Quantification of Paeoniflorin by Fully Validated LC–MS/MS Method: Its Application to Pharmacokinetic Interaction between Paeoniflorin and Verapamil
Abstract
1. Introduction
2. Results and Discussion
2.1. Method Development
2.2. Method Validation
2.2.1. Selectivity and Interference
2.2.2. Standard Curve and Linearity
2.2.3. Precision and Accuracy
2.2.4. Carryover Effect
2.2.5. Matrix Effect and Recovery
2.2.6. Stability
2.2.7. Dilution Integrity
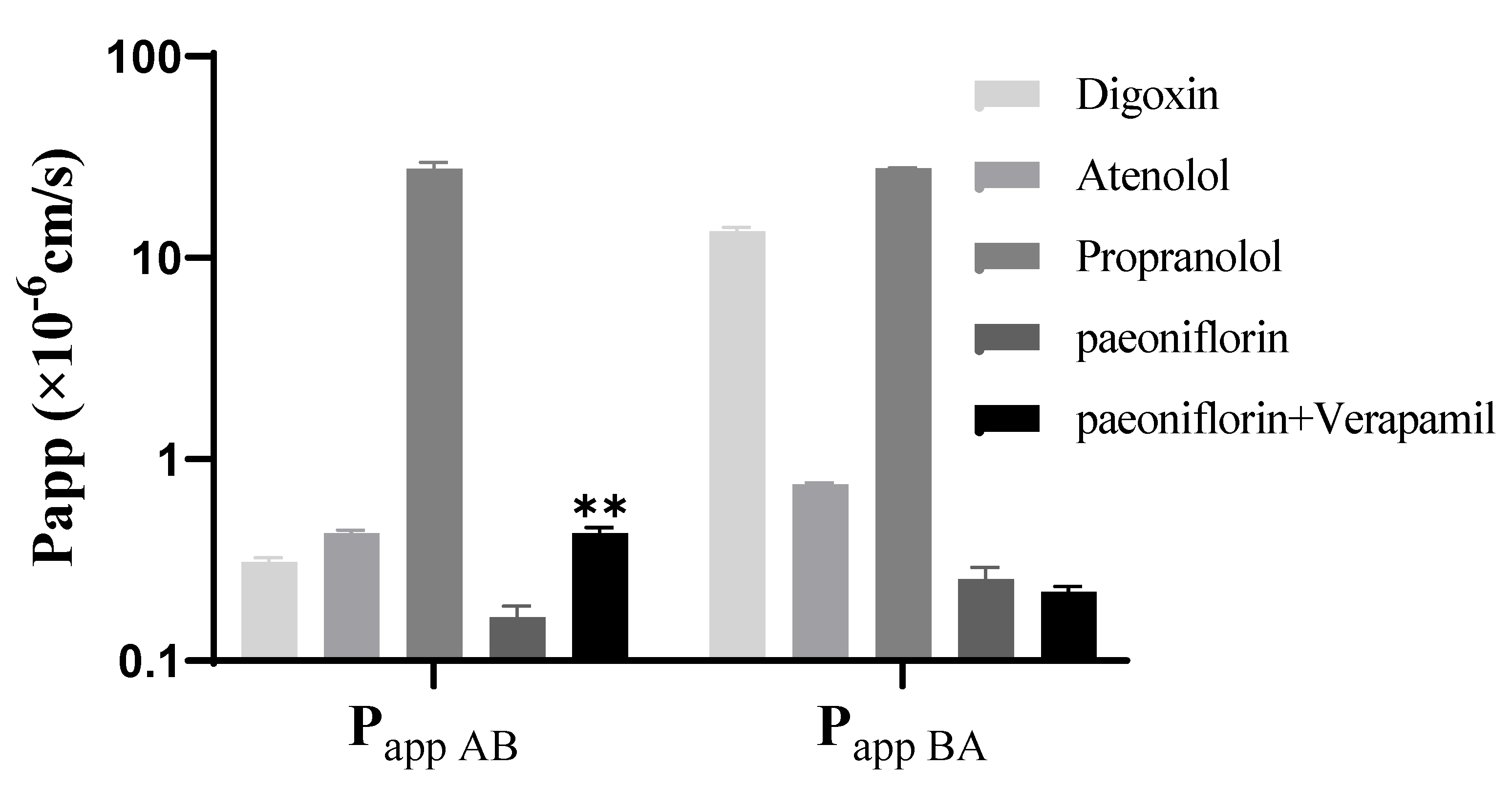
2.3. Effect of Verapamil on the Transport of Paeoniflorin
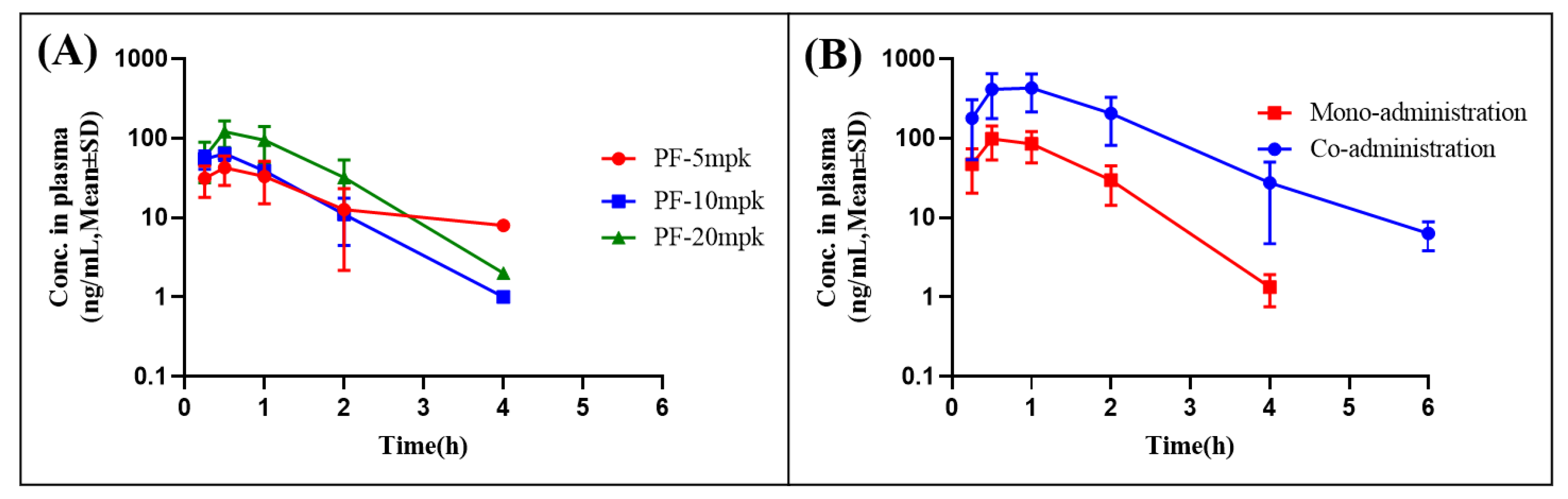
2.4. Pharmacokinetic Study
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Animals
3.3. LC-MS/MS Conditions
3.4. Sample Preparation
3.5. Method Validation
3.5.1. Stock and Working Solutions, Calibration Standards, and Quality Control Samples
3.5.2. Specificity
3.5.3. Linearity and Sensitivity
3.5.4. Carryover Effect
3.5.5. Accuracy and Precision
3.5.6. Matrix Effects and Extraction Recovery
3.5.7. Stability
3.5.8. Dilution Factor
3.6. Cell Culture and Caco−2 Transwell Model
3.7. Pharmacokinetic Study
3.8. Data Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Silman, A.J.; Pearson, J.E. Epidemiology and genetics of rheumatoid arthritis. Arthritis Res. 2002, 4, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.J.; Anzaghe, M.; Schulke, S. Update on the Pathomechanism, Diagnosis, and Treatment Options for Rheumatoid Arthritis. Cells 2020, 9, 880. [Google Scholar] [CrossRef] [PubMed]
- Sparks, J.A. Rheumatoid Arthritis. Ann. Intern. Med. 2019, 170, ITC1–ITC16. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, A.; Tiwari, M.K.; Ghangrekar, M.M. A review on environmental occurrence, toxicity and microbial degradation of Non-Steroidal Anti-Inflammatory Drugs (NSAIDs). J. Environ. Manag. 2021, 300, 113694. [Google Scholar] [CrossRef] [PubMed]
- Shin, S. Safety of celecoxib versus traditional nonsteroidal anti-inflammatory drugs in older patients with arthritis. J. Pain Res. 2018, 11, 3211–3219. [Google Scholar] [CrossRef]
- Dona, I.; Perez-Sanchez, N.; Eguiluz-Gracia, I.; Munoz-Cano, R.; Bartra, J.; Torres, M.J.; Cornejo-Garcia, J.A. Progress in understanding hypersensitivity reactions to nonsteroidal anti-inflammatory drugs. Allergy 2020, 75, 561–575. [Google Scholar] [CrossRef]
- Schjerning, A.M.; McGettigan, P.; Gislason, G. Cardiovascular effects and safety of (non-aspirin) NSAIDs. Nat. Rev. Cardiol. 2020, 17, 574–584. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs over the Nearly Four Decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef]
- Rengasamy, K.R.R.; Khan, H.; Gowrishankar, S.; Lagoa, R.J.L.; Mahomoodally, F.M.; Khan, Z.; Suroowan, S.; Tewari, D.; Zengin, G.; Hassan, S.T.S.; et al. The role of flavonoids in autoimmune diseases: Therapeutic updates. Pharmacol. Ther. 2019, 194, 107–131. [Google Scholar] [CrossRef]
- Sung, S.; Kwon, D.; Um, E.; Kim, B. Could Polyphenols Help in the Control of Rheumatoid Arthritis? Molecules 2019, 24, 1589. [Google Scholar] [CrossRef]
- Shep, D.; Khanwelkar, C.; Gade, P.; Karad, S. Safety and efficacy of curcumin versus diclofenac in knee osteoarthritis: A randomized open-label parallel-arm study. Trials 2019, 20, 214. [Google Scholar] [CrossRef]
- Xu, H.M.; Wei, W.; Jia, X.Y.; Chang, Y.; Zhang, L. Effects and mechanisms of total glucosides of paeony on adjuvant arthritis in rats. J. Ethnopharmacol. 2007, 109, 442–448. [Google Scholar] [CrossRef]
- He, D.Y.; Dai, S.M. Anti-inflammatory and immunomodulatory effects of Paeonia Lactiflora pall., a traditional chinese herbal medicine. Front. Pharmacol. 2011, 2, 10. [Google Scholar] [CrossRef]
- Luo, J.; Jin, D.E.; Yang, G.Y.; Zhang, Y.Z.; Wang, J.M.; Kong, W.P.; Tao, Q.W. Total glucosides of paeony for rheumatoid arthritis: A systematic review of randomized controlled trials. Complement. Ther. Med. 2017, 34, 46–56. [Google Scholar] [CrossRef]
- Zhang, L.; Yu, J.; Wang, C.; Wei, W. The effects of total glucosides of paeony (TGP) and paeoniflorin (Pae) on inflammatory-immune responses in rheumatoid arthritis (RA). Funct. Plant Biol. 2019, 46, 107–117. [Google Scholar] [CrossRef]
- Ma, J.; Meng, Q.; Zhan, J.; Wang, H.; Fan, W.; Wang, Y.; Zhang, S.; Bian, H.; Zheng, F. Paeoniflorin Suppresses Rheumatoid Arthritis Development via Modulating the Circ-FAM120A/miR-671-5p/MDM4 Axis. Inflammation 2021, 44, 2309–2322. [Google Scholar] [CrossRef]
- Li, H.; Cao, X.; Liu, Y.; Liu, T.; Wang, M.; Ren, X.; Dou, Z. Establishment of modified biopharmaceutics classification system absorption model for oral Traditional Chinese Medicine (Sanye Tablet). J. Ethnopharmacol. 2019, 244, 112148. [Google Scholar] [CrossRef]
- Sharom, F.J. The P-glycoprotein multidrug transporter. Essays Biochem. 2011, 50, 161–178. [Google Scholar] [CrossRef]
- Hsiu, S.L.; Lin, Y.T.; Wen, K.C.; Hou, Y.C.; Chao, P.D. A deglucosylated metabolite of paeoniflorin of the root of Paeonia lactiflora and its pharmacokinetics in rats. Planta Med. 2003, 69, 1113–1118. [Google Scholar]
- Liu, C.X.; Yi, X.L.; Si, D.Y.; Xiao, X.F.; He, X.; Li, Y.Z. Herb-drug interactions involving drug metabolizing enzymes and transporters. Curr. Drug Metab. 2011, 12, 835–849. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, J.; Wang, L.; Chen, L.; Wu, Q. Absorption and interaction of the main constituents from the traditional Chinese drug pair Shaoyao-Gancao via a Caco-2 cell monolayer model. Molecules 2012, 17, 14908–14917. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Song, X.; Dong, G. Effects of verapamil on the pharmacokinetics of puerarin in rats. Xenobiotica 2019, 49, 1178–1182. [Google Scholar] [CrossRef] [PubMed]
- Miyake, T.; Tsutsui, H.; Haraya, K.; Tachibana, T.; Morimoto, K.; Takehara, S.; Ayabe, M.; Kobayashi, K.; Kazuki, Y. Quantitative prediction of P-glycoprotein-mediated drug-drug interactions and intestinal absorption using humanized mice. Br. J. Pharmacol. 2021, 178, 4335–4351. [Google Scholar] [CrossRef] [PubMed]
- Tong, L.; Wan, M.; Zhou, D.; Gao, J.; Zhu, Y.; Bi, K. LC-MS/MS determination and pharmacokinetic study of albiflorin and paeoniflorin in rat plasma after oral administration of Radix Paeoniae Alba extract and Tang-Min-Ling-Wan. Biomed. Chromatogr. 2010, 24, 1324–1331. [Google Scholar] [CrossRef]
- Li, X.; Shi, F.; Gu, P.; Liu, L.; He, H.; Ding, L. A sensitive LC-MS/MS method for simultaneous determination of amygdalin and paeoniflorin in human plasma and its application. J. Pharm. Biomed. Anal. 2014, 92, 160–164. [Google Scholar] [CrossRef]
- Ashri, N.Y.; Abdel-Rehim, M. Sample treatment based on extraction techniques in biological matrices. Bioanalysis 2011, 3, 2003–2018. [Google Scholar]
- He, R.; Xu, Y.; Peng, J.; Ma, T.; Li, J.; Gong, M. The effects of 18beta-glycyrrhetinic acid and glycyrrhizin on intestinal absorption of paeoniflorin using the everted rat gut sac model. J. Nat. Med. 2017, 71, 198–207. [Google Scholar] [CrossRef]
- Sun, H.; Wang, J.; Lv, J. Effects of glycyrrhizin on the pharmacokinetics of paeoniflorin in rats and its potential mechanism. Pharm. Biol. 2019, 57, 550–554. [Google Scholar] [CrossRef]



| Compound | Q1 | Q3 | Dwell Time (msec) | RT (min) | DP (V) | CE (V) | EP (eV) | CXP (V) |
|---|---|---|---|---|---|---|---|---|
| Paeoniflorin | 498.1 | 179.1 | 100 | 2.3 | 80 | 26 | 10 | 14 |
| Tolbutamide | 271.1 | 154.9 | 100 | 2.1 | 80 | 24 | 10 | 14 |
| Scan Type | MRM | |||||||
| Ionization Model | positive | |||||||
| Ion Source | ESI | |||||||
| CAD | Medium | |||||||
| Curtain Gas | 40 psi | |||||||
| GS1 | 55 psi | |||||||
| GS2 | 55 psi | |||||||
| Ion Spray Voltage | 5000 V | |||||||
| Sample | Analyte Peak Area | IS Peak Area | Analyte Peak | IS Peak Area (%) |
|---|---|---|---|---|
| Area (%) | ||||
| Selectivity Blank-1 | 156 | 9730 | 7.16 | 0.42 |
| Selectivity Blank-2 | 315 | 11,400 | 14.46 | 0.49 |
| Selectivity Blank-3 | 348 | 9580 | 15.98 | 0.41 |
| Selectivity Blank-4 | 267 | 10,100 | 12.26 | 0.43 |
| Selectivity Blank-5 | 277 | 10,000 | 12.72 | 0.43 |
| Selectivity Blank-6 | 153 | 9410 | 7.02 | 0.40 |
| Mean LLOQ | 2178 | 2,328,333 | 11.60 | 0.43 |
| Only IS-1 | 0.000124 | 2,490,000 | 0.00 | / |
| Only IS-2 | 0.0000245 | 2,480,000 | 0.00 | / |
| Only IS-3 | 0.000114 | 2,490,000 | 0.00 | / |
| Only ULOQ-1 | 4,770,000 | 14,000 | / | 0.56 |
| Only ULOQ-2 | 4,730,000 | 11,700 | / | 0.47 |
| Only ULOQ-3 | 4,760,000 | 11,700 | / | 0.47 |
| Nominal Conc. (ng/mL) | Intra-Batch (n = 3) | ||
|---|---|---|---|
| Measured Conc. (ng/mL) | Precision (RSD%) | Accuracy (RE%) | |
| 1.0 | 1.02 ± 0.01 | 1.1 | 2.3 |
| 2.0 | 1.94 ± 0.02 | 1.0 | −3.0 |
| 5.0 | 4.81 ± 0.36 | 7.5 | −3.9 |
| 50 | 45.9 ± 2.1 | 4.5 | −8.3 |
| 200 | 195 ± 6.4 | 3.3 | −2.3 |
| 1000 | 980 ± 25.0 | 2.5 | −2.0 |
| 1600 | 1723 ± 51.3 | 3.0 | 7.7 |
| 2000 | 2183 ± 20.8 | 1.0 | 9.2 |
| Nominal Conc. (ng/mL) | Intra-Batch (n = 6) | Inter-Batch (n = 3 × 6) | ||||
|---|---|---|---|---|---|---|
| Measured Conc. (ng/mL) | Precision (RSD%) | Accuracy (RE%) | Measured Conc. (ng/mL) | Precision (RSD%) | Accuracy (RE%) | |
| 1.0 | 1.1 ± 0.09 | 8.1 | 8.2 | 1.0 ± 0.1 | 7.7 | 5.0 |
| 3.0 | 3.2 ± 0.22 | 6.8 | 6.7 | 3.0 ± 0.3 | 10.0 | 0.6 |
| 150 | 140.8 ± 8.5 | 6.0 | −6.1 | 141.9 ± 10.4 | 7.3 | −5.4 |
| 1500 | 1570 ± 78.2 | 5.0 | 4.7 | 1551.8 ± 89.0 | 5.7 | 3.5 |
| Nominal Conc. (ng/mL) | Extraction Recovery (%) | Matrix Effect (%) | ||
|---|---|---|---|---|
| Mean ± SD | RSD% | Mean ± SD | RSD% | |
| 3.0 | 97.0 ± 2.1 | 2.2 | 104.0 ± 8.6 | 8.3 |
| 150 | 93.0 ± 4.9 | 5.3 | 111.3± 5.5 | 4.9 |
| 1500 | 98.0 ± 3.9 | 4.0 | 99.3 ± 4.2 | 4.2 |
| Item | Storage Conditions | Nominal Conc. (ng/mL) | Measured Conc. (ng/mL) | Precision (RSD%) | Accuracy (RE%) |
|---|---|---|---|---|---|
| Bench-top Stability | Room temperature for 16 h | 3.0 | 3.01 ± 0.21 | 7.1 | 0.3 |
| 1500 | 1538 ± 52.0 | 3.0 | 2.6 | ||
| Autosampler Stability | 10 °C for 72 h | 3.0 | 2.91 ± 0.16 | 5.4 | −3.1 |
| 1500 | 1535 ± 54.7 | 3.6 | 2.3 | ||
| Freeze-thaw Stability | 4 Cycles at −20 °C | 3.0 | 3.14 ± 0.17 | 5.5 | 4.6 |
| 1500 | 1662 ± 44.5 | 2.7 | 10.8 | ||
| Long-term Stability | −20 °C for 90 days | 3.0 | 2.86 ± 0.20 | 6.0 | −4.8 |
| 1500 | 1500 ± 37.4 | 2.5 | 0.0 |
| Dilution Factor | Nominal Conc. (ng/mL) | Measured Conc. (ng/mL) | Precision (RSD%) | Accuracy (RE%) |
|---|---|---|---|---|
| 10 | 15,000 | 14,917 ± 417 | 2.8 | −0.6 |
| Parameter | Unit | Intravenous | Mono-Administration | Co-Administration | ||
|---|---|---|---|---|---|---|
| 2 mg/kg | 5 mg/kg | 10 mg/kg | 20 mg/kg | 20 mg/kg | ||
| Tmax | h | — | 0.5 ± 0.0 | 0.5 ± 0.0 | 0.7 ± 0.3 | 0.7 ± 0.3 |
| Cmax 1 or C0 2 | ng·mL−1 | 11,230.0 ± 149.0 | 42.3 ± 17.5 | 63.4 ± 11.4 | 100.2 ± 42.5 | 463.6 ± 236.8 ** |
| AUC0–t | ng·h·mL−1 | 2401.0 ± 201.9 | 64.7 ± 44.0 | 82.3 ± 18.4 | 148.6 ± 64.8 | 882.3 ± 490.6 * |
| AUC0–∞ | ng·h·mL−1 | 2402.3 ± 201.3 | 74.7 ± 49.1 | 84.0 ± 17.0 | 156.9 ± 65.3 | 892.0 ± 487.4 * |
| T1/2 | h | 0.9 ± 0.4 | 0.8 ± 0.4 | 0.5 ± 0.1 | 0.5 ± 0.1 | 0.7 ± 0.1 |
| MRT0–∞ | h | 0.2 ± 0.0 | 1.3 ± 0.5 | 1.0 ± 0.2 | 1.2 ± 0.1 | 1.5 ± 0.1 |
| Vz/F 1 or Vz 2 | L·kg−1 | 0.2 ± 0.0 | 85.7 ± 21.1 | 89.0 ± 7.1 | 112 ± 45.7 | 31.5 ± 20.3 ** |
| CL/F 1 or CL 2 | L·h−1·kg−1 | 0.8 ± 0.1 | 98.2 ± 77.9 | 123 ± 27.9 | 145 ± 54.9 | 29.2 ± 16.4 ** |
| F | % | — | 1.3 | 0.7 | 0.6 | 3.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bao, B.; Zhao, Y.; Gong, H.; Shi, S.; Wang, H.; Wang, S. Quantification of Paeoniflorin by Fully Validated LC–MS/MS Method: Its Application to Pharmacokinetic Interaction between Paeoniflorin and Verapamil. Molecules 2022, 27, 8337. https://doi.org/10.3390/molecules27238337
Bao B, Zhao Y, Gong H, Shi S, Wang H, Wang S. Quantification of Paeoniflorin by Fully Validated LC–MS/MS Method: Its Application to Pharmacokinetic Interaction between Paeoniflorin and Verapamil. Molecules. 2022; 27(23):8337. https://doi.org/10.3390/molecules27238337
Chicago/Turabian StyleBao, Bin, Yonglin Zhao, Huan Gong, Songshan Shi, Huijun Wang, and Shunchun Wang. 2022. "Quantification of Paeoniflorin by Fully Validated LC–MS/MS Method: Its Application to Pharmacokinetic Interaction between Paeoniflorin and Verapamil" Molecules 27, no. 23: 8337. https://doi.org/10.3390/molecules27238337
APA StyleBao, B., Zhao, Y., Gong, H., Shi, S., Wang, H., & Wang, S. (2022). Quantification of Paeoniflorin by Fully Validated LC–MS/MS Method: Its Application to Pharmacokinetic Interaction between Paeoniflorin and Verapamil. Molecules, 27(23), 8337. https://doi.org/10.3390/molecules27238337








