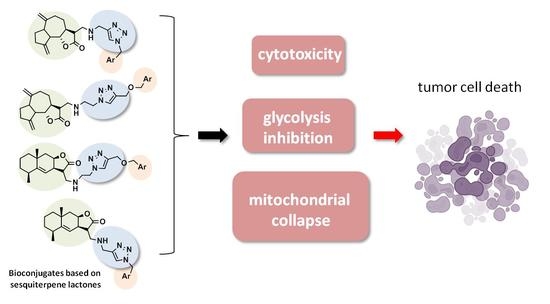
Design of Conjugates Based on Sesquiterpene Lactones with Polyalkoxybenzenes by “Click” Chemistry to Create Potential Anticancer Agents
Abstract
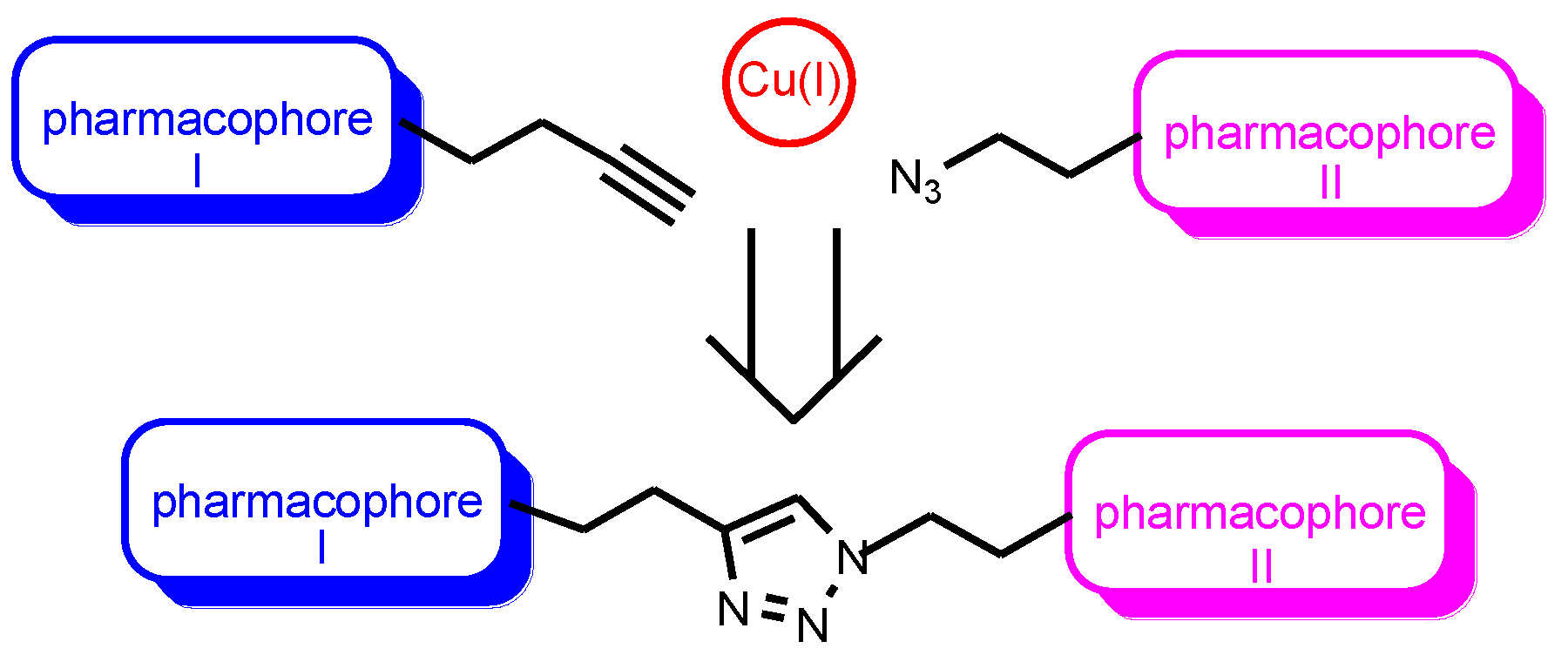
1. Introduction
2. Results and Discussion
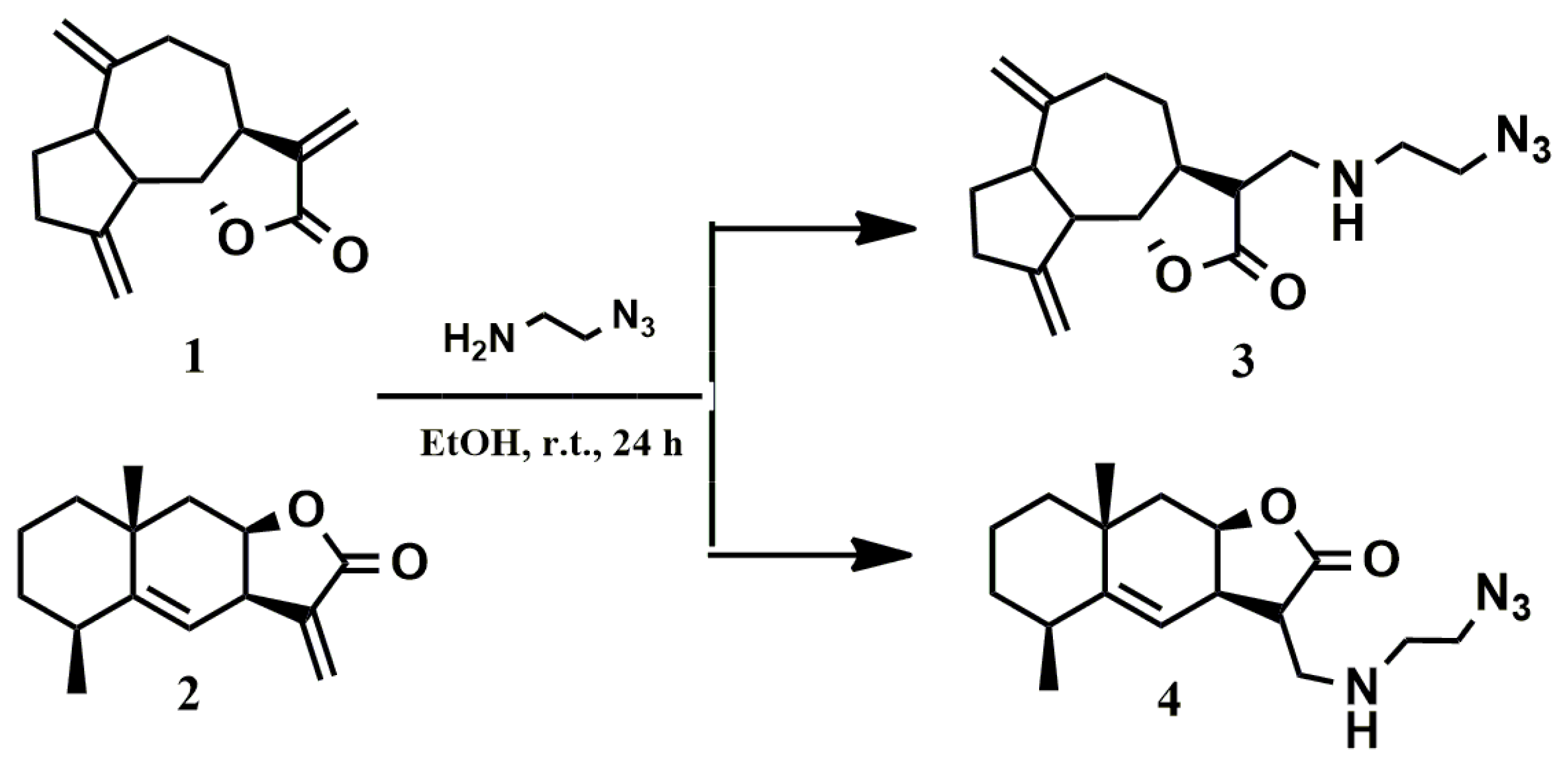
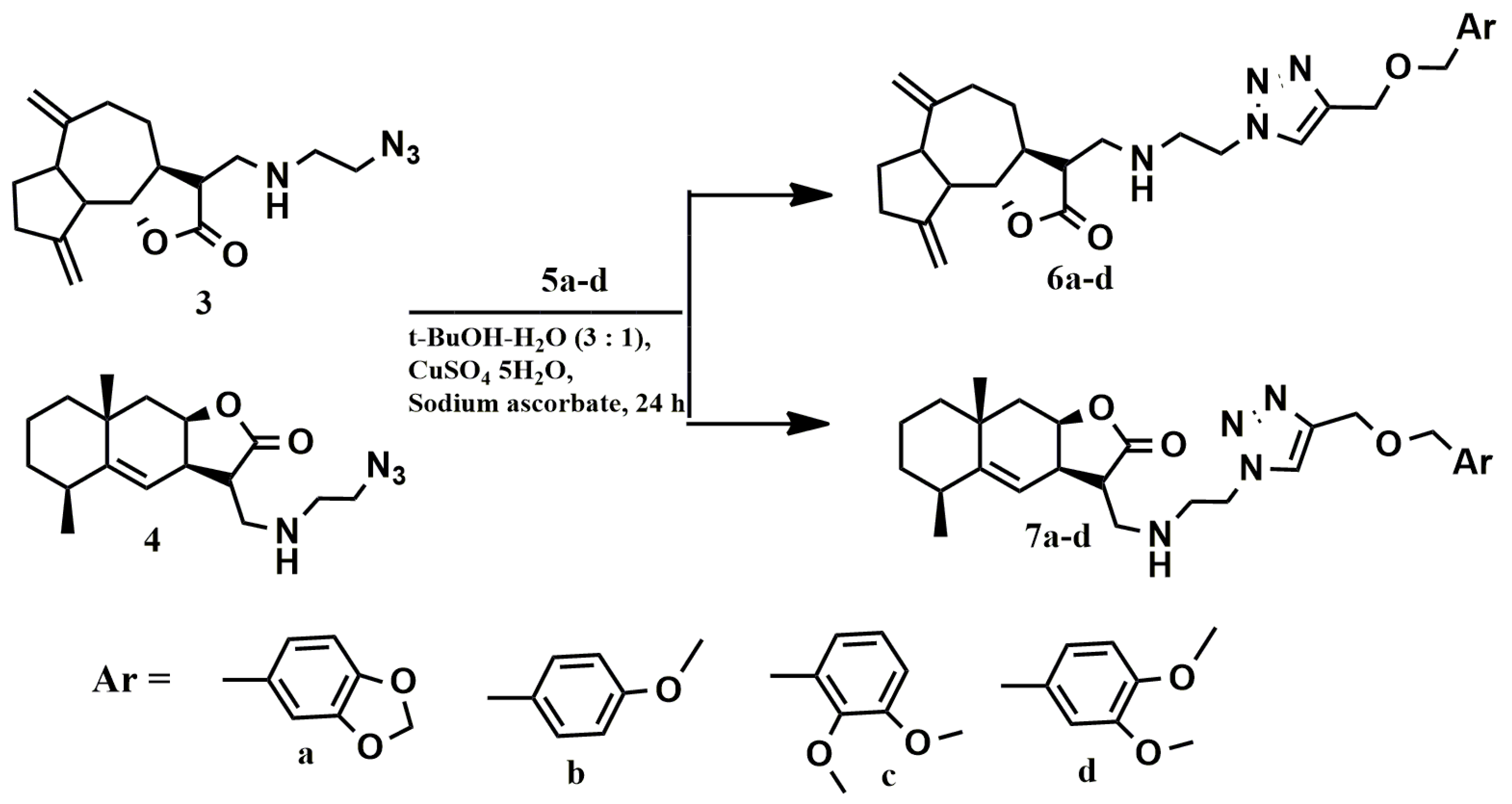
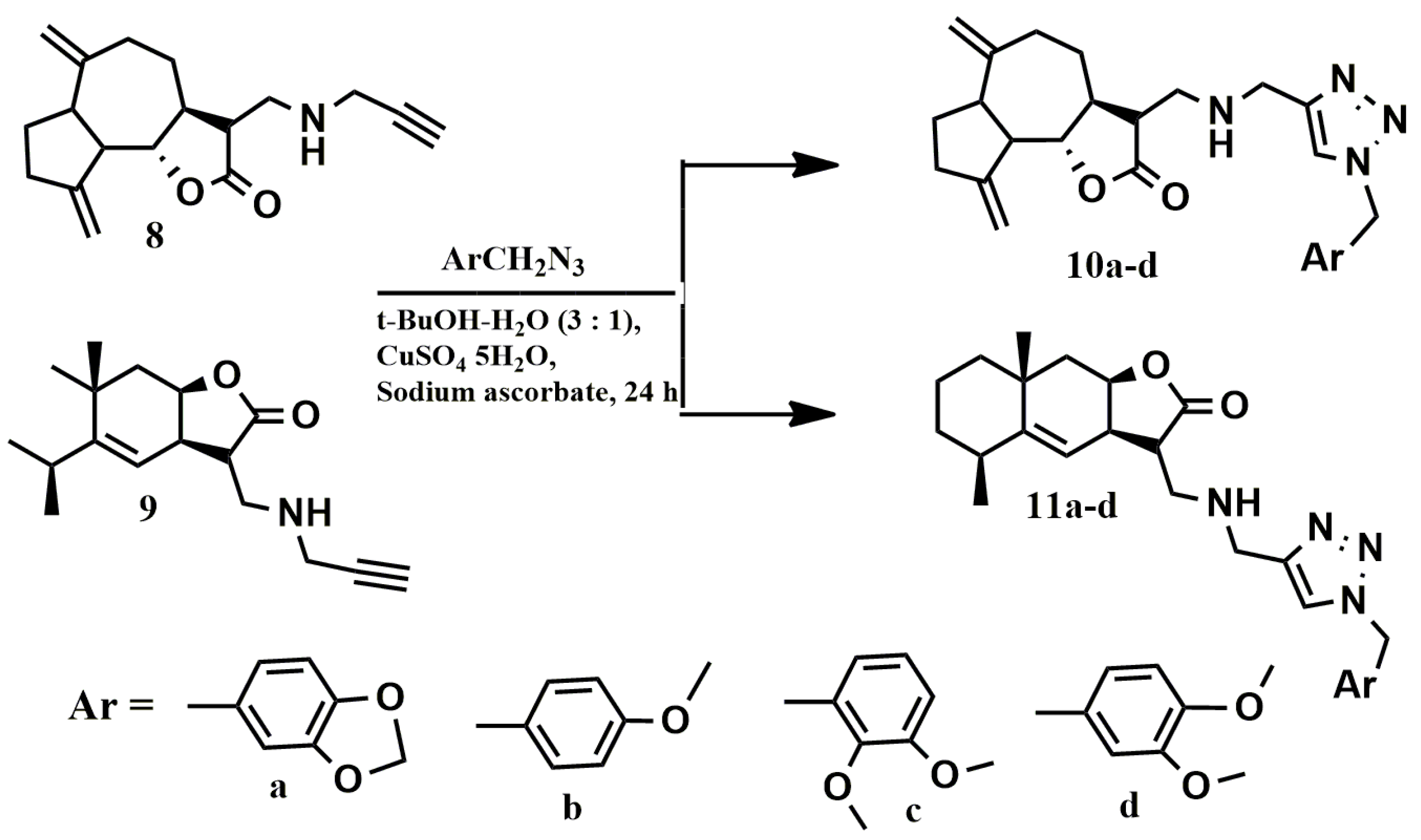
2.1. Chemistry
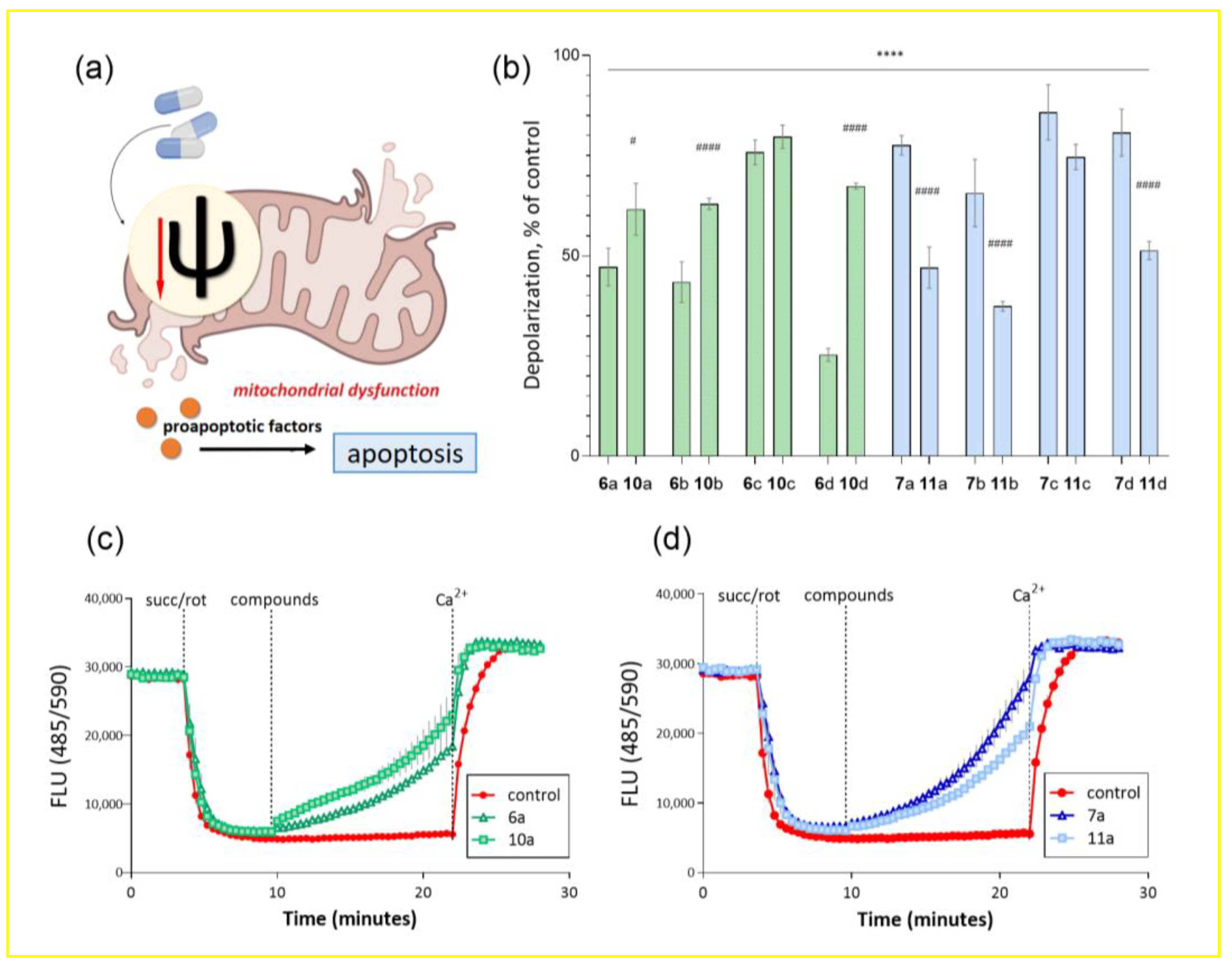
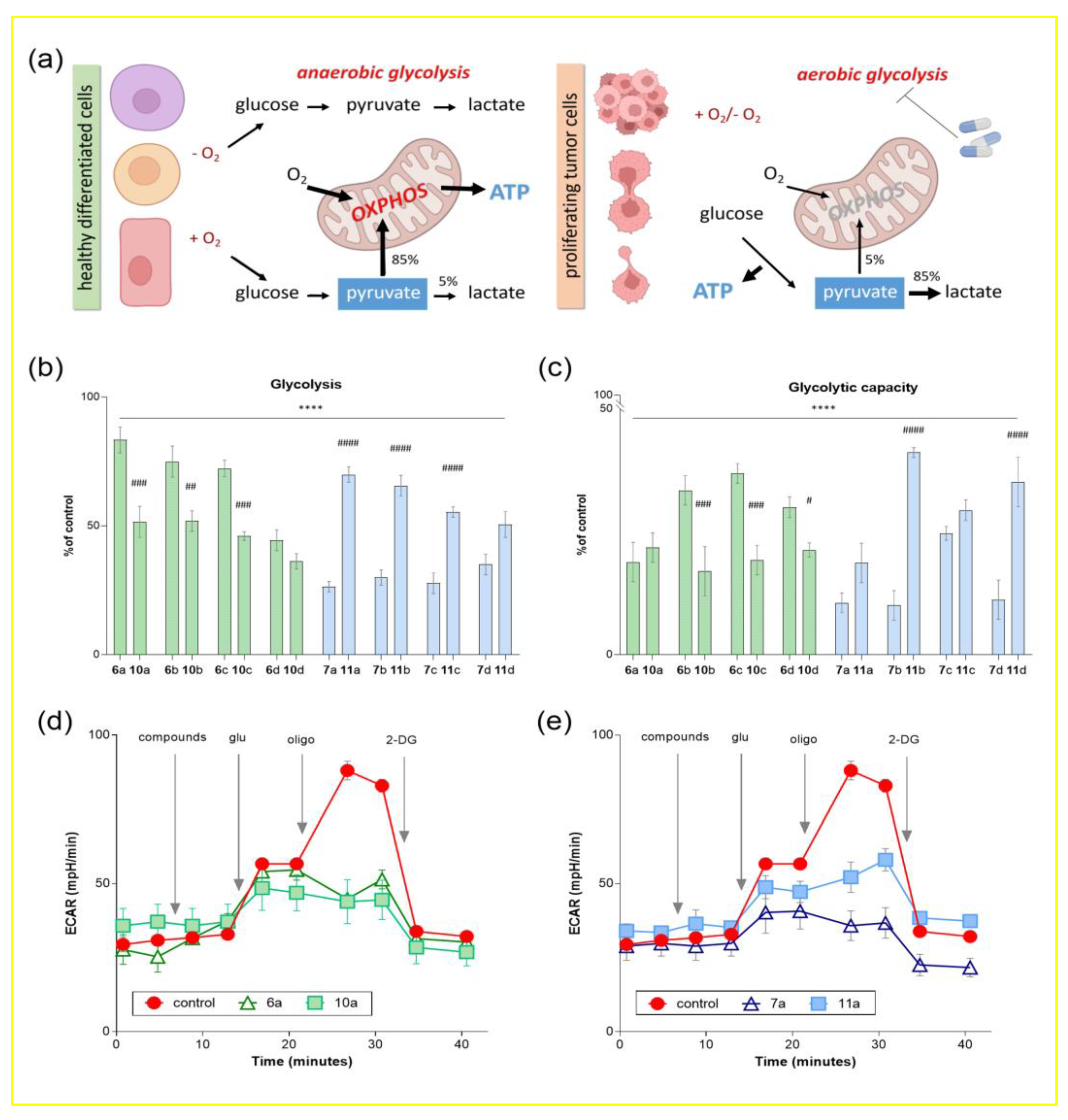
2.2. Biological Evaluation
2.3. Evaluation of Synthesized Compounds Pharmacokinetic Parameters
3. Materials and Methods
3.1. Reagents and Materials
3.2. General Procedure for the Synthesis of Azides (3, 4)
3.3. General Procedure for the Synthesis of Alkynes (5a–d)
3.4. General Procedure for the “Click” Reactions
3.5. Animals
3.6. Isolation of Rat Liver Mitochondria
3.7. Determination of Mitochondrial Membrane Potential
3.8. Cell Culture
3.9. Determination of Cell Viability
3.10. Glycolysis Flux Assay
3.11. Evaluation of Synthesized Compounds Pharmacokinetic Parameters
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Kingston, D.G.I. Taxol and its analogs. In Anticancer Agents from Natural Products, 2nd ed.; Cragg, G.M., Kingston, D.G.I., Newman, D.J., Eds.; CRC Press: Boca Raton, FL, USA, 2012; pp. 123–175. [Google Scholar] [CrossRef]
- Schiff, P.B.; Horwitz, S.B. Taxol stabilizes microtubules in mouse fibroblast cells. Proc. Natl. Acad. Sci. USA 1980, 77, 1561–1565. [Google Scholar] [CrossRef] [PubMed]
- Klayman, D.L. Qinghaosu (artemisinin): An antimalarial drug from China. Science 1985, 228, 1049–1055. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, P.M.; Posner, G.H. A medicinal chemistry perspective on artemisinin and related endoperoxides. J. Med. Chem. 2004, 47, 2945–2964. [Google Scholar] [CrossRef] [PubMed]
- Curry, E.A.; Murry, D.J.; Yoder, C.; Fife, K.; Armstrong, V.; Nakshatri, H.; O’Connell, M.; Sweeney, C.J. Phase I dose escalation trial of feverfew with standardized doses of parthenolide in patients with cancer. Investig. New Drugs 2004, 22, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Mahalingam, D.; Wilding, G.; Denmeade, S.; Sarantopoulas, J.; Cosgrove, D.; Cetnar, J.; Azad, N.; Bruce, J.; Kurman, M.; Allgood, V.E.; et al. Mipsagargin, a novel thapsigargin-based PSMA-activated prodrug: Results of a first-in-man phase I clinical trial in patients with refractory, advanced or metastatic solid tumours. Br. J. Cancer 2016, 114, 986–994. [Google Scholar] [CrossRef] [PubMed]
- Zhumakayeva, A.; Rakhimov, K.; Sirota, V.; Arystan, L.; Madiyarov, A.; Adekenov, S. Long-term results of combination therapy for locally advanced breast cancer. Georgian Med. News 2018, 282, 30–35. [Google Scholar]
- Zhang, S.; Won, Y.-K.; Ong, C.-N.; Shen, H.M. Anti-cancer potential of sesquiterpene lactones: Bioactivity and molecular mechanisms. Curr. Med. Chem.-Anti-Cancer Agents 2005, 5, 239–249. [Google Scholar] [CrossRef]
- Dandawate, P.R.; Subramaniam, D.; Jensen, R.A.; Anant, S. Targeting cancer stem cells and signaling pathways by phytochemicals: Novel approach for breast cancer therapy. Semin. Cancer Biol. 2016, 40–41, 192–208. [Google Scholar] [CrossRef]
- Siveen, K.S.; Uddin, S.; Mohammad, R.M. Targeting acute myeloid leukemia stem cell signaling by natural products. Mol. Cancer 2017, 16, 13. [Google Scholar] [CrossRef]
- Xu, Y.-Z.; Gu, X.-Y.; Peng, S.-J.; Fang, J.-G.; Zhang, Y.-M.; Huang, D.-J.; Chen, J.-J.; Gao, K. Design, synthesis and biological evaluation of novel sesquiterpene mustards as potential anticancer agents. Eur. J. Med. Chem. 2015, 94, 284–297. [Google Scholar] [CrossRef]
- Babaei, G.; Aliarab, A.; Abroon, S.; Rasmi, Y.; Aziz, S.G. Application of sesquiterpene lactone: A new promising way for cancer therapy based on anticancer activity. Biomed. Pharmacother. 2018, 106, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Lu, Y.; Ding, Y.; Zhai, J.; Li, Q.; Ma, W.; Yang, M.; Fan, H.; Long, J.; Tong, Z.; et al. Guaianolide sesquiterpene lactones, a source to discover agents that selectively inhibit acute myelogenous leukemia stem and progenitor cells. J. Med. Chem. 2012, 55, 8757–8769. [Google Scholar] [CrossRef]
- Ghantous, A.; Gali-Muhtasib, H.; Vuorela, H.; Saliba, N.A.; Darwiche, N. What made sesquiterpene lactones reach cancer clinical trials? Drug Discov. Today 2010, 15, 668–678. [Google Scholar] [CrossRef] [PubMed]
- Neganova, M.; Klochkov, S.G.; Pukhov, S.A.; Afanasieva, S.V.; Aleksandrova, Y.R.; Yandulova, E.Y.; Avila-Rodriguez, M.; Mikhaleva, L.M.; Nikolenko, V.N.; Somasundaram, S.G.; et al. Synthesis and cytotoxic activity of azine derivatives of 6-hydroxyxanthanodiene. Curr. Cancer Drug Targets 2020, 20, 666–674. [Google Scholar] [CrossRef] [PubMed]
- Semakov, A.V.; Klochkov, S.G. Addition products of thiophenol and selenophenol to Inula helenium lactones. Chem. Nat. Comp. 2020, 56, 254–256. [Google Scholar] [CrossRef]
- Semakov, A.V.; Anikina, L.V.; Afanasyeva, S.V.; Pukhov, S.A.; Klochkov, S.G. Synthesis and antiproliferative activity of conjugates of anthracycline antibiotics with sesquiterpene lactones of the elecampane. Russ. J. Bioorg. Chem. 2018, 44, 538–546. [Google Scholar] [CrossRef]
- Taleghani, A.; Nasseri, M.A.; Iranshahi, M. Synthesis of dual-action parthenolide prodrugs as potent anticancer agents. Bioorg. Chem. 2017, 71, 128–134. [Google Scholar] [CrossRef]
- Artyushin, O.I.; Sharova, E.V.; Vinogradova, N.M.; Genkina, G.K.; Moiseeva, F.F.; Klemenkova, Z.S.; Orshanskaya, I.R.; Shtro, A.A.; Kadyrova, R.A.; Zarubaev, V.V.; et al. Synthesis of camphecene derivatives using click chemistry methodology and study of their antiviral activity. Bioorg. Med. Chem. Lett. 2017, 27, 2181–2184. [Google Scholar] [CrossRef]
- Artyushin, O.I.; Moiseeva, A.A.; Zarubaev, V.V.; Slita, A.V.; Galochkina, A.V.; Muryleva, A.A.; Borisevich, S.S.; Yarovaya, O.I.; Salakhutdinov, N.F.; Brel, V.K. Synthesis of Camphecene and Cytisine Conjugates Using Click Chemistry Methodology and Study of Their Antiviral Activity. Chem. Biodivers. 2019, 16, e1900340. [Google Scholar] [CrossRef]
- Neganova, M.E.; Aleksandrova, Y.R.; Nikolaeva, N.S.; Brel, V.K. Synthesis and biological testing of 3,5-bis(arylidene)-4-piperidone conjugates with 2,5-dihydro-5H-1,2-oxaphospholenes. Bioorg. Med. Chem. Lett. 2022, 74, 128940. [Google Scholar] [CrossRef]
- Rasul, A.; Khan, M.; Ali, M.; Li, J.; Li, X. Targeting apoptosis pathways in cancer with alantolactone and isoalantolactone. Sci. World J. 2013, 9, 248532. [Google Scholar] [CrossRef] [PubMed]
- Kretschmer, N.; Rinner, B.; Stuendl, N.; Kaltenegger, H.; Wolf, E.; Kunert, O.; Boechzelt, H.; Leithner, A.; Bauer, R.; Lohberger, B. Effect of costunolide and dehydrocostus lactone on cell cycle, apoptosis, and ABC transporter expression in human soft tissue sarcoma cells. Planta Med. 2012, 78, 1749–1756. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.-M.; Syu, W.-J.; Don, M.-J.; Lu, J.-J.; Lee, G.-H. Cytotoxic sesquiterpene lactones from the root of Saussurea lappa. J. Nat. Prod. 2003, 66, 1175–1180. [Google Scholar] [CrossRef]
- de Lima, C.A.; de Souza Bueno, I.L.; Vasconcelos, S.N.S.; Sciani, J.M.; Ruiz, A.L.T.G.; Foglio, M.A.; de Carvalho, J.E.; Longato, G.B. Reversal of ovarian cancer cell lines multidrug resistance phenotype by the association of apiole with chemotherapies. Pharmaceuticals 2020, 13, 327. [Google Scholar] [CrossRef]
- Martins, C.; Doran, C.; Silva, I.C.; Miranda, C.; Rueff, J.; Rodrigues, A.S. Myristicin from nutmeg induces apoptosis via the mitochondrial pathway and down regulates genes of the DNA damage response pathways in human leukaemia K562 cells. Chem. Biol. Interact. 2014, 218, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Lien, H.-M.; Kuo, P.-T.; Huang, C.-L.; Kao, J.-Y.; Lin, H.; Yang, D.-Y.; Lai, Y.-Y. Study of the anti-proliferative activity of 5-substituted 4,7-dimethoxy-1,3-benzodioxole derivatives of SY-1 from Antrodia camphorata on human COLO 205 colon cancer cells. J. Evid.-Based Complement. Altern. Med. 2011, 2011, 450529. [Google Scholar]
- Bozorova, K.; Zhao, J.; Aisa, H.A. 1,2,3-Triazole-containing hybrids as leads in medicinal chemistry: A recent overview. Bioorg. Med. Chem. 2019, 27, 3511–3531. [Google Scholar] [CrossRef]
- Kolb, H.C.; Finn, M.G.; Sharpless, K.B. Click Chemistry: Diverse Chemical Function from a Few Good Reactions. Angew. Chem. Int. Ed. 2001, 40, 2004–2021. [Google Scholar] [CrossRef]
- Lahann, J. (Ed.) Click Chemistry: A Universal Ligation Strategy for Biotechnology and Materials Science; John Wiley & Sons Ltd.: Chichester, UK, 2009. [Google Scholar]
- Guo, H.-Y.; Chen, Z.-A.; Shen, Q.-K.; Quan, Z.-S. Application of triazoles in the structural modification of natural products. J. Enzyme Inhib. Med. Chem. 2021, 36, 1115–1144. [Google Scholar] [CrossRef]
- Kolb, H.C.; Sharpless, K.B. The Growing Impact of Click Chemistry on Drug Discovery. Drug Discov. Today 2003, 8, 1128–1137. [Google Scholar] [CrossRef]
- Rostovtsev, V.V.; Green, L.G.; Fokin, V.V.; Sharpless, K. A Stepwise Huisgen Cycloaddition Process: Copper(I)-Catalyzed Regioselective “Ligation” of Azides and Terminal Alkynes. Angew. Chem. Int. Ed. 2002, 41, 2596–2599. [Google Scholar] [CrossRef]
- Artyushin, O.I.; Sharova, E.V.; Nikolaeva, N.S.; Aleksandrova, Y.R.; Semakov, A.V.; Neganova, M.E.; Brel, V.K. Modification of sesquiterpene lactones—dehydrocostus lactone and alantolactone—by click chemistry method. Cytotoxic activity of the obtained conjugates. Russ. J. Gen. Chem. 2022, 92, 960–968. [Google Scholar] [CrossRef]
- He, W.; Lai, R.; Lin, Q.; Huang, Y.; Wang, L. Arglabin is a plant sesquiterpene lactone that exerts potent anticancer effects on human oral squamous cancer cells via mitochondrial apoptosis and downregulation of the mTOR/PI3K/Akt signaling pathway to inhibit tumor growth in vivo. J. BUON 2018, 23, 1679–1685. [Google Scholar] [PubMed]
- Fulda, S. Targeting apoptosis for anticancer therapy. Semin. Cancer Biol. 2015, 31, 84–88. [Google Scholar] [CrossRef]
- Yan, X.; Wang, L.; Yang, X.; Qiu, Y.; Tian, X.; Lv, Y.; Tian, F.; Song, G.; Wang, T. Fluoride induces apoptosis in H9c2 car- diomyocytes via the mitochondrial pathway. Chemosphere 2017, 182, 159–165. [Google Scholar] [CrossRef]
- Bonora, M.; Pinton, P. The mitochondrial permeability transition pore and cancer: Molecular mechanisms involved in cell death. Front. Oncol. 2014, 4, 302. [Google Scholar] [CrossRef]
- Huo, H.; Zhou, Z.; Qin, J.; Liu, W.; Wang, B.; Gu, Y. Erastin Disrupts Mitochondrial Permeability Transition Pore (mPTP) and Induces Apoptotic Death of Colorectal Cancer Cells. PLoS ONE 2016, 11, e0154605. [Google Scholar] [CrossRef]
- Wu, Z.; Xu, X.; Dai, L.; Wang, Y.; Yang, B.; Zhao, H.; Lou, C. Eupalinolide J induces apoptosis, cell cycle arrest, mitochondrial membrane potential disruption and DNA damage in human prostate cancer cells. J. Toxicol. Sci. 2020, 45, 15–23. [Google Scholar] [CrossRef]
- Stine, Z.E.; Schug, Z.T.; Salvino, J.M.; Dang, C.V. Targeting cancer metabolism in the era of precision oncology. Nat. Rev. Drug Discov. 2022, 21, 141–162. [Google Scholar] [CrossRef]
- Elia, I.; Haigis, M.C. Metabolites and the tumour microenvironment: From cellular mechanisms to systemic metabolism. Nat. Metab. 2021, 3, 21–32. [Google Scholar] [CrossRef]
- Ganapathy-Kanniappan, S. Taming tumor glycolysis and poten- tial implications for immunotherapy. Front. Oncol. 2017, 7, 36. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Wu, J.; Zhao, Q.; Fu, S.; Jin, J. Emerging roles of aerobic glycolysis in breast cancer. Clin. Transl. Oncol. 2019, 22, 631–646. [Google Scholar] [CrossRef] [PubMed]
- Shankar Babu, M.; Mahanta, S.; Lakhter, A.J.; Hato, T.; Paul, S.; Naidu, S.R. Lapachol inhibits glycolysis in cancer cells by tar- geting pyruvate kinase M2. PLoS ONE. 2018, 13, e0191419. [Google Scholar] [CrossRef] [PubMed]
- Semakov, A.V.; Anikina, L.V.; Klochkov, S.G. Synthesis and cytotoxic activity of the products of addition of thiophenol to sesquiterpene lactones. Russ. J. Bioorg. Chem. 2021, 47, 906–917. [Google Scholar] [CrossRef]
- Semakov, A.V.; Klochkov, S.G. Methods of preparative isolation of isoalantholactone and alantholactone from ele-campane root. Chem. Plant Raw Mater. 2020, 3, 145–154. [Google Scholar] [CrossRef]
- Fang, L.; Trigiante, G.; Crespo-Otero, R.; Philpott, M.P.; Jones, C.R.; Watkinson, M. An alternative modular ‘click-SNAr-click’ approach to develop subcellular localised fluorescent probes to image mobile Zn2+. Org. Biomol. Chem. 2019, 17, 10013–10019. [Google Scholar] [CrossRef]
- Yang, Z.; Wang, J. Enantioselective Palladium-catalyzed hydrophosphinylation of allenes with phosphine oxides: Access to chiral allylic phosphine oxides. Angew. Chem. Int. Ed. 2021, 60, 27288–27292. [Google Scholar] [CrossRef]
- Koufaki, M.; Fotopoulou, T.; Kapetanou, M.; Heropoulos, G.A.; Gonos, E.S.; Chondrogianni, N. Microwave-assisted synthesis of 3,5-disubstituted isoxazoles and evaluation of their anti-ageing activity. Eur. J. Med. Chem. 2014, 83, 508–515. [Google Scholar] [CrossRef]
- Baur, J.A.; Sinclair, D.A. Therapeutic potential of resveratrol: The in vivo evidence. Nat. Rev. Drug Discov. 2006, 5, 493–506. [Google Scholar] [CrossRef]
- Roger, L.R.L. Proteins and Albumin. Lab. Med. 2014, 45, e25–e41. [Google Scholar] [CrossRef]
- Chowdhury, S.R.; Djordjevic, J.; Albensi, B.C.; Fernyhough, P. Simultaneous evaluation of substrate-dependent oxygen consumption rates and mitochondrial membrane potential by TMRM and safranin in cortical mitochondria. Biosci. Rep. 2015, 36, e00286. [Google Scholar] [CrossRef] [PubMed]
- Neganova, M.; Semakov, A.; Aleksandrova, Y.; Yandulova, E.; Pukhov, S.; Anikina, L.; Klochkov, S. N-Alkylation of Anthracycline Antibiotics by Natural Sesquiterpene Lactones as a Way to Obtain Antitumor Agents with Reduced Side Effects. Biomedicines 2021, 9, 547. [Google Scholar] [CrossRef] [PubMed]
- Wilkins, H.M.; Koppel, S.; Carl, S.M.; Ramanujan, S.; Weidling, I.; Michaelis, M.L.; Michaelis, E.K.; Swerdlow, R.H. Oxaloacetate enhances neuronal cell bioenergetic fluxes and infrastructure. J. Neurochem. 2016, 137, 76–87. [Google Scholar] [CrossRef] [PubMed]
- Schrödinger, L. L. C. Available online: https://www.schrodinger.com/. (accessed on 29 November 2022).

| Compounds | IC50, µM | ||||
|---|---|---|---|---|---|
| SH-SY5Y | HeLa | Hep-2 | A549 | Hek 293 | |
| 6a | 37.35 ± 0.28 | 62.40 ± 0.05 | 88.12 ± 2.66 | 85.65 ± 0.38 | 56.98 ± 1.24 |
| 6b | 36.32 ± 2.38 | 61.52 ± 0.12 | >100 | >100 | 56.42 ± 1.03 |
| 6c | 94.90 ± 1.08 | 82.4 ± 1.14 | >100 | >100 | 59.75 ± 1.37 |
| 6d | 57.77 ± 1.71 | >100 | >100 | >100 | 69.47 ± 0.54 |
| 7a | 33.59 ± 1.27 | 38.96 ± 1.27 | 55.68 ± 0.52 | 29.34 ± 1.37 | 38.87 ± 1.23 |
| 7b | 41.74 ± 1.73 | 55.14 ± 0.94 | 67.16 ± 0.14 | 47.33 ± 1.52 | 42.31 ± 1.09 |
| 7c | 39.98 ± 1.32 | 46.55 ± 0.81 | 62.56 ± 0.72 | 53.11 ± 0.25 | 46.25 ± 0.22 |
| 7d | 45.17 ± 1.24 | 81.01 ± 3.17 | 91.05 ± 2.85 | 83.52 ± 1.09 | 35.39 ± 0.29 |
| 10a | 19.31 ± 0.57 | 18.02 ± 0.32 | >100 | >100 | 58.04 ± 0.07 |
| 10b | 16.14 ± 0.46 | 16.60 ± 0.59 | 26.28 ± 0.38 | 25.20 ± 3.44 | 56.65 ± 0.05 |
| 10c | 89.47 ± 2.57 | 79.59 ± 0.33 | >100 | >100 | 66.29 ± 0.08 |
| 10d | 21.83 ± 0.02 | 26.40 ± 2.39 | 82.16 ± 1.15 | >100 | >100 |
| 11a | 71.78 ± 2.00 | 88.86 ± 0.21 | >100 | 95.93 ± 1.50 | 61.43 ± 0.10 |
| 11b | 60.38 ± 0.39 | 72.79 ± 0.07 | >100 | 70.33 ± 1.17 | 49.55 ± 0.33 |
| 11c | >100 | 88.22 ± 1.27 | >100 | >100 | 67.91 ± 0.65 |
| 11d | 56.08 ± 1.06 | 89.35 ± 0.40 | >100 | 91.22 ± 1.54 | 59.58 ± 1.84 |
| Arglabin | 34.02 ± 2.48 | 20.00 ± 0.00 [35] | 29.92 ± 0.93 | - | 78.00 ± 2.05 |
| Title | #Stars | QP logPo/w | QP logS | QPP Caco | QP logBB | % Human Oral Absorption | Rule of Five | Rule of Three |
|---|---|---|---|---|---|---|---|---|
| 6a | 1 | 3.969 | −3.8 | 366.597 | −0.465 | 83.12 | 1 | 1 |
| 6b | 1 | 4.625 | −5.66 | 187.542 | −1.027 | 94.711 | 0 | 1 |
| 6c | 2 | 4.754 | −5.889 | 189.541 | −1.117 | 82.592 | 1 | 2 |
| 6d | 1 | 4.719 | −5.574 | 257.607 | −0.919 | 84.771 | 1 | 1 |
| 7a | 0 | 4.19 | −5.204 | 297.515 | −0.711 | 82.789 | 1 | 1 |
| 7b | 0 | 4.287 | −4.964 | 199.479 | −0.932 | 93.208 | 0 | 1 |
| 7c | 1 | 4.512 | −5.48 | 182.961 | −1.103 | 80.897 | 1 | 1 |
| 7d | 2 | 4.649 | −5.46 | 391.813 | −0.696 | 87.616 | 1 | 1 |
| 10a | 0 | 3.3 | −2.088 | 405.689 | −0.058 | 92.952 | 0 | 1 |
| 10b | 1 | 4.397 | −5.221 | 221.865 | −0.651 | 94.684 | 0 | 1 |
| 10c | 0 | 3.743 | −4.252 | 281.813 | −0.411 | 92.714 | 0 | 0 |
| 10d | 1 | 4.408 | −5.071 | 200.677 | −0.745 | 93.963 | 0 | 1 |
| 11a | 1 | 4.309 | −5.32 | 193.287 | −0.736 | 93.093 | 0 | 1 |
| 11b | 0 | 4.338 | −5.255 | 221.334 | −0.736 | 94.317 | 0 | 1 |
| 11c | 0 | 4.258 | −4.825 | 241.762 | −0.645 | 94.534 | 0 | 1 |
| 11d | 1 | 4.613 | −5.401 | 318.736 | −0.561 | 100 | 0 | 1 |
| Range of values | Less is better | (−2 ÷ 6.5) | (−6.5 ÷ 0.5) | >500 | (−3 ÷ 1.2) | >80—large <20—small | <4 | <3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Neganova, M.E.; Smirnova, E.V.; Sharova, E.V.; Artyushin, O.I.; Aleksandrova, Y.R.; Yandulova, E.Y.; Nikolaeva, N.S.; Brel, V.K. Design of Conjugates Based on Sesquiterpene Lactones with Polyalkoxybenzenes by “Click” Chemistry to Create Potential Anticancer Agents. Molecules 2022, 27, 8411. https://doi.org/10.3390/molecules27238411
Neganova ME, Smirnova EV, Sharova EV, Artyushin OI, Aleksandrova YR, Yandulova EY, Nikolaeva NS, Brel VK. Design of Conjugates Based on Sesquiterpene Lactones with Polyalkoxybenzenes by “Click” Chemistry to Create Potential Anticancer Agents. Molecules. 2022; 27(23):8411. https://doi.org/10.3390/molecules27238411
Chicago/Turabian StyleNeganova, Margarita E., Ekaterina V. Smirnova, Elena V. Sharova, Oleg I. Artyushin, Yulia R. Aleksandrova, Ekaterina Yu. Yandulova, Natalia S. Nikolaeva, and Valery K. Brel. 2022. "Design of Conjugates Based on Sesquiterpene Lactones with Polyalkoxybenzenes by “Click” Chemistry to Create Potential Anticancer Agents" Molecules 27, no. 23: 8411. https://doi.org/10.3390/molecules27238411
APA StyleNeganova, M. E., Smirnova, E. V., Sharova, E. V., Artyushin, O. I., Aleksandrova, Y. R., Yandulova, E. Y., Nikolaeva, N. S., & Brel, V. K. (2022). Design of Conjugates Based on Sesquiterpene Lactones with Polyalkoxybenzenes by “Click” Chemistry to Create Potential Anticancer Agents. Molecules, 27(23), 8411. https://doi.org/10.3390/molecules27238411