Microfluidic Synthesis of the Tumor Microenvironment-Responsive Nanosystem for Type-I Photodynamic Therapy
Abstract
:1. Introduction
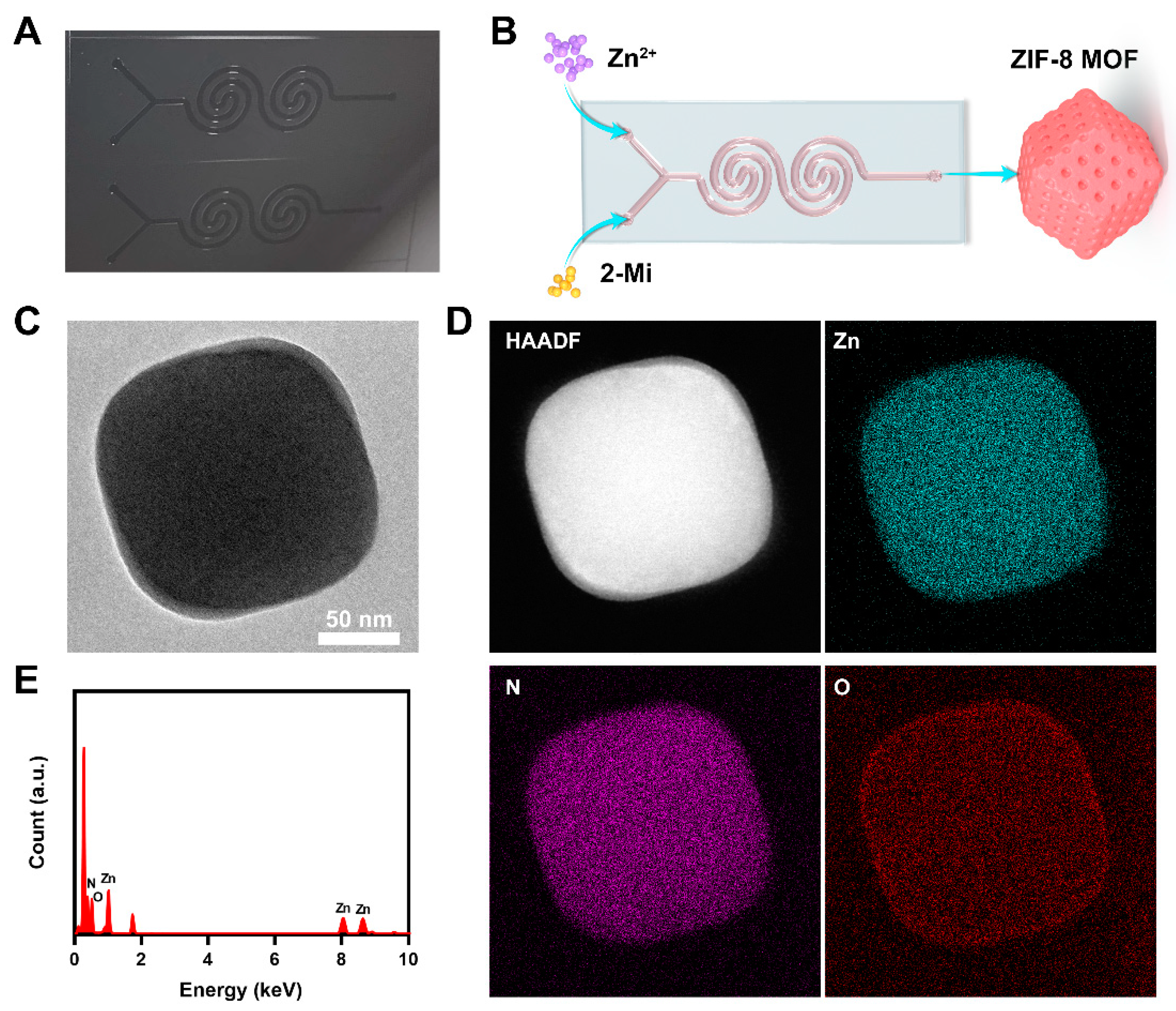
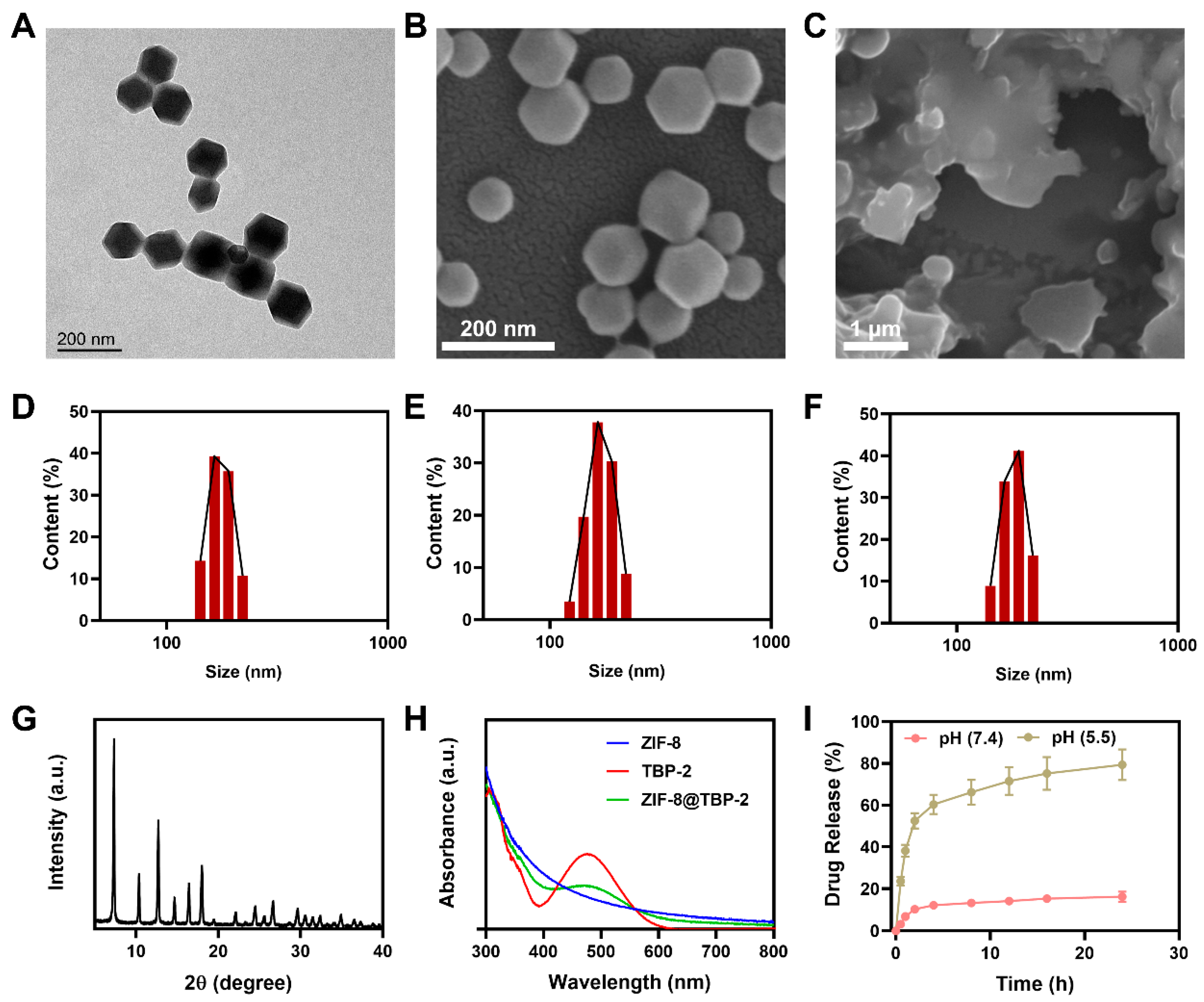
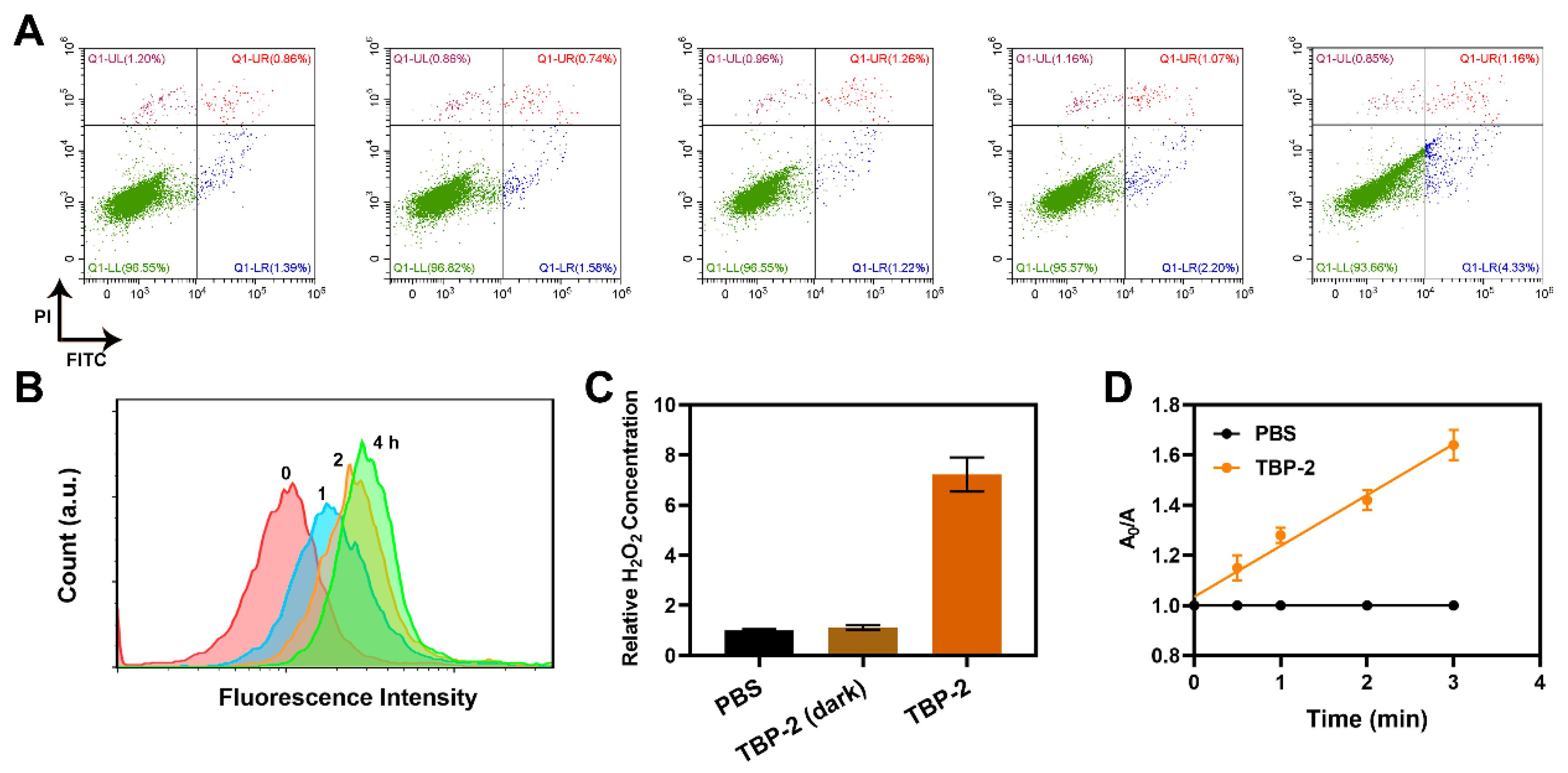
2. Results and Discussion
3. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Ho, D. Artificial intelligence in cancer therapy. Science 2020, 367, 982–983. [Google Scholar] [CrossRef] [PubMed]
- Ai, K.; Huang, J.; Xiao, Z.; Yang, Y.; Bai, Y.; Peng, J. Localized surface plasmon resonance properties and biomedical applications of copper selenide nanomaterials. Mater. Today Chem. 2021, 20, 100402. [Google Scholar] [CrossRef]
- Zhu, Y.; Shi, H.; Li, T.; Yu, J.; Guo, Z.; Cheng, J.; Liu, Y. A Dual Functional Nanoreactor for Synergistic Starvation and Photodynamic Therapy. ACS Appl. Mater. Interfaces 2020, 12, 18309–18318. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Gong, L.; Zhu, X.; Zhu, S.; Wu, X.; Xue, T.; Yan, L.; Du, J.; Gu, Z. Transformable Gallium-Based Liquid Metal Nanoparticles for Tumor Radiotherapy Sensitization. Adv. Healthc. Mater. 2022, 11, e2102584. [Google Scholar] [CrossRef]
- Wang, Z.; Shao, D.; Chang, Z.; Lu, M.; Wang, Y.; Yue, J.; Yang, D.; Li, M.; Xu, Q.; Dong, W.F. Janus Gold Nanoplatform for Synergetic Chemoradiotherapy and Computed Tomography Imaging of Hepatocellular Carcinoma. ACS Nano 2017, 11, 12732–12741. [Google Scholar] [CrossRef]
- Zhu, D.; Zhang, J.; Luo, G.; Duo, Y.; Tang, B.Z. Bright Bacterium for Hypoxia-Tolerant Photodynamic Therapy Against Orthotopic Colon Tumors by an Interventional Method. Adv. Sci. 2021, 8, e2004769. [Google Scholar] [CrossRef]
- Zhang, T.; Zhang, J.; Wang, F.B.; Cao, H.; Zhu, D.; Chen, X.; Xu, C.; Yang, X.; Huang, W.; Wang, Z.; et al. Mitochondria-Targeting Phototheranostics by Aggregation-Induced NIR-II Emission Luminogens: Modulating Intramolecular Motion by Electron Acceptor Engineering for Multi-Modal Synergistic Therapy. Adv. Funct. Mater. 2022, 32, 2110526. [Google Scholar] [CrossRef]
- Li, Y.; Wu, Q.; Kang, M.; Song, N.; Wang, D.; Tang, B.Z. Boosting the photodynamic therapy efficiency by using stimuli-responsive and AIE-featured nanoparticles. Biomaterials 2020, 232, 119749. [Google Scholar] [CrossRef]
- Zhu, D.; Zhang, T.; Li, Y.; Huang, C.; Suo, M.; Xia, L.; Xu, Y.; Li, G.; Tang, B.Z. Tumor-derived exosomes co-delivering aggregation-induced emission luminogens and proton pump inhibitors for tumor glutamine starvation therapy and enhanced type-I photodynamic therapy. Biomaterials 2022, 283, 121462. [Google Scholar] [CrossRef]
- Zhu-Ge, X.; Xi, D.-M.; Zhang, S.-S. Multimodal tumor therapy based on chemodynamic therapy. Chin. J. Anal. Chem. 2022, 50, 100121. [Google Scholar] [CrossRef]
- Zhao, T.; Wu, W.; Sui, L.; Huang, Q.; Nan, Y.; Liu, J.; Ai, K. Reactive oxygen species-based nanomaterials for the treatment of myocardial ischemia reperfusion injuries. Bioact. Mater. 2022, 7, 47–72. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Gong, Z.; Li, Z.; Wang, J.; Zhang, J.; Zhao, Z.; Zhang, P.; Zheng, S.; Miron, R.J.; Yuan, Q.; et al. Target Reprogramming Lysosomes of CD8+ T Cells by a Mineralized Metal-Organic Framework for Cancer Immunotherapy. Adv. Mater. 2021, 33, e2100616. [Google Scholar] [CrossRef]
- Su, L.; Wu, Q.; Tan, L.; Huang, Z.; Fu, C.; Ren, X.; Xia, N.; Chen, Z.; Ma, X.; Lan, X.; et al. High Biocompatible ZIF-8 Coated by ZrO2 for Chemo-microwave Thermal Tumor Synergistic Therapy. ACS Appl. Mater. Interfaces 2019, 11, 10520–10531. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Zhang, H.; Wu, J.; Zhai, G.; Li, Z.; Luan, Y.; Garg, S. CuS@MOF-Based Well-Designed Quercetin Delivery System for Chemo-Photothermal Therapy. ACS Appl. Mater. Interfaces 2018, 10, 34513–34523. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Xu, Z.; Jiang, Y.; Law, W.C.; Dong, B.; Zeng, X.; Ma, M.; Xu, G.; Zou, J.; Yang, C. Metal organic framework-coated gold nanorod as an on-demand drug delivery platform for chemo-photothermal cancer therapy. J. Nanobiotechnol. 2021, 19, 219. [Google Scholar] [CrossRef] [PubMed]
- Ren, S.Z.; Wang, B.; Zhu, X.H.; Zhu, D.; Liu, M.; Li, S.K.; Yang, Y.S.; Wang, Z.C.; Zhu, H.L. Oxygen Self-Sufficient Core-Shell Metal-Organic Framework-Based Smart Nanoplatform for Enhanced Synergistic Chemotherapy and Photodynamic Therapy. ACS Appl. Mater. Interfaces 2020, 12, 24662–24674. [Google Scholar] [CrossRef] [PubMed]
- Geng, S.; Lou, R.; Yin, Q.; Li, S.; Yang, R.; Zhou, J. Reshaping the tumor microenvironment for increasing the distribution of glucose oxidase in tumor and inhibiting metastasis. J. Mater. Chem. B 2021, 9, 1424–1431. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Z.; Zeng, W.; Wang, Z.; Liu, C.; Zeng, N.; Zhong, K.; Jiang, D.; Wu, Y. A Novel H2O2 Generator for Tumor Chemotherapy-Enhanced CO Gas Therapy. Front. Oncol. 2021, 11, 738567. [Google Scholar] [CrossRef]
- Balachandran, Y.L.; Li, X.; Jiang, X. Integrated Microfluidic Synthesis of Aptamer Functionalized Biozeolitic Imidazolate Framework (BioZIF-8) Targeting Lymph Node and Tumor. Nano Lett. 2021, 21, 1335–1344. [Google Scholar] [CrossRef]
- Wang, J.; Sui, L.; Huang, J.; Miao, L.; Nie, Y.; Wang, K.; Yang, Z.; Huang, Q.; Gong, X.; Nan, Y.; et al. MoS2-based nanocomposites for cancer diagnosis and therapy. Bioact. Mater. 2021, 6, 4209–4242. [Google Scholar] [CrossRef]
- Huang, J.; Huang, Q.; Liu, M.; Chen, Q.; Ai, K. Emerging Bismuth Chalcogenides Based Nanodrugs for Cancer Radiotherapy. Front. Pharmacol. 2022, 13, 844037. [Google Scholar] [CrossRef] [PubMed]
- Lengert, E.V.; Trushina, D.B.; Soldatov, M.; Ermakov, A.V. Microfluidic Synthesis and Analysis of Bioinspired Structures Based on CaCO3 for Potential Applications as Drug Delivery Carriers. Pharmaceutics 2022, 14, 139. [Google Scholar] [CrossRef] [PubMed]
- Cai, Q.; Castagnola, V.; Boselli, L.; Moura, A.; Lopez, H.; Zhang, W.; de Araujo, J.M.; Dawson, K.A. A microfluidic approach for synthesis and kinetic profiling of branched gold nanostructures. Nanoscale Horiz. 2022, 7, 288–298. [Google Scholar] [CrossRef] [PubMed]
- Hao, N.; Xu, Z.; Nie, Y.; Jin, C.; Closson, A.B.; Zhang, M.; Zhang, J.X.J. Microfluidics-enabled rational design of ZnO micro-/nanoparticles with enhanced photocatalysis, cytotoxicity, and piezoelectric properties. Chem. Eng. J. 2019, 378, 122222. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Ma, P.; Kim, D.H.; Liu, B.F.; Demirci, U. Towards Microfluidic-Based Exosome Isolation and Detection for Tumor Therapy. Nano Today 2021, 37, 101066. [Google Scholar] [CrossRef]
- Cheng, R.; Jiang, L.; Gao, H.; Liu, Z.; Makila, E.; Wang, S.; Saiding, Q.; Xiang, L.; Tang, X.; Shi, M.; et al. A pH-Responsive Cluster Metal-organic Framework Nanoparticle for Enhanced Tumor Accumulation and Antitumor Effect. Adv. Mater. 2022, 34, e2203915. [Google Scholar] [CrossRef]
- Liu, Y.; Luo, J.; Liu, Y.; Liu, W.; Yu, G.; Huang, Y.; Yang, Y.; Chen, X.; Chen, T. Brain-Targeted Biomimetic Nanodecoys with Neuroprotective Effects for Precise Therapy of Parkinson’s Disease. ACS Cent. Sci. 2022, 8, 1336–1349. [Google Scholar] [CrossRef]
- Zhu, D.; Chen, H.; Huang, C.; Li, G.; Wang, X.; Jiang, W.; Fan, K. H2O2 Self-Producing Single-Atom Nanozyme Hydrogels as Light-Controlled Oxidative Stress Amplifier for Enhanced Synergistic Therapy by Transforming “Cold” Tumors. Adv. Funct. Mater. 2022, 32, 2110268. [Google Scholar] [CrossRef]
- Liu, Y.; Luo, J.; Chen, X.; Liu, W.; Chen, T. Cell Membrane Coating Technology: A Promising Strategy for Biomedical Applications. Nano-Micro Lett. 2019, 11, 100. [Google Scholar] [CrossRef] [Green Version]
- Yang, R.; Ouyang, Z.; Guo, H.; Qu, J.; Xia, J.; Shen, M.; Shi, X. Microfluidic synthesis of intelligent nanoclusters of ultrasmall iron oxide nanoparticles with improved tumor microenvironment regulation for dynamic MR imaging-guided tumor photothermo-chemo-chemodynamic therapy. Nano Today 2022, 46, 101615. [Google Scholar] [CrossRef]
- Zhu, D.; Ling, R.; Chen, H.; Lyu, M.; Qian, H.; Wu, K.; Li, G.; Wang, X. Biomimetic copper single-atom nanozyme system for self-enhanced nanocatalytic tumor therapy. Nano Res. 2022, 15, 7320–7328. [Google Scholar] [CrossRef]
- Ye, P.; Li, F.; Zou, J.; Luo, Y.; Wang, S.; Lu, G.; Zhang, F.; Chen, C.; Long, J.; Jia, R.; et al. In Situ Generation of Gold Nanoparticles on Bacteria-Derived Magnetosomes for Imaging-Guided Starving/Chemodynamic/Photothermal Synergistic Therapy against Cancer. Adv. Funct. Mater. 2022, 32, 2110063. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, C.; Chen, M.; Du, L.; Xiang, J.; Jiang, D.; Liu, W. Microfluidic Synthesis of the Tumor Microenvironment-Responsive Nanosystem for Type-I Photodynamic Therapy. Molecules 2022, 27, 8386. https://doi.org/10.3390/molecules27238386
Huang C, Chen M, Du L, Xiang J, Jiang D, Liu W. Microfluidic Synthesis of the Tumor Microenvironment-Responsive Nanosystem for Type-I Photodynamic Therapy. Molecules. 2022; 27(23):8386. https://doi.org/10.3390/molecules27238386
Chicago/Turabian StyleHuang, Chunyu, Mingzhu Chen, Liang Du, Jingfeng Xiang, Dazhen Jiang, and Wei Liu. 2022. "Microfluidic Synthesis of the Tumor Microenvironment-Responsive Nanosystem for Type-I Photodynamic Therapy" Molecules 27, no. 23: 8386. https://doi.org/10.3390/molecules27238386
APA StyleHuang, C., Chen, M., Du, L., Xiang, J., Jiang, D., & Liu, W. (2022). Microfluidic Synthesis of the Tumor Microenvironment-Responsive Nanosystem for Type-I Photodynamic Therapy. Molecules, 27(23), 8386. https://doi.org/10.3390/molecules27238386





