Automated Optimized Synthesis of [18F]FLT Using Non-Basic Phase-Transfer Catalyst with Reduced Precursor Amount
Abstract
:1. Introduction
2. Results and Discussion
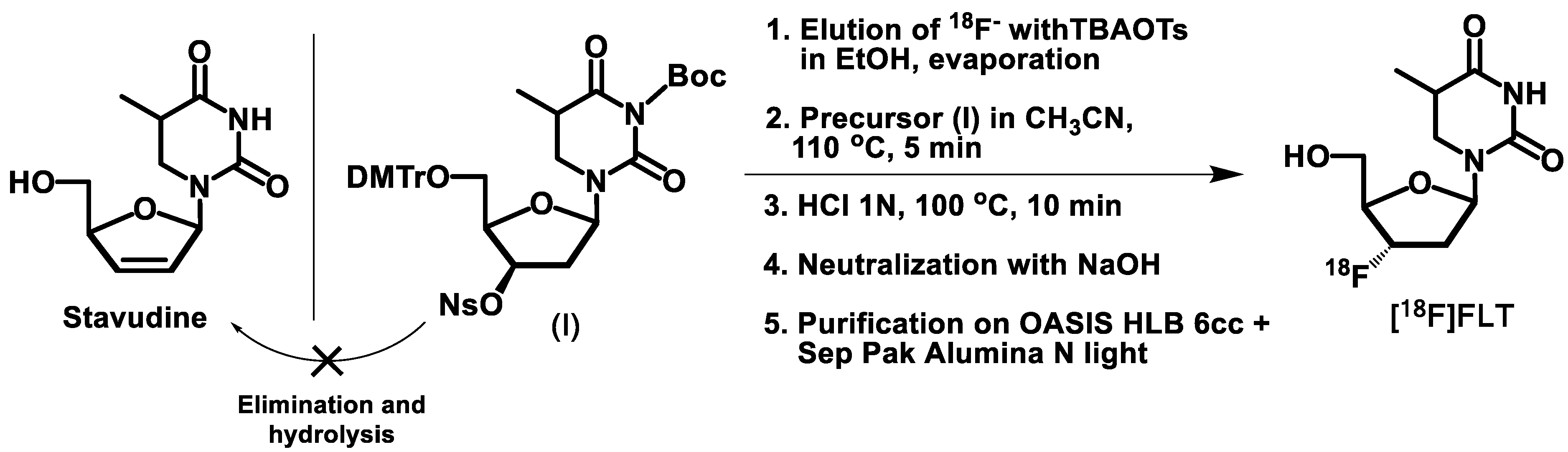
2.1. Optimization of the [18F]FLT Radiosynthesis under Remote-Controlled Operation
2.2. Automated Synthesis of [18F]FLT
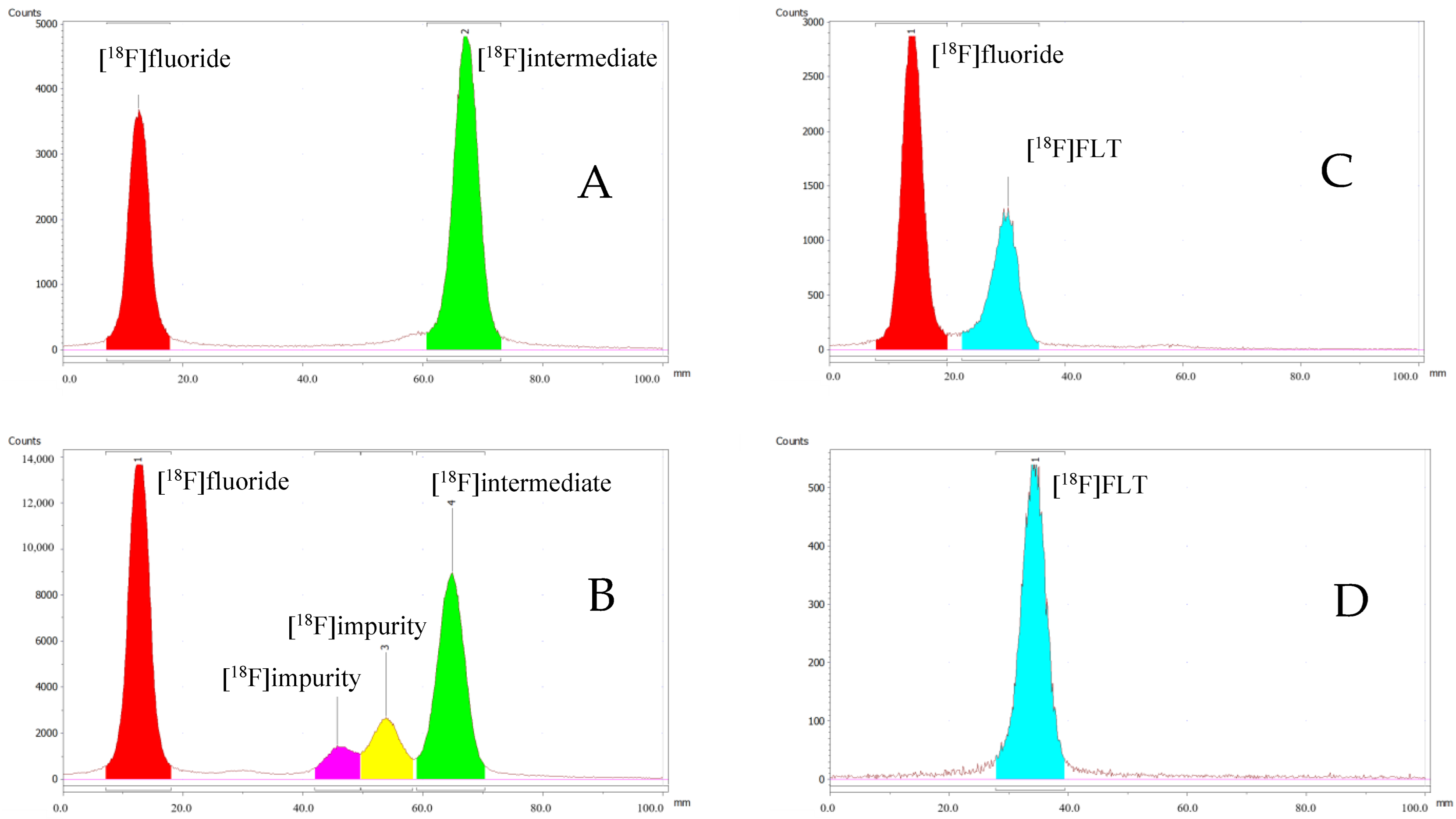
2.3. Quality Control
3. Experimental
3.1. Materials and Methods
3.2. Production of [18F]Fluoride
3.3. Remote-Controlled Radiosynthesis of [18F]FLT
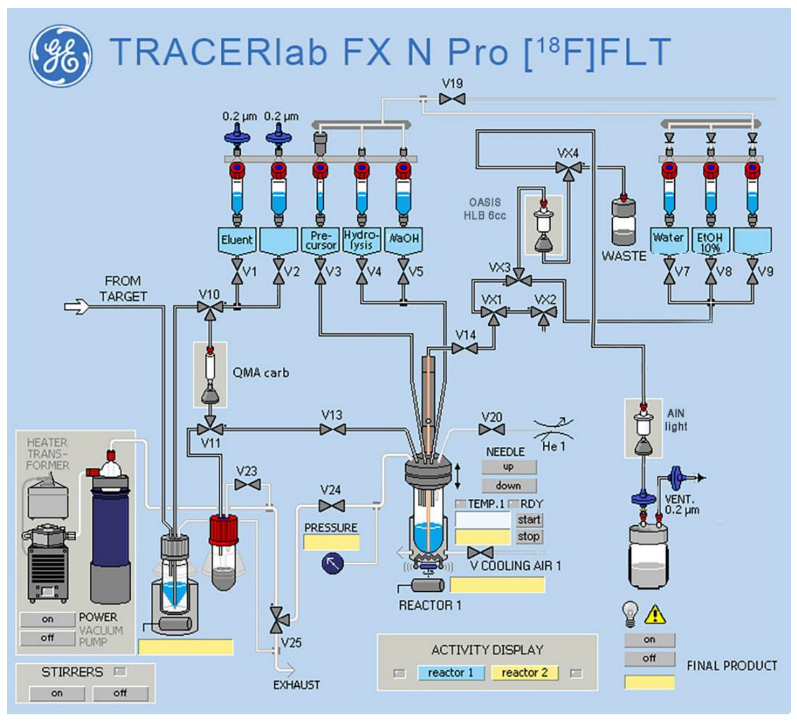
3.4. Automated Radiosynthesis of [18F]FLT
- Vial 1—4.0 mg of TBAOTs in 2 mL of anhydrous ethanol;
- Vial 2—empty;
- Vial 3—4.0 mg of precursor I in 1 mL CH3CN;
- Vial 4—1.0 mL 1N HCl;
- Vial 5—10 mL of H2O and 2.6 mL of 0.3 N NaOH
- Vial 7—5 mL water;
- Vial 8—8 mL of 10% ethanol.
- Step 1: 18F-fluoride preparation.
- Step 2: Nucleophilic fluorination.
- Step 3: Acidic hydrolysis.
- Step 4: Neutralization.
- Step 5. Purification and formulation.
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Sample Availability
References
- Shields, A.F.; Grierson, J.R.; Dohmen, B.M.; Machulla, H.J.; Stayanoff, J.C.; Lawhorn-Crews, J.M.; Obradovich, J.E.; Muzik, O.; Mangner, T.J. Imaging proliferation in vivo with [F-18]FLT and positron emission tomography. Nat. Med. 1998, 4, 1334–1336. [Google Scholar] [CrossRef] [PubMed]
- Bertagna, F.; Biasiotto, G.; Guibbini, R. The role of F-18-fluorothymidine PET in oncology. Clin. Trans. Imaging 2013, 1, 77–97. [Google Scholar] [CrossRef] [Green Version]
- Peck, M.; Pollack, H.A.; Friesen, A.; Muzi, M.; Shoner, S.C.; Shankland, E.G.; Fink, J.R.; Armstrong, J.O.; Link, J.M.; Krohn, K.A. Applications of PET imaging with the proliferation marker [18F]FLT. Q. J. Nucl. Med. Mol. Imaging 2015, 59, 95–104. [Google Scholar] [PubMed]
- Shen, G.; Ma, H.; Pang, F.; Ren, P.; Kuang, A. Correlations of 18F-FDG and 18F-FLT uptake on PET with Ki-67 expression in patients with lung cancer: A meta-analysis. Acta Radiol. 2018, 59, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Contractor, K.B.; Kenny, L.M.; Stebbing, J.; Rosso, L.; Ahmad, R.; Jacob, J.; Challapalli, A.; Turkheimer, F.; Al-Nahhas, A.; Sharma, R.; et al. [18F]-3’Deoxy-3’-fluorothymidine positron emission tomography and breast cancer response to docetaxel. Clin. Cancer Res. 2011, 17, 7664–7672. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kishino, T.; Hoshikawa, H.; Nishiyama, Y.; Yamamoto, Y.; Mori, N. Usefulness of 3′-deoxy-3′-18F-fluorothymidine PET for predicting early response to chemoradiotherapy in head and neck cancer. J. Nucl. Med. 2012, 53, 1521–1527. [Google Scholar] [CrossRef] [Green Version]
- Nikaki, A.; Angelidis, G.; Efthimiadou, R.; Tsougos, I.; Valotassiou, V.; Fountas, K.; Prasopoulos, V.; Georgoulias, P. 18F-fluorothymidine PET imaging in gliomas: An update. Ann. Nucl. Med. 2017, 31, 495–505. [Google Scholar] [CrossRef] [Green Version]
- Saga, T.; Koizumi, M.; Inubushi, M.; Yoshikawa, K.; Tanimoto, K.; Fukumura, T.; Miyamoto, T.; Nakajima, M.; Yamamoto, N.; Baba, M. PET/CT with 3’-deoxy-3’-[18F]fluorothymidine for lung cancer patients receiving carbon-ion radiotherapy. Nucl. Med. Commun. 2011, 32, 348–355. [Google Scholar] [CrossRef]
- Leimgruber, A.; Moller, A.; Everitt, S.J.; Chabrot, M.; Ball, D.L.; Solomon, B.; MacManus, M.; Hicks, R.J. Effect of platinum-based chemoradiotherapy on cellular proliferation in bone marrow and spleen, estimated by (18)F-FLT PET/CT in patients with locally advanced non-small cell lung cancer. J. Nucl. Med. 2014, 55, 1075–1080. [Google Scholar] [CrossRef] [Green Version]
- Bollineni, V.R.; Kramer, G.M.; Jansma, E.P.; Liu, Y.; Oyen, W.J. A systematic review on [(18)F]FLT-PET uptake as a measure of treatment response in cancer patients. Eur. J. Cancer 2016, 55, 81–97. [Google Scholar] [CrossRef]
- Szyszko, T.A.; Dunn, J.T.; Phillips, M.M.; Bomalaski, J.; Sheaff, M.T.; Ellis, S.; Pike, L.; Goh, V.; Cook, G.J.R.; Szlosarek, P.W. Role of 3′-Deoxy-3′-[18F]Fluorothymidine Positron Emission Tomography-Computed Tomography as a Predictive Biomarker in Argininosuccinate Synthetase 1-Deficient Thoracic Cancers Treated With Pegargiminase. JTO Clin. Res. Rep. 2022, 3, 100382. [Google Scholar] [CrossRef] [PubMed]
- Saidijam, M.; Afshar, S.; Ahmad, I.; Patching, S.G. Nucleoside transporters in PET imaging of proliferating cancer cell using 3′-deoxy-3′-[18F]fluoro-L-thymidine. J. Diagn. Imaging Ther. 2018, 5, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Yun, M.; Oh, S.J.; Ha, H.-J.; Ryu, J.S.; Moon, D.H. High radiochemical yield synthesis of 3′-deoxy-3′-[18F]fluorothymidine using (5′-O-dimethoxytrityl-2′-deoxy-3′-O-nosyl-β-D-threo pentofuranosyl)thymine and its 3-N-BOC-protected analogue as a labeling precursor. Nucl. Med. Biol. 2003, 30, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Martin, S.J.; Eisenbarth, J.A.; Wagner-Utermann, U.; Mier, W.; Henze, M.; Pritzkow, H.; Haberkorn, U.; Eisenhut, M. A new precursor for the radiosynthesis of [18F]FLT. Nucl. Med. Biol. 2002, 29, 263–273. [Google Scholar] [CrossRef]
- Oh, S.J.; Mosdzianowski, C.; Chi, D.Y.; Kim, J.Y.; Kang, S.H.; Ryu, J.S.; Yeo, J.S.; Moon, D.H. Fully automated synthesis system of 3-deoxy-3-[18F]fluorothymidine. Nucl. Med. Biol. 2004, 31, 803–809. [Google Scholar] [CrossRef]
- Pascali, C.; Bogni, A.; Fugazza, L.; Cucchi, C.; Crispu, O.; Laera, L.; Iwata, R.; Maiocchi, G.; Crippa, F.; Bombardieri, E. Simple preparation and purification of ethanol-free solutions of 3’-deoxy-3’-[18F]fluorothymidine by means of disposable solid-phase extraction cartridges. Nucl. Med. Biol. 2012, 39, 540–550. [Google Scholar] [CrossRef]
- Kim, D.W.; Ahn, D.-S.; Oh, Y.-H.; Lee, S.; Kil, H.S.; Oh, S.J.; Lee, S.J.; Kim, J.S.; Ryu, J.S.; Moon, D.H.; et al. A new class of SN2 reactions catalyzed by protic solvents: Facile fluorination for isotopic labeling of diagnostic molecules. J. Am. Chem. Soc. 2006, 128, 16394–16397. [Google Scholar] [CrossRef]
- Lee, S.J.; Oh, S.J.; Chi, D.Y.; Kil, H.S.; Kim, E.N.; Ryu, J.S.; Moon, D.H. Simple and highly efficient synthesis of 3′-deoxy-3′-[18F]fluorothymidine using nucleophilic fluorination catalyzed by protic solvent. Eur. J. Nucl. Med. Mol. Imaging 2007, 34, 1406–1409. [Google Scholar] [CrossRef]
- Marchand, P.; Ouadi, A.; Pellicioli, M.; Schuler, J.; Laquerriere, P.; Boisson, F.; Brasse, D. Automated and efficient radiosynthesis of [18F]FLT using a low amount of precursor. Nucl. Med. Biol. 2016, 43, 520–527. [Google Scholar] [CrossRef]
- Cheung, Y.-Y.; Nickels, M.L.; McKinley, E.T.; Buck, J.R.; Manning, H.C. High-yielding, automated production of 3′-deoxy-3′-[18F]fluorothymidine using a modified Bioscan Coincidence FDG reaction module. Appl. Radiat. Isot. 2015, 97, 47–51. [Google Scholar] [CrossRef]
- Nascimento, L.T.C.; Silveira, M.B.; Ferreira, S.M.Z.M.D.; Silva, J.B. Comparison between Two Ethanolic Solutions for 3′-Deoxy-3′-[18F]Fluorothymidine Elution. Adv. Chem. Eng. Sci. 2017, 7, 23–33. [Google Scholar] [CrossRef] [Green Version]
- Mitra, A.; Chakraborty, A.; Upadhye, T.; Tawate, M.; Lad, S.; Sahu, S.; Rajesh, C.; Bagul, S.; Pawar, Y.; Ray, M.K.; et al. Automated radiochemical synthesis of pharmaceutical grade [18F]FLT using 3-N-Boc-5′-O-dimethoxytrityl-3′-O-nosyl-thymidine precursor and its Sep-Pak® purification employing selective elution from reversed phase. J. Label. Compd. Radiopharm. 2022, 65, 206–222. [Google Scholar] [CrossRef] [PubMed]
- Alovudine (18F) Injection. European Pharmacopoeia 9.0. Available online: https://file.wuxuwang.com/yaopinbz/EP8.0_2_00538.pdf (accessed on 21 October 2022).
- Orlovskaya, V.; Fedorova, O.; Nadporojskii, M.; Krasikova, R. A fully automated azeotropic drying free synthesis of O-(2-[18F]fluoroethyl)-L-tyrosine ([18F]FET) using tetrabutylammonium tosylate. Appl. Radiat. Isot. 2019, 152, 135–139. [Google Scholar] [CrossRef] [PubMed]
- Fedorova, O.; Orlovskaya, V.; Nadporojskii, M.; Krasikova, R. Automated synthesis of the 16α-[18F]fluoroestradiol ([18F]FES): Minimization of precursor amount and resulting benefits. Radiochim. Acta 2020, 108, 979–988. [Google Scholar] [CrossRef]
- Orlovskaya, V.; Antuganov, D.; Fedorova, O.; Timofeev, V.; Krasikova, R. Tetrabutyl ammonium tosylate as inert phase-transfer catalyst: The key to high efficiency SN2 radiofluorinations. Appl. Radiat. Isot. 2020, 163, 109195. [Google Scholar] [CrossRef] [PubMed]
- Fedorova, O.; Nikolaeva, V.; Krasikova, R. Automated SPE-based synthesis of 16α-[18F]fluoroestradiol without HPLC purification step. Appl. Radiat. Isot. 2018, 141, 57–63. [Google Scholar] [CrossRef]
- Nascimento, L.T.C.; Silva, J.B.; Silveira, M.B.; Santos, P.F.; Faria, T. Synthesis and quality control of [18F]fluorothymidine. In Proceedings of the International Nuclear Atlantic Conference-INAC 2013, Recife, PE, Brazil, 24–29 November 2013. ISBN: 978-85-19914-05-2. [Google Scholar]
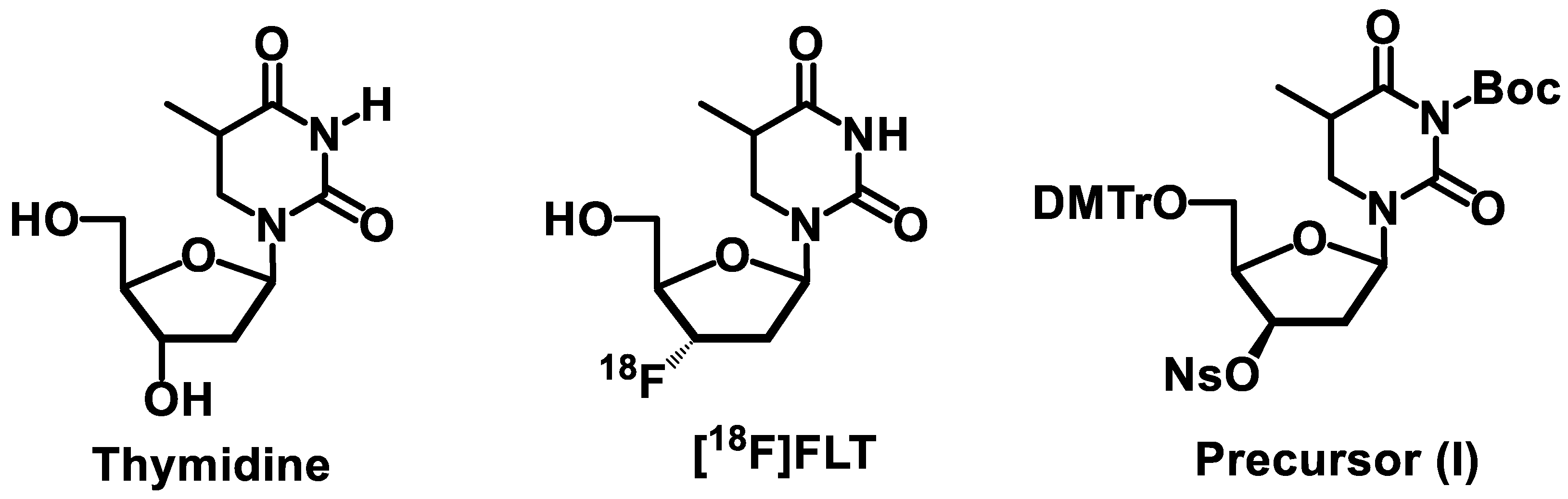

| I, mg | PTC | Automation Platform | SPE Purification Cartridges | RCY, %/Synthesis Time, min | Thymine Thymidine, Stavudine, μg/mL | Ref. |
|---|---|---|---|---|---|---|
| 20 | TBAHCO3 | Modular-Lab, Eckert & Ziegler | Maxi Clean IC-H, two tC18 Sep-Pak Light, Alumina N Light | 37/39 | 28 15 5 | [16] |
| 25 | TBAHCO3 | GE Bioscan Coincidence FDG (cassette based) | PS-H, WAX, HLB | 16–39/ND | ND | [20] |
| 40 | K2.2.2/K2CO3 | GE TRACERlab MX FDG (cassette based) | PS-H+, HLB Plus Short, Alumina Plus Long, | 6.5/35 | 57 110 28 | [28] |
| 25 | TBAHCO3 | GE TRACERlab MX FDG (cassette based) | PS-H+, Oasis WAX, Oasis HLB, AluminaN | 27/54 | 0.089–0.898 0.129 to 0.176 0.023 to 0.061 | [21] |
| 20 | TBAHCO3 | GE TRACERlab FX | Light Alumina-N, PS-H+, WAX plus, HLB plus | 10/45 | <1.2 | [22] |
| 4 | TBAOTs | GE TRACERlab FX N Pro | Oasis HLB 6cc, Alumina N Plus Light | 16/52 | 0.3 0.9 0.6 | This work |
| Entry | Module Component | Activity % |
|---|---|---|
| 1 | Start | 100 |
| 2 | Transfer vial for [18O]H2O | 9.0 ± 0.8 |
| 3 | QMA carb 46 mg (after elution) | 5.0 ± 1.3 |
| 4 | Reactor | 3.2 ± 1.2 |
| 5 | OASIS HLB 6cc | 6.5 ± 1.3 |
| 6 | Alumina N Plus Light | 0.9 ± 0.3 |
| 7 | Combined waste | 45.7 ± 5.0 |
| 8 | Sterile filter + needle | 2.0 ± 0.1 |
| 9 | Uncontrolled losses of radioactivity | 11.7 ± 2.2 |
| 10 | [18F]FLT | 16.0 ± 2.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fedorova, O.S.; Orlovskaya, V.V.; Krasikova, R.N. Automated Optimized Synthesis of [18F]FLT Using Non-Basic Phase-Transfer Catalyst with Reduced Precursor Amount. Molecules 2022, 27, 8323. https://doi.org/10.3390/molecules27238323
Fedorova OS, Orlovskaya VV, Krasikova RN. Automated Optimized Synthesis of [18F]FLT Using Non-Basic Phase-Transfer Catalyst with Reduced Precursor Amount. Molecules. 2022; 27(23):8323. https://doi.org/10.3390/molecules27238323
Chicago/Turabian StyleFedorova, Olga S., Viktoriya V. Orlovskaya, and Raisa N. Krasikova. 2022. "Automated Optimized Synthesis of [18F]FLT Using Non-Basic Phase-Transfer Catalyst with Reduced Precursor Amount" Molecules 27, no. 23: 8323. https://doi.org/10.3390/molecules27238323
APA StyleFedorova, O. S., Orlovskaya, V. V., & Krasikova, R. N. (2022). Automated Optimized Synthesis of [18F]FLT Using Non-Basic Phase-Transfer Catalyst with Reduced Precursor Amount. Molecules, 27(23), 8323. https://doi.org/10.3390/molecules27238323







