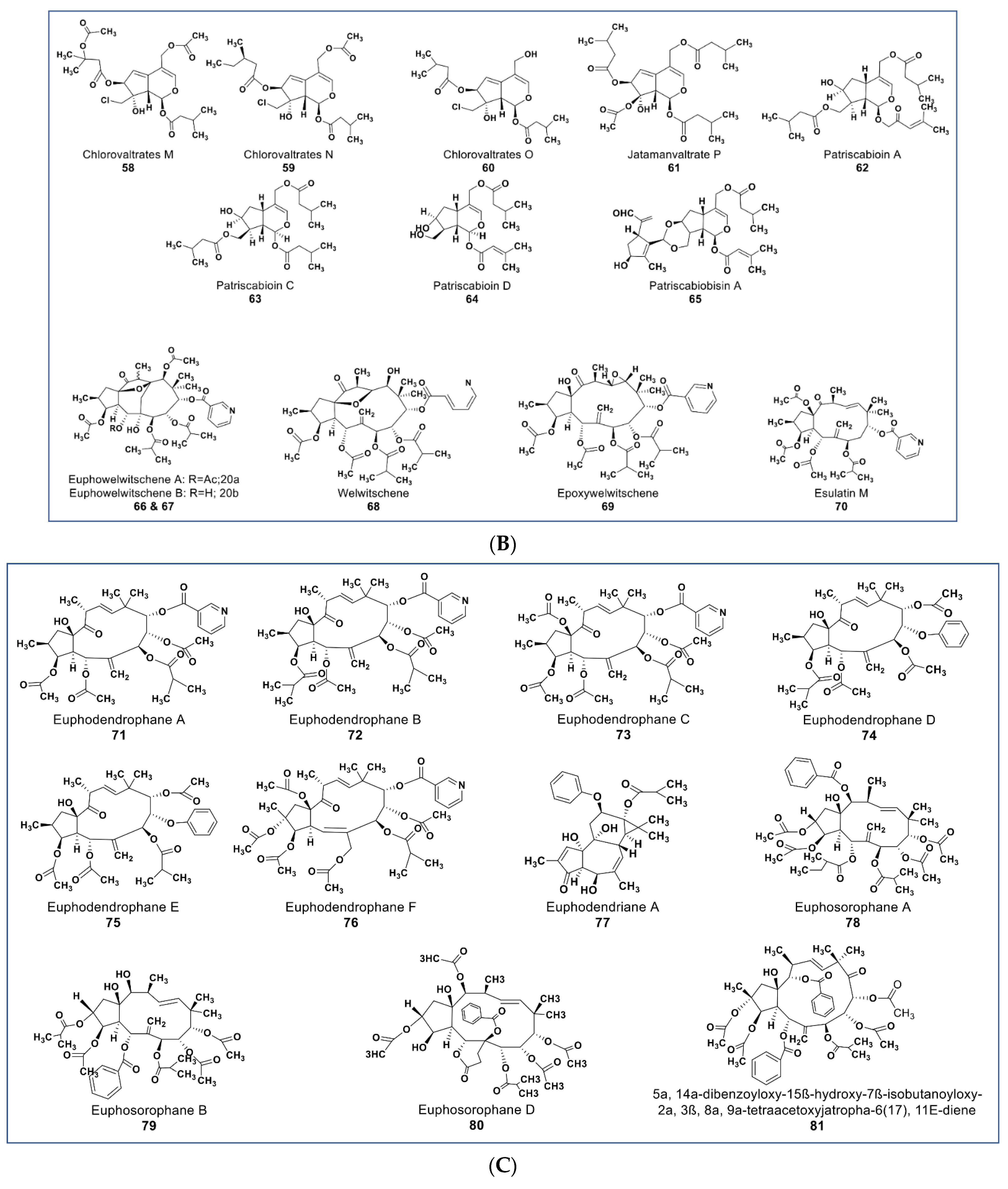
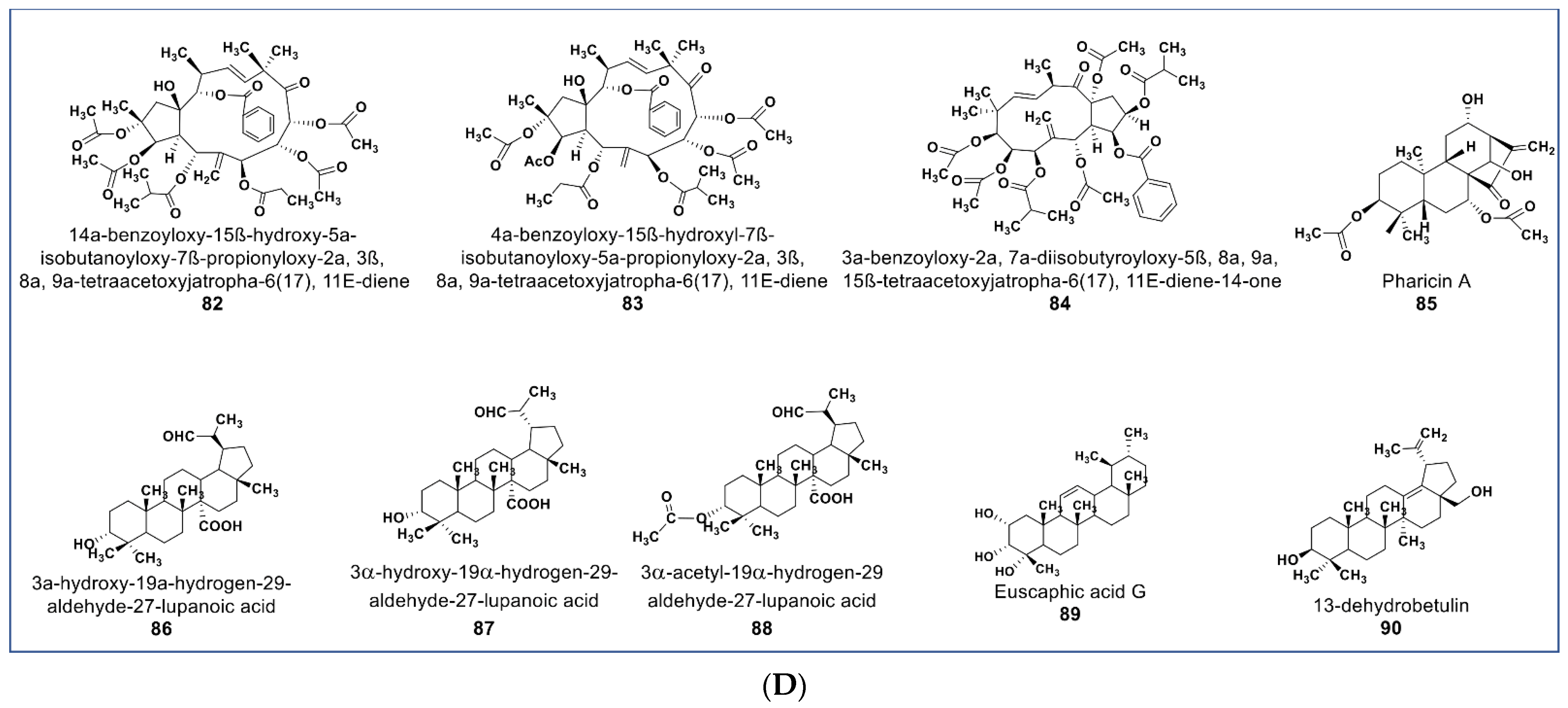
(A): Representative members of new terpenoid phytochemicals from various plant sources (compounds 43 to 57). (B): Representative members of new terpenoid phytochemicals from various plant sources (compounds 58 to 70). (C): Representative members of new terpenoid phytochemicals from various plant sources (compounds 71 to 81). (D): Representative members of new terpenoid phytochemicals from various plant sources (compounds 82 to 90).
Compound 61 (Figure 6B), one of two new iridoids (named Jatamanvaltrate P) isolated from Valeriana jatamansi, showed selective anticancer properties in vivo and in vitro [94]. It inhibited the growth of triple-negative breast cancer (TNBC) and MCF-7 cancer cell lines in a concentration-dependent manner (IC50 values 4.05–7.05 µM, respectively). Additionally, 61 induced apoptosis and cell cycle arrest in treated cells. It also triggered autophagosome formation, indicated by the upregulation of LC3-II level in treated cells [95]. Some new sets of novel iridoids and bisiridoids were also reported from Patrinia scabiosaefolia. Three iridoids (compounds 62–64) and one bisiridoid (compound 65) showed selective cytotoxicity against HL-60, SMMC-7721, MCF-7, and SW-480, with IC50 values ranging from 1.2 to 23.9 µM [96].
Among the major challenges facing cancer chemotherapy is the resistance and cross-resistance of cancer cells to anticancer agents. This menace results from the overexpression of ATP-binding cassette (ABC) transporter proteins such as P-glycoprotein (P-gp) and breast cancer resistance protein (BCRP, ABCG2) “drug efflux transport” [97]. These membrane proteins are responsible for the excretion of drugs from the cell, resulting in low intracellular accumulation and consequent inefficacy of the drug, even at lethal concentration [98,99]. Examples of drugs against which cells have developed cross-resistance are taxanes [100], anthracyclines [101], vinca alkaloids [102], platinum compounds [103], and mitoxantrone [104]. Available MDR modulator drugs such as verapamil [105], cyclosporine [106], dexverapamil [107], tariquidar [108], and zosuquidar [109] show high toxicity and pharmacokinetic interactions with anticancer drugs [110].
Reports from various experiments have shown promising phytochemicals that are more potent than the currently available MDR modulators. Recently, some new jatrophane diterpenoids (compounds 66–70, Figure 6B) were isolated from Euphorbia welwitschia [111]. These compounds were reported to be stronger MDR modulators than verapamil [111]. At 2 µM, compounds 68, 69, and 70 showed distinct MDR phenotype reversal [112]. Compounds 69 and 70 (Figure 6B) led to selective MDR reversal in EPG85-257RDB-, EPP85-181RNOV-, and EPP85-181RDB-resistant cell lines. Compound 70 showed only minor activity against EPG85-180RDB [111,112].
Some new diterpenoids from Euphorbia dendroides (compounds 71–77, Figure 6C) inhibited the growth of a resistant NCI-H460/R lung carcinoma cell line [113] Compounds 71 and 72 both showed strong anti-MDR activities, possessed strong synergy with paxlitatel and doxorubicin, and reversed the resistance to paclitaxel in the NCI-H460/R cell line [114]. Some potent MDR reversal compounds were also isolated from Euphorbia sororia (compounds 78–84, Figure 6C,D) [115,116,117]. Compounds 78–80 showed low cytotoxicity at 10 µM against a sensitive MCF-7 cancer cell line but had higher modulation of P-glycoprotein (P-gp) transporter in doxorubicin-resistant MCF-7/ADR cells than verapamil [113].
A novel mitotic arrest inducer belonging to ent-kaurene diterpenoids (compound 85, Figure 6D) was purified from Isodon xerophilous. It was suggested that the compound’s antimitotic activity was via abnormal activation of the mitotic spindle checkpoint protein BubR1 [117]. More importantly, the compound also induced mitotic arrest in paclitaxel-resistant Jurkat and U2OS cancer cell lines [117]. Novel anticancer triterpenes include the three rarely found triterpene derivatives of C-27-carboxylated-lupine (compounds 86–88, Figure 6D) isolated from Potentilla discolor. These compounds demonstrate a higher antiproliferative effect than matrine (a known anticancer agent) against HepG2, MCF7, and T-84 cell lines. In contrast, they seem to be nontoxic against the HL-7702 noncancerous liver cell line at 35 µM [118]. A pentacyclic triterpene (compound 89, Figure 6D) isolated from Glechoma longituba induced apoptosis and cell cycle arrest in NCI-H460 lung carcinoma by targeting the NF-κB/AP-1 signaling pathway [119]. Compound 90 (Figure 6D), a newly isolated betulin derivative from the stem of Ziziphusspina christi, showed antiproliferative property against a HepG2 cancer cell line [120].
8. Novel Alkaloids
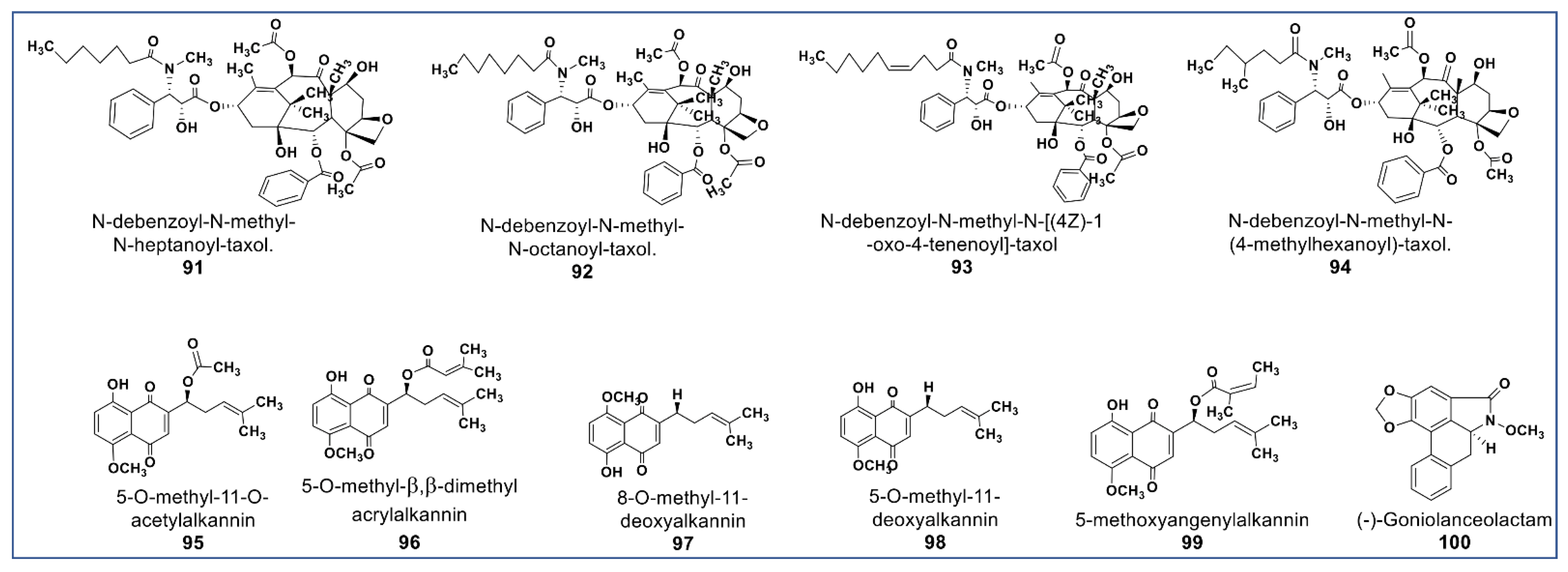
The main phytochemical class, well known for its wide pharmaceutical spectrum, is the alkaloids [121]. Anticancer alkaloids comprise a large number of chemical structures [18]. Prominent among anticancer alkaloids are paclitaxel [122], pyrrolizidines [123], indole [124], quinoline [125], tropane [126], and acronycine alkaloids [127]. Figure 7 shows the chemical structures of some of the newly identified anticancer alkaloids. There are some new taxane derivatives isolated from the ethanol extract of Taxus wallichiana. Compounds 91–94 (Figure 7) induced a tubulin effect similar to paclitaxel and had antiproliferative activities at IC50 values between 0.077 and 7.48 µM against MCF -7, A549, 3-AO, and normal HUVEC cells [128].
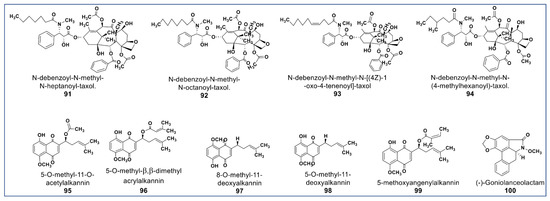
Figure 7.
Representative members of new alkaloid phytochemicals from various plant sources (compounds 91 to 100).
Another phytochemical group of pharmacological importance are the naphthoquinones [129]. Some naphthoquinones have been reported as typical endoplasmic reticulum stressors (ERS) [130,131]. Studies have shown that ERS is involved in the activation of inositol-regulating enzyme 1 (IRE1), a representative of unfolded protein regulators (UPRs), which are responsible for proteostasis [132,133]. Persistent ERS induces IRE1 to activate ASK1 (apoptosis signal-regulating kinase 1) and subsequently, activation of the downstream c-Jun N-terminal kinases (JNK) phosphorylation, which induces cell death [134]. Naphthoquinones are potent anti-invasive agents acting on EMT (endothelial mesothelial transition) of cancer stem cells and STAT3 signaling cascades [135,136,137]. Their modulation of ROS enzymes such as ubiquitin-specific protease-2 (USP2) and NADPH Quinone Oxidoreductase 1 (NQO1) gave them selectivity against cancer cells [138]. Compounds 95–98 (Figure 7) isolated from Alkanna cappadocica showed intriguing cytotoxic activities against a panel of cancer cells including HT-29, MDA-MB 231, PC-3, AU565, HepG2, LNCaP, MCF7, HeLa, SK-BR-3, DU 145, Saos, and Hep3B at low micromolar concentration. Compound 95 showed significantly higher activity in these cell lines than doxorubicin and etoposide. [139]. Moreover, a new phytochemical from Alkanna tinctoria (Compound 99, Figure 7) was isolated and evaluated for its cytotoxicity against HCT-116 and SW-480 (IC50: 4.4 and 9.6 µM, respectively) [123]. A novel naphthoquinone alkaloid (Compound 100, Figure 7) isolated from Goniothalamus lanceolatus showed selective antiproliferative propery that was more potent than 5-fluorouracil when tested against lung and colon carcinoma cells [140].
9. Novel Chalcones
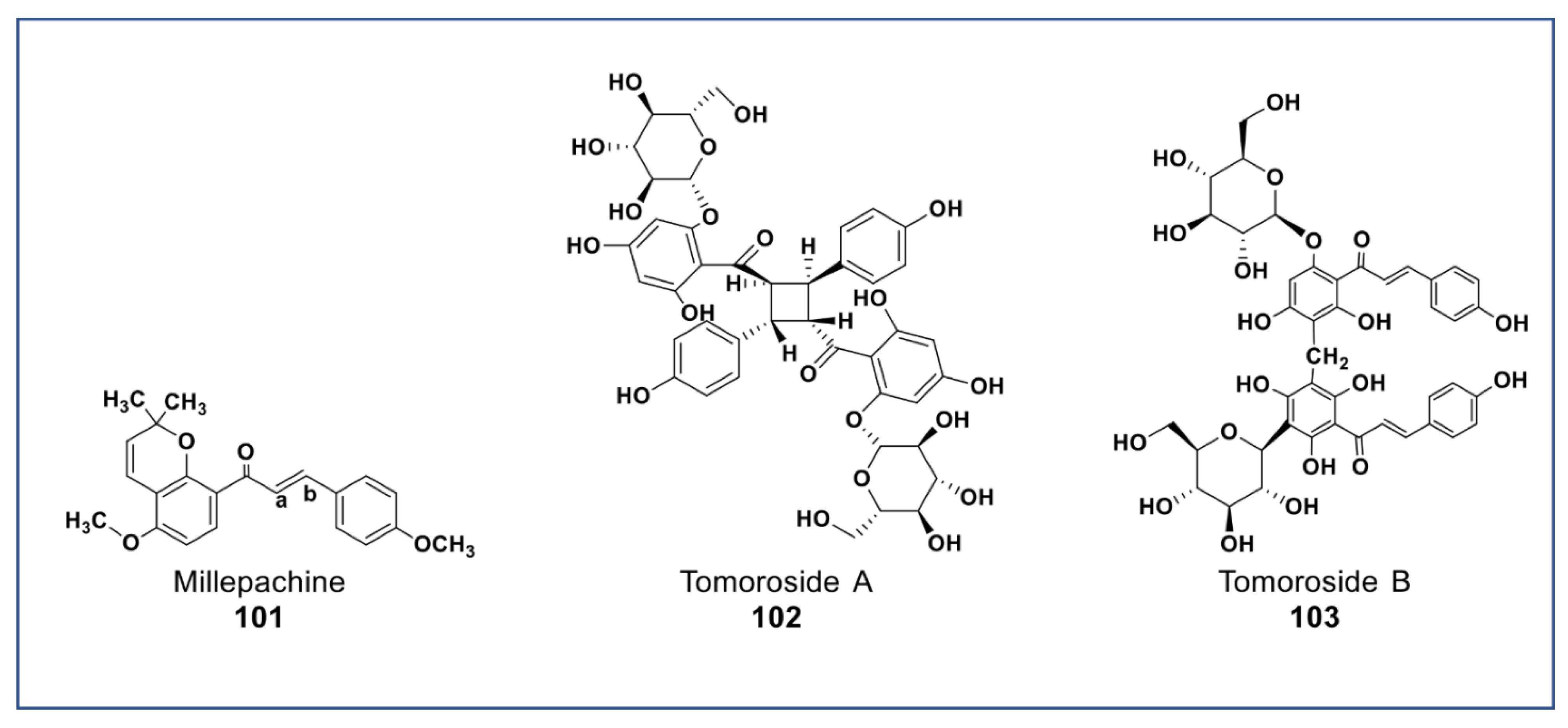
A new chalcone isolated from Millettia pachycarpa (compound 101, Figure 8) showed more potent antiproliferative effects (at 2 µM) against HeLa cell than cisplatin [141]. Recently, two newly synthesized derivatives of compound 101 have been reported to have a very potent cytotoxicity than compound 18 in vitro and in vivo (at nanomolar concentration) against many cancer cell lines. These compounds induced apoptosis via G2/M arrest, inhibited tububin polymerization, repressed MDR phenotype, and had little cytotoxicity against normal cells [142,143]. Two new chalcone dimers (compound 102 and 103, Figure 8) isolated from Helichrysum zivojinii inhibited the growth of both sensitive and resistant lung carcinoma NCI-H460, NCI-H460/R, and HaCaT cells [144]. Compound 102 suppressed the topoisomerase IIα and significantly enhanced doxorubicin activity (a typical anticancer drug that suppresses topoisomerase IIα) when combined. Compounds 103 increased the efficacy of tipifarnib (farnesyltransferase inhibitor used in the treatment of leukemia) against MDR cancer cells [144].
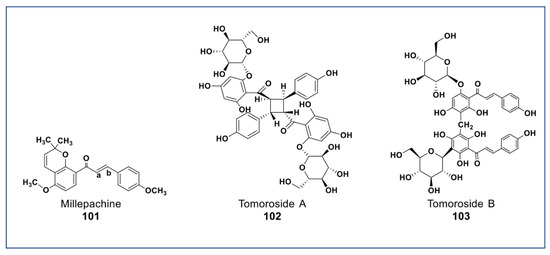
Figure 8.
Representative members of new chalcone phytochemicals from various plant sources (compounds 101 to 103).
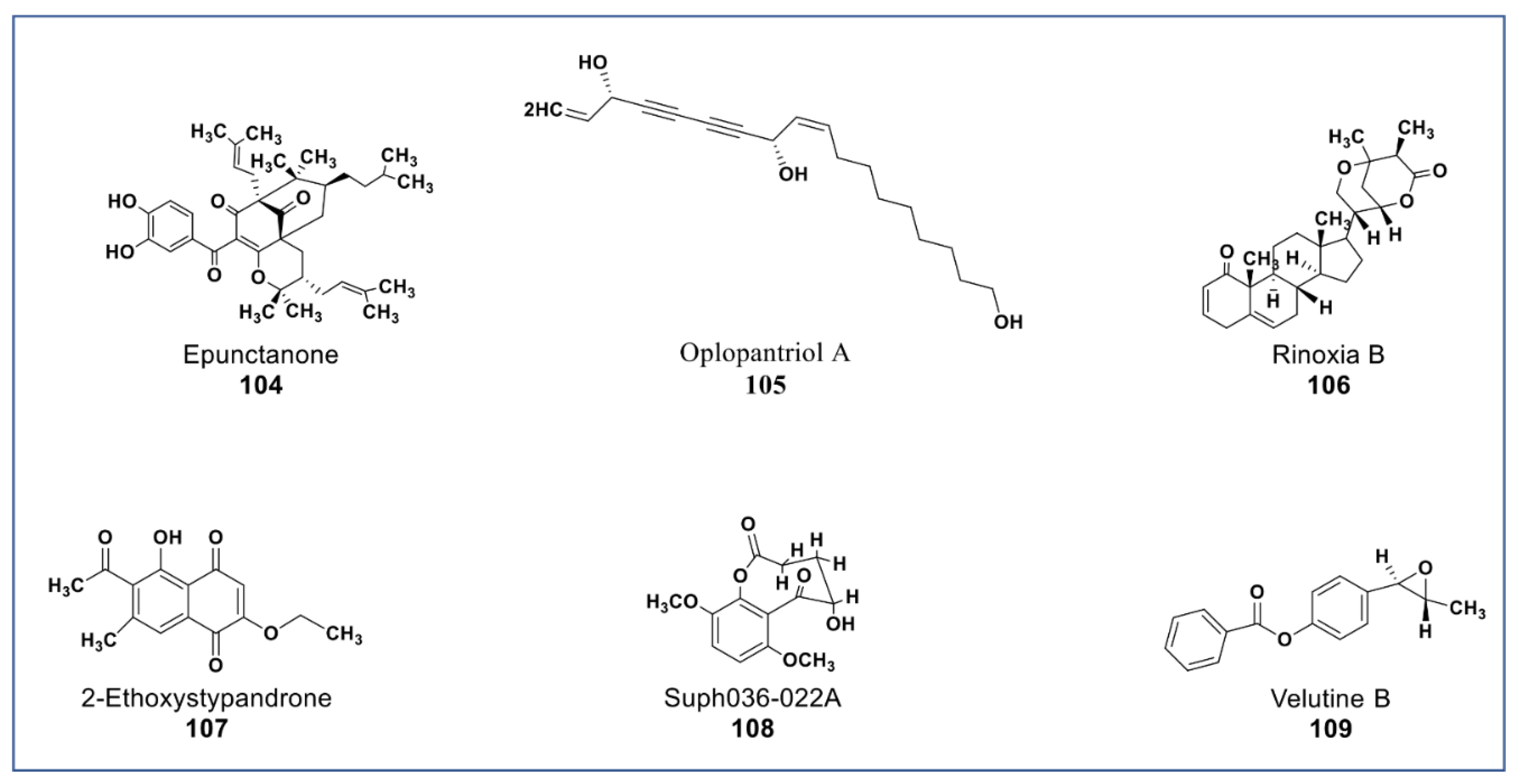
A novel antiproliferative phytochemical belonging to polyprenylated benzophenone (Compound 104, Figure 9) was isolated from Garcinia epunctata [145]. The compound showed anti-MDR effects with relative reversal (RR) values of 0.5, 0.73, and 0.86 in HCT116 (p53−/−), CEM/ADR5000, and U87MG.ΔEGFR glioblastoma cell lines, respectively, and a collateral resistance (CR) value of 0.22 in MDA-MB-231-BCRP cancer cell [146]. A novel polyene (compound 105, Figure 9) isolated from the root bark of Oplopanax horridus [147] was found to inhibit growth of HCT-116, MCF-7, and SW-480 cancer cells at <10 µM [148]. A new phytosterol (compound 106, Figure 9) belonging to the withanolides was recently isolated from Datura inoxia. This phytosterol was reported to inhibit the growth of HCT15 lung carcinoma at 4 µM [149]. A juglone analogue (compound 107, Figure 9) isolated from the root of Polygonum cuspidatum inhibited the growth of hepatocellular carcinoma (HCC) and HCC cancer stem cells via the blockage of the STAT3 signaling pathway [150]. New phytochemicals isolated from Brugmansia suaveolens (compounds 108 and 109, Figure 9) showed immunomodulatory potentials against PBMC-immunostimulated MCF7, A549, and HL-60 cell lines via enhanced IL-2 and IFN-γ secretions and IL-1β reduction [151,152].
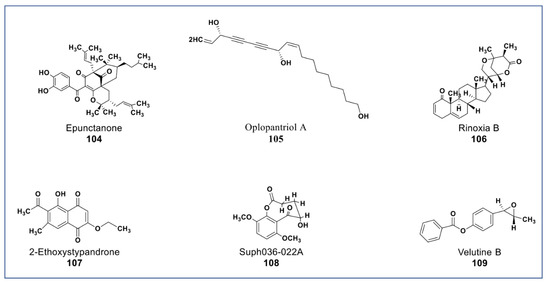
Figure 9.
New phytochemicals belonging to different chemical classes. Polyenes, phytosterols, and a juglone analogue from various plant sources (compounds 104 to 109).
Finally, polysaccharides from various plants have been reported to have anticancer effects due to their scavenging, antioxidant, and reducing ability, as well as their cytotoxicity [153,154]. A newly isolated polysaccharide from fractions of Meliato osendan water extract, named pMTPS-3, inhibited the growth of BGC-823 gastric cancer cells at 400 µg/mL [155]. Another novel polysaccharide, named APP3a, was isolated from Auricularia polytrichais. APP3a was reported to be a strong scavenger of free radicals, including hydroxyl, superoxide, and DPPH radicals, along with its strong reducing power [156].
10. Conclusions
In summary, phytochemicals in chemotherapy have always had a promising future. Many of the phytochemicals reported have, in one way or the other, laid the foundation for resolving the chemotherapy-related setbacks earlier mentioned. Some have shown selective and higher potency than some of the existing anticancer drugs; some work in synergy with existing anticancer drugs, increasing their effectiveness; and some reverse multiple-drug-resistance phenotypes (a worrying situation in chemotherapy). However, it must be said that new compounds are often declared as potential anticancer agents, although only tests with established cell lines are available. This initially only identifies the compounds as cytotoxic. There are usually no attempts to determine whether they really have the potential to become a cancer drug in vivo. More extensive investigations would have to be carried out much earlier, e.g., with xenografts in mice. However, since the effort is great, more advanced models would also have to be established in vitro to identify promising compounds at an early stage. The available information about the biological activities, the cellular targets, and the names of the sensitive and nonsensitive cell lines to the 109 compounds included in this review are summarized in Supplementary Table S1.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules27238307/s1, Supplementary Table S1: Chemical classes and biological activities of the phytochemicals.
Author Contributions
S.O.A. and W.S.N.A. performed the literature search and wrote the first draft of the manuscript; A.Y.B.-H.-A. revised the chemical structures; B.A.A.-D., F.S., M.A.M.A.-S. and Y.A.E. revised and edited the manuscript; M.J. helped in reviewing and editing the revised version of the manuscript; Y.A.E. reviewed and edited the revised version of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Nurgali, K.; Jagoe, R.T.; Abalo, R. Editorial: Adverse Effects of Cancer Chemotherapy: Anything New to Improve Tolerance and Reduce Sequelae? Front. Pharmacol. 2018, 9, 245. [Google Scholar] [CrossRef]
- Wang, C.; Wang, X.; Chen, Y.; Fang, Z. In-vitro photothermal therapy using plant extract polyphenols functionalized graphene sheets for treatment of lung cancer. J. Photochem. Photobiol. B Biol. 2020, 204, 111587. [Google Scholar] [CrossRef]
- Demain, A.L.; Vaishnav, P. Natural products for cancer chemotherapy. Microb. Biotechnol. 2011, 4, 687–699. [Google Scholar] [CrossRef]
- Crosbie, W.; Kamdar, H.; Belcher, J. A controlled trial of vinblastine sulphate in the treatment of cancer of the lung. Br. J. Dis. Chest 1966, 60, 28–35. [Google Scholar] [CrossRef]
- Lichtman, S.M.; Niedzwiecki, D.; Barcos, M.; Carlisle, T.L.; Cooper, M.R.; Johnson, J.L.; Peterson, B.A. Phase II study of infusional chemotherapy with doxorubicin, vincristine and etoposide plus cyclophosphamide and prednisone (I-CHOPE) in resistant diffuse aggressive non-Hodgkin’s lymphoma: CALGB 9255. Cancer and Leukemia Group B. Ann. Oncol. 2000, 11, 1141–1146. [Google Scholar] [CrossRef]
- Xiang, X.-S.; Su, Y.; Li, G.-L.; Ma, L.; Zhou, C.-S.; Ma, R.-F. Phase II Study of Preoperative Intra-Arterial Epirubicin, Etoposide, and Oxaliplatin Combined with Oral S-1 Chemotherapy for the Treatment of Borrmann Type 4 Gastric Cancer. J. Gastric Cancer 2020, 20, 395–407. [Google Scholar] [CrossRef]
- Ettinger, D.S.; Finkelstein, D.M.; Ritch, P.S.; Lincoln, S.T.; Blum, R.H. Study of either ifosfamide or teniposide compared to a standard chemotherapy for extensive disease small cell lung cancer: An Eastern Cooperative Oncology Group randomized study (E1588). Lung Cancer 2002, 37, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Lissoni, P.; Fugamalli, E.; Malugani, F.; Ardizzoia, A.; Secondino, S.; Tancini, G.; Gardani, G. Chemotherapy and Angiogenesis in Advanced Cancer: Vascular Endothelial Growth Factor (VEGF) Decline as Predictor of Disease Control during Taxol Therapy in Metastatic Breast Cancer. Int. J. Biol. Markers 2000, 15, 308–311. [Google Scholar] [CrossRef]
- Perry, M.C.; Ihde, D.C.; Herndon, J.E., 2nd; Grossbard, M.L.; Grethein, S.J.; Atkins, J.N.; Vokes, E.E.; Green, M.R. Paclitaxel/ifosfamide or navelbine/ifosfamide chemotherapy for advanced non-small cell lung cancer: CALGB 9532. Lung Cancer 2000, 28, 63–68. [Google Scholar] [CrossRef]
- Laurie, S.A.; Kris, M.G. Single-Agent Docetaxel (Taxotere) in the Treatment of Advanced Non–Small-Cell Lung Cancer: Clinical Concepts and Commentary. Clin. Lung Cancer 2000, 1 (Suppl. S1), S5–S9. [Google Scholar] [CrossRef] [PubMed]
- Sessa, C.; Wanders, J.; Roelvink, M.; Dombernowsky, P.; Nielsen, D.; Morant, R.; Drings, P.; Wissel, P.; Hanauske, A.R. Second-line treatment of small-cell lung cancer with the camptothecin-derivative GI147211: A study of the EORTC Early Clinical Studies Group (ECSG). Ann. Oncol. 2000, 11, 207–210. [Google Scholar] [CrossRef] [PubMed]
- Asbury, R.F.; Lipsitz, S.; Graham, D.; Falkson, C.I.; Baez, L.; Benson, A.B., 3rd. Treatment of squamous cell esophageal cancer with topotecan: An Eastern Cooperative Oncology Group Study (E2293). Am. J. Clin. Oncol. 2000, 23, 45–46. [Google Scholar] [CrossRef]
- Takimoto, C.H.; Morrison, G.; Harold, N.; Quinn, M.; Monahan, B.P.; Band, R.A.; Cottrell, J.; Guemei, A.; Llorens, V.; Hehman, H.; et al. Phase I and Pharmacologic Study of Irinotecan Administered as a 96-Hour Infusion Weekly to Adult Cancer Patients. J. Clin. Oncol. 2000, 18, 659–667. [Google Scholar] [CrossRef]
- Habtemariam, S.; Lentini, G. Plant-Derived Anticancer Agents: Lessons from the Pharmacology of Geniposide and Its Aglycone, Genipin. Biomedicines 2018, 6, 39. [Google Scholar] [CrossRef]
- Atanasov, A.G.; Waltenberger, B.; Pferschy-Wenzig, E.-M.; Linder, T.; Wawrosch, C.; Uhrin, P.; Temml, V.; Wang, L.; Schwaiger, S.; Heiss, E.H.; et al. Discovery and resupply of pharmacologically active plant-derived natural products: A review. Biotechnol. Adv. 2015, 33, 1582–1614. [Google Scholar] [CrossRef]
- Kuruppu, A.I.; Paranagama, P.; De Silva, R. Anticancer potential of natural products a review focusing on Sri Lankan plants. Front. Biosci. 2019, 11, 161–177. [Google Scholar] [CrossRef]
- Balunas, M.J.; Kinghorn, A.D. Drug discovery from medicinal plants. Life Sci. 2005, 78, 431–441. [Google Scholar] [CrossRef]
- Suffness, M.; Cordell, G.A. Antitumor alkaloids. In The Alkaloids: Chemistry and Pharmacology; Elsevier: Amsterdam, The Netherlands, 1985; pp. 1–355. [Google Scholar]
- Lahlou, M. The Success of Natural Products in Drug Discovery. Pharmacol. Pharm. 2013, 4, 17–31. [Google Scholar] [CrossRef]
- Shakya, A.K. Medicinal plants: Future source of new drugs. Int. J. Herb. Med. 2016, 4, 59–64. [Google Scholar]
- Katiyar, C.; Kanjilal, S.; Gupta, A.; Katiyar, S. Drug discovery from plant sources: An integrated approach. Ayu 2012, 33, 9–10. [Google Scholar] [CrossRef]
- Saxena, M.; Saxena, J.; Nema, R.; Singh, D.; Gupta, A. Phytochemistry of medicinal plants. J. Pharmacogn. 2013, 5, 1. [Google Scholar]
- Bernhoft, A.J.A.B. A Brief Review on Bioactive Compounds in Plants; The Norwegian Academy of Science and Letters: Oslo, Norway, 2010; pp. 11–17. [Google Scholar]
- You, J.S.; Jones, P.A. Cancer genetics and epigenetics: Two sides of the same coin? Cancer Cell 2012, 22, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Polakis, P. Wnt signaling and cancer. Genes Dev. 2000, 14, 1837–1851. [Google Scholar] [CrossRef] [PubMed]
- Toftgard, R. Hedgehog signalling in cancer. Cell Mol. Life Sci. 2000, 57, 1720–1731. [Google Scholar] [CrossRef] [PubMed]
- Jang, M.S.; Zlobin, A.; Kast, W.M.; Miele, L. Notch signaling as a target in multimodality cancer therapy. Curr. Opin. Mol. Ther. 2000, 2, 55–65. [Google Scholar]
- Liu, A.M.; Xu, Z.; Luk, J. An update on targeting Hippo-YAP signaling in liver cancer. Expert Opin. Ther. Targets 2012, 16, 243–247. [Google Scholar] [CrossRef]
- Du, W.; Hong, J.; Wang, Y.C.; Zhang, Y.J.; Wang, P.; Su, W.Y.; Lin, Y.W.; Lu, R.; Zou, W.P.; Xiong, H.; et al. Inhibition of JAK2/STAT3 signalling induces colorectal cancer cell apoptosis via mitochondrial pathway. J. Cell Mol. Med. 2012, 16, 1878–1888. [Google Scholar] [CrossRef]
- Ettl, T.; Schwarz-Furlan, S.; Haubner, F.; Müller, S.; Zenk, J.; Gosau, M.; Reichert, T.E.; Zeitler, K. The PI3K/AKT/mTOR signalling pathway is active in salivary gland cancer and implies different functions and prognoses depending on cell localisation. Oral Oncol. 2012, 48, 822–830. [Google Scholar] [CrossRef]
- Pópulo, H.; Lopes, J.M.; Soares, P. The mTOR Signalling Pathway in Human Cancer. Int. J. Mol. Sci. 2012, 13, 1886–1918. [Google Scholar] [CrossRef]
- Strillacci, A.; Valerii, M.C.; Sansone, P.; Caggiano, C.; Sgromo, A.; Vittori, L.; Fiorentino, M.; Poggioli, G.; Rizzello, F.; Campieri, M.; et al. Loss of miR-101 expression promotes Wnt/beta-catenin signalling pathway activation and malignancy in colon cancer cells. J. Pathol. 2013, 229, 379–389. [Google Scholar] [CrossRef]
- Yang, C.-L.; Liu, Y.-Y.; Ma, Y.-G.; Xue, Y.-X.; Liu, D.-G.; Ren, Y.; Liu, X.-B.; Li, Y.; Li, Z. Curcumin blocks small cell lung cancer cells migration, invasion, angiogenesis, cell cycle and neoplasia through janus kinase-stat3 signalling pathway. PLoS ONE 2012, 7, e37960. [Google Scholar] [CrossRef] [PubMed]
- Kontomanolis, E.N.; Koutras, A.; Syllaios, A.; Schizas, D.; Mastoraki, A.; Garmpis, N.; Diakosavvas, M.; Angelou, K.; Tsatsaris, G.; Pagkalos, A.; et al. Role of Oncogenes and Tumor-suppressor Genes in Carcinogenesis: A Review. Anticancer Res. 2020, 40, 6009–6015. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.-H.; Wu, C.-F.; Rajasekaran, N.; Shin, Y.K. Loss of Tumor Suppressor Gene Function in Human Cancer: An Overview. Cell. Physiol. Biochem. 2018, 51, 2647–2693. [Google Scholar] [CrossRef]
- Cheng, F.; Liu, C.; Jiang, J.; Lu, W.; Li, W.; Liu, G.; Zhou, W.-X.; Huang, J.; Tang, Y. Prediction of Drug-Target Interactions and Drug Repositioning via Network-Based Inference. PLoS Comput. Biol. 2012, 8, e1002503. [Google Scholar] [CrossRef]
- Schenone, M.; Dančík, V.; Wagner, B.K.; Clemons, P.A. Target identification and mechanism of action in chemical biology and drug discovery. Nat. Chem. Biol. 2013, 9, 232–240. [Google Scholar] [CrossRef] [PubMed]
- Buolamwini, J.K.; Adjei, A.A. Novel Anticancer Drug Protocols; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2003. [Google Scholar]
- Heiden, M.G.V. Targeting cancer metabolism: A therapeutic window opens. Nat. Rev. Drug Discov. 2011, 10, 671–684. [Google Scholar] [CrossRef] [PubMed]
- van Leeuwen, I.M. Cyclotherapy: Opening a therapeutic window in cancer treatment. Oncotarget 2012, 3, 596–600. [Google Scholar] [CrossRef] [PubMed]
- Fendt, S.-M. Is there a therapeutic window for metabolism-based cancer therapies? Front. Endocrinol. 2017, 8, 150. [Google Scholar] [CrossRef]
- Dandawate, P.R.; Subramaniam, D.; Jensen, R.A.; Anant, S. Targeting cancer stem cells and signaling pathways by phytochemicals: Novel approach for breast cancer therapy. Semin. Cancer Biol. 2016, 40–41, 192–208. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Meng, J.; Chen, S.; Wang, X.; Yin, S.; Zhang, Q.; Liu, H.; Qin, R.; Li, Z.; Zhong, W.; et al. YY1 Complex Promotes Quaking Expression via Super-Enhancer Binding during EMT of Hepatocellular Carcinoma. Cancer Res. 2019, 79, 1451–1464. [Google Scholar] [CrossRef]
- Liu, T.; Zuo, L.; Guo, D.; Chai, X.; Xu, J.; Cui, Z.; Wang, Z.; Hou, C. Ginsenoside Rg3 regulates DNA damage in non-small cell lung cancer cells by activating VRK1/P53BP1 pathway. Biomed Pharmacother. 2019, 120, 109483. [Google Scholar] [CrossRef] [PubMed]
- Geng, X.; Wang, F.; Tian, D.; Huang, L.; Streator, E.; Zhu, J.; Kurihara, H.; He, R.; Yao, X.; Zhang, Y.; et al. Cardiac glycosides inhibit cancer through Na/K-ATPase-dependent cell death induction. Biochem. Pharmacol. 2020, 182, 114226. [Google Scholar] [CrossRef]
- Tian, D.-M.; Qiao, J.; Bao, Y.-Z.; Liu, J.; Zhang, X.-K.; Sun, X.-L.; Zhang, Y.-W.; Yao, X.-S.; Tang, J.-S. Design and synthesis of biotinylated cardiac glycosides for probing Nur77 protein inducting pathway. Bioorganic Med. Chem. Lett. 2019, 29, 707–712. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zhang, Z.-X.; Hu, H.; Li, D.; Qiu, G.; Hu, X.; He, X. Novel indole C-glycosides from Isatis indigotica and their potential cytotoxic activity. Fitoterapia 2011, 82, 288–292. [Google Scholar] [CrossRef] [PubMed]
- Zilla, M.K.; Nayak, D.; Amin, H.; Nalli, Y.; Rah, B.; Chakraborty, S.; Kitchlu, S.; Goswami, A.; Ali, A. 4′-Demethyl-deoxypodophyllotoxin glucoside isolated from Podophyllum hexandrum exhibits potential anticancer activities by altering Chk-2 signaling pathway in MCF-7 breast cancer cells. Chem. Interactions 2014, 224, 100–107. [Google Scholar] [CrossRef]
- Hussain, H.; Raees, M.A.; Rehman, N.U.; Al-Rawahi, A.; Csuk, R.; Khan, H.Y.; Abbas, G.; Al-Broumi, M.A.; Green, I.R.; Elyassi, A.; et al. Nizwaside: A new anticancer pregnane glycoside from the sap of Desmidorchis flava. Arch. Pharmacal Res. 2015, 38, 2137–2142. [Google Scholar] [CrossRef] [PubMed]
- Raees, M.A.; Hussain, H.; Rehman, N.U.; Khan, H.Y.; Abbas, G.; Al-Rawahi, A.; Elyassi, A.; Al-Amri, I.S.; Green, I.R.; Al-Broumi, M.A.; et al. Desmiflavasides A and B: Two new bioactive pregnane glycosides from the sap of Desmidorchis flava. Phytochem. Lett. 2015, 12, 153–157. [Google Scholar] [CrossRef]
- Raees, M.A.; Hussain, H.; Al-Rawahi, A.; Csuk, R.; Muhammad, S.A.; Khan, H.Y.; Rehman, N.U.; Abbas, G.; Al-Broumi, M.A.; Green, I.R.; et al. Anti-proliferative and computational studies of two new pregnane glycosides from Desmidorchis flava. Bioorganic Chem. 2016, 67, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.R.; Tian, H.Y.; Tan, Y.F.; Chung, T.Y.; Sun, X.H.; Xia, X.; Ye, W.C.; Middleton, D.A.; Fedosova, N.; Esmann, M.; et al. Structures, chemotaxonomic significance, cytotoxic and Na+,K+-ATPase inhibitory activities of new cardenolides from Asclepias curassavica. Org. Biomol. Chem. 2014, 12, 8919–8929. [Google Scholar] [CrossRef]
- Chen, G.-Q.; Dai, W.; Xu, H.-Z.; Li, D. Pharicin a, a Novel Natural Compound That Induces Mitotic Arrest and Catastrophe of Cancer Cells by Perturbing Microtubule Dynamics and the Spindle Checkpoint. Blood 2008, 112, 4723. [Google Scholar] [CrossRef]
- Li, J.-Z.; Qing, C.; Chen, C.-X.; Hao, X.-J.; Liu, H.-Y. Cytotoxicity of cardenolides and cardenolide glycosides from Asclepias curassavica. Bioorganic Med. Chem. Lett. 2009, 19, 1956–1959. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Sun, H.; Ye, Y.; Chen, F.; Tu, J.; Pan, Y. Three New Immunomodulating C21-Steroidal Glycosides from the Stems of Stephanotis mucronata. Chem. Biodivers. 2005, 2, 1701–1711. [Google Scholar] [CrossRef] [PubMed]
- Yuan, W.-Q.; Zhang, R.-R.; Wang, J.; Ma, Y.; Li, W.-X.; Jiang, R.W.; Cai, S.-H. Asclepiasterol, a novel C21 steroidal glycoside derived from Asclepias curassavica, reverses tumor multidrug resistance by down-regulating P-glycoprotein expression. Oncotarget 2016, 7, 31466–31483. [Google Scholar] [CrossRef] [PubMed]
- Yue, S.J.; Qu, C.; Zhang, P.X.; Tang, Y.P.; Jin, Y.; Jiang, J.S.; Yang, Y.N.; Zhang, P.C.; Duan, J.A. Carthorquinosides A and B, Quinochalcone C-Glycosides with Diverse Dimeric Skeletons from Carthamus tinctorius. J. Nat. Prod. 2016, 79, 2644–2651. [Google Scholar] [CrossRef] [PubMed]
- Xue, R.; Han, N.; Xia, M.; Ye, C.; Hao, Z.; Wang, L.; Wang, Y.; Yang, J.; Saiki, I.; Yin, J. TXA9, a cardiac glycoside from Streptocaulon juventas, exerts a potent anti-tumor activity against human non-small cell lung cancer cells in vitro and in vivo. Steroids 2015, 94, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Chirumbolo, S. Plant polyphenolic compounds as potential antimicrobial drugs. J. Med. Microbiol. 2011, 60 Pt 10, 1562–1563. [Google Scholar] [CrossRef]
- Oruganti, L.; Meriga, B. Plant Polyphenolic Compounds Potentiates Therapeutic Efficiency of Anticancer Chemotherapeutic Drugs: A Review. Endocr. Metab. Immune. Disord. Drug Targets. 2021, 21, 246–252. [Google Scholar] [CrossRef]
- Huang, W.-Y.; Cai, Y.-Z.; Zhang, Y. Natural Phenolic Compounds from Medicinal Herbs and Dietary Plants: Potential Use for Cancer Prevention. Nutr. Cancer 2009, 62, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Yahfoufi, N.; Alsadi, N.; Jambi, M.; Matar, C. The Immunomodulatory and Anti-Inflammatory Role of Polyphenols. Nutrients 2018, 10, 1618. [Google Scholar] [CrossRef]
- Sun, L.-R.; Zhou, W.; Zhang, H.-M.; Guo, Q.-S.; Yang, W.; Li, B.-J.; Sun, Z.-H.; Gao, S.-H.; Cui, R.-J. Modulation of Multiple Signaling Pathways of the Plant-Derived Natural Products in Cancer. Front. Oncol. 2019, 9, 1153. [Google Scholar] [CrossRef]
- Arbizu-Berrocal, S.H.; Kim, H.; Fang, C.; Krenek, K.A.; Talcott, S.T.; Mertens-Talcott, S.U. Polyphenols from mango (Mangifera indica L.) modulate PI3K/AKT/mTOR-associated micro-RNAs and reduce inflammation in non-cancer and induce cell death in breast cancer cells. J. Funct. Foods 2019, 55, 9–16. [Google Scholar] [CrossRef]
- Krajka-Kuźniak, V.; Cykowiak, M.; Szaefer, H.; Kleszcz, R.; Baer-Dubowska, W. Combination of xanthohumol and phenethyl isothiocyanate inhibits NF-κB and activates Nrf2 in pancreatic cancer cells. Toxicol. In Vitro 2020, 65, 104799. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.; Gwak, J.; Park, S.; Yang, C.S. Green tea polyphenol EGCG suppresses W nt/β-catenin signaling by promoting GSK-3β-and PP2A-independent β-catenin phosphorylation/degradation. Biofactors 2014, 40, 586–595. [Google Scholar] [CrossRef] [PubMed]
- Ohnuma, T.; Matsumoto, T.; Itoi, A.; Kawana, A.; Nishiyama, T.; Ogura, K.; Hiratsuka, A. Enhanced sensitivity of A549 cells to the cytotoxic action of anticancer drugs via suppression of Nrf2 by procyanidins from Cinnamomi Cortex extract. Biochem. Biophys. Res. Commun. 2011, 413, 623–629. [Google Scholar] [CrossRef] [PubMed]
- AlAjmi, M.F.; Rehman, M.T.; Hussain, A.; Rather, G.M.J. Pharmacoinformatics approach for the identification of Polo-like kinase-1 inhibitors from natural sources as anti-cancer agents. Int. J. Biol. Macromol. 2018, 116, 173–181. [Google Scholar] [CrossRef]
- Fang, W.; Liu, S.; Nie, Y. Anticancer Activity of Chamaejasmine: Effect on Tubulin Protein. Molecules 2011, 16, 6243–6254. [Google Scholar] [CrossRef]
- Woo, S.U.; Jang, H.R.; Chin, Y.W.; Yim, H. 7-O-Methylwogonin from Scutellaria baicalensis Disturbs Mitotic Progression by Inhibiting Plk1 Activity in Hep3B Cells. Planta Med. 2019, 85, 217–224. [Google Scholar] [CrossRef]
- Touil, Y.S.; Fellous, A.; Scherman, D.; Chabot, G.G. Flavonoid-Induced Morphological Modifications of Endothelial Cells through Microtubule Stabilization. Nutr. Cancer 2009, 61, 310–321. [Google Scholar] [CrossRef]
- Lim, C.-K.; Hemaroopini, S.; Say, Y.-H.; Jong, V.Y.-M. Cytotoxic Compounds from the Stem Bark of Calophyllum soulattri. Nat. Prod. Commun. 2017, 12, 1934578X1701200922. [Google Scholar] [CrossRef]
- Li, K.K.; Liu, C.L.; Tam, J.C.; Kwok, H.F.; Lau, C.P.; Leung, P.C.; Ko, C.H.; Ye, C.X. In vitro and in vivo mechanistic study of a novel proanthocyanidin, GC-(4→8)-GCG from cocoa tea (Camellia ptilophylla) in antiangiogenesis. J. Nutr. Biochem. 2014, 25, 319–328. [Google Scholar] [CrossRef]
- Haggag, E.G.; Kamal, A.M.; Abdelhady, M.I.S.; El-Sayed, M.M.; El-Wakil, E.A.; Abd-El-Hamed, S.S. Antioxidant and cytotoxic activity of polyphenolic compounds isolated from the leaves of Leucenia leucocephala. Pharm. Biol. 2011, 49, 1103–1113. [Google Scholar] [CrossRef] [PubMed]
- Rub, R.; Pati, M.; Siddiqui, A.; Moghe, A.; Shaikh, N. Characterization of anticancer principles of Celosia argentea (Amaranthaceae). Pharmacogn. Res. 2016, 8, 97–104. [Google Scholar] [CrossRef]
- Ge, L.; Li, J.; Wan, H.; Zhang, K.; Wu, W.; Zou, X.; Wu, S.; Zhou, B.; Tian, J.; Zeng, X. Novel flavonoids from Lonicera japonica flower buds and validation of their anti-hepatoma and hepatoprotective activity in vitro studies. Ind. Crops Prod. 2018, 125, 114–122. [Google Scholar] [CrossRef]
- Gong, G.; Chen, H.; Kam, H.; Chan, G.; Tang, Y.X.; Wu, M.; Tan, H.; Tse, Y.C.; Xu, H.X.; Lee, S.M. In Vivo Screening of Xanthones from Garcinia oligantha Identified Oliganthin H as a Novel Natural Inhibitor of Convulsions. J. Nat. Prod. 2020, 83, 3706–3716. [Google Scholar] [CrossRef] [PubMed]
- Na, Z.; Bin Hu, H.; Fan, Q.F. A novel caged-prenylxanthone from Garcinia bracteata. Chin. Chem. Lett. 2010, 21, 443–445. [Google Scholar] [CrossRef]
- Tao, S.-J.; Guan, S.-H.; Li, X.-N.; Guo, D.-A. A Highly Rearranged Pentaprenylxanthonoid from the Resin of Garcinia hanburyi. Helvetica Chim. Acta 2010, 93, 1395–1400. [Google Scholar] [CrossRef]
- Xu, D.; Lao, Y.; Xu, N.; Hu, H.; Fu, W.; Tan, H.; Gu, Y.; Song, Z.; Cao, P.; Xu, H. Identification and Characterization of Anticancer Compounds Targeting Apoptosis and Autophagy from Chinese Native Garcinia Species. Planta Medica 2015, 81, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Na, Z.; Hu, H.-B.; Xu, Y.-K. Cytotoxic caged xanthones from the fruits of Garcinia bracteata. Chem. Nat. Compd. 2013, 49, 505–506. [Google Scholar] [CrossRef]
- Zheng, Z.; Tan, J.; Zhang, J.; Wu, M.; Chen, G.; Li, Z.; Shi, X.; Fu, W.; Zhou, H.; Lao, Y.J.F. The natural compound neobractatin inhibits cell proliferation mainly by regulating the RNA binding protein CELF6. Food Funct. 2022; advance article. [Google Scholar] [CrossRef]
- Zhang, J.; Zheng, Z.; Wu, M.; Zhang, L.; Wang, J.; Fu, W.; Xu, N.; Zhao, Z.; Lao, Y.; Xu, H. The natural compound neobractatin inhibits tumor metastasis by upregulating the RNA-binding-protein MBNL2. Cell Death Dis. 2019, 10, 554. [Google Scholar] [CrossRef]
- Tang, Y.-X.; Fu, W.-W.; Xi, Z.-C.; Yang, J.-L.; Zheng, C.-W.; Lu, Y.; Shen, Z.-W.; Xu, H.-X. Xanthone derivatives from the leaves of Garcinia oligantha. Eur. J. Med. Chem. 2019, 181, 111536. [Google Scholar] [CrossRef]
- Tang, Y.-X.; Fu, W.-W.; Wu, R.; Tan, H.-S.; Shen, Z.-W.; Xu, H.-X. Bioassay-Guided Isolation of Prenylated Xanthone Derivatives from the Leaves of Garcinia oligantha. J. Nat. Prod. 2016, 79, 1752–1761. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Fu, W.; Xiang, Q.; Tang, Y.; Wu, R.; Zheng, C.; Lu, Y.; Zhou, H.; Xu, H. Cytotoxic xanthone derivatives from the twigs of Garcinia oligantha. Phytochemistry 2020, 174, 112329. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.-M.; Yu, T.; Cui, M.-Z.; Pu, J.-X.; Du, X.; Han, Q.-B.; Hu, Q.-F.; Liu, T.-C.; Luo, K.Q.; Xu, H.-X. Identification and evaluation of apoptotic compounds from Garcinia oligantha. Bioorganic Med. Chem. Lett. 2012, 22, 2350–2353. [Google Scholar] [CrossRef]
- Mah, S.H.; Ee, G.C.L.; Teh, S.S.; Rahmani, M.; Lim, Y.M.; Go, R. Phylattrin, a New Cytotoxic Xanthone from Calophyllum soulattri. Molecules 2012, 17, 8303–8311. [Google Scholar] [CrossRef]
- Lee, H.J.; Jue, S.S.; Kang, S.K.; Bae, W.J.; Kim, Y.C.; Kim, E.C. Cudraxanthone H Induces Growth Inhibition and Apoptosis in Oral Cancer Cells via NF-kappaB and PIN1 Pathways. Am. J. Chin. Med. 2015, 43, 1439–1452. [Google Scholar] [CrossRef]
- Mathioudaki, A.; Berzesta, A.; Kypriotakis, Z.; Skaltsa, H.; Heilmann, J. Phenolic metabolites from Hypericum kelleri Bald. an endemic species of Crete (Greece). Phytochemistry 2018, 146, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Bounthanh, C.; Bergmann, C.; Beck, J.P.; Haag-Berrurier, M.; Anton, R. Valepotriates, a New Class of Cytotoxic and Antitumor Agents. Planta Medica 1981, 41, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Chen, T.; Fu, P.; Ye, J.; Yang, X.-W.; Shan, L.; Li, H.-L.; Liu, R.-H.; Shen, Y.-H.; Xu, X.-K. Three decomposition products of valepotriates from Valeriana jatamansi and their cytotoxic activity. J. Asian Nat. Prod. Res. 2015, 17, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Zhang, Z.X.; Chen, T.; Ye, J.; Dai, W.X.; Shan, L.; Su, J.; Shen, Y.H.; Li, H.L.; Liu, R.H.; et al. Characterization of chlorinated valepotriates from Valeriana jatamansi. Phytochemistry 2013, 85, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.-J.; Qiu, X.-Q.; Zhu, J.-Y.; Ma, X.-Q.; Lin, B.; Zheng, C.-J.; Qin, L.-P. Two New Iridoids from the Root and Rhizome of Valeriana jatamansi Jones. Helvetica Chim. Acta 2014, 97, 722–726. [Google Scholar] [CrossRef]
- Yang, B.; Zhu, R.; Tian, S.; Wang, Y.; Lou, S.; Zhao, H. Jatamanvaltrate P induces cell cycle arrest, apoptosis and autophagy in human breast cancer cells in vitro and in vivo. Biomed. Pharmacother. 2017, 89, 1027–1036. [Google Scholar] [CrossRef]
- Liu, Z.-H.; Hou, B.; Yang, L.; Ma, R.-J.; Li, J.-Y.; Hu, J.-M.; Zhou, J. Iridoids and bis-iridoids from Patrinia scabiosaefolia. RSC Adv. 2017, 7, 24940–24949. [Google Scholar] [CrossRef]
- Mansoori, B.; Mohammadi, A.; Davudian, S.; Shirjang, S.; Baradaran, B. The Different Mechanisms of Cancer Drug Resistance: A Brief Review. Adv. Pharm. Bull. 2017, 7, 339–348. [Google Scholar] [CrossRef]
- Wang, J.-Q.; Yang, Y.; Cai, C.-Y.; Teng, Q.-X.; Cui, Q.; Lin, J.; Assaraf, Y.G.; Chen, Z.-S. Multidrug resistance proteins (MRPs): Structure, function and the overcoming of cancer multidrug resistance. Drug Resist. Updat. 2021, 54, 100743. [Google Scholar] [CrossRef] [PubMed]
- Gradilone, A.; Raimondi, C.; Naso, G.; Silvestri, I.; Repetto, L.; Palazzo, A.; Gianni, W.; Frati, L.; Cortesi, E.; Gazzaniga, P. How circulating tumor cells escape from multidrug resistance: Translating molecular mechanisms in metastatic breast cancer treatment. Am. J. Clin. Oncol. 2011, 34, 625–627. [Google Scholar] [CrossRef]
- Wang, S.; Qiu, J.; Shi, Z.; Wang, Y.; Chen, M. Nanoscale drug delivery for taxanes based on the mechanism of multidrug resistance of cancer. Biotechnol. Adv. 2015, 33, 224–241. [Google Scholar] [CrossRef]
- Zhao, H.; Yu, Z.; Zhao, L.; He, M.; Ren, J.; Wu, H.; Chen, Q.; Yao, W.; Wei, M. HDAC2 overexpression is a poor prognostic factor of breast cancer patients with increased multidrug resistance-associated protein expression who received anthracyclines therapy. Jpn. J. Clin. Oncol. 2016, 46, 893–902. [Google Scholar] [CrossRef]
- Attaoua, C.; Vincent, L.-A.; Jaoued, A.A.; Hadj-Kaddour, K.; Baï, Q.; De Vos, J.; Vian, L.; Cuq, P. Differential involvement of glutathione S-transferase mu 1 and multidrug resistance protein 1 in melanoma acquired resistance to vinca alkaloids. Fundam. Clin. Pharmacol. 2015, 29, 62–71. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, T.C.; Ozols, R.F.; Dabrow, M.B. Multidrug resistance to alkylating agents and platinum compounds: State of our knowledge. Oncology 1990, 4, 101–106, discussion 106, 109. [Google Scholar]
- Amerigos Daddy, J.C.K.; Chen, M.; Raza, F.; Xiao, Y.; Su, Z.; Ping, Q. Co-Encapsulation of Mitoxantrone and beta-Elemene in Solid Lipid Nanoparticles to Overcome Multidrug Resistance in Leukemia. Pharmaceutics 2020, 12, 191. [Google Scholar] [CrossRef] [PubMed]
- Dietel, M.; Boss, H.; Reymann, A.; Pest, S.; Seidel, A. In vivo reversibility of multidrug resistance by the MDR-modulator dexniguldipine (niguldipine derivative B859-35) and by verapamil. J. Exp. Ther. Oncol. 1996, 1, 23–29. [Google Scholar] [PubMed]
- Pawarode, A.; Shukla, S.; Minderman, H.; Fricke, S.M.; Pinder, E.M.; O’Loughlin, K.L.; Ambudkar, S.V.; Baer, M.R. Differential effects of the immunosuppressive agents cyclosporin A, tacrolimus and sirolimus on drug transport by multidrug resistance proteins. Cancer Chemother. Pharmacol. 2007, 60, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Pea, F.; Damiani, D.; Michieli, M.; Ermacora, A.; Baraldo, M.; Russo, D.; Fanin, R.; Baccarani, M.; Furlanut, M. Multidrug resistance modulation in vivo: The effect of cyclosporin A alone or with dexverapamil on idarubicin pharmacokinetics in acute leukemia. Eur. J. Clin. Pharmacol. 1999, 55, 361–368. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Zhou, S.; Li, Y.; Parshad, B.; Li, W.; Haag, R. Novel dendritic polyglycerol-conjugated, mesoporous silica-based targeting nanocarriers for co-delivery of doxorubicin and tariquidar to overcome multidrug resistance in breast cancer stem cells. J. Control. Release 2021, 330, 1106–1117. [Google Scholar] [CrossRef]
- Abu Ajaj, K.; Graeser, R.; Kratz, F. Zosuquidar and an albumin-binding prodrug of zosuquidar reverse multidrug resistance in breast cancer cells of doxorubicin and an albumin-binding prodrug of doxorubicin. Breast Cancer Res. Treat. 2012, 134, 117–129. [Google Scholar] [CrossRef]
- Krishna, R.; Mayer, L.D. Multidrug resistance (MDR) in cancer: Mechanisms, reversal using modulators of MDR and the role of MDR modulators in influencing the pharmacokinetics of anticancer drugs. Eur. J. Pharm. Sci. 2000, 11, 265–283. [Google Scholar] [CrossRef]
- Reis, M.A.; André, V.; Duarte, M.T.; Lage, H.; Ferreira, M.-J.U. 12,17-Cyclojatrophane and Jatrophane Constituents of Euphorbia welwitschii. J. Nat. Prod. 2015, 78, 2684–2690. [Google Scholar] [CrossRef]
- Reis, M.A.; Ahmed, O.B.; Spengler, G.; Molnár, J.; Lage, H.; Ferreira, M.-J.U. Jatrophane diterpenes and cancer multidrug resistance–ABCB1 efflux modulation and selective cell death induction. Phytomedicine 2016, 23, 968–978. [Google Scholar] [CrossRef]
- Hu, R.; Gao, J.; Rozimamat, R.; Aisa, H.A. Jatrophane diterpenoids from Euphorbia sororia as potent modulators against P-glycoprotein-based multidrug resistance. Eur. J. Med. Chem. 2018, 146, 157–170. [Google Scholar] [CrossRef] [PubMed]
- Aljančić, I.S.; Pešić, M.; Milosavljević, S.M.; Todorović, N.M.; Jadranin, M.; Milosavljević, G.; Povrenović, D.; Banković, J.; Tanić, N.; Marković, I.D.; et al. Isolation and Biological Evaluation of Jatrophane Diterpenoids from Euphorbia dendroides. J. Nat. Prod. 2011, 74, 1613–1620. [Google Scholar] [CrossRef]
- Huang, Y.; Aisa, H.A. Jatrophane diterpenoids from Fructus Euphorbia sororia. Phytochem. Lett. 2010, 3, 176–180. [Google Scholar] [CrossRef]
- Lu, D.; Liu, Y.; Aisa, H.A. Jatrophane diterpenoid esters from Euphorbia sororia serving as multidrug resistance reversal agents. Fitoterapia 2014, 92, 244–251. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.-Z.; Huang, Y.; Wu, Y.-L.; Zhao, Y.; Xiao, W.-L.; Lin, Q.-S.; Sun, H.-D.; Dai, W.; Chen, G.-Q. Pharicin A, a novel natural ent-kaurene diterpenoid, induces mitotic arrest and mitotic catastrophe of cancer cells by interfering with BubR1 function. Cell Cycle 2010, 9, 2897–2907. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Liu, C.; Huang, R.-Z.; Chen, H.-F.; Liao, Z.-X.; Sun, J.-Y.; Xia, X.-K.; Wang, F.-X. Three new C-27-carboxylated-lupane-triterpenoid derivatives from Potentilla discolor Bunge and their in vitro antitumor activities. PLoS ONE 2017, 12, e0175502. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, X.-L.; Qin, F.; Huang, R.-Z.; Liang, D.; Wang, C.-G.; Wang, H.-S.; Liao, Z.-X. NF-κB inhibitory and cytotoxic activities of hexacyclic triterpene acid constituents from Glechoma longituba. Phytomedicine 2019, 63, 153037. [Google Scholar] [CrossRef] [PubMed]
- Soliman, S.; Hamoda, A.M.; El-Shorbagi, A.-N.A.; El-Keblawy, A.A. Novel betulin derivative is responsible for the anticancer folk use of Ziziphus spina-christi from the hot environmental habitat of UAE. J. Ethnopharmacol. 2019, 231, 403–408. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.-J.; Bao, J.-L.; Chen, X.-P.; Huang, M.; Wang, Y.-T. Alkaloids Isolated from Natural Herbs as the Anticancer Agents. Evid.-Based Complement. Altern. Med. 2012, 2012, 485042. [Google Scholar] [CrossRef] [PubMed]
- Rowinsky, E.K.; Cazenave, L.A.; Donehower, R.C. Taxol: A Novel Investigational Antimicrotubule Agent. JNCI J. Natl. Cancer Inst. 1990, 82, 1247–1259. [Google Scholar] [CrossRef]
- Culvenor, C. Tumor-Inhibitory Activity of Pyrrolizidine Alkaloids. J. Pharm. Sci. 1968, 57, 1112–1117. [Google Scholar] [CrossRef]
- Wall, M.E. Camptothecin and taxol: Discovery to clinic. Med. Res. Rev. 1998, 18, 299–314. [Google Scholar] [CrossRef]
- Shinkre, B.A.; Raisch, K.; Fan, L.; Velu, S.E. Analogs of the marine alkaloid makaluvamines: Synthesis, topoisomerase II inhibition, and anticancer activity. Bioorganic Med. Chem. Lett. 2007, 17, 2890–2893. [Google Scholar] [CrossRef] [PubMed]
- Kaur, R.; Arora, S. Alkaloids-important therapeutic secondary metabolites of plant origin. J. Crit. Rev. 2015, 2, 1–8. [Google Scholar]
- Guilbaud, N.; Léonce, S.; Tillequin, F.; Koch, M.; Hickman, J.A.; Pierré, A. Acronycine derivatives as promising antitumor agents. Anticancer. Drugs 2002, 13, 445–449. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, J.; Wang, H.; Ye, W. Novel taxane derivatives from Taxus wallichiana with high anticancer potency on tumor cells. Chem. Biol. Drug Des. 2016, 88, 556–561. [Google Scholar] [CrossRef] [PubMed]
- Wellington, K.W. Understanding cancer and the anticancer activities of naphthoquinones—A review. RSC Adv. 2015, 5, 20309–20338. [Google Scholar] [CrossRef]
- Binoy, A.; Nedungadi, D.; Katiyar, N.; Bose, C.; Shankarappa, S.A.; Nair, B.G.; Mishra, N. Plumbagin induces paraptosis in cancer cells by disrupting the sulfhydryl homeostasis and proteasomal function. J. Chemico.-Biol. Interact. 2019, 310, 108733. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wang, P.; Xue, Y.-X.; Li, Z.; Qu, C.-B.; Liu, Y.-H. Enhanced antitumor effect of shikonin by inhibiting Endoplasmic Reticulum Stress via JNK/c-Jun pathway in human glioblastoma stem cells. Biochem. Biophys. Res. Commun. 2015, 466, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Rahman, S.; Archana, A.; Dutta, D.; Kumar, V.; Kim, J.; Jan, A.T.; Minakshi, R. The onus of cannabinoids in interrupting the molecular odyssey of breast cancer: A critical perspective on UPR(ER) and beyond. Saudi Pharm. J. 2019, 27, 437–445. [Google Scholar] [CrossRef] [PubMed]
- Eugene, S.P.; Reddy, V.S.; Trinath, J. Endoplasmic Reticulum Stress and Intestinal Inflammation: A Perilous Union. Front. Immunol. 2020, 11, 543022. [Google Scholar] [CrossRef] [PubMed]
- Lien, J.-C.; Huang, C.-C.; Lu, T.-J.; Tseng, C.-H.; Sung, P.-J.; Lee, H.-Z.; Bao, B.-Y.; Kuo, Y.-H. Naphthoquinone Derivative PPE8 Induces Endoplasmic Reticulum Stress in p53 Null H1299 Cells. Oxidative Med. Cell. Longev. 2015, 2015, 453679. [Google Scholar] [CrossRef]
- Cao, H.-H.; Liu, D.-Y.; Lai, Y.-C.; Chen, Y.-Y.; Yu, L.-Z.; Shao, M.; Liu, J.-S. Inhibition of the STAT3 Signaling Pathway Contributes to the Anti-Melanoma Activities of Shikonin. Front. Pharmacol. 2020, 11, 748. [Google Scholar] [CrossRef] [PubMed]
- Dwivedi, A.R.; Thakur, A.; Kumar, V.; Skvortsova, I.; Kumar, V. Targeting Cancer Stem Cells Pathways for the Effective Treatment of Cancer. Curr. Drug Targets 2020, 21, 258–278. [Google Scholar] [CrossRef] [PubMed]
- Pradubyat, N.; Sakunrangsit, N.; Mutirangura, A.; Ketchart, W. NADPH: Quinone oxidoreductase 1 (NQO1) mediated anti-cancer effects of plumbagin in endocrine resistant MCF7 breast cancer cells. Phytomedicine 2019, 66, 153133. [Google Scholar] [CrossRef] [PubMed]
- Gopinath, P.; Mahammed, A.; Ohayon, S.; Gross, Z.; Brik, A. Understanding and predicting the potency of ROS-based enzyme inhibitors, exemplified by naphthoquinones and ubiquitin specific protease-2. Chem. Sci. 2016, 7, 7079–7086. [Google Scholar] [CrossRef] [PubMed]
- Sevimli-Gur, C.; Akgun, I.H.; Deliloglu-Gurhan, I.; Korkmaz, K.S.; Bedir, E. Cytotoxic naphthoquinones from Alkanna cappadocica (perpendicular). J. Nat. Prod. 2010, 73, 860–864. [Google Scholar] [CrossRef]
- Rasol, N.E.; Ahmad, F.B.; Lim, X.-Y.; Chung, F.F.-L.; Leong, C.-O.; Mai, C.-W.; Bihud, N.V.; Zaki, H.M.; Ismail, N.H. Cytotoxic lactam and naphthoquinone alkaloids from roots of Goniothalamus lanceolatus Miq. Phytochem. Lett. 2018, 24, 51–55. [Google Scholar] [CrossRef]
- Ye, H.; Fu, A.; Wu, W.; Li, Y.; Wang, G.; Tang, M.; Li, S.; He, S.; Zhong, S.; Lai, H.; et al. Cytotoxic and apoptotic effects of constituents from Millettia pachycarpa Benth. Fitoterapia 2012, 83, 1402–1408. [Google Scholar] [CrossRef]
- Yan, J.; Zhuang, Q.; Li, Z.; Xiong, Y.; He, M.; Kang, C.; Zhang, Q.; Han, L.; Liang, E.; Liu, H.; et al. MIL-1, a novel antitumor agent derived from natural product millepachine, acts as tubulin polymerization inhibitor for the treatment of hepatocellular carcinoma. Eur. J. Pharmacol. 2021, 898, 173975. [Google Scholar] [CrossRef] [PubMed]
- An, B.; Zhang, S.; Yan, J.; Huang, L.; Li, X. Synthesis, in vitro and in vivo evaluation of new hybrids of millepachine and phenstatin as potent tubulin polymerization inhibitors. Org. Biomol. Chem. 2016, 15, 852–862. [Google Scholar] [CrossRef]
- Aljančić, I.S.; Vučković, I.; Jadranin, M.; Pešić, M.; Đorđević, I.; Podolski-Renić, A.; Stojković, S.; Menković, N.; Vajs, V.E.; Milosavljević, S.M. Two structurally distinct chalcone dimers from Helichrysum zivojinii and their activities in cancer cell lines. Phytochemistry 2014, 98, 190–196. [Google Scholar] [CrossRef]
- Fotso, G.W.; Ntumy, A.N.; Ngachussi, E.; Dube, M.; Mapitse, R.; Kapche, G.D.; Andrae-Marobela, K.; Ngadjui, B.T.; Abegaz, B.M. Epunctanone, a new benzophenone, and further secondary metabolites from Garcinia epunctata Stapf (Guttiferae). Helv. Chim. Acta 2014, 97, 957–964. [Google Scholar] [CrossRef]
- Mbaveng, A.T.; Fotso, G.W.; Ngnintedo, D.; Kuete, V.; Ngadjui, B.T.; Keumedjio, F.; Andrae-Marobela, K.; Efferth, T. Cytotoxicity of epunctanone and four other phytochemicals isolated from the medicinal plants Garcinia epunctata and Ptycholobium contortum towards multi-factorial drug resistant cancer cells. Phytomedicine 2018, 48, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.-H.; Zhang, Q.-W.; Wang, C.-Z.; Yuan, C.-S.; Li, S.-P. Isolation and Identification of Two New Polyynes from a North American Ethnic Medicinal Plant—Oplopanax horridus (Smith) Miq. Molecules 2010, 15, 1089–1096. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.-Z.; Zhang, Z.; Huang, W.-H.; Du, G.-J.; Wen, X.-D.; Calway, T.; Yu, C.; Nass, R.; Zhao, J.; Du, W.; et al. Identification of potential anticancer compounds from Oplopanax horridus. Phytomedicine 2013, 20, 999–1006. [Google Scholar] [CrossRef]
- Gajendran, B.; Durai, P.; Varier, K.M.; Chinnasamy, A. A novel phytosterol isolated from Datura inoxia, RinoxiaB is a potential cure colon cancer agent by targeting BAX/Bcl2 pathway. Bioorganic Med. Chem. 2019, 28, 115242. [Google Scholar] [CrossRef]
- Li, W.; Zhang, Q.; Chen, K.; Sima, Z.; Liu, J.; Yu, Q.; Liu, J. 2-Ethoxystypandrone, a novel small-molecule STAT3 signaling inhibitor from Polygonum cuspidatum, inhibits cell growth and induces apoptosis of HCC cells and HCC Cancer stem cells. BMC Complement. Altern. Med. 2019, 19, 38. [Google Scholar] [CrossRef] [PubMed]
- Abreu, L.S.; Nascimento, Y.M.D.; Espirito-Santo, R.F.D.; Meira, C.S.; Santos, I.P.; Brandão, R.B.; Souto, A.L.; Guedes, M.L.S.; Soares, M.B.P.; Villarreal, C.F.; et al. Phenylpropanoids from Croton velutinus with cytotoxic, trypanocidal and anti-inflammatory activities. Fitoterapia 2020, 145, 104632. [Google Scholar] [CrossRef]
- Kumar, S.; Gupta, A.; Saini, R.V.; Kumar, A.; Dhar, K.L.; Mahindroo, N.J.B.; Chemistry, M. Immunomodulation-mediated anticancer activity of a novel compound from Brugmansia suaveolens leaves. Bioorganic Med. Chem. 2020, 28, 115552. [Google Scholar] [CrossRef]
- Zong, A.; Cao, H.; Wang, F. Anticancer polysaccharides from natural resources: A review of recent research. Carbohydr. Polym. 2012, 90, 1395–1410. [Google Scholar] [CrossRef]
- Sreelekha, T.T.; Joseph, M.M.; Aravind, S.R.; Varghese, S.; Mini, S. Evaluation of antioxidant, antitumor and immunomodulatory properties of polysaccharide isolated from fruit rind of Punica granatum. Mol. Med. Rep. 2011, 5, 489–496. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Ji, P.; Gong, X.; Li, W.; Cheng, J.; Qian, H.; Song, X. Physico-chemical characterization, antioxidant and anticancer activities in vitro of a novel polysaccharide from Melia toosendan Sieb. Et Zucc fruit. Int. J. Biol. Macromol. 2011, 49, 422–427. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Xue, Y. Purification, chemical characterization and antioxidant activities of a novel polysaccharide from Auricularia polytricha. Int. J. Biol. Macromol. 2018, 120 Pt A, 1087–1092. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).