A Polyoxometalate-Encapsulated Metal–Organic Framework Nanoplatform for Synergistic Photothermal–Chemotherapy and Anti-Inflammation of Ovarian Cancer
Abstract
1. Introduction
2. Results and Discussions
2.1. Characterization of ZCPHs
2.2. Photothermal Performance of ZCPHs
2.3. pH-Responsive CQ Release In Vitro
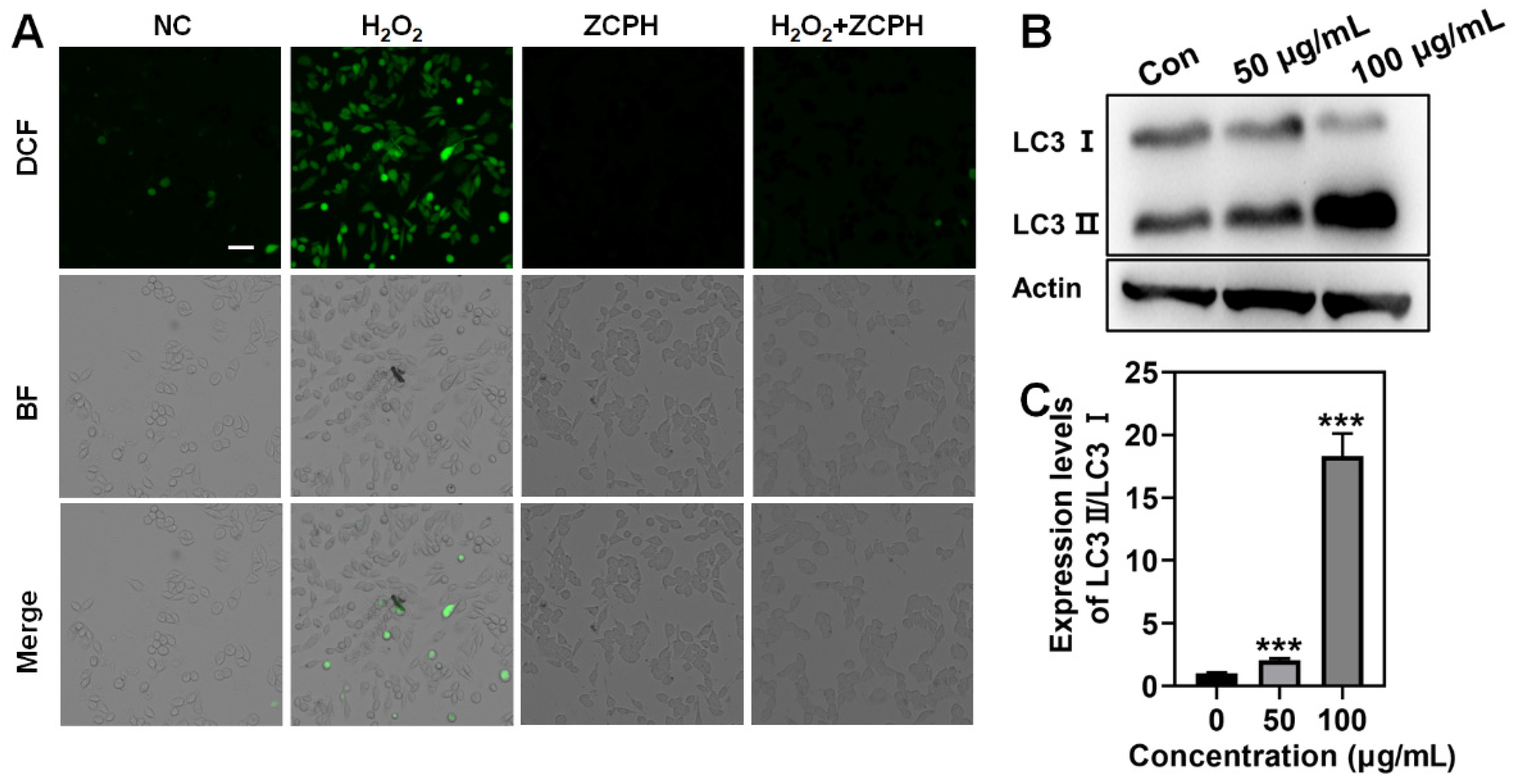
2.4. Scavenging ROS with ZCPHs
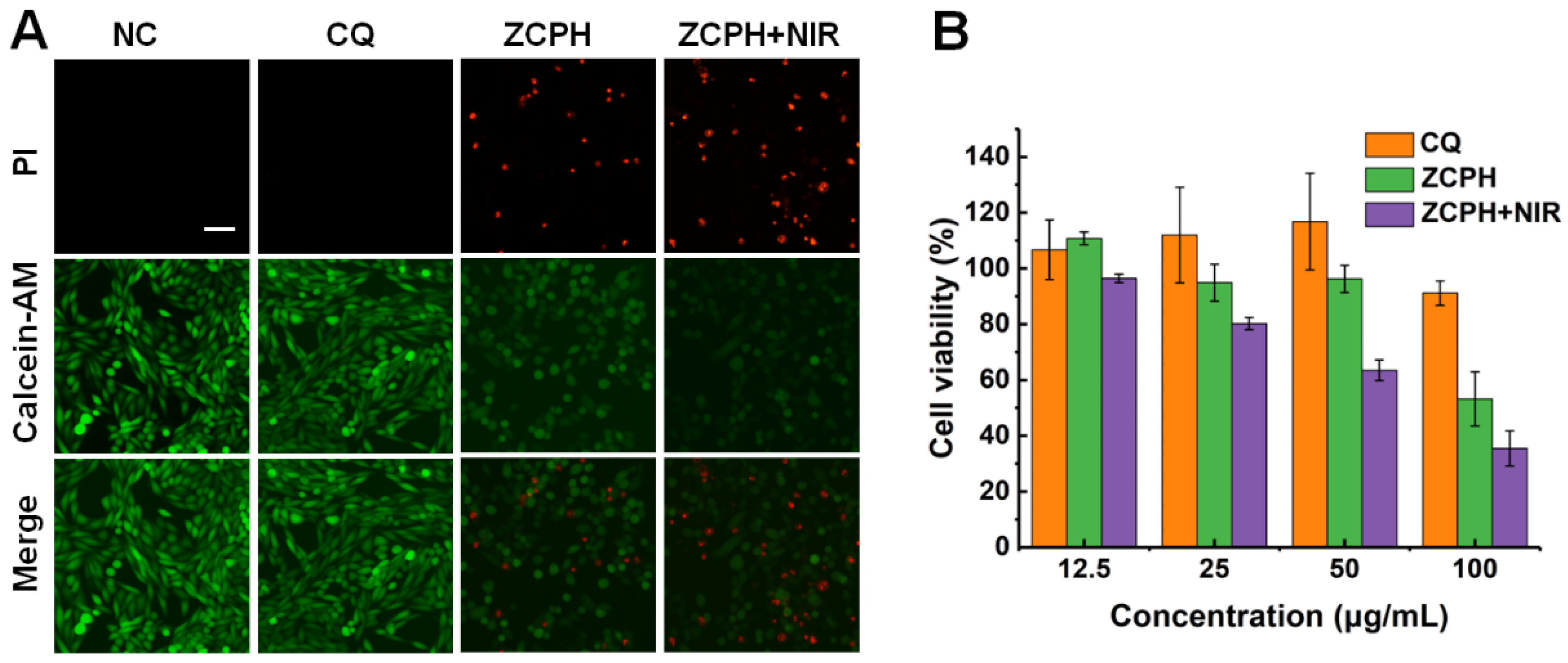
2.5. In Vitro Combined Therapy
2.6. Autophagy Efficiency In Vitro
3. Materials and Methods
3.1. Materials
3.2. Preparation of POM Clusters
3.3. Preparation of ZCP NPs
3.4. Preparation of ZCPH NPs
3.5. Characterization
3.6. In Vitro PTT
3.7. Acid-Responsive Release of CQ
3.8. Cell Culture
3.9. In Vitro Cytotoxicity Study
3.10. Live/Dead Cell Staining
3.11. ROS Scavenging with ZCPHs
3.12. Western Blotting
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, S.; Wu, W.; Liu, Y.; Wang, C.; Xu, Q.; Lv, Q.; Huang, R.; Li, X. Targeted peptide-modified oxidized mesoporous carbon nanospheres for chemo-thermo combined therapy of ovarian cancer in vitro. Drug Deliv. 2022, 29, 1947–1952. [Google Scholar] [CrossRef] [PubMed]
- Zahedi, P.; Yoganathan, R.; Piquette-Miller, M.; Allen, C. Recent advances in drug delivery strategies for treatment of ovarian cancer. Expert Opin. Drug Deliv. 2012, 9, 567–583. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Cheng, Y.; Wu, J.; Wang, Q.; Wang, W.; Yang, J.; Zhao, Z.; Lou, X.; Xia, F.; Wang, S.; et al. Modular Peptide Probe for Pre/Intra/Postoperative Therapeutic to Reduce Recurrence in Ovarian Cancer. ACS Nano 2020, 14, 14698–14714. [Google Scholar] [CrossRef] [PubMed]
- Ni, N.; Zhang, X.; Ma, Y.; Yuan, J.; Wang, D.; Ma, G.; Dong, J.; Sun, X. Biodegradable two-dimensional nanomaterials for cancer theranostics. Coord. Chem. Rev. 2022, 458, 214415. [Google Scholar] [CrossRef]
- Chen, J.; Ning, C.; Zhou, Z.; Yu, P.; Zhu, Y.; Tan, G.; Mao, C. Nanomaterials as photothermal therapeutic agents. Prog. Mater. Sci. 2019, 99, 1–26. [Google Scholar] [CrossRef]
- Liu, Y.; Bhattarai, P.; Dai, Z.; Chen, X. Photothermal therapy and photoacoustic imaging via nanotheranostics in fighting cancer. Chem. Soc. Rev. 2019, 48, 2053–2108. [Google Scholar] [CrossRef]
- Rejinold, N.S.; Choi, G.; Choy, J.H. Recent Developments on Semiconducting Polymer Nanoparticles as Smart Photo-Therapeutic Agents for Cancer Treatments—A Review. Polymers 2021, 13, 981. [Google Scholar] [CrossRef]
- Liu, J.; Kang, L.; Smith, S.; Wang, C. Transmembrane MUC18 Targeted Polydopamine Nanoparticles and a Mild Photothermal Effect Synergistically Disrupt Actin Cytoskeleton and Migration of Cancer Cells. Nano Lett. 2021, 21, 9609–9618. [Google Scholar] [CrossRef]
- Zhou, J.; Zhao, W.; Miao, Z.; Wang, J.; Ma, Y.; Wu, H.; Sun, T.; Qian, H.; Zha, Z. Folin-Ciocalteu Assay Inspired Polyoxometalate Nanoclusters as a Renal Clearable Agent for Non-Inflammatory Photothermal Cancer Therapy. ACS Nano 2020, 14, 2126–2136. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, Y.; Xue, Y.; Wu, Y.; Wang, Q.; Xue, L.; Su, Z.; Zhang, C. Transforming Weakness into Strength: Photothermal-Therapy-Induced Inflammation Enhanced Cytopharmaceutical Chemotherapy as a Combination Anticancer Treatment. Adv. Mater. 2019, 31, e1805936. [Google Scholar] [CrossRef]
- Han, Q.; Wang, X.; Jia, X.; Cai, S.; Liang, W.; Qin, Y.; Yang, R.; Wang, C. CpG loaded MoS2 nanosheets as multifunctional agents for photothermal enhanced cancer immunotherapy. Nanoscale 2017, 9, 5927–5934. [Google Scholar] [CrossRef] [PubMed]
- Miao, Z.; Jiang, S.; Ding, M.; Sun, S.; Ma, Y.; Younis, M.R.; He, G.; Wang, J.; Lin, J.; Cao, Z.; et al. Ultrasmall Rhodium Nanozyme with RONS Scavenging and Photothermal Activities for Anti-Inflammation and Antitumor Theranostics of Colon Diseases. Nano Lett. 2020, 20, 3079–3089. [Google Scholar] [CrossRef] [PubMed]
- Pedone, D.; Moglianetti, M.; De Luca, E.; Bardi, G.; Pompa, P.P. Platinum nanoparticles in nanobiomedicine. Chem. Soc. Rev. 2017, 46, 4951–4975. [Google Scholar] [CrossRef]
- Sun, Q.; Bi, H.; Wang, Z.; Li, C.; Wang, X.; Xu, J.; Zhu, H.; Zhao, R.; He, F.; Gai, S.; et al. Hyaluronic acid-targeted and pH-responsive drug delivery system based on metal-organic frameworks for efficient antitumor therapy. Biomaterials 2019, 223, 119473. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Hu, Y.; Ding, W.; Li, F.; Lin, J.; Wu, M.; Wu, J.; Wen, L.P.; Qiu, B.; Wei, P.F.; et al. Rationally designed rapamycin-encapsulated ZIF-8 nanosystem for overcoming chemotherapy resistance. Biomaterials 2020, 258, 120308. [Google Scholar] [CrossRef]
- Ni, N.; Wang, W.; Sun, Y.; Sun, X.; Leong, D.T. Inducible endothelial leakiness in nanotherapeutic applications. Biomaterials 2022, 287, 121640. [Google Scholar] [CrossRef]
- Čolović, M.B.; Lacković, M.; Lalatović, J.; Mougharbel, A.S.; Kortz, U.; Krstić, D.Z. Polyoxometalates in Biomedicine: Update and Overview. Curr. Med. Chem. 2020, 27, 362–379. [Google Scholar] [CrossRef]
- Chang, D.; Li, Y.; Chen, Y.; Wang, X.; Zang, D.; Liu, T. Polyoxometalates-based nanocomposites for application in antitumor and antibacterial. Nanoscale Adv. 2022, 4, 3689–3706. [Google Scholar] [CrossRef]
- Liu, J.; Huang, M.; Zhang, X.; Hua, Z.; Feng, Z.; Dong, Y.; Sun, T.; Sun, X.; Chen, C. Polyoxometalate nanomaterials for enhanced reactive oxygen species theranostics. Coord. Chem. Rev. 2022, 472, 214785. [Google Scholar] [CrossRef]
- Yongge, W. Polyoxometalates: An interdisciplinary journal focused on all aspects of polyoxometalates. Polyoxometalates 2022, 1, 9140014. [Google Scholar] [CrossRef]
- Tang, W.; Fan, W.; Wang, Z.; Zhang, W.; Zhou, S.; Liu, Y.; Yang, Z.; Shao, E.; Zhang, G.; Jacobson, O.; et al. Acidity/Reducibility Dual-Responsive Hollow Mesoporous Organosilica Nanoplatforms for Tumor-Specific Self-Assembly and Synergistic Therapy. ACS Nano 2018, 12, 12269–12283. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Bu, W.; Ni, D.; Zuo, C.; Cheng, C.; Li, Q.; Zhang, L.; Wang, Z.; Shi, J. A Polyoxometalate Cluster Paradigm with Self-Adaptive Electronic Structure for Acidity/Reducibility-Specific Photothermal Conversion. J. Am. Chem. Soc. 2016, 138, 8156–8164. [Google Scholar] [CrossRef]
- Ni, D.; Jiang, D.; Kutyreff, C.J.; Lai, J.; Yan, Y.; Barnhart, T.E.; Yu, B.; Im, H.J.; Kang, L.; Cho, S.Y.; et al. Molybdenum-based nanoclusters act as antioxidants and ameliorate acute kidney injury in mice. Nat. Commun. 2018, 9, 5421. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.G.; Codogno, P.; Zhang, H. Machinery, regulation and pathophysiological implications of autophagosome maturation. Nat. Rev. Mol. Cell Biol. 2021, 22, 733–750. [Google Scholar] [CrossRef] [PubMed]
- Nakatogawa, H. Mechanisms governing autophagosome biogenesis. Nat. Rev. Mol. Cell Biol. 2020, 21, 439–458. [Google Scholar] [CrossRef] [PubMed]
- Feng, Q.; Yang, X.; Hao, Y.; Wang, N.; Feng, X.; Hou, L.; Zhang, Z. Cancer Cell Membrane-Biomimetic Nanoplatform for Enhanced Sonodynamic Therapy on Breast Cancer via Autophagy Regulation Strategy. ACS Appl. Mater. Interfaces 2019, 11, 32729–32738. [Google Scholar] [CrossRef]
- Mauthe, M.; Orhon, I.; Rocchi, C.; Zhou, X.; Luhr, M.; Hijlkema, K.J.; Coppes, R.P.; Engedal, N.; Mari, M.; Reggiori, F. Chloroquine inhibits autophagic flux by decreasing autophagosome-lysosome fusion. Autophagy 2018, 14, 1435–1455. [Google Scholar] [CrossRef]
- Zhang, X.; Tang, J.; Li, C.; Lu, Y.; Cheng, L.; Liu, J. A targeting black phosphorus nanoparticle based immune cells nano-regulator for photodynamic/photothermal and photo-immunotherapy. Bioact. Mater. 2021, 6, 472–489. [Google Scholar] [CrossRef]
- Song, Y.; Sun, Y.; Tang, M.; Yue, Z.; Ni, J.; Zhao, J.; Wang, W.; Sun, T.; Shi, L.; Wang, L. Polyoxometalate Modified by Zeolite Imidazole Framework for the pH-Responsive Electrodynamic/Chemodynamic Therapy. ACS Appl. Mater. Interfaces 2022, 14, 4914–4920. [Google Scholar] [CrossRef]
- Huang, C.X.; Chen, H.J.; Li, F.; Wang, W.N.; Li, D.D.; Yang, X.Z.; Miao, Z.H.; Zha, Z.B.; Lu, Y.; Qian, H.S. Controlled synthesis of upconverting nanoparticles/CuS yolk-shell nanoparticles for in vitro synergistic photothermal and photodynamic therapy of cancer cells. J. Mater. Chem. B 2017, 5, 9487–9496. [Google Scholar] [CrossRef]
- Tanida, I.; Ueno, T.; Kominami, E. LC3 and Autophagy. Methods Mol. Biol. 2008, 445, 77–88. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Ding, L.; Yao, H.; Chen, Y.; Shi, J. A Metal-Organic Framework (MOF) Fenton Nanoagent-Enabled Nanocatalytic Cancer Therapy in Synergy with Autophagy Inhibition. Adv. Mater. 2020, 32, e1907152. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, D.; Wang, Y.; Zhang, X.; Lv, Q.; Ma, G.; Gao, Y.; Liu, S.; Wang, C.; Li, C.; Sun, X.; et al. A Polyoxometalate-Encapsulated Metal–Organic Framework Nanoplatform for Synergistic Photothermal–Chemotherapy and Anti-Inflammation of Ovarian Cancer. Molecules 2022, 27, 8350. https://doi.org/10.3390/molecules27238350
Wang D, Wang Y, Zhang X, Lv Q, Ma G, Gao Y, Liu S, Wang C, Li C, Sun X, et al. A Polyoxometalate-Encapsulated Metal–Organic Framework Nanoplatform for Synergistic Photothermal–Chemotherapy and Anti-Inflammation of Ovarian Cancer. Molecules. 2022; 27(23):8350. https://doi.org/10.3390/molecules27238350
Chicago/Turabian StyleWang, Diqing, Yuqi Wang, Xinyu Zhang, Qian Lv, Guiqi Ma, Yuan Gao, Shuangqing Liu, Chenyu Wang, Changzhong Li, Xiao Sun, and et al. 2022. "A Polyoxometalate-Encapsulated Metal–Organic Framework Nanoplatform for Synergistic Photothermal–Chemotherapy and Anti-Inflammation of Ovarian Cancer" Molecules 27, no. 23: 8350. https://doi.org/10.3390/molecules27238350
APA StyleWang, D., Wang, Y., Zhang, X., Lv, Q., Ma, G., Gao, Y., Liu, S., Wang, C., Li, C., Sun, X., & Wan, J. (2022). A Polyoxometalate-Encapsulated Metal–Organic Framework Nanoplatform for Synergistic Photothermal–Chemotherapy and Anti-Inflammation of Ovarian Cancer. Molecules, 27(23), 8350. https://doi.org/10.3390/molecules27238350




