Tackling the Future Pandemics: Broad-Spectrum Antiviral Agents (BSAAs) Based on A-Type Proanthocyanidins
Abstract
1. Introduction
2. A-Type Proanthocyanidins’ Chemistry and Where They Can Be Found
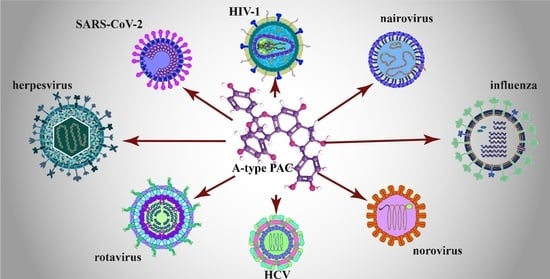
3. The Broad-Spectrum Antiviral Activity of A-Type PACs
3.1. Herpes Simplex Virus
3.2. Human Immunodeficiency Virus (HIV)
3.3. Chronic Hepatitis Viruses
3.4. Enteric Viruses
3.5. Respiratory Viruses
3.6. Non-Respiratory Emerging and Highly Pathogenic Viruses
4. Biological Activities of PAC-As Other than the Antiviral Effects
4.1. Antioxidant Activity
4.2. Antibacterial Activity
4.3. Antidiabetic and Hypoglycemic Activity
4.4. Lipid Lowering Effects and Cardiovascular Protection
4.5. Immunomodulatory Activity
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- To, K.K.; Sridhar, S.; Chiu, K.H.; Hung, D.L.; Li, X.; Hung, I.F.; Tam, A.R.; Chung, T.W.; Chan, J.F.; Zhang, A.J.; et al. Lessons learned 1 year after SARS-CoV-2 emergence leading to COVID-19 pandemic. Emerg. Microbes Infect. 2021, 10, 507–535. [Google Scholar] [CrossRef] [PubMed]
- Drosten, C.; Gunther, S.; Preiser, W.; van der Werf, S.; Brodt, H.R.; Becker, S.; Rabenau, H.; Panning, M.; Kolesnikova, L.; Fouchier, R.A.; et al. Identification of a novel coronavirus in patients with severe acute respiratory syndrome. N. Engl. J. Med. 2003, 348, 1967–1976. [Google Scholar] [CrossRef] [PubMed]
- Chafekar, A.; Fielding, B.C. Mers-cov: Understanding the latest human coronavirus threat. Viruses 2018, 10, 93. [Google Scholar] [CrossRef] [PubMed]
- Adalja, A.; Inglesby, T. Broad-spectrum antiviral agents: A crucial pandemic tool. Expert Rev. Anti Infect. Ther. 2019, 17, 467–470. [Google Scholar] [CrossRef] [PubMed]
- Dolgin, E. Stocking the shelves for the next pandemic. Nature 2021, 592, 340–343. [Google Scholar] [CrossRef]
- Geraghty, R.J.; Aliota, M.T.; Bonnac, L.F. Broad-spectrum antiviral strategies and nucleoside analogues. Viruses 2021, 13, 667. [Google Scholar] [CrossRef]
- Andersen, P.I.; Ianevski, A.; Lysvand, H.; Vitkauskiene, A.; Oksenych, V.; Bjørås, M.; Telling, K.; Lutsar, I.; Dumpis, U.; Irie, Y.; et al. Discovery and development of safe-in-man broad-spectrum antiviral agents. Int. J. Infect. Dis. 2020, 93, 268–276. [Google Scholar] [CrossRef]
- Owen, L.; Laird, K.; Shivkumar, M. Antiviral plant-derived natural products to combat RNA viruses: Targets throughout the viral life cycle. Lett. Appl. Microbiol. 2022, 75, 476–499. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the 30 years from 1981 to 2010. J. Nat. Prod. 2012, 75, 311–335. [Google Scholar] [CrossRef]
- Martinez, J.P.; Sasse, F.; Brönstrup, M.; Diez, J.; Meyerhans, A. Antiviral drug discovery: Broad-spectrum drugs from nature. Nat. Prod. Rep. 2015, 32, 29–48. [Google Scholar] [CrossRef]
- Mohan, S.; Elhassan Taha, M.M.; Makeen, H.A.; Alhazmi, H.A.; Al Bratty, M.; Sultana, S.; Ahsan, W.; Najmi, A.; Khalid, A. Bioactive natural antivirals: An updated review of the available plants and isolated molecules. Molecules 2020, 25, 4878. [Google Scholar] [CrossRef] [PubMed]
- Musarra-Pizzo, M.; Pennisi, R.; Ben-Amor, I.; Mandalari, G.; Sciortino, M.T. Antiviral activity exerted by natural products against human viruses. Viruses 2021, 13, 828. [Google Scholar] [CrossRef] [PubMed]
- Pappas, E.; Schaich, K.M. Phytochemicals of cranberries and cranberry products: Characterization, potential health effects, and processing stability. Crit. Rev. Food Sci. Nutr. 2009, 49, 741–781. [Google Scholar] [CrossRef] [PubMed]
- Foo, L.Y.; Lu, Y.; Howell, A.B.; Vorsa, N. The structure of cranberry proanthocyanidins which inhibit adherence of uropathogenic p-fimbriated Escherichia coli in vitro. Phytochemistry 2000, 54, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Foo, L.Y.; Lu, Y.R.; Howell, A.B.; Vorsa, N. A-type proanthocyanidin trimers from cranberry that inhibit adherence of uropathogenic p-fimbriated Escherichia coli. J. Nat. Prod. 2000, 63, 1225–1228. [Google Scholar] [CrossRef]
- Maffei, M.E. Plant Bioactive Molecules; Cambridge Scholars Publishing: Newcastle upon Tyne, UK, 2018. [Google Scholar]
- Alejo-Armijo, A.; Salido, S.; Altarejos, J. Synthesis of a-type proanthocyanidins and their analogues: A comprehensive review. J. Agric. Food Chem. 2020, 68, 8104–8118. [Google Scholar] [CrossRef]
- Mannino, G.; Gentile, C.; Ertani, A.; Serio, G.; Bertea, C.M. Anthocyanins: Biosynthesis, distribution, ecological role, and use of biostimulants to increase their content in plant foods—A review. Agriculture 2021, 11, 212. [Google Scholar] [CrossRef]
- Mannino, G.; Chinigò, G.; Serio, G.; Genova, T.; Gentile, C.; Munaron, L.; Bertea, C.M. Proanthocyanidins and where to find them: A meta-analytic approach to investigate their chemistry, biosynthesis, distribution, and effect on human health. Antioxidants 2021, 10, 1229. [Google Scholar] [CrossRef]
- Feliciano, R.P.; Shea, M.P.; Shanmuganayagam, D.; Krueger, C.G.; Howell, A.B.; Reed, J.D. Comparison of isolated cranberry (Vaccinium macrocarpon ait.) proanthocyanidins to catechin and procyanidins a2 and b2 for use as standards in the 4-(dimethylamino)cinnamaldehyde assay. J. Agric. Food Chem. 2012, 60, 4578–4585. [Google Scholar] [CrossRef]
- Rue, E.A.; Glinski, J.A.; Glinski, V.B.; van Breemen, R.B. Ion mobility-mass spectrometry for the separation and analysis of procyanidins. J. Mass Spectrom. 2020, 55, e4377. [Google Scholar] [CrossRef]
- Reed, J.D.; Krueger, C.G.; Vestling, M.M. Maldi-tof mass spectrometry of oligomeric food polyphenols. Phytochemistry 2005, 66, 2248–2263. [Google Scholar] [CrossRef] [PubMed]
- Krueger, C.G.; Reed, J.D.; Feliciano, R.P.; Howell, A.B. Quantifying and characterizing proanthocyanidins in cranberries in relation to urinary tract health. Anal. Bioanal. Chem. 2013, 405, 4385–4395. [Google Scholar] [CrossRef] [PubMed]
- Gentile, C.; Mannino, G.; Palazzolo, E.; Gianguzzi, G.; Perrone, A.; Serio, G.; Farina, V. Pomological, sensorial, nutritional and nutraceutical profile of seven cultivars of cherimoya (Annona cherimola mill). Foods 2021, 10, 35. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F. Proanthocyanidins in cereals and pseudocereals. Crit. Rev. Food Sci. Nutr. 2019, 59, 1521–1533. [Google Scholar] [CrossRef]
- de Carvalho, M.V.O.; de Oliveira, L.d.L.; Costa, A.M. Effect of training system and climate conditions on phytochemicals of Passiflora setacea, a wild passiflora from Brazilian savannah. Food Chem. 2018, 266, 350–358. [Google Scholar] [CrossRef]
- Mannino, G.; Maffei, M.E. Metabolomics-based profiling, antioxidant power, and uropathogenic bacterial anti-adhesion activity of sp4(tm), a formulation with a high content of type-a proanthocyanidins. Antioxidants 2022, 11, 1234. [Google Scholar] [CrossRef]
- Cáceres-Mella, A.; Peña-Neira, Á.; Narváez-Bastias, J.; Jara-Campos, C.; López-Solís, R.; Canals, J.M. Comparison of analytical methods for measuring proanthocyanidins in wines and their relationship with perceived astringency. Int. J. Food Sci. Technol. 2013, 48, 2588–2594. [Google Scholar] [CrossRef]
- Li, H.-J.; Deinzer, M.L. Tandem mass spectrometry for sequencing proanthocyanidins. Anal. Chem. 2007, 79, 1739–1748. [Google Scholar] [CrossRef]
- Krueger, C.G.; Chesmore, N.; Chen, X.; Parker, J.; Khoo, C.; Marais, J.P.J.; Shanmuganayagam, D.; Crump, P.; Reed, J.D. Critical reevaluation of the 4-(dimethylamino)cinnamaldehyde assay: Cranberry proanthocyanidin standard is superior to procyanidin a2 dimer for accurate quantification of proanthocyanidins in cranberry products. J. Funct. Foods 2016, 22, 13–19. [Google Scholar] [CrossRef]
- Shahat, A.A.; Ahmed, H.H.; Hassan, R.A.; Hussein, A.A. Antooxidant activity of proanthocyanidins from adansonia digitata fruit. Asian Pac. J. Trop. Med. 2008, 1, 55–59. [Google Scholar]
- Ogawa, S.; Kimura, H.; Niimi, A.; Katsube, T.; Jisaka, M.; Yokota, K. Fractionation and structural characterization of polyphenolic antioxidants from seed shells of Japanese horse chestnut (Aesculus turbinata blume). J. Agric. Food Chem. 2008, 56, 12046–12051. [Google Scholar] [CrossRef] [PubMed]
- Karonen, M.; Bin Imran, I.; Engstrom, M.T.; Salminen, J.P. Characterization of natural and alkaline-oxidized proanthocyanidins in plant extracts by ultrahigh-resolution uhplc-ms/ms. Molecules 2021, 26, 1873. [Google Scholar] [CrossRef] [PubMed]
- Bansode, R.R.; Randolph, P.; Ahmedna, M.; Williams, L.L.; Yu, J.M. Bioavailability and hypolipidemic effects of peanut skin polyphenols. J. Med. Food 2015, 18, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Yan, F.F.; Chen, L.; Chen, W.B.; Zhao, L.; Lu, Q.; Liu, R. Protective effect of procyanidin A-type dimers against H2O2-induced oxidative stress in prostate DU145 cells through the MAPKs signaling pathway. Life Sci. 2021, 266, 118908. [Google Scholar] [CrossRef] [PubMed]
- Enomoto, H.; Nirasawa, T. Localization of flavan-3-ol species in peanut testa by mass spectrometry imaging. Molecules 2020, 25, 2373. [Google Scholar] [CrossRef] [PubMed]
- Rush, M.D.; Rue, E.A.; Wong, A.; Kowalski, P.; Glinsk, J.A.; van Breemen, R.B. Rapid determination of procyanidins using maldi-tof/tof mass spectrometry. J. Agric. Food Chem. 2018, 66, 11355–11361. [Google Scholar] [CrossRef]
- Appeldoorn, M.M.; Sanders, M.; Vincken, J.P.; Cheynier, V.; Le Guerneve, C.; Hollman, P.C.H.; Gruppen, H. Efficient isolation of major procyanidin a-type dimers from peanut skins and b-type dimers from grape seeds. Food Chem. 2009, 117, 713–720. [Google Scholar] [CrossRef]
- Appeldoorn, M.M.; Vincken, J.P.; Sanders, M.; Hollman, P.C.H.; Gruppen, H. Combined normal-phase and reversed-phase liquid chromatography/esi-ms as a tool to determine the molecular diversity of a-type procyanidins in peanut skins. J. Agric. Food Chem. 2009, 57, 6007–6013. [Google Scholar] [CrossRef]
- Yu, J.M.; Ahmedna, M.; Goktepe, P. Peanut skin phenolics: Extraction, identification, antioxidant activity, and potential applications, Symposium on Antioxidant Measurement and Applications. In Proceedings of the 229th ACS National Meeting, San Diego, CA, USA, 13–17 March 2005; Amer Chemical Soc.: San Diego, CA, USA, 2007; pp. 226–241. [Google Scholar]
- Dudek, M.K.; Glinski, V.B.; Davey, M.H.; Sliva, D.; Kazmierski, S.; Glinski, J.A. Trimeric and tetrameric a-type procyanidins from peanut skins. J. Nat. Prod. 2017, 80, 415–426. [Google Scholar] [CrossRef]
- Yu, J.M.; Ahmedna, M.; Goktepe, I.; Dai, J.A. Peanut skin procyanidins: Composition and antioxidant activities as affected by processing. J. Food Compos. Anal. 2006, 19, 364–371. [Google Scholar] [CrossRef]
- Huang, P.-L.; Chi, C.-W.; Liu, T.-Y. Areca nut procyanidins ameliorate streptozocin-induced hyperglycemia by regulating gluconeogenesis. Food Chem. Toxicol. 2013, 55, 137–143. [Google Scholar] [CrossRef]
- Ropiak, H.M.; Ramsay, A.; Mueller-Harvey, I. Condensed tannins in extracts from European medicinal plants and herbal products. J. Pharm. Biomed. Anal. 2016, 121, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Yuan, P.L.; Chen, K.X.; Jia, Q.; Li, Y.M. Oxidative conversion of b- to a-type procyanidin trimer: Evidence for quinone methide mechanism. Food Chem. 2014, 154, 315–322. [Google Scholar] [CrossRef]
- Wang, T.; Sun, P.; Chen, L.; Huang, Q.; Chen, K.X.; Jia, Q.; Li, Y.M.; Wang, H.Y. Cinnamtannin d-1 protects pancreatic beta-cells from palmitic acid-induced apoptosis by attenuating oxidative stress. J. Agric. Food Chem. 2014, 62, 5038–5045. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Sun, P.; Wang, T.; Chen, K.X.; Jia, Q.; Wang, H.Y.; Li, Y.M. Diverse mechanisms of antidialbetic effects of the different procyanidin oligomer types of two different cinnamon species on db/db mice. J. Agric. Food Chem. 2012, 60, 9144–9150. [Google Scholar] [CrossRef]
- Lu, Z.L.; Jia, Q.; Wang, R.; Wu, X.M.; Wu, Y.C.; Huang, C.G.; Li, Y.M. Hypoglycemic activities of a- and b-type procyanidin oligomer-rich extracts from different cinnamon barks. Phytomedicine 2011, 18, 298–302. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.Y.; Zhu, B.R.; Jia, Q.; Li, Y.M.; Wang, T.; Wang, H.Y. Cinnamtannin d1 protects pancreatic beta-cells from glucolipotoxicity-induced apoptosis by enhancement of autophagy in vitro and in vivo. J. Agric. Food Chem. 2020, 68, 12617–12630. [Google Scholar] [CrossRef] [PubMed]
- Vasudevan, S.; Selvan, G.T.; Bhaskaran, S.; Hari, N.; Solomon, A.P. Reciprocal cooperation of type a procyanidin and nitrofurantoin against multi-drug resistant (mdr) upec: A ph-dependent study. Front. Cell. Infect. Microbiol. 2020, 10, 421. [Google Scholar] [CrossRef]
- Kandhare, A.D.; Aswar, U.M.; Mohan, V.; Thakurdesai, P.A. Ameliorative effects of type-a procyanidins polyphenols from cinnamon bark in compound 48/80-induced mast cell degranulation. Anat. Cell Biol. 2017, 50, 275–283. [Google Scholar] [CrossRef]
- Lin, W.L.; Guu, S.Y.; Tsai, C.C.; Prakash, E.; Viswaraman, M.; Chen, H.B.; Chang, C.F. Derivation of cinnamon blocks leukocyte attachment by interacting with sialosides. PLoS ONE 2015, 10, e0130389. [Google Scholar] [CrossRef]
- Aswar, U.M.; Kandhare, A.D.; Mohan, V.; Thakurdesai, P.A. Anti-allergic effect of intranasal administration of type-a procyanidin polyphenols based standardized extract of cinnamon bark in ovalbumin sensitized balb/c mice. Phytother. Res. 2015, 29, 423–433. [Google Scholar] [CrossRef] [PubMed]
- Panickar, K.S.; Polansky, M.M.; Graves, D.J.; Urban, J.F.; Anderson, R.A. A procyanidin type a trimer from cinnamon extract attenuates glial cell swelling and the reduction in glutamate uptake following ischemia-like injury in vitro. Neuroscience 2012, 202, 87–98. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.M.; Zhang, K.Q.; Nam, S.; Anderson, R.A.; Jove, R.; Wen, W. Novel angiogenesis inhibitory activity in cinnamon extract blocks vegfr2 kinase and downstream signaling. Carcinogenesis 2010, 31, 481–488. [Google Scholar] [CrossRef] [PubMed]
- Anderson, R.A.; Broadhurst, C.L.; Polansky, M.M.; Schmidt, W.F.; Khan, A.; Flanagan, V.P.; Schoene, N.W.; Graves, D.J. Isolation and characterization of polyphenol type-a polymers from cinnamon with insulin-like biological activity. J. Agric. Food Chem. 2004, 52, 65–70. [Google Scholar] [CrossRef]
- Chen, L.; Chen, L.; Wang, T.; Yuan, P.L.; Chen, K.X.; Jia, Q.; Wang, H.Y.; Li, Y.M. Preparation of methylated products of a-type procyanidin trimers in cinnamon bark and their protective effects on pancreatic beta-cell. J. Food Sci. 2016, 81, C1062–C1069. [Google Scholar] [CrossRef]
- Xu, Q.; Chen, Z.Y.; Zhu, B.R.; Wang, G.R.; Jia, Q.; Li, Y.M.; Wu, X.J. A-type cinnamon procyanidin oligomers protect against 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced neurotoxicity in mice through inhibiting the p38 mitogen-activated protein kinase/p53/bcl-2 associated x protein signaling pathway. J. Nutr. 2020, 150, 1731–1737. [Google Scholar] [CrossRef]
- Wong-Paz, J.E.; Guyot, S.; Aguilar-Zarate, P.; Muniz-Marquez, D.B.; Contreras-Esquivel, J.C.; Aguilar, C.N. Structural characterization of native and oxidized procyanidins (condensed tannins) from coffee pulp (Coffea arabica) using phloroglucinolysis and thioglycolysis-hplc-esi-ms. Food Chem. 2021, 340, 127830. [Google Scholar] [CrossRef]
- Chai, W.M.; Chen, C.M.; Gao, Y.S.; Feng, H.L.; Ding, Y.M.; Shi, Y.; Zhou, H.T.; Chen, Q.X. Structural analysis of proanthocyanidins isolated from fruit stone of chinese hawthorn with potent antityrosinase and antioxidant activity. J. Agric. Food Chem. 2014, 62, 123–129. [Google Scholar] [CrossRef]
- Soong, Y.Y.; Barlow, P.J. Isolation and structure elucidation of phenolic compounds from longan (Dimocarpus longan lour.) seed by high-performance liquid chromatography-electrospray ionization mass spectrometry. J. Chromatogr. A 2005, 1085, 270–277. [Google Scholar] [CrossRef]
- Fu, C.L.; Yang, X.N.; Lai, S.J.; Liu, C.; Huang, S.R.; Yang, H.S. Structure, antioxidant and alpha-amylase inhibitory activities of longan pericarp proanthocyanidins. J. Funct. Food. 2015, 14, 23–32. [Google Scholar] [CrossRef]
- Lin, L.C.; Kuo, Y.C.; Chou, C.J. Immunomodulatory proanthocyanidins from ecdysanthera utilis. J. Nat. Prod. 2002, 65, 505–508. [Google Scholar] [CrossRef]
- Yoshimura, M.; Amakura, Y.; Hyuga, S.; Hyuga, M.; Nakamori, S.; Maruyama, T.; Oshima, N.; Uchiyama, N.; Yang, J.; Oka, H.; et al. Quality evaluation and characterization of fractions with biological activity from ephedra herb extract and ephedrine alkaloids-free ephedra herb extract. Chem. Pharm. Bull. 2020, 68, 140–149. [Google Scholar] [CrossRef] [PubMed]
- Orejola, J.; Matsuo, Y.; Saito, Y.; Tanaka, T. Characterization of proanthocyanidin oligomers of ephedra sinica. Molecules 2017, 22, 1308. [Google Scholar] [CrossRef] [PubMed]
- Michel, P.; Owczarek, A.; Kosno, M.; Gontarek, D.; Matczak, M.; Olszewska, M.A. Variation in polyphenolic profile and in vitro antioxidant activity of eastern teaberry (Gaultheria procumbens L.) leaves following foliar development. Phytochem. Lett. 2017, 20, 356–364. [Google Scholar] [CrossRef]
- Michel, P.; Dobrowolska, A.; Kicel, A.; Owczarek, A.; Bazylko, A.; Granica, S.; Piwowarski, J.P.; Olszewska, M.A. Polyphenolic profile, antioxidant and anti-inflammatory activity of eastern teaberry (Gaultheria procumbens L.) leaf extracts. Molecules 2014, 19, 20498–20520. [Google Scholar] [CrossRef] [PubMed]
- Idowu, T.O.; Ogundaini, A.O.; Salau, A.O.; Obuotor, E.M.; Bezabih, M.; Abegaz, B.M. Doubly linked, a-type proanthocyanidin turner and other constituents of ixora coccinea leaves and their antioxidant and antibacterial properties. Phytochemistry 2010, 71, 2092–2098. [Google Scholar] [CrossRef]
- Alejo-Armijo, A.; Ortega-Vidal, J.; Salido, S.; Altarejos, J. Recovery and seasonal variation of cinnamtannin b-1 from laurel (Laurus nobilis L.) pruning wood wastes. Chem. Biodivers. 2022, 19, e202100807. [Google Scholar] [CrossRef]
- Sui, Y.; Zheng, Y.; Li, X.P.; Li, S.Y.; Xie, B.J.; Sun, Z.D. Characterization and preparation of oligomeric procyanidins from litchi chinensis pericarp. Fitoterapia 2016, 112, 168–174. [Google Scholar] [CrossRef]
- Li, W.; Liang, H.; Zhang, M.W.; Zhang, R.F.; Deng, Y.Y.; Wei, Z.C.; Zhang, Y.; Tang, X.J. Phenolic profiles and antioxidant activity of litchi (Litchi chinensis Sonn.) fruit pericarp from different commercially available cultivars. Molecules 2012, 17, 14954–14967. [Google Scholar] [CrossRef] [PubMed]
- Li, S.Y.; Xiao, J.; Chen, L.; Hu, C.L.; Chen, P.; Xie, B.J.; Sun, Z.D. Identification of a-series oligomeric procyanidins from pericarp of litchi chinensis by ft-icr-ms and lc-ms. Food Chem. 2012, 135, 31–38. [Google Scholar] [CrossRef]
- Su, D.; Luo, M.; Liu, H.; Qi, X.; Zeng, Q.; He, S.; Fen, S.; Zhang, J. The effect of simulated digestion on the composition of phenolic compounds and antioxidant activities in lychee pulp of different cultivars. Int. J. Food Sci. Technol. 2019, 54, 3042–3050. [Google Scholar] [CrossRef]
- Chen, Y.F.; Li, H.Q.; Zhang, S.; Yang, C.Y.; Mai, Z.Y.; Hu, X.Y.; Gao, Z.H.; Deng, H. Anti-myocardial ischemia effect and components of litchi pericarp extracts. Phytother. Res. 2017, 31, 1384–1391. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.J.; Chang, Y.Y.; Hsu, C.L.; Liu, C.W.; Wang, Y.; Chen, Y.C. Protective effect of a litchi (Litchi chinensis Sonn.)-flower-water-extract on cardiovascular health in a high-fat/cholesterol-dietary hamsters. Food Chem. 2010, 119, 1457–1464. [Google Scholar] [CrossRef]
- Li, S.Y.; Liu, Y.; Liu, G.; He, J.R.; Qin, X.G.; Yang, H.C.; Hu, Z.Z.; Lamikanra, O. Effect of the a-type linkage on the pharmacokinetics and intestinal metabolism of litchi pericarp oligomeric procyanidins. J. Agric. Food Chem. 2017, 65, 1893–1899. [Google Scholar] [CrossRef]
- Wu, Q.; Li, S.Y.; Xiao, J.; Sui, Y.; Xie, B.J.; Sun, Z.D. Analysis of distribution and pharmacokinetics of litchi pericarp procyanidins in rat plasma and organs by using liquid chromatography-tandem mass spectrometry. Eur. Food Res. Technol. 2017, 243, 167–176. [Google Scholar] [CrossRef]
- Lv, Q.; Luo, F.L.; Zhao, X.Y.; Liu, Y.; Hu, G.B.; Sun, C.D.; Li, X.; Chen, K.S. Identification of proanthocyanidins from litchi (Litchi chinensis Sonn.) pulp by lc-esi-q-tof-ms and their antioxidant activity. PLoS ONE 2015, 10, e0120480. [Google Scholar] [CrossRef]
- Reichel, M.; Triani, R.; Wellhofer, J.; Sruamsiri, P.; Carle, R.; Neidhart, S. Vital characteristics of litchi (Litchi chinensis Sonn.) pericarp that define postharvest concepts for Thai cultivars. Food Bioprocess Technol. 2013, 6, 1191–1206. [Google Scholar] [CrossRef]
- Zhou, H.C.; Lin, Y.M.; Li, Y.Y.; Li, M.; Wei, S.D.; Chai, W.M.; Tam, N.F.Y. Antioxidant properties of polymeric proanthocyanidins from fruit stones and pericarps of litchi chinensis Sonn. Food Res. Int. 2011, 44, 613–620. [Google Scholar] [CrossRef]
- Liu, L.; Xie, B.J.; Cao, S.Q.; Yang, E.N.; Xu, X.; Guo, S.S. A-type procyanidins from litchi chinensis pericarp with antioxidant activity. Food Chem. 2007, 105, 1446–1451. [Google Scholar] [CrossRef]
- Li, S.Y.; Sui, Y.; Xiao, J.; Wu, Q.; Hu, B.; Xie, B.J.; Sun, Z.D. Absorption and urinary excretion of a-type procyanidin oligomers from litchi chinensis pericarp in rats by selected ion monitoring liquid chromatography-mass spectrometry. Food Chem. 2013, 138, 1536–1542. [Google Scholar] [CrossRef]
- Li, S.Y.; Chen, L.; Yang, T.; Wu, Q.; Lv, Z.J.; Xie, B.J.; Sun, Z.D. Increasing antioxidant activity of procyanidin extracts from the pericarp of litchi chinensis processing waste by two probiotic bacteria bioconversions. J. Agric. Food Chem. 2013, 61, 2506–2512. [Google Scholar] [CrossRef] [PubMed]
- Engemann, A.; Hubner, F.; Rzeppa, S.; Humpf, H.U. Intestinal metabolism of two a-type procyanidins using the pig cecum model: Detailed structure elucidation of unknown catabolites with fourier transform mass spectrometry (ftms). J. Agric. Food Chem. 2012, 60, 749–757. [Google Scholar] [CrossRef]
- Sivakumaran, S.; Rumball, W.; Lane, G.A.; Fraser, K.; Foo, L.Y.; Yu, M.; Meagher, L.P. Variation of proanthocyanidins in lotus species. J. Chem. Ecol. 2006, 32, 1797–1816. [Google Scholar] [CrossRef]
- Navarro, M.; Moreira, I.; Arnaez, E.; Quesada, S.; Azofeifa, G.; Vargas, F.; Alvarado, D.; Chen, P. Polyphenolic characterization and antioxidant activity of malus domestica and prunus domestica cultivars from costa rica. Foods 2018, 7, 15. [Google Scholar] [CrossRef]
- Ferreira, L.; Leao, V.D.; de Melo, C.M.; Machado, T.D.; Amaral, A.C.F.; da Silva, L.L.; Simas, N.K.; Muzitano, M.F.; Leal, I.C.R.; Raimundo, J.M. Ethyl acetate fraction and isolated phenolics derivatives from mandevilla moricandiana identified by uhplc-dad-esi-msn with pharmacological potential for the improvement of obesity-induced endothelial dysfunction. Pharmaceutics 2021, 13, 1173. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.B.; Zhang, L.; Zhao, L.; Yan, F.F.; Zhu, X.L.; Lu, Q.; Liu, R. Metabolomic profiles of a-type procyanidin dimer and trimer with gut microbiota in vitro. J. Funct. Food. 2021, 85, 104637. [Google Scholar] [CrossRef]
- Yang, S.Y.; Zhang, Y.Y.; Li, W.; You, B.Y.; Yu, J.W.; Huang, X.X.; Yang, R.L. Gut microbiota composition affects procyanidin a2-attenuated atherosclerosis in apoe(-/-) mice by modulating the bioavailability of its microbial metabolites. J. Agric. Food Chem. 2021, 69, 6989–6999. [Google Scholar] [CrossRef] [PubMed]
- Alejo-Armijo, A.; Glibota, N.; Frias, M.P.; Altarejos, J.; Galvez, A.; Salido, S.; Ortega-Morente, E. Synthesis and evaluation of antimicrobial and antibiofilm properties of a-type procyanidin analogues against resistant bacteria in food. J. Agric. Food Chem. 2018, 66, 2151–2158. [Google Scholar] [CrossRef]
- Ge, Z.Z.; Dong, X.Q.; Zhu, W.; Zhang, Y.; Li, C.M. Metabolites and changes in antioxidant activity of a-type and b-type proanthocyanidin dimers after incubation with rat intestinal microbiota. J. Agric. Food Chem. 2015, 63, 8991–8998. [Google Scholar] [CrossRef]
- Ou, K.Q.; Sarnoski, P.; Schneider, K.R.; Song, K.J.; Khoo, C.; Gu, L.W. Microbial catabolism of procyanidins by human gut microbiota. Mol. Nutr. Food Res. 2014, 58, 2196–2205. [Google Scholar] [CrossRef]
- Machado, K.N.; Barbosa, A.D.; de Freitas, A.A.; Alvarenga, L.F.; de Padua, R.M.; Faraco, A.A.G.; Braga, F.C.; Vianna-Soares, C.D.; Castilho, R.O. Tnf-alpha inhibition, antioxidant effects and chemical analysis of extracts and fraction from Brazilian guarana seed powder. Food Chem. 2021, 355, 129563. [Google Scholar] [CrossRef] [PubMed]
- da Silva, G.S.; Canuto, K.M.; Ribeiro, P.R.V.; de Brito, E.S.; Nascimento, M.M.; Zocolo, G.J.; Coutinho, J.P.; de Jesus, R.M. Chemical profiling of guarana seeds (Paullinia cupana) from different geographical origins using uplc-qtof-ms combined with chemometrics. Food Res. Int. 2017, 102, 700–709. [Google Scholar] [CrossRef] [PubMed]
- Spiegler, V. Anthelmintic a-type procyanidins and further characterization of the phenolic composition of a root extract from Paullinia pinnata. Molecules 2020, 25, 2287. [Google Scholar] [CrossRef] [PubMed]
- Spiegler, V.; Liebau, E.; Peppler, C.; Raue, K.; Werne, S.; Strube, C.; Heckendorn, F.; Agyare, C.; Stark, T.; Hofmann, T.; et al. A hydroalcoholic extract from Paullinia pinnata L. Roots exerts anthelmintic activity against free-living and parasitic nematodes. Planta Med. 2016, 82, 1173–1179. [Google Scholar] [CrossRef]
- Janecki, A.; Conrad, A.; Engels, I.; Frank, U.; Kolodziej, H. Evaluation of an aqueous-ethanolic extract from pelargonium sidoides (eps((r)) 7630) for its activity against group a-streptococci adhesion to human hep-2 epithelial cells. J. Ethnopharmacol. 2011, 133, 147–152. [Google Scholar] [CrossRef]
- Lopez-Cobo, A.; Gomez-Caravaca, A.M.; Pasini, F.; Caboni, M.F.; Segura-Carretero, A.; Fernandez-Gutierrez, A. Hplc-dad-esi-qtof-ms and hplc-fld-ms as valuable tools for the determination of phenolic and other polar compounds in the edible part and by-products of avocado. LWT Food Sci. Technol. 2016, 73, 505–513. [Google Scholar] [CrossRef]
- Gu, L.W.; Kelm, M.A.; Hammerstone, J.F.; Beecher, G.; Holden, J.; Haytowitz, D.; Prior, R.L. Screening of foods containing proanthocyanidins and their structural characterization using lc-ms/ms and thiolytic degradation. J. Agric. Food Chem. 2003, 51, 7513–7521. [Google Scholar] [CrossRef]
- Sirisena, S.; Zabaras, D.; Ng, K.; Ajlouni, S. Characterization of date (Deglet Nour) seed free and bound polyphenols by high-performance liquid chromatography-mass spectrometry. J. Food Sci. 2017, 82, 333–340. [Google Scholar] [CrossRef]
- Zhou, B.; Alania, Y.; Reis, M.C.; McAlpine, J.B.; Bedran-Russo, A.K.; Pauli, G.F.; Chen, S.N. Rare a-type, spiro-type, and highly oligomeric proanthocyanidins from Pinus massoniana. Org. Lett. 2020, 22, 5304–5308. [Google Scholar] [CrossRef]
- Ramos, P.A.B.; Pereira, C.; Gomes, A.P.; Neto, R.T.; Almeida, A.; Santos, S.A.O.; Silva, A.M.S.; Silvestre, A.J.D. Chemical characterisation, antioxidant and antibacterial activities of Pinus pinaster ait. And Pinus pinea L. Bark polar extracts: Prospecting forestry by-products as renewable sources of bioactive compounds. Appl. Sci. 2022, 12, 784. [Google Scholar] [CrossRef]
- Glavnik, V.; Vovk, I. High performance thin-layer chromatography-mass spectrometry methods on diol stationary phase for the analyses of flavan-3-ols and proanthocyanidins in invasive Japanese knotweed. J. Chromatogr. A 2019, 1598, 196–208. [Google Scholar] [CrossRef] [PubMed]
- Li, S.Y.; Yang, Y.J.; Li, J.S.; Zhu, Z.Z.; Lorenzo, J.M.; Barba, F.J. Increasing yield and antioxidative performance of litchi pericarp procyanidins in baked food by ultrasound-assisted extraction coupled with enzymatic treatment. Molecules 2018, 23, 2089. [Google Scholar] [CrossRef] [PubMed]
- Nunes, C.; Guyot, S.; Marnet, N.; Barros, A.S.; Saraiva, J.A.; Renard, C.; Coimbra, M.A. Characterization of plum procyanidins by thiolytic depolymerization. J. Agric. Food Chem. 2008, 56, 5188–5196. [Google Scholar] [CrossRef]
- Prodanov, M.; Garrido, I.; Vacas, V.; Lebron-Aguilar, R.; Duenas, M.; Gomez-Cordoves, C.; Bartolome, B. Ultrafiltration as alternative purification procedure for the characterization of low and high molecular-mass phenolics from almond skins. Anal. Chim. Acta 2008, 609, 241–251. [Google Scholar] [CrossRef] [PubMed]
- Bolling, B.W.; Chen, C.Y.; McKay, D.L.; Blumberg, J.B. Tree nut phytochemicals: Composition, antioxidant capacity, bioactivity, impact factors. A systematic review of almonds, brazils, cashews, hazelnuts, macadamias, pecans, pine nuts, pistachios and walnuts. Nutr. Res. Rev. 2011, 24, 244–275. [Google Scholar] [CrossRef] [PubMed]
- Irizar, A.C.; Fernandez, M.F.; Gonzalez, A.G.; Ravelo, A.G. Constituents of prunus-spinosa. J. Nat. Prod. 1992, 55, 450–454. [Google Scholar] [CrossRef]
- Pham, H.N.; Michalet, S.; Bodillis, J.; Nguyen, T.D.; Nguyen, T.K.O.; Le, T.P.Q.; Haddad, M.; Nazaret, S.; Dijoux-Franca, M.G. Impact of metal stress on the production of secondary metabolites in pteris Vittata L. and associated rhizosphere bacterial communities. Environ. Sci. Pollut. Res. 2017, 24, 16735–16750. [Google Scholar] [CrossRef]
- Wei, M.K.; Chai, W.M.; Yang, Q.; Wang, R.; Peng, Y.Y. Novel insights into the inhibitory effect and mechanism of proanthocyanidins from Pyracantha fortuneana fruit on alpha-glucosidase. J. Food Sci. 2017, 82, 2260–2268. [Google Scholar] [CrossRef]
- Jeong, D.E.; Cho, J.Y.; Lee, Y.G.; Jeong, H.Y.; Lee, H.J.; Moon, J.H. Isolation of five proanthocyanidins from pear (Pyrus pyrifolia nakai) fruit peels. Food Sci. Biotechnol. 2017, 26, 1209–1215. [Google Scholar] [CrossRef]
- Zhang, L.L.; Wang, Y.M.; Wu, D.M.; Xu, M.; Chen, J.H. Study on the structure of mangrove polyflavonoid tannins with maldi-tof mass spectrometry and NMR. In Proceedings of the 2nd International Conference on Chemical Engineering and Advanced Materials (CEAM 2012), Guangzhou, China, 13–15 July 2012; Trans Tech Publications Ltd.: Stafa-Zurich, Switzerland, 2012; pp. 1988–1993. [Google Scholar]
- Kandil, F.E.; Grace, M.H.; Seigler, D.S.; Cheeseman, J.M. Polyphenolics in Rhizophora mangle L. Leaves and their changes during leaf development and senescence. Trees Struct. Funct. 2004, 18, 518–528. [Google Scholar] [CrossRef]
- Louis, A.; Petereit, F.; Lechtenberg, M.; Deters, A.; Hensel, A. Phytochemical characterization of rhododendron ferrugineum and in vitro assessment of an aqueous extract on cell toxicity. Planta Med. 2010, 76, 1550–1557. [Google Scholar] [CrossRef] [PubMed]
- Way, T.D.; Tsai, S.J.; Wang, C.M.; Jhan, Y.L.; Ho, C.T.; Chou, C.H. Cinnamtannin d1 from rhododendron formosanum induces autophagy via the inhibition of akt/mtor and activation of erk1/2 in non-small-cell lung carcinoma cells. J. Agric. Food Chem. 2015, 63, 10407–10417. [Google Scholar] [CrossRef]
- Wang, C.M.; Hsu, Y.M.; Jhan, Y.L.; Tsai, S.J.; Lin, S.X.; Su, C.H.; Chou, C.H. Structure elucidation of procyanidins isolated from rhododendron formosanum and their anti-oxidative and anti-bacterial activities. Molecules 2015, 20, 12787–12803. [Google Scholar] [CrossRef] [PubMed]
- Antolak, H.; Czyzowska, A.; Kregiel, D. Black currant (Ribes nigrum L.) and bilberry (Vaccinium myrtillus L.) fruit juices inhibit adhesion of Asaia spp. Biomed Res. Int. 2016, 2016, 3671306. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, E.; Franceschi, P.; Feller, A.; Herrera, L.; Palmieri, L.; Arapitsas, P.; Riccadonna, S.; Martens, S. Discovery of a-type procyanidin dimers in yellow raspberries by untargeted metabolomics and correlation based data analysis. Metabolomics 2016, 12, 144. [Google Scholar] [CrossRef] [PubMed]
- Spencer, P.; Sivakumaran, S.; Fraser, K.; Foo, L.Y.; Lane, G.A.; Edwards, P.J.B.; Meagher, L.P. Isolation and characterisation of procyanidins from Rumex obtusifolius. Phytochem. Anal. 2007, 18, 193–203. [Google Scholar] [CrossRef]
- Li, W.T.; Liu, J.; Guan, R.G.; Chen, J.P.; Yang, D.P.; Zhao, Z.M.; Wang, D.M. Chemical characterization of procyanidins from Spatholobus suberectus and their antioxidative and anticancer activities. J. Funct. Food. 2015, 12, 468–477. [Google Scholar] [CrossRef]
- Hurst, W.J.; Stanley, B.; Glinski, J.A.; Davey, M.; Payne, M.J.; Stuart, D.A. Characterization of primary standards for use in the hplc analysis of the procyanidin content of cocoa and chocolate containing products. Molecules 2009, 14, 4136–4146. [Google Scholar] [CrossRef]
- De Taeye, C.; Caullet, G.; Evina, V.J.E.; Collin, S. Procyanidin a2 and its degradation products in raw, fermented, and roasted cocoa. J. Agric. Food Chem. 2017, 65, 1715–1723. [Google Scholar] [CrossRef]
- Appeldoorn, M.M.; Vincken, J.P.; Gruppen, H.; Hollman, P.C.H. Procyanidin dimers a1, a2, and b2 are absorbed without conjugation or methylation from the small intestine of rats. J. Nutr. 2009, 139, 1469–1473. [Google Scholar] [CrossRef]
- De Bruyne, T.; Pieters, L.; Witvrouw, M.; De Clercq, E.; Vanden Berghe, D.; Vlietinck, A.J. Biological evaluation of proanthocyanidin dimers and related polyphenols. J. Nat. Prod. 1999, 62, 954–958. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, Y.; Fujita, Y.; Ohnishi, S.; Tanaka, T.; Hirabaru, H.; Kai, T.; Sakaida, H.; Nishizono, S.; Kouno, I. Chemical constituents of the leaves of rabbiteye blueberry (Vaccinium ashei) and characterisation of polymeric proanthocyanidins containing phenylpropanoid units and a-type linkages. Food Chem. 2010, 121, 1073–1079. [Google Scholar] [CrossRef]
- Esquivel-Alvarado, D.; Munoz-Arrieta, R.; Alfaro-Viquez, E.; Madrigal-Carballo, S.; Krueger, C.G.; Reed, J.D. Composition of anthocyanins and proanthocyanidins in three tropical vaccinium species from costa rica. J. Agric. Food Chem. 2020, 68, 2872–2879. [Google Scholar] [CrossRef] [PubMed]
- Toomik, P.; Pussa, T.; Raal, A. Variability of procyanidin type a- and -b trimers content in aerial parts of some vaccinium species and cultivars. Nat. Prod. Commun. 2014, 9, 815–816. [Google Scholar] [CrossRef]
- Sintara, M.; Wang, Y.F.; Li, L.; Liu, H.Y.; Cunningham, D.G.; Prior, R.R.; Chen, P.; Chang, T.; Wu, X.L. Quantification of cranberry proanthocyanidins by normal-phase high-performance liquid chromatography using relative response factors. Phytochem. Anal. 2020, 31, 874–883. [Google Scholar] [CrossRef]
- Gardana, C.; Scialpi, A.; Fachechi, C.; Simonetti, P. Identification of markers for the authentication of cranberry extract and cranberry-based food supplements. Heliyon 2020, 6, e03863. [Google Scholar] [CrossRef]
- Gao, C.; Cunningham, D.G.; Liu, H.Y.; Khoo, C.; Gu, L.W. Development of a thiolysis hplc method for the analysis of procyanidins in cranberry products. J. Agric. Food Chem. 2018, 66, 2159–2167. [Google Scholar] [CrossRef]
- van Dooren, I.; Foubert, K.; Theunis, M.; Naessens, T.; Pieters, L.; Apers, S. Advantages of a validated uplc-ms/ms standard addition method for the quantification of a-type dimeric and trimeric proanthocyanidins in cranberry extracts in comparison with well-known quantification methods. J. Pharm. Biomed. Anal. 2018, 148, 32–41. [Google Scholar] [CrossRef]
- Wang, Y.F.; Singh, A.P.; Hurst, W.J.; Glinski, J.A.; Koo, H.; Vorsa, N. Influence of degree-of-polymerization and linkage on the quantification of proanthocyanidins using 4-dimethylaminocinnamaldehyde (dmac) assay. J. Agric. Food Chem. 2016, 64, 2190–2199. [Google Scholar] [CrossRef]
- Carpenter, J.L.; Caruso, F.L.; Tata, A.; Vorsa, N.; Neto, C.C. Variation in proanthocyanidin content and composition among commonly grown north American cranberry cultivars (Vaccinium macrocarpon). J. Sci. Food Agric. 2014, 94, 2738–2745. [Google Scholar] [CrossRef]
- Lee, J. Proanthocyanidin a2 purification and quantification of American cranberry (vaccinium macrocarpon ait.) products. J. Funct. Food. 2013, 5, 144–153. [Google Scholar] [CrossRef]
- Jungfer, E.; Zimmermann, B.F.; Ruttkat, A.; Galensa, R. Comparing procyanidins in selected vaccinium species by uhplc-ms2 with regard to authenticity and health effects. J. Agric. Food Chem. 2012, 60, 9688–9696. [Google Scholar] [CrossRef] [PubMed]
- White, B.L.; Howard, L.R.; Prior, R.L. Release of bound procyanidins from cranberry pomace by alkaline hydrolysis. J. Agric. Food Chem. 2010, 58, 7572–7579. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Patan, F.; Bartolome, B.; Martin-Alvarez, P.J.; Anderson, M.; Howell, A.; Monagas, M. Comprehensive assessment of the quality of commercial cranberry products. Phenolic characterization and in vitro bioactivity. J. Agric. Food Chem. 2012, 60, 3396–3408. [Google Scholar] [CrossRef] [PubMed]
- Severo, C.; Anjos, I.; Souza, V.G.L.; Canejo, J.P.; Bronze, M.R.; Fernando, A.L.; Coelhoso, I.; Bettencourt, A.F.; Ribeiro, I.A.C. Development of cranberry extract films for the enhancement of food packaging antimicrobial properties. Food Packag. Shelf Life 2021, 28, 100646. [Google Scholar] [CrossRef]
- Botto, H.; Neuzillet, Y. Effectiveness of a cranberry (Vaccinium macrocarpon) preparation in reducing asymptomatic bacteriuria in patients with an ileal enterocystoplasty. Scand. J. Urol. Nephrol. 2010, 44, 165–168. [Google Scholar] [CrossRef] [PubMed]
- Baranowska, M.; Bartoszek, A. Antioxidant and antimicrobial properties of bioactive phytochemicals from cranberry. Postep. Hig. Med. Dosw. 2016, 70, 1460–1468. [Google Scholar] [CrossRef] [PubMed]
- Faggian, M.; Bernabe, G.; Valente, M.; Francescato, S.; Baratto, G.; Brun, P.; Castagliuolo, I.; Dall’Acqua, S.; Peron, G. Characterization of pacs profile and bioactivity of a novel nutraceutical combining cranberry extracts with different pac-a oligomers, d-mannose and ascorbic acid: An in vivo/ex vivo evaluation of dual mechanism of action on intestinal barrier and urinary epithelium. Food Res. Int. 2021, 149, 110649. [Google Scholar]
- Jepson, R.G.; Williams, G.; Craig, J.C. Cranberries for preventing urinary tract infections. Cochrane Database Syst. Rev. 2012, 10, CD001321. [Google Scholar] [CrossRef]
- Ermel, G.; Georgeault, S.; Inisan, C.; Besnard, M. Inhibition of adhesion of uropathogenic Escherichia coli bacteria to uroepithelial cells by extracts from cranberry. J. Med. Food 2012, 15, 126–134. [Google Scholar] [CrossRef]
- Fu, Z.; Liska, D.; Talan, D.; Chung, M. Cranberry reduces the risk of urinary tract infection recurrence in otherwise healthy women: A systematic review and meta-analysis. J. Nutr. 2017, 147, 2282–2288. [Google Scholar] [CrossRef] [PubMed]
- Howell, A.B.; Reed, J.D.; Krueger, C.G.; Winterbottom, R.; Cunningham, D.G.; Leahy, M. A-type cranberry proanthocyanidins and uropathogenic bacterial anti-adhesion activity. Phytochemistry 2005, 66, 2281–2291. [Google Scholar] [CrossRef] [PubMed]
- Nemzer, B.V.; Al-Taher, F.; Yashin, A.; Revelsky, I.; Yashin, Y. Cranberry: Chemical composition, antioxidant activity and impact on human health: Overview. Molecules 2022, 27, 1503. [Google Scholar] [CrossRef] [PubMed]
- González de Llano, D.; Moreno-Arribas, M.V.; Bartolomé, B. Cranberry polyphenols and prevention against urinary tract infections: Relevant considerations. Molecules 2020, 25, 3523. [Google Scholar]
- Jiao, J.J.; Wei, Y.; Chen, J.N.; Chen, X.Y.; Zhang, Y. Anti-aging and redox state regulation effects of a-type proanthocyanidins-rich cranberry concentrate and its comparison with grape seed extract in mice. J. Funct. Food. 2017, 30, 63–73. [Google Scholar] [CrossRef]
- Liu, H.W.; Zou, T.; Gao, J.M.; Gu, L.W. Depolymerization of cranberry procyanidins using (+)-catechin, (-)-epicatechin, and (-)-epigallocatechin gallate as chain breakers. Food Chem. 2013, 141, 488–494. [Google Scholar] [CrossRef]
- Ou, K.Q.; Percival, S.S.; Zou, T.; Khoo, C.; Gu, L.W. Transport of cranberry a-type procyanidin dimers, trimers, and tetramers across monolayers of human intestinal epithelial caco-2 cells. J. Agric. Food Chem. 2012, 60, 1390–1396. [Google Scholar] [CrossRef]
- Maatta-Riihinen, K.R.; Kahkonen, M.P.; Torronen, A.R.; Heinonen, I.M. Catechins and procyanidins in berries of vaccinium species and their antioxidant activity. J. Agric. Food Chem. 2005, 53, 8485–8491. [Google Scholar] [CrossRef]
- Reed, J. Cranberry flavonoids, atherosclerosis and cardiovascular health. Crit. Rev. Food Sci. Nutr. 2002, 42, 301–316. [Google Scholar] [CrossRef]
- Weh, K.M.; Clarke, J.; Kresty, L.A. Cranberries and cancer: An update of preclinical studies evaluating the cancer inhibitory potential of cranberry and cranberry derived constituents. Antioxidants 2016, 5, 27. [Google Scholar] [CrossRef]
- Zhao, Y.; Wu, X.L.; Yu, L.L.; Chen, P. Retention of polyphenols in blueberries (Vaccinium corymbosum) after different cooking methods, using uhplc-dad-ms based metabolomics. J. Food Compos. Anal. 2017, 56, 55–66. [Google Scholar] [CrossRef]
- Merghem, R.; Jay, M.; Brun, N.; Voirin, B. Qualitative analysis and hplc isolation and identification of procyanidins from vicia faba. Phytochem. Anal. 2004, 15, 95–99. [Google Scholar] [CrossRef] [PubMed]
- Passos, C.P.; Cardoso, S.M.; Domingues, M.R.M.; Domingues, P.; Silva, C.M.; Coimbra, M.A. Evidence for galloylated type-a procyanidins in grape seeds. Food Chem. 2007, 105, 1457–1467. [Google Scholar] [CrossRef]
- Zhao, L.; Wen, L.M.; Lu, Q.; Liu, R. Interaction mechanism between alpha-glucosidase and a-type trimer procyanidin revealed by integrated spectroscopic analysis techniques. Int. J. Biol. Macromol. 2020, 143, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Vaid, M.; Prasad, R.; Singh, T.; Katiyar, S.K. Dietary grape seed proanthocyanidins inactivate regulatory t cells by promoting ner-dependent DNA repair in dendritic cells in uvb-exposed skin. Oncotarget 2017, 8, 49625–49636. [Google Scholar] [CrossRef][Green Version]
- Zhou, D.Y.; Du, Q.; Li, R.R.; Huang, M.; Zhang, Q.; Wei, G.Z. Grape seed proanthocyanidin extract attenuates airway inflammation and hyperresponsiveness in a murine model of asthma by downregulating inducible nitric oxide synthase. Planta Med. 2011, 77, 1575–1581. [Google Scholar] [CrossRef] [PubMed]
- Roychowdhury, S.; Wolf, G.; Keilhoff, G.; Bagchi, D.; Horn, T. Protection of primary glial cells by grape seed proanthocyanidin extract against nitrosative/oxidative stress. Nitric Oxide 2001, 5, 137–149. [Google Scholar] [CrossRef] [PubMed]
- Natella, F.; Belelli, F.; Gentili, V.; Ursini, F.; Scaccini, C. Grape seed proanthocyanidins prevent plasma postprandial oxidative stress in humans. J. Agric. Food Chem. 2002, 50, 7720–7725. [Google Scholar] [CrossRef]
- Quesada, H.; del Bas, J.M.; Pajuelo, D.; Diaz, S.; Fernandez-Larrea, J.; Pinent, M.; Arola, L.; Salvado, M.J.; Blade, C. Grape seed proanthocyanidins correct dyslipidemia associated with a high-fat diet in rats and repress genes controlling lipogenesis and vldl assembling in liver. Int. J. Obes. 2009, 33, 1007–1012. [Google Scholar] [CrossRef] [PubMed]
- Razavi, S.M.; Gholamin, S.; Eskandari, A.; Mohsenian, N.; Ghorbanihaghjo, A.; Delazar, A.; Rashtchizadeh, N.; Keshtkar-Jahromi, M.; Argani, H. Red grape seed extract improves lipid profiles and decreases oxidized low-density lipoprotein in patients with mild hyperlipidemia. J. Med. Food 2013, 16, 255–258. [Google Scholar] [CrossRef]
- Lu, R.H.; Qin, C.B.; Yang, F.; Zhang, W.Y.; Zhang, Y.R.; Yang, G.K.; Yang, L.P.; Meng, X.L.; Yan, X.; Nie, G.X. Grape seed proanthocyanidin extract ameliorates hepatic lipid accumulation and inflammation in grass carp (Ctenopharyngodon idella). Fish Physiol. Biochem. 2020, 46, 1665–1677. [Google Scholar] [CrossRef]
- Vinson, J.A.; Mandarano, M.A.; Shuta, D.L.; Bagchi, M.; Bagchi, D. Beneficial effects of a novel ih636 grape seed proanthocyanidin extract and a niacin-bound chromium in a hamster atherosclerosis model. Mol. Cell. Biochem. 2002, 240, 99–103. [Google Scholar] [CrossRef] [PubMed]
- Yamakoshi, J.; Kataoka, S.; Koga, T.; Ariga, T. Proanthocyanidin-rich extract from grape seeds attenuates the development of aortic atherosclerosis in cholesterol-fed rabbits. Atherosclerosis 1999, 142, 139–149. [Google Scholar] [CrossRef] [PubMed]
- Zhong, L.; Luo, N.; Zhong, X.; Xu, T.; Hao, P. The immunoregulatory effects of natural products on psoriasis via its action on th17 cells versus regulatory t cells balance. Int. Immunopharmacol. 2022, 110, 109032. [Google Scholar] [CrossRef] [PubMed]
- Park, M.-K.; Park, J.-S.; Cho, M.-L.; Oh, H.-J.; Heo, Y.-J.; Woo, Y.-J.; Heo, Y.-M.; Park, M.-J.; Park, H.-S.; Park, S.-H.; et al. Grape seed proanthocyanidin extract (gspe) differentially regulates foxp3+ regulatory and il-17+ pathogenic t cell in autoimmune arthritis. Immunol. Lett. 2011, 135, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Katiyar, S.K.; Pal, H.C.; Prasad, R. Dietary proanthocyanidins prevent ultraviolet radiation-induced non-melanoma skin cancer through enhanced repair of damaged DNA-dependent activation of immune sensitivity. Semin. Cancer Biol. 2017, 46, 138–145. [Google Scholar] [CrossRef]
- Katiyar, S.K. Dietary proanthocyanidins inhibit uv radiation-induced skin tumor development through functional activation of the immune system. Mol. Nutr. Food Res. 2016, 60, 1374–1382. [Google Scholar] [CrossRef]
- Katiyar, S.K. Proanthocyanidins from grape seeds inhibit uv-radiation-induced immune suppression in mice: Detection and analysis of molecular and cellular targets. Photochem. Photobiol. 2015, 91, 156–162. [Google Scholar] [CrossRef]
- Percival, S.S. Grape consumption supports immunity in animals and humans. J. Nutr. 2009, 139, 1801S–1805S. [Google Scholar] [CrossRef]
- Narusaka, M.; Hatanaka, T.; Narusaka, Y. Inactivation of plant and animal viruses by proanthocyanidins from alpinia zerumbet extract. Plant Biotechnol. 2021, 38, 453–455. [Google Scholar] [CrossRef]
- Morimoto, H.; Hatanaka, T.; Narusaka, M.; Narusaka, Y. Molecular investigation of proanthocyanidin from alpinia zerumbet against the influenza a virus. Fitoterapia 2022, 158, 105141. [Google Scholar] [CrossRef]
- Mateos-Martín, M.L.; Fuguet, E.; Jiménez-Ardón, A.; Herrero-Uribe, L.; Tamayo-Castillo, G.; Torres, J.L. Identification of polyphenols from antiviral chamaecrista nictitans extract using high-resolution lc-esi-ms/ms. Anal. Bioanal. Chem. 2014, 406, 5501–5506. [Google Scholar] [CrossRef] [PubMed]
- Fink, R.C.; Roschek, B., Jr.; Alberte, R.S. Hiv type-1 entry inhibitors with a new mode of action. Antivir. Chem. Chemother. 2009, 19, 243–255. [Google Scholar] [CrossRef]
- Connell, B.J.; Chang, S.Y.; Prakash, E.; Yousfi, R.; Mohan, V.; Posch, W.; Wilflingseder, D.; Moog, C.; Kodama, E.N.; Clayette, P.; et al. A cinnamon-derived procyanidin compound displays anti-hiv-1 activity by blocking heparan sulfate- and co-receptor- binding sites on gp120 and reverses t cell exhaustion via impeding tim-3 and pd-1 upregulation. PLoS ONE 2016, 11, e0165386. [Google Scholar] [CrossRef]
- Fauvelle, C.; Lambotin, M.; Heydmann, L.; Prakash, E.; Bhaskaran, S.; Vishwaraman, M.; Baumert, T.F.; Moog, C. A cinnamon-derived procyanidin type a compound inhibits hepatitis c virus cell entry. Hepatol. Int. 2017, 11, 440–445. [Google Scholar] [CrossRef]
- Zhuang, M.; Jiang, H.; Suzuki, Y.; Li, X.; Xiao, P.; Tanaka, T.; Ling, H.; Yang, B.; Saitoh, H.; Zhang, L.; et al. Procyanidins and butanol extract of cinnamomi cortex inhibit SARS-CoV infection. Antivir. Res. 2009, 82, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Tietjen, I.; Ntie-Kang, F.; Mwimanzi, P.; Onguéné, P.A.; Scull, M.A.; Idowu, T.O.; Ogundaini, A.O.; Meva’a, L.M.; Abegaz, B.M.; Rice, C.M.; et al. Screening of the pan-african natural product library identifies ixoratannin a-2 and boldine as novel hiv-1 inhibitors. PLoS ONE 2015, 10, e0121099. [Google Scholar] [CrossRef]
- Xu, X.; Xie, H.; Wang, Y.; Wei, X. A-type proanthocyanidins from lychee seeds and their antioxidant and antiviral activities. J. Agric. Food Chem. 2010, 58, 11667–11672. [Google Scholar] [CrossRef] [PubMed]
- Feng, W.Y.; Tanaka, R.; Inagaki, Y.; Saitoh, Y.; Chang, M.O.; Amet, T.; Yamamoto, N.; Yamaoka, S.; Yoshinaka, Y. Pycnogenol, a procyanidin-rich extract from french maritime pine, inhibits intracellular replication of hiv-1 as well as its binding to host cells. Jpn. J. Infect. Dis. 2008, 61, 279–285. [Google Scholar]
- Ezzikouri, S.; Nishimura, T.; Kohara, M.; Benjelloun, S.; Kino, Y.; Inoue, K.; Matsumori, A.; Tsukiyama-Kohara, K. Inhibitory effects of pycnogenol® on hepatitis c virus replication. Antivir. Res. 2015, 113, 93–102. [Google Scholar] [CrossRef]
- Suedee, A.; Tewtrakul, S.; Panichayupakaranant, P. Anti-hiv-1 integrase compound from pometia pinnata leaves. Pharm. Biol. 2013, 51, 1256–1261. [Google Scholar] [CrossRef] [PubMed]
- Mirandola, M.; Salvati, M.V.; Rodigari, C.; Appelberg, K.S.; Mirazimi, A.; Maffei, M.E.; Gribaudo, G.; Salata, C. Cranberry (Vaccinium macrocarpon) extract impairs nairovirus infection by inhibiting the attachment to target cells. Pathogens 2021, 10, 1025. [Google Scholar] [CrossRef] [PubMed]
- Luganini, A.; Terlizzi, M.E.; Catucci, G.; Gilardi, G.; Maffei, M.E.; Gribaudo, G. The cranberry extract oximacro(®) exerts in vitro virucidal activity against influenza virus by interfering with hemagglutinin. Front. Microbiol. 2018, 9, 1826. [Google Scholar] [CrossRef] [PubMed]
- Turmagambetova, A.S.; Sokolova, N.S.; Bogoyavlenskiy, A.P.; Berezin, V.E.; Lila, M.A.; Cheng, D.M.; Dushenkov, V. New functionally-enhanced soy proteins as food ingredients with anti-viral activity. Virusdisease 2015, 26, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Terlizzi, M.E.; Occhipinti, A.; Luganini, A.; Maffei, M.E.; Gribaudo, G. Inhibition of herpes simplex type 1 and type 2 infections by oximacro(®), a cranberry extract with a high content of a-type proanthocyanidins (pacs-a). Antivir. Res. 2016, 132, 154–164. [Google Scholar] [CrossRef]
- Su, X.W.; Howell, A.B.; D’Souza, D.H. Antiviral effects of cranberry juice and cranberry proanthocyanidins on foodborne viral surrogates—A time dependence study in vitro. Food Microbiol. 2010, 27, 985–991. [Google Scholar] [CrossRef]
- Su, X.; Howell, A.B.; D’Souza, D.H. The effect of cranberry juice and cranberry proanthocyanidins on the infectivity of human enteric viral surrogates. Food Microbiol. 2010, 27, 535–540. [Google Scholar] [CrossRef]
- Lipson, S.M.; Cohen, P.; Zhou, J.; Burdowski, A.; Stotzky, G. Cranberry cocktail juice, cranberry concentrates, and proanthocyanidins reduce reovirus infectivity titers in african green monkey kidney epithelial cell cultures. Mol. Nutr. Food Res. 2007, 51, 752–758. [Google Scholar] [CrossRef]
- Lipson, S.M.; Sethi, L.; Cohen, P.; Gordon, R.E.; Tan, I.P.; Burdowski, A.; Stotzky, G. Antiviral effects on bacteriophages and rotavirus by cranberry juice. Phytomedicine 2007, 14, 23–30. [Google Scholar] [CrossRef]
- Lipson, S.M.; Ozen, F.S.; Karthikeyan, L.; Gordon, R.E. Effect of ph on anti-rotavirus activity by comestible juices and proanthocyanidins in a cell-free assay system. Food Environ. Virol. 2012, 4, 168–178. [Google Scholar] [CrossRef]
- Sugamoto, K.; Tanaka, Y.L.; Saito, A.; Goto, Y.; Nakayama, T.; Okabayashi, T.; Kunitake, H.; Morishita, K. Highly polymerized proanthocyanidins (pac) components from blueberry leaf and stem significantly inhibit SARS-CoV-2 infection via inhibition of ace2 and viral 3clpro enzymes. Biochem. Biophys. Res. Commun. 2022, 615, 56–62. [Google Scholar] [CrossRef]
- Joshi, S.S.; Howell, A.B.; D’Souza, D.H. Reduction of enteric viruses by blueberry juice and blueberry proanthocyanidins. Food Environ. Virol. 2016, 8, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Takeshita, M.; Ishida, Y.; Akamatsu, E.; Ohmori, Y.; Sudoh, M.; Uto, H.; Tsubouchi, H.; Kataoka, H. Proanthocyanidin from blueberry leaves suppresses expression of subgenomic hepatitis c virus RNA. J. Biol. Chem. 2009, 284, 21165–21176. [Google Scholar] [CrossRef] [PubMed]
- Nair, M.P.; Kandaswami, C.; Mahajan, S.; Nair, H.N.; Chawda, R.; Shanahan, T.; Schwartz, S.A. Grape seed extract proanthocyanidins downregulate hiv-1 entry coreceptors, ccr2b, ccr3 and ccr5 gene expression by normal peripheral blood mononuclear cells. Biol. Res. 2002, 35, 421–431. [Google Scholar] [CrossRef]
- McCormick, I.; James, C.; Welton, N.J.; Mayaud, P.; Turner, K.M.E.; Gottlieb, S.L.; Foster, A.; Looker, K.J. Incidence of herpes simplex virus keratitis and other ocular disease: Global review and estimates. Ophthalmic Epidemiol. 2022, 29, 353–362. [Google Scholar] [CrossRef] [PubMed]
- James, C.; Harfouche, M.; Welton, N.J.; Turner, K.M.; Abu-Raddad, L.J.; Gottlieb, S.L.; Looker, K.J. Herpes simplex virus: Global infection prevalence and incidence estimates, 2016. Bull. World Health Organ. 2020, 98, 315–329. [Google Scholar] [CrossRef] [PubMed]
- Whitley, R.; Baines, J. Clinical management of herpes simplex virus infections: Past, present, and future. F1000Research 2018, 7, F1000. [Google Scholar] [CrossRef]
- Son, M.; Lee, M.; Sung, G.H.; Lee, T.; Shin, Y.S.; Cho, H.; Lieberman, P.M.; Kang, H. Bioactive activities of natural products against herpesvirus infection. J. Microbiol. 2013, 51, 545–551. [Google Scholar] [CrossRef]
- Hassan, S.T.; Masarčíková, R.; Berchová, K. Bioactive natural products with anti-herpes simplex virus properties. J. Pharm. Pharmacol. 2015, 67, 1325–1336. [Google Scholar] [CrossRef]
- Khan, M.T.; Ather, A.; Thompson, K.D.; Gambari, R. Extracts and molecules from medicinal plants against herpes simplex viruses. Antivir. Res. 2005, 67, 107–119. [Google Scholar] [CrossRef]
- Gescher, K.; Hensel, A.; Hafezi, W.; Derksen, A.; Kühn, J. Oligomeric proanthocyanidins from rumex acetosa l. Inhibit the attachment of herpes simplex virus type-1. Antivir. Res. 2011, 89, 9–18. [Google Scholar] [CrossRef]
- Gescher, K.; Kühn, J.; Lorentzen, E.; Hafezi, W.; Derksen, A.; Deters, A.; Hensel, A. Proanthocyanidin-enriched extract from myrothamnus flabellifolia welw. Exerts antiviral activity against herpes simplex virus type 1 by inhibition of viral adsorption and penetration. J. Ethnopharmacol. 2011, 134, 468–474. [Google Scholar] [CrossRef] [PubMed]
- Freitas, A.M.; Almeida, M.T.R.; Andrighetti-Fröhner, C.R.; Cardozo, F.T.G.S.; Barardi, C.R.M.; Farias, M.R.; Simões, C.M.O. Antiviral activity-guided fractionation from araucaria angustifolia leaves extract. J. Ethnopharmacol. 2009, 126, 512–517. [Google Scholar] [CrossRef] [PubMed]
- Shahat, A.A.; Cos, P.; De Bruyne, T.; Apers, S.; Hammouda, F.M.; Ismail, S.I.; Azzam, S.; Claeys, M.; Goovaerts, E.; Pieters, L.; et al. Antiviral and antioxidant activity of flavonoids and proanthocyanidins from Crataegus sinaica. Planta Med. 2002, 68, 539–541. [Google Scholar] [CrossRef] [PubMed]
- Erdelmeier, C.A.; Cinatl, J., Jr.; Rabenau, H.; Doerr, H.W.; Biber, A.; Koch, E. Antiviral and antiphlogistic activities of hamamelis virginiana bark. Planta Med. 1996, 62, 241–245. [Google Scholar] [CrossRef] [PubMed]
- Danaher, R.J.; Wang, C.; Dai, J.; Mumper, R.J.; Miller, C.S. Antiviral effects of blackberry extract against herpes simplex virus type 1. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2011, 112, e31–e35. [Google Scholar] [CrossRef][Green Version]
- Cheng, H.Y.; Lin, C.C.; Lin, T.C. Antiviral properties of prodelphinidin b-2 3’-o-gallate from green tea leaf. Antivir. Chem. Chemother. 2002, 13, 223–229. [Google Scholar] [CrossRef]
- Connolly, S.A.; Jardetzky, T.S.; Longnecker, R. The structural basis of herpesvirus entry. Nature reviews. Microbiology 2021, 19, 110–121. [Google Scholar]
- Waymack, J.R.; Sundareshan, V. Acquired Immune Deficiency Syndrome; StatPearls Publishing LLC.: Tampa, FL, USA, 2022. [Google Scholar]
- Laila, U.; Akram, M.; Shariati, M.A.; Hashmi, A.M.; Akhtar, N.; Tahir, I.M.; Ghauri, A.O.; Munir, N.; Riaz, M.; Akhter, N.; et al. Role of medicinal plants in hiv/aids therapy. Clin. Exp. Pharmacol. Physiol. 2019, 46, 1063–1073. [Google Scholar] [CrossRef]
- Olubiyi, O.O.; Idowu, T.O.; Ogundaini, A.O.; Orhuah, G. Computational prospecting for the pharmacological mechanism of activity: Hiv-1 inhibition by ixoratannin a-2. Curr. Comput. Aided Drug Des. 2020, 16, 376–388. [Google Scholar] [CrossRef]
- Zai, W.; Hu, K.; Ye, J.; Ding, J.; Huang, C.; Li, Y.; Fang, Z.; Wu, M.; Wang, C.; Chen, J.; et al. Long-term hepatitis b virus infection induces cytopathic effects in primary human hepatocytes, and can be partially reversed by antiviral therapy. Microbiol. Spectr. 2022, 10, e0132821. [Google Scholar] [CrossRef]
- Jose-Abrego, A.; Rivera-Iñiguez, I.; Torres-Reyes, L.A.; Roman, S. Anti-hepatitis b virus activity of food nutrients and potential mechanisms of action. Ann. Hepatol. 2022, 100766. [Google Scholar] [CrossRef] [PubMed]
- Tsukuda, S.; Watashi, K.; Hojima, T.; Isogawa, M.; Iwamoto, M.; Omagari, K.; Suzuki, R.; Aizaki, H.; Kojima, S.; Sugiyama, M.; et al. A new class of hepatitis b and d virus entry inhibitors, proanthocyanidin and its analogs, that directly act on the viral large surface proteins. Hepatology 2017, 65, 1104–1116. [Google Scholar] [CrossRef] [PubMed]
- Ishida, Y.; Takeshita, M.; Kataoka, H. Functional foods effective for hepatitis c: Identification of oligomeric proanthocyanidin and its action mechanism. World J. Hepatol. 2014, 6, 870–879. [Google Scholar] [CrossRef]
- Li, S.; Kodama, E.N.; Inoue, Y.; Tani, H.; Matsuura, Y.; Zhang, J.; Tanaka, T.; Hattori, T. Procyanidin b1 purified from cinnamomi cortex suppresses hepatitis c virus replication. Antivir. Chem. Chemother. 2010, 20, 239–248. [Google Scholar] [CrossRef] [PubMed]
- Bishop, R.; Kirkwood, C. Enteric viruses. In Encyclopedia of Virology, 3rd ed.; Mahy, B.W.J., Van Regenmortel, M.H.V., Eds.; Academic Press: London, UK, 2008; pp. 116–123. [Google Scholar]
- Eckardt, A.J.; Baumgart, D.C. Viral gastroenteritis in adults. Recent Pat. Anti Infect. Drug Discov. 2011, 6, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Fong, T.T.; Lipp, E.K. Enteric viruses of humans and animals in aquatic environments: Health risks, detection, and potential water quality assessment tools. Microbiol. Mol. Biol. Rev. 2005, 69, 357–371. [Google Scholar] [CrossRef] [PubMed]
- Bernier, C.; Goetz, C.; Jubinville, E.; Jean, J. The new face of berries: A review of their antiviral proprieties. Foods 2021, 11, 102. [Google Scholar] [CrossRef]
- Iwasawa, A.; Niwano, Y.; Mokudai, T.; Kohno, M. Antiviral activity of proanthocyanidin against feline calicivirus used as a surrogate for noroviruses, and coxsackievirus used as a representative enteric virus. Biocontrol Sci. 2009, 14, 107–111. [Google Scholar] [CrossRef]
- Clark, A.; Black, R.; Tate, J.; Roose, A.; Kotloff, K.; Lam, D.; Blackwelder, W.; Parashar, U.; Lanata, C.; Kang, G.; et al. Estimating global, regional and national rotavirus deaths in children aged < 5 years: Current approaches, new analyses and proposed improvements. PLoS ONE 2017, 12, e0183392. [Google Scholar]
- Esona, M.D.; Gautam, R. Rotavirus. Clin. Lab. Med. 2015, 35, 363–391. [Google Scholar] [CrossRef]
- Green, K.Y. Caliciviridae. In The Noroviruses, 6th ed.; Wolters Kluwer Health/Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2013; Volume 1. [Google Scholar]
- Lopman, B.A.; Steele, D.; Kirkwood, C.D.; Parashar, U.D. The vast and varied global burden of norovirus: Prospects for prevention and control. PLoS Med. 2016, 13, e1001999. [Google Scholar] [CrossRef]
- de Graaf, M.; van Beek, J.; Koopmans, M.P. Human norovirus transmission and evolution in a changing world. Nat. Reviews. Microbiol. 2016, 14, 421–433. [Google Scholar] [CrossRef] [PubMed]
- Karst, S.M.; Wobus, C.E.; Goodfellow, I.G.; Green, K.Y.; Virgin, H.W. Advances in norovirus biology. Cell Host Microbe 2014, 15, 668–680. [Google Scholar] [CrossRef] [PubMed]
- Estes, M.K.; Ettayebi, K.; Tenge, V.R.; Murakami, K.; Karandikar, U.; Lin, S.C.; Ayyar, B.V.; Cortes-Penfield, N.W.; Haga, K.; Neill, F.H.; et al. Human norovirus cultivation in nontransformed stem cell-derived human intestinal enteroid cultures: Success and challenges. Viruses 2019, 11, 638. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Bae, S.Y.; Oh, M.; Seok, J.H.; Kim, S.; Chung, Y.B.; Gowda, K.G.; Mun, J.Y.; Chung, M.S.; Kim, K.H. Antiviral effects of black raspberry (Rubus coreanus) seed extract and its polyphenolic compounds on norovirus surrogates. Biosci. Biotechnol. Biochem. 2016, 80, 1196–1204. [Google Scholar] [CrossRef]
- Joshi, S.; Howell, A.B.; D’Souza, D.H. Blueberry proanthocyanidins against human norovirus surrogates in model foods and under simulated gastric conditions. Food Microbiol. 2017, 63, 263–267. [Google Scholar] [CrossRef] [PubMed]
- Kutter, J.S.; Spronken, M.I.; Fraaij, P.L.; Fouchier, R.A.; Herfst, S. Transmission routes of respiratory viruses among humans. Curr. Opin. Virol. 2018, 28, 142–151. [Google Scholar] [CrossRef]
- Hodinka, R.L. Respiratory RNA viruses. Microbiol. Spectr. 2016, 4. [Google Scholar] [CrossRef]
- Moriyama, M.; Hugentobler, W.J.; Iwasaki, A. Seasonality of respiratory viral infections. Annu. Rev. Virol. 2020, 7, 83–101. [Google Scholar] [CrossRef]
- Roschek, B., Jr.; Fink, R.C.; McMichael, M.D.; Li, D.; Alberte, R.S. Elderberry flavonoids bind to and prevent h1n1 infection in vitro. Phytochemistry 2009, 70, 1255–1261. [Google Scholar] [CrossRef]
- Ikuta, K.; Hashimoto, K.; Kaneko, H.; Mori, S.; Ohashi, K.; Suzutani, T. Anti-viral and anti-bacterial activities of an extract of blackcurrants (Ribes nigrum L.). Microbiol. Immunol. 2012, 56, 805–809. [Google Scholar] [CrossRef]
- Lin, L.T.; Hsu, W.C.; Lin, C.C. Antiviral natural products and herbal medicines. J. Tradit. Complement. Med. 2014, 4, 24–35. [Google Scholar] [CrossRef] [PubMed]
- Global Influenza Strategy 2019–2030; World Health Organization: Geneva, Switzerland, 2019.
- Krammer, F.; Smith, G.J.D.; Fouchier, R.A.M.; Peiris, M.; Kedzierska, K.; Doherty, P.C.; Palese, P.; Shaw, M.L.; Treanor, J.; Webster, R.G.; et al. Influenza. Nat. Rev. Dis. Prim. 2018, 4, 3. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Available online: https://www.Who.Int/en/news-room/fact-sheets/detail/influenza-(seasonal) (accessed on 22 September 2022).
- Derksen, A.; Hensel, A.; Hafezi, W.; Herrmann, F.; Schmidt, T.J.; Ehrhardt, C.; Ludwig, S.; Kühn, J. 3-o-galloylated procyanidins from rumex acetosa l. Inhibit the attachment of influenza a virus. PLoS ONE 2014, 9, e110089. [Google Scholar] [CrossRef] [PubMed]
- Sekizawa, H.; Ikuta, K.; Mizuta, K.; Takechi, S.; Suzutani, T. Relationship between polyphenol content and anti-influenza viral effects of berries. J. Sci. Food Agric. 2013, 93, 2239–2241. [Google Scholar] [CrossRef]
- World Health Organization. Available online: https://covid19.Who.Int (accessed on 23 September 2022).
- Hu, B.; Guo, H.; Zhou, P.; Shi, Z.L. Characteristics of SARS-CoV-2 and COVID-19. Nat. Rev. Microbiol. 2021, 19, 141–154. [Google Scholar] [CrossRef]
- Martella, V.; Elia, G.; Buonavoglia, C. Canine distemper virus. Vet. Clin. N. Am. Small Anim. Pract. 2008, 38, 787–797. [Google Scholar] [CrossRef]
- Gallina, L.; Dal Pozzo, F.; Galligioni, V.; Bombardelli, E.; Scagliarini, A. Inhibition of viral RNA synthesis in canine distemper virus infection by proanthocyanidin a2. Antivir. Res. 2011, 92, 447–452. [Google Scholar] [CrossRef]
- Lunney, J.K.; Fang, Y.; Ladinig, A.; Chen, N.; Li, Y.; Rowland, B.; Renukaradhya, G.J. Porcine reproductive and respiratory syndrome virus (prrsv): Pathogenesis and interaction with the immune system. Annu. Rev. Anim. Biosci. 2016, 4, 129–154. [Google Scholar] [CrossRef]
- Zhang, M.; Wu, Q.; Chen, Y.; Duan, M.; Tian, G.; Deng, X.; Sun, Y.; Zhou, T.; Zhang, G.; Chen, W.; et al. Inhibition of proanthocyanidin a2 on porcine reproductive and respiratory syndrome virus replication in vitro. PLoS ONE 2018, 13, e0193309. [Google Scholar] [CrossRef]
- Salata, C.; Calistri, A.; Parolin, C.; Palù, G. Coronaviruses: A paradigm of new emerging zoonotic diseases. Pathog. Dis. 2019, 77, ftaa006. [Google Scholar] [CrossRef]
- Salata, C.; Calistri, A.; Parolin, C.; Baritussio, A.; Palù, G. Antiviral activity of cationic amphiphilic drugs. Expert Rev. Anti Infect. Ther. 2017, 15, 483–492. [Google Scholar] [CrossRef]
- Salata, C.; Calistri, A.; Alvisi, G.; Celestino, M.; Parolin, C.; Palù, G. Ebola virus entry: From molecular characterization to drug discovery. Viruses 2019, 11, 274. [Google Scholar] [CrossRef]
- Liu, C.H.; Hu, Y.T.; Wong, S.H.; Lin, L.T. Therapeutic strategies against ebola virus infection. Viruses 2022, 14, 579. [Google Scholar] [CrossRef]
- Hansen, F.; Feldmann, H.; Jarvis, M.A. Targeting ebola virus replication through pharmaceutical intervention. Expert Opin. Investig. Drugs 2021, 30, 201–226. [Google Scholar] [CrossRef]
- Du, R.; Cui, Q.; Caffrey, M.; Rong, L. Ebola virus entry inhibitors. Adv. Exp. Med. Biol. 2022, 1366, 155–170. [Google Scholar]
- Cui, Q.; Du, R.; Anantpadma, M.; Schafer, A.; Hou, L.; Tian, J.; Davey, R.A.; Cheng, H.; Rong, L. Identification of ellagic acid from plant rhodiola rosea l. As an anti-ebola virus entry inhibitor. Viruses 2018, 10, 152. [Google Scholar] [CrossRef] [PubMed]
- Reid, S.P.; Shurtleff, A.C.; Costantino, J.A.; Tritsch, S.R.; Retterer, C.; Spurgers, K.B.; Bavari, S. Hspa5 is an essential host factor for ebola virus infection. Antivir. Res. 2014, 109, 171–174. [Google Scholar] [CrossRef]
- Tsang, N.Y.; Li, W.F.; Varhegyi, E.; Rong, L.; Zhang, H.J. Ebola entry inhibitors discovered from Maesa perlarius. Int. J. Mol. Sci. 2022, 23, 2620. [Google Scholar] [CrossRef]
- Altamish, M.; Khan, M.; Baig, M.S.; Pathak, B.; Rani, V.; Akhtar, J.; Khan, A.A.; Ahmad, S.; Krishnan, A. Therapeutic potential of medicinal plants against dengue infection: A mechanistic viewpoint. ACS Omega 2022, 7, 24048–24065. [Google Scholar] [CrossRef]
- Kimmel, E.M.; Jerome, M.; Holderness, J.; Snyder, D.; Kemoli, S.; Jutila, M.A.; Hedges, J.F. Oligomeric procyanidins stimulate innate antiviral immunity in dengue virus infected human PBMCS. Antivir. Res. 2011, 90, 80–86. [Google Scholar] [CrossRef]
- Andreolla, A.P.; Borges, A.A.; Bordignon, J.; Duarte Dos Santos, C.N. Mayaro virus: The state-of-the-art for antiviral drug development. Viruses 2022, 14, 1787. [Google Scholar] [CrossRef]
- Mello, M.V.P.; Domingos, T.F.S.; Ferreira, D.F.; Ribeiro, M.M.J.; Ribeiro, T.P.; Rodrigues, C.R.; Souza, A.M.T. Antiviral drug discovery and development for mayaro fever—What do we have so far? Mini Rev. Med. Chem. 2020, 20, 921–928. [Google Scholar] [CrossRef]
- Ferraz, A.C.; Almeida, L.T.; da Silva Caetano, C.C.; da Silva Menegatto, M.B.; Souza Lima, R.L.; de Senna, J.P.N.; de Oliveira Cardoso, J.M.; Perucci, L.O.; Talvani, A.; Geraldo de Lima, W.; et al. Hepatoprotective, antioxidant, anti-inflammatory, and antiviral activities of silymarin against mayaro virus infection. Antivir. Res. 2021, 194, 105168. [Google Scholar] [CrossRef]
- Kuehnert, P.A.; Stefan, C.P.; Badger, C.V.; Ricks, K.M. Crimean-congo hemorrhagic fever virus (cchfv): A silent but widespread threat. Curr. Trop. Med. Rep. 2021, 8, 141–147. [Google Scholar] [CrossRef]
- Shahhosseini, N.; Wong, G.; Babuadze, G.; Camp, J.V.; Ergonul, O.; Kobinger, G.P.; Chinikar, S.; Nowotny, N. Crimean-congo hemorrhagic fever virus in Asia, Africa and Europe. Microorganisms 2021, 9, 1907. [Google Scholar] [CrossRef]
- Mehand, M.S.; Al-Shorbaji, F.; Millett, P.; Murgue, B. The who r&d blueprint: 2018 review of emerging infectious diseases requiring urgent research and development efforts. Antivir. Res. 2018, 159, 63–67. [Google Scholar]
- Dai, S.; Deng, F.; Wang, H.; Ning, Y. Crimean-congo hemorrhagic fever virus: Current advances and future prospects of antiviral strategies. Viruses 2021, 13, 1195. [Google Scholar] [CrossRef]
- Harding, S.A. Condensed tannins: Arbiters of abiotic stress tolerance? Tree Physiol. 2019, 39, 341–344. [Google Scholar] [CrossRef]
- Liu, H.Y.; Howell, A.B.; Zhang, D.J.; Khoo, C. A randomized, double-blind, placebo-controlled pilot study to assess bacterial anti-adhesive activity in human urine following consumption of a cranberry supplement. Food Funct. 2019, 10, 7645–7652. [Google Scholar] [CrossRef]
- Terlizzi, M.E.; Gribaudo, G.; Maffei, M.E. Uropathogenic Escherichia coli (upec) infections: Virulence factors, bladder responses, antibiotic, and non-antibiotic antimicrobial strategies. Front. Microbiol. 2017, 8, 1566. [Google Scholar] [CrossRef]
- Bladé, C.; Arola, L.; Salvadó, M.-J. Hypolipidemic effects of proanthocyanidins and their underlying biochemical and molecular mechanisms. Mol. Nutr. Food Res. 2010, 54, 37–59. [Google Scholar] [CrossRef]
- Zhang, Y.Y.; Li, X.L.; Li, T.Y.; Li, M.Y.; Huang, R.M.; Li, W.; Yang, R.L. 3-(4-hydroxyphenyl)propionic acid, a major microbial metabolite of procyanidin a2, shows similar suppression of macrophage foam cell formation as its parent molecule. RSC Adv. 2018, 8, 6242–6250. [Google Scholar] [CrossRef]
- Andersen-Civil, A.I.S.; Arora, P.; Williams, A.R. Regulation of enteric infection and immunity by dietary proanthocyanidins. Front. Immunol. 2021, 12, 637603. [Google Scholar] [CrossRef]
- Martinez-Micaelo, N.; González-Abuín, N.; Terra, X.; Richart, C.; Ardèvol, A.; Pinent, M.; Blay, M. Omega-3 docosahexaenoic acid and procyanidins inhibit cyclo-oxygenase activity and attenuate nf-κb activation through a p105/p50 regulatory mechanism in macrophage inflammation. Biochem. J. 2011, 441, 653–663. [Google Scholar] [CrossRef]
- Anhê, F.F.; Varin, T.V.; Le Barz, M.; Desjardins, Y.; Levy, E.; Roy, D.; Marette, A. Gut microbiota dysbiosis in obesity-linked metabolic diseases and prebiotic potential of polyphenol-rich extracts. Curr. Obes. Rep. 2015, 4, 389–400. [Google Scholar] [CrossRef]
- Zheng, Y.Y.; Viswanathan, B.; Kesarwani, P.; Mehrotra, S. Dietary agents in cancer prevention: An immunological perspective. Photochem. Photobiol. 2012, 88, 1083–1098. [Google Scholar] [CrossRef]
- Kim, H.; Kim, J.Y.; Song, H.S.; Park, K.U.; Mun, K.C.; Ha, E. Grape seed proanthocyanidin extract inhibits interleukin-17-induced interleukin-6 production via mapk pathway in human pulmonary epithelial cells. Naunyn Schmiedebergs Arch. Pharmacol. 2011, 383, 555–562. [Google Scholar] [CrossRef]
- Dhanalakshmi, A.; Agarwal, R.; Agarwal, C. Inhibition of nf-kappa b pathway in grape seed extract-induced apoptotic death of human prostate carcinoma du145 cells. Int. J. Oncol. 2003, 23, 721–727. [Google Scholar]
- Chen, F.Z.; Wang, H.; Zhao, J.; Yan, J.J.; Meng, H.Y.; Zhan, H.L.; Chen, L.W.; Yuan, L.B. Grape seed proanthocyanidin inhibits monocrotaline-induced pulmonary arterial hypertension via attenuating inflammation: In vivo and in vitro studies. J. Nutr. Biochem. 2019, 67, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Charlton, A.J.; Baxter, N.J.; Khan, M.L.; Moir, A.J.; Haslam, E.; Davies, A.P.; Williamson, M.P. Polyphenol/peptide binding and precipitation. J. Agric. Food Chem. 2002, 50, 1593–1601. [Google Scholar] [CrossRef] [PubMed]
- Ebraihimnejad, H.; Burkholz, T.; Jacob, C. Flavanols and proanthocyanidins. In Recent Advances in Redox Active Plant and Microbial Products; Jacob, C., Kirsch, G., Slusarenko, A., Winyard, P.G., Burkholz, T., Eds.; Springer: London, UK, 2014; pp. 211–234. [Google Scholar]
- Ali, S.; Alam, M.; Khatoon, F.; Fatima, U.; Elasbali, A.M.; Adnan, M.; Islam, A.; Hassan, M.I.; Snoussi, M.; De Feo, V. Natural products can be used in therapeutic management of COVID-19: Probable mechanistic insights. Biomed. Pharmacother. 2022, 147, 112658. [Google Scholar] [CrossRef] [PubMed]
- Karim, Q.A.; Baxter, C. Microbicides for the prevention of sexually transmitted HIV infection. Expert Rev. Anti Infect. Ther. 2013, 11, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Obiero, J.; Mwethera, P.G.; Wiysonge, C.S. Topical microbicides for prevention of sexually transmitted infections. Cochrane Database Syst. Rev. 2012, 3, CD007961. [Google Scholar] [CrossRef]
- Sibille, G.; Pavan, M.; Mannino, G.; Frasson, I.; Salata, C.; Luganini, A.; Maffei, M.E.; Gribaudo, G. Manuscript in preparation. 2022. [Google Scholar]
- Howell, A.B.; Botto, H.; Combescure, C.; Blanc-Potard, A.B.; Gausa, L.; Matsumoto, T.; Tenke, P.; Sotto, A.; Lavigne, J.P. Dosage effect on uropathogenic Escherichia coli anti-adhesion activity in urine following consumption of cranberry powder standardized for proanthocyanidin content: A multicentric randomized double blind study. BMC Infect. Dis. 2010, 10, 94. [Google Scholar] [CrossRef] [PubMed]
- Blumberg, J.B.; Camesano, T.A.; Cassidy, A.; Kris-Etherton, P.; Howell, A.; Manach, C.; Ostertag, L.M.; Sies, H.; Skulas-Ray, A.; Vita, J.A. Cranberries and their bioactive constituents in human health. Adv. Nutr. 2013, 4, 618–632. [Google Scholar] [CrossRef]
- Silverman, R.; Keller, J.M.; Glassman, A.; Chalkidou, K. Tackling the Triple Transition in Global Health Procurement. Available online: https://www.Cgdev.Org/better-health-procurement (accessed on 12 October 2022).
- Schweitzer, S.O.; Comanor, W.S. Prices of pharmaceuticals in poor countries are much lower than in wealthy countries. Health Aff. 2011, 30, 1553–1561. [Google Scholar] [CrossRef]



| Natural Source | A-Type PACs | Properties | References |
|---|---|---|---|
| Adansonia digitata | dimers | antioxidant | [31] |
| Aesculus turbinata | procyanidins * | antioxidant | [32] |
| Aglaonema commutatum var. maculatum | trimers | chemical composition | [33] |
| Aglaonema crispum | dimers | chemical composition | [33] |
| Arachis hypogaea | dimers, trimers | cardiovascular diseases, dyslipidemia antioxidant prevention of pathogen infection chemical composition anti-inflammatory antioxidant | [34] [35] [36] [21,37,38,39,40] [41] [42] |
| Areca catechu | dimer | hypoglycemic | [43] |
| Calluna vulgaris | dimers, trimers | chemical composition | [44] |
| Cinnamomum cassia | dimers, oligomers | oxidative conversion of B- to A-procyanidins antidiabetic | [45] [46,47,48] |
| Cinnamomum japonica | oligomers | hypoglycemic | [48] |
| Cinnamomum tamala | oligomers | antidiabetic | [47,49] |
| Cinnamomum zeylanicum | trimers, tetramers | multidrug resistance, biofilm inhibitory activity antiasthmatic, antiallergic anti-inflammatory antiallergic attenuation of the reduction in glutamate uptake anti- vascular endothelial growth factor (VEGF) antidiabetic prevention of neurodegeneration | [50] [51] [52] [53] [54] [55] [56,57] [58] |
| Coffea arabica | trimers | chemical composition | [59] |
| Crataegus pinnatifida var. major | procyanidins | antioxidant | [60] |
| Dimocarpus longan | dimer | health-beneficial bioactivity | [61] |
| procyanidins | antioxidant | [62] | |
| Ecdysanthera utilis | monomers, dimers | immunomodulator | [63] |
| Ephedra equisetina | procyanidins | chemical composition | [64] |
| Ephedra intermedia | procyanidins | chemical composition | [64] |
| Ephedra sinica | procyanidins | chemical composition | [64,65] |
| Gaultheria procumbens | trimers | antioxidant | [66,67] |
| Ixora coccinea | dimers | antioxidant, antibacterial | [68] |
| Laurus nobilis | trimers | antioxidant | [69] |
| Litchi chinensis | dimers, trimers | chemical composition antioxidant cardioprotection alteration of oligomers in the gastrointestinal system bioavailability antioxidant absorption and urinary excretion bacterial bioconversions | [70,71,72] [73] [74,75] [76] [77] [78,79,80,81] [82] [83,84] |
| Lotus americanus | procyanidins | chemical composition | [85] |
| Malus domestica | dimers | antioxidant | [86] |
| Mandevilla moricandiana | trimers | antioxidant | [87] |
| Microbiota (faecal and gut) | procyanidins | Inability to cleave A-type linkages preventing of biofilm formation antioxidant degradation by human gut microbiota | [88,89] [90] [91] [92] |
| Paullinia cupana | trimers | Anti-inflammatory, antioxidant | [93,94] |
| Paullinia pinnata | trimers, tetramers | antihelminthic | [95,96] |
| Pelargonium sidoides | trimers | antiadhesive | [97] |
| Persea americana | dimers, trimers, tetramers, procyanidins | chemical composition | [98,99] |
| Pheonix dactylifera | dimers | chemical composition | [100] |
| Pinus massoniana | trimers, tetramers | increased modulus of elasticity of dentin | [101] |
| Pinus pinaster | dimers | antioxidant, bactericidal | [102] |
| Polygonum cuspidatum | dimers | chemical composition | [103,104] |
| Prunus domestica | dimers | chemical composition antioxidant | [99,105] [86] |
| Prunus dulcis | procyanidins | chemical composition | [106,107] |
| Prunus spinosa | dimers, trimers | chemical composition | [44,108] |
| Pteris vittata | procyanidins | antioxidant | [109] |
| Pyracantha fortuneana | procyanidins | antidiabetic | [110] |
| Pyrus pyrifolia | trimers | chemical composition | [111] |
| Rhizophora apiculata | monomers | chemical composition | [112] |
| Rhizophora mangle | procyanidins | chemical composition | [113] |
| Rhododendron ferrugineum | trimers | vitality and the proliferation rates of epithelial HaCaT keratinocytes | [114] |
| Rhododendron formosanum | trimers | induction of autophagy antioxidant | [115] [116] |
| Ribes nigrum | dimers | bacterial growth and cell adhesion | [117] |
| Rubus idaeus | procyanidins | chemical composition | [118] |
| Rumex obtusifolius | trimers | chemical composition | [119] |
| Spatholobus suberectus | procyanidins | antioxidants, inhibitor of breast cancer | [120] |
| Tectaria macrodonta | trimers | chemical composition | [33] |
| Theobroma cacao | procyanidins | chemical composition absorption antioxidant | [37,121,122] [123] [124] |
| Vaccinium ashei | dimers, dodecamers | chemical composition | [125] |
| Vaccinium consanguineum, | monomers, dimers, trimers, tetramers, procyanidins | chemical composition | [126] |
| Vaccinium corymbosum | trimers | antidiabetic | [127] |
| Vaccinium floribundum | monomers, dimers, trimers, tetramers, procyanidins | chemical composition | [126] |
| Vaccinium macrocarpon | monomers, dimers, trimers, tetramers, procyanidins | chemical composition urinary tract infections (UTIs) antiaging bioavailability transported across Caco-2 cells antioxidant cardiovascular health immune system | [30,128,129,130,131,132,133,134,135,136] [14,15,23,137,138,139,140,141,142,143,144,145,146,147] [148] [149] [150] [151] [152] [153] |
| Vaccinium myrtillus | dimers, trimers | antidiabetic chemical composition bacterial growth and cell adhesion | [127] [44,154] [117] |
| Vaccinium oxycoccus | monomers, dimers, trimers, tetramers, procyanidins | chemical composition | [135] |
| Vaccinium poasanum | monomers, dimers, trimers, tetramers, procyanidins | chemical composition | [126] |
| Vaccinium vitis-idaea | dimers, trimers | chemical composition | [44,135] |
| Vicia faba | dimers | chemical composition | [155] |
| Vitis vinifera | dimers, trimers, tetramers, procyanidins | chemical composition inhibition of alpha-glucosidase promotion of DNA repair in dendritic cells in UVB-exposed skin. decreases the progression of airway inflammation antioxidant control of lipid metabolism immune system | [156] [157] [158] [159] [160] [161,162,163,164,165,166] [167,168,169,170,171,172] |
| Natural Source | A-Type PACs | Virus and Mechanism of Action | References |
|---|---|---|---|
| Alpinia zerumbet | procyanidins * | influenza A virus, inhibition of attachment, virucidal | [173,174] |
| Chamaecrista nictitans | procyanidins | HSV-1 and HSV-2, NA | [175] |
| Cinnamomum cassia | dimers, oligomers | HIV-1, interaction with envelope glycoproteins | [176] |
| Cinnamomum zeylanicum | trimers, tetramers | HIV-1, inhibition of attachment HCV, inhibition of attachment SARS-CoV, virucidal | [177] [178] [179] |
| Ixora coccinea | trimers | HIV-1, inhibition of Vpu activity; HCV, NA | [180] |
| Litchi chinensis | dimers, trimers | HSV-1 and Coxsackie virus B3, NA | [181] |
| Pinus maritima | procyanidins | HIV-1, inhibition of entry and replication HCV, inhibition of replication | [182] [183] |
| Pomelia pinnata | dimers | HIV-1, inhibition of integrase activity | [184] |
| Sambucus nigra | dimers | HIV-1, interaction with envelope glycoproteins | [176] |
| Theobroma cacao | dimers | HSV and HIV, NA | [124] |
| Vaccinium macrocarpon | monomers, dimers, trimers, tetramers, procyanidins | nairovirus, inhibition of attachment | [185] |
| influenza A and B virus, inhibition of attachment | [186,187] | ||
| and entry, virucidal | |||
| HSV-1 and HSV-2, inhibition of entry | [188] | ||
| human norovirus surrogates: murine norovirus (MNV-1), feline calicivirus (FCV-F9), virucidal | [189,190] | ||
| reovirus, NA | [191] | ||
| rotavirus, inhibition of attachment, interaction with capsid proteins | [192,193] | ||
| Vaccinium myrtillus | dimers, trimers | SARS-CoV-2, inhibition of entry and replication | [194] |
| HA, NA | [195] | ||
| HCV, inhibition of replication | [196] | ||
| Vitis vinifera | dimers, trimers, tetramers, procyanidins | rotavirus, affecting virion integrity HIV-1, inhibition of entry by down-modulation of co-receptors | [193] [197] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maffei, M.E.; Salata, C.; Gribaudo, G. Tackling the Future Pandemics: Broad-Spectrum Antiviral Agents (BSAAs) Based on A-Type Proanthocyanidins. Molecules 2022, 27, 8353. https://doi.org/10.3390/molecules27238353
Maffei ME, Salata C, Gribaudo G. Tackling the Future Pandemics: Broad-Spectrum Antiviral Agents (BSAAs) Based on A-Type Proanthocyanidins. Molecules. 2022; 27(23):8353. https://doi.org/10.3390/molecules27238353
Chicago/Turabian StyleMaffei, Massimo E., Cristiano Salata, and Giorgio Gribaudo. 2022. "Tackling the Future Pandemics: Broad-Spectrum Antiviral Agents (BSAAs) Based on A-Type Proanthocyanidins" Molecules 27, no. 23: 8353. https://doi.org/10.3390/molecules27238353
APA StyleMaffei, M. E., Salata, C., & Gribaudo, G. (2022). Tackling the Future Pandemics: Broad-Spectrum Antiviral Agents (BSAAs) Based on A-Type Proanthocyanidins. Molecules, 27(23), 8353. https://doi.org/10.3390/molecules27238353