An Improved Synthetic Method for Sensitive Iodine Containing Tricyclic Flavonoids
Abstract
1. Introduction
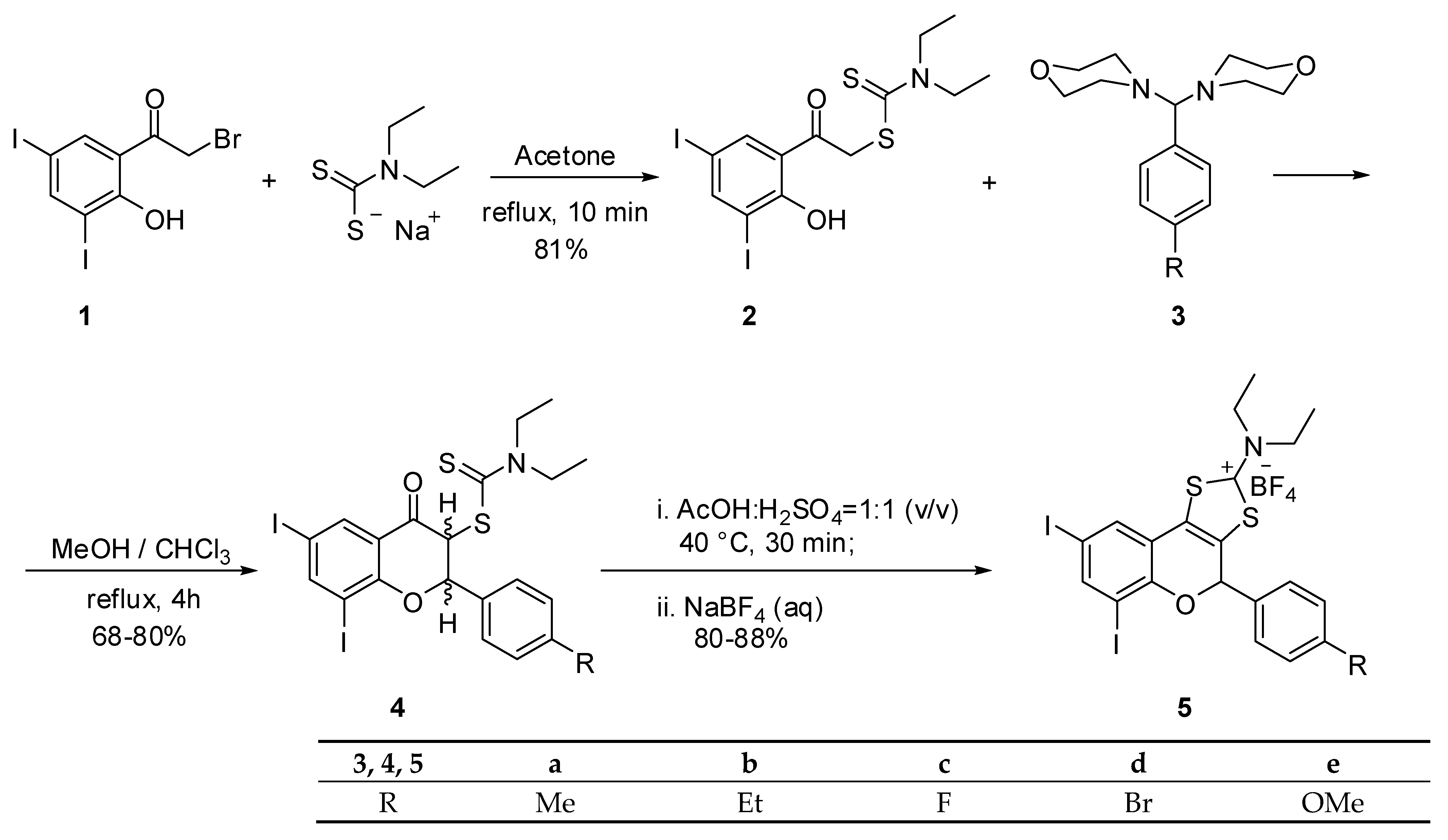
2. Results and Discussion
3. Materials and Methods
3.1. Chemistry
3.1.1. 1-(2-Hydroxy-3,5-diiodophenyl)-1-oxoethan-2-yl N,N-diethylamino-1-carbodithioate (2)
3.1.2. General Procedure for 6,8-Diiodo-2-(4-methylphenyl)-4-oxochroman-3-yl N,N-diethyldithiocarbamate (4a)
3.1.3. 6,8-Diiodo-2-(4-ethylphenyl)-4-oxochroman-3-yl N,N-diethyldithiocarbamate (4b)
3.1.4. 6,8-Diiodo-2-(4-fluorophenyl)-4-oxochroman-3-yl N,N-diethyldithiocarbamate (4c)
3.1.5. 6,8-Diiodo-2-(4-bromophenyl)-4-oxochroman-3-yl N,N-diethyldithiocarbamate (4d)
3.1.6. 6,8-Diiodo-2-(4-methoxyphenyl)-4-oxochroman-3-yl N,N-diethyldithiocarbamate (4e)
3.1.7. General Procedure for 2-N,N-diethylamino-6,8-diiodo-4-(4-methylphenyl)-4H-1,3-dithiol[4,5-c]chromen-2-ylium tetrafluoroborate (5a)
3.1.8. 2-N,N-Diethylamino-6,8-diiodo-4-(4-ethylphenyl)-4H-1,3-dithiol[4,5-c]chromen-2-ylium tetrafluoroborate (5b)
3.1.9. 2-N,N-diethylamino-6,8-diiodo-4-(4-fluorophenyl)-4H-1,3-dithiol[4,5-c]chromen-2-ylium tetrafluoroborate (5c)
3.1.10. 2-N,N-diethylamino-6,8-diiodo-4-(4-bromophenyl)-4H-1,3-dithiol[4,5-c]chromen-2-ylium tetrafluoroborate (5d)
3.1.11. 2-N,N-diethylamino-6,8-diiodo-4-(4-methoxyphenyl)-4H-1,3-dithiol[4,5-c]chromen-2-ylium tetrafluoroborate (5e)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Williams, C.A.; Grayer, J. Anthocyanins and other flavonoids. Nat. Prod. Rep. 2004, 21, 539–573. [Google Scholar] [CrossRef] [PubMed]
- Cushnie, T.P.T.; Lamb, A.J. Recent advances in understanding the antibacterial properties of flavonoids. Int. J. Antimicrob. Agents 2011, 38, 99–107. [Google Scholar] [CrossRef]
- Jae, M.S.; Kwang, H.L.; Baik, L.S. Antiviral effect of catechins in green tea on influenza virus. Antivir. Res. 2005, 68, 66–74. [Google Scholar] [CrossRef]
- Rana, A.C.; Gulliya, B. Chemistry and Pharmacology of Flavonoids- A Review. Ind. J. Pharm. Ed. Res. 2019, 53, 8–20. [Google Scholar] [CrossRef]
- Rauha, J.P.; Remes, S.; Heinonen, M.; Hopia, A.; Kahkonen, M.; Kujala, T.; Pihlaja, K.; Vuorela, H.; Vuorela, P. Antimicrobial effects of Finnish plant extracts containing flavonoids and other phenolic compounds. Int. J. Food Microbiol. 2000, 56, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Suzaki, S.; Nishikawa, T.; Kihara, M.; Shibata, H.; Higuti, T. Phytochemical flavones isolated from Scutellaria barbata and antibacterial activity against methicillin-resistant Staphylococcus aureus. J. Ethnopharmacol. 2000, 72, 483–488. [Google Scholar] [CrossRef] [PubMed]
- Ikigai, H.; Nakae, T.; Hara, Y.; Shimamura, T. Bactericidal catechins damage the lipid bilayer. Biochim. Biophys. Acta 1993, 1147, 132–136. [Google Scholar] [CrossRef]
- Zhao, W.H.; Hu, Z.Q.; Okubo, S.; Hara, Y.; Shimamura, T. Mechanism of synergy between epigallocatechin gallate and beta-lactams against methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 2001, 45, 1737–1742. [Google Scholar] [CrossRef]
- Manner, S.; Skogman, M.; Goeres, D.; Vuorela, P.; Fallarero, A. Systematic exploration of natural and synthetic flavonoids for the inhibition of Staphylococcus aureus biofilms. Int. J. Mol. Sci. 2013, 14, 19434–19451. [Google Scholar] [CrossRef]
- Sarbu, L.G.; Bahrin, L.G.; Babii, C.; Stefan, M.; Birsa, M.L. Synthetic flavonoids with antimicrobial activity: A review. J. Appl. Microbiol. 2019, 127, 1282–1290. [Google Scholar] [CrossRef]
- Aslam, B.; Wang, W.; Arshad, M.I.; Khurshid, M.; Muzammil, S.; Rasool, M.H.; Nisar, M.A.; Alvi, R.F.; Aslam, M.A.; Qamar, M.U.; et al. Antibiotic resistance: A rundown of a global crisis. Infect. Drug Resist. 2018, 11, 1645–1658. [Google Scholar] [CrossRef] [PubMed]
- Bahrin, L.G.; Jones, P.G.; Hopf, H. Tricyclic flavonoids with 1,3-dithiolium substructure. Beilstein J. Org. Chem. 2012, 8, 1999–2003. [Google Scholar] [CrossRef] [PubMed]
- Bahrin, L.G.; Apostu, M.O.; Birsa, M.L.; Stefan, M. The antibacterial properties of sulfur containing flavonoids. Bioorg. Med. Chem. Lett. 2014, 24, 2315–2318. [Google Scholar] [CrossRef] [PubMed]
- Bahrin, L.G.; Hopf, H.; Jones, P.G.; Sarbu, L.G.; Babii, C.; Mihai, A.C.; Stefan, M.; Birsa, M.L. Antibacterial structure–activity relationship studies of several tricyclic sulfur-containing flavonoids. Beilstein J. Org. Chem. 2016, 12, 1065–1071. [Google Scholar] [CrossRef] [PubMed]
- Babii, C.; Mihalache, G.; Bahrin, L.G.; Neagu, A.-N.; Gostin, I.; Mihai, C.T.; Sarbu, L.G.; Birsa, M.L.; Stefan, M. A novel synthetic flavonoid with potent antibacterial properties: In vitro activity and proposed mode of action. PLoS ONE 2018, 13, e0194898. [Google Scholar] [CrossRef]
- Sarbu, L.G.; Shova, S.; Peptanariu, D.; Sandu, I.A.; Birsa, M.L.; Bahrin, L.G. The Cytotoxic Properties of Some Tricyclic 1,3-Dithiolium Flavonoids. Molecules 2019, 24, 154–162. [Google Scholar] [CrossRef]
- Bahrin, L.G.; Sarbu, L.G.; Hopf, H.; Jones, P.G.; Babii, C.; Stefan, M.; Birsa, M.L. The influence of halogen substituents on the biological properties of sulfur-containing flavonoids. Bioorg. Med. Chem. 2016, 24, 3166–3173. [Google Scholar] [CrossRef]
- Sandulache, A.; Cascaval, A.; Toniutti, N.; Giumanini, A.G. New flavones by a novel synthetic route. Tetrahedron 1997, 53, 9813–9822. [Google Scholar] [CrossRef]
- Seliger, H.; Happ, E.; Cascaval, A.; Birsa, M.L.; Nicolaescu, T.; Poinescu, I.; Cojocariu, C. Synthesis and characterization of new photostabilizers from 2,4-dihydroxybenzophenone. Eur. Polym. J. 1999, 35, 827–833. [Google Scholar] [CrossRef]
- Birsa, M.L. Synthesis of some new substituted flavanones and related 4-chromanones by a novel synthetic method. Synth. Commun. 2002, 32, 115–118. [Google Scholar] [CrossRef]
- Birsa, M.L.; Ganju, D. Synthesis and UV/Vis spectroscopic properties of new [2-(N,N-dialkylamino)-1,3-dithiolium-4-yl]phenolates. J. Phys. Org. Chem. 2003, 16, 207–212. [Google Scholar] [CrossRef]
- Liu, S.-Y.; Wang, D.-G.; Zhong, A.-G.; Wen, H.-R. One-step rapid synthesis of π-conjugated large oligomers via C–H activation coupling. Org. Chem. Front. 2018, 5, 653–661. [Google Scholar] [CrossRef]
- Wang, Y.-F.; Wang, C.-J.; Feng, Q.-Z.; Zhai, J.-J.; Qi, S.-S.; Zhong, A.-G.; Chu, M.-M.; Xu, D.-Q. Copper-catalyzed asymmetric 1,6-conjugate addition of in situ generated para-quinone methides with β-ketoesters. Chem. Commun. 2022, 58, 6653–6656. [Google Scholar] [CrossRef] [PubMed]
- Bahrin, L.G.; Asaftei, I.V.; Sandu, I.G.; Sarbu, L.G. Synthesis of (4-Methylpiperazin-1-yl)carbodithioates and of their 1,3-Dithiolium Derivatives. Rev. Chim. Buchar. 2014, 65, 1046–1048. [Google Scholar]
- Birsa, M.L. A new approach to preparation of 1,3-dithiolium salts. Synth. Commun. 2001, 31, 1271–1275. [Google Scholar] [CrossRef]
- Birsa, M.L.; Asaftei, I.V. Solvatochromism of mesoionic iodo(1,3-dithiol-2-ylium-4-yl)phenolates. Monat. Chem. 2008, 139, 1433–1438. [Google Scholar] [CrossRef]

| 4 | a | b | c | d | e |
|---|---|---|---|---|---|
| 3J anti, Hz | 6.2 | 6.3 | 7.3 | 7.1 | 6.7 |
| 3J syn, Hz | 4.1 | 4.3 | 3.8 | 3.6 | 3.7 |
| anti/syn ratio | 77:23 | 95:5 | 77:23 | 67:33 | 76:24 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Birsa, M.L.; Sarbu, L.G. An Improved Synthetic Method for Sensitive Iodine Containing Tricyclic Flavonoids. Molecules 2022, 27, 8430. https://doi.org/10.3390/molecules27238430
Birsa ML, Sarbu LG. An Improved Synthetic Method for Sensitive Iodine Containing Tricyclic Flavonoids. Molecules. 2022; 27(23):8430. https://doi.org/10.3390/molecules27238430
Chicago/Turabian StyleBirsa, Mihail Lucian, and Laura G. Sarbu. 2022. "An Improved Synthetic Method for Sensitive Iodine Containing Tricyclic Flavonoids" Molecules 27, no. 23: 8430. https://doi.org/10.3390/molecules27238430
APA StyleBirsa, M. L., & Sarbu, L. G. (2022). An Improved Synthetic Method for Sensitive Iodine Containing Tricyclic Flavonoids. Molecules, 27(23), 8430. https://doi.org/10.3390/molecules27238430







