Copper-Catalyzed Difluoroalkylation Reaction
Abstract
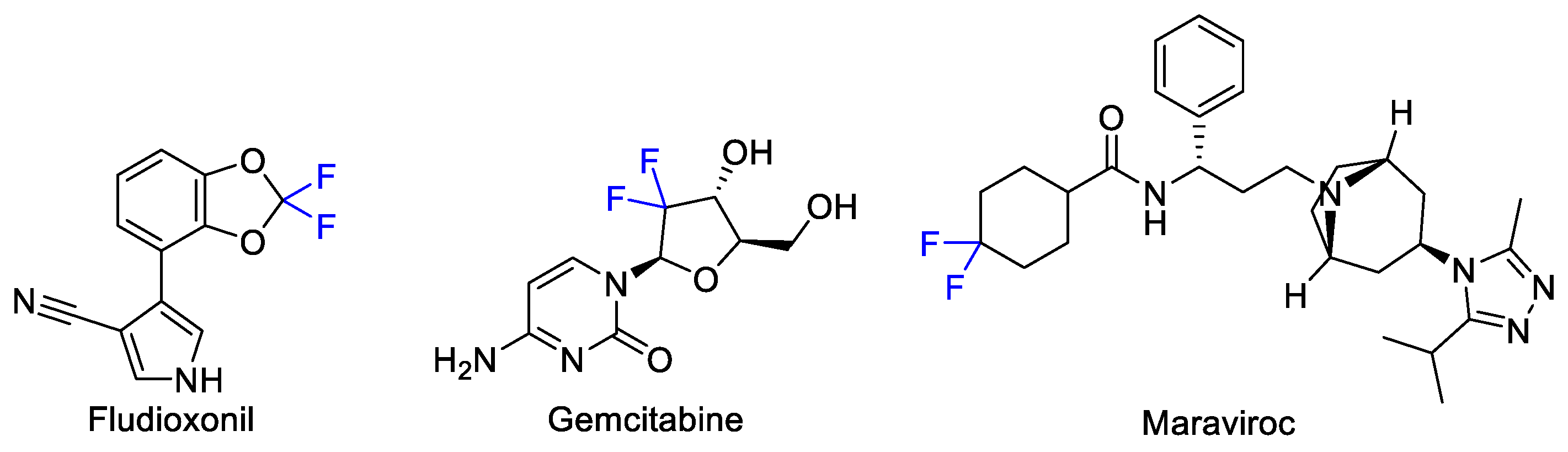
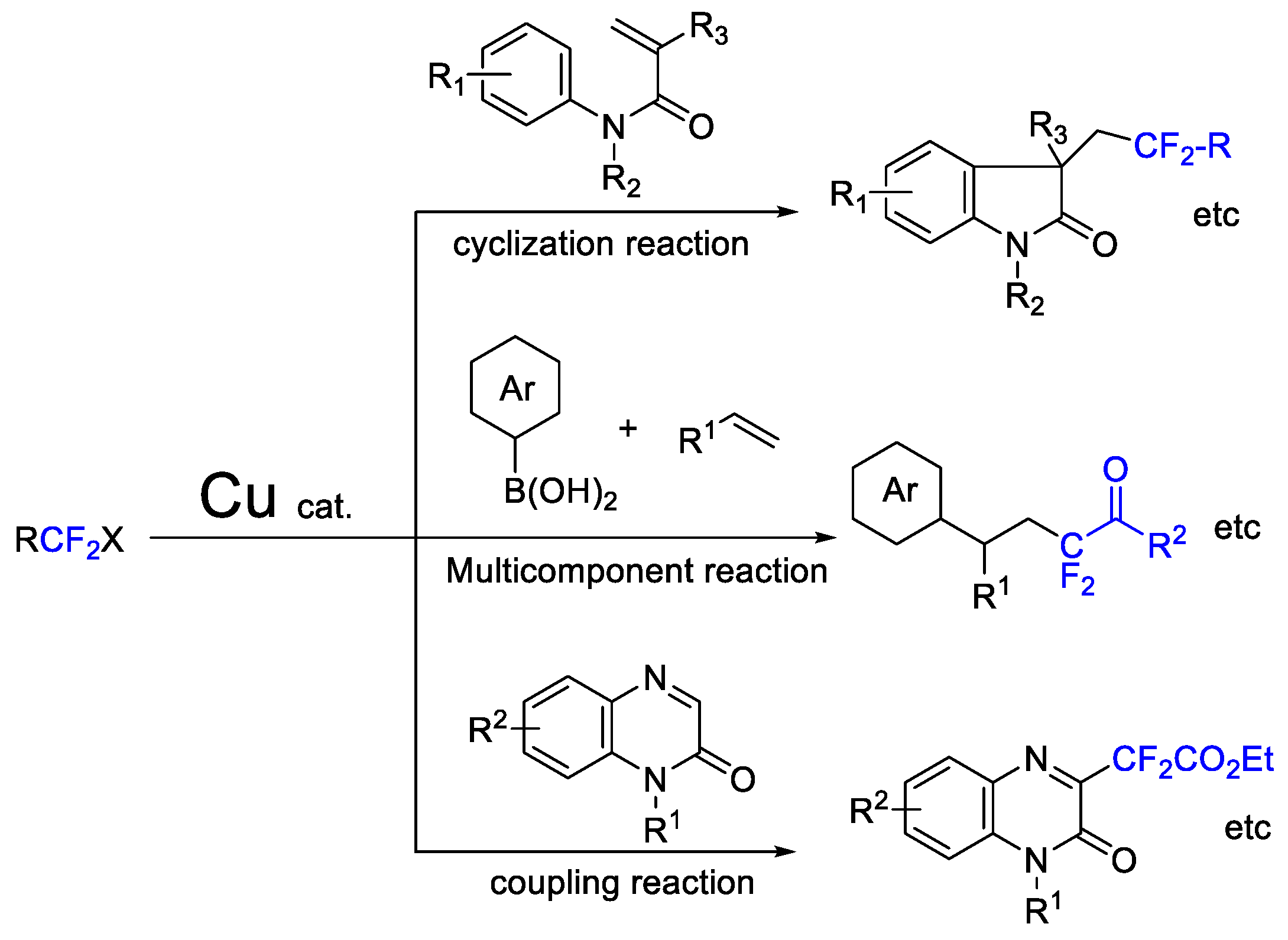
1. Introduction
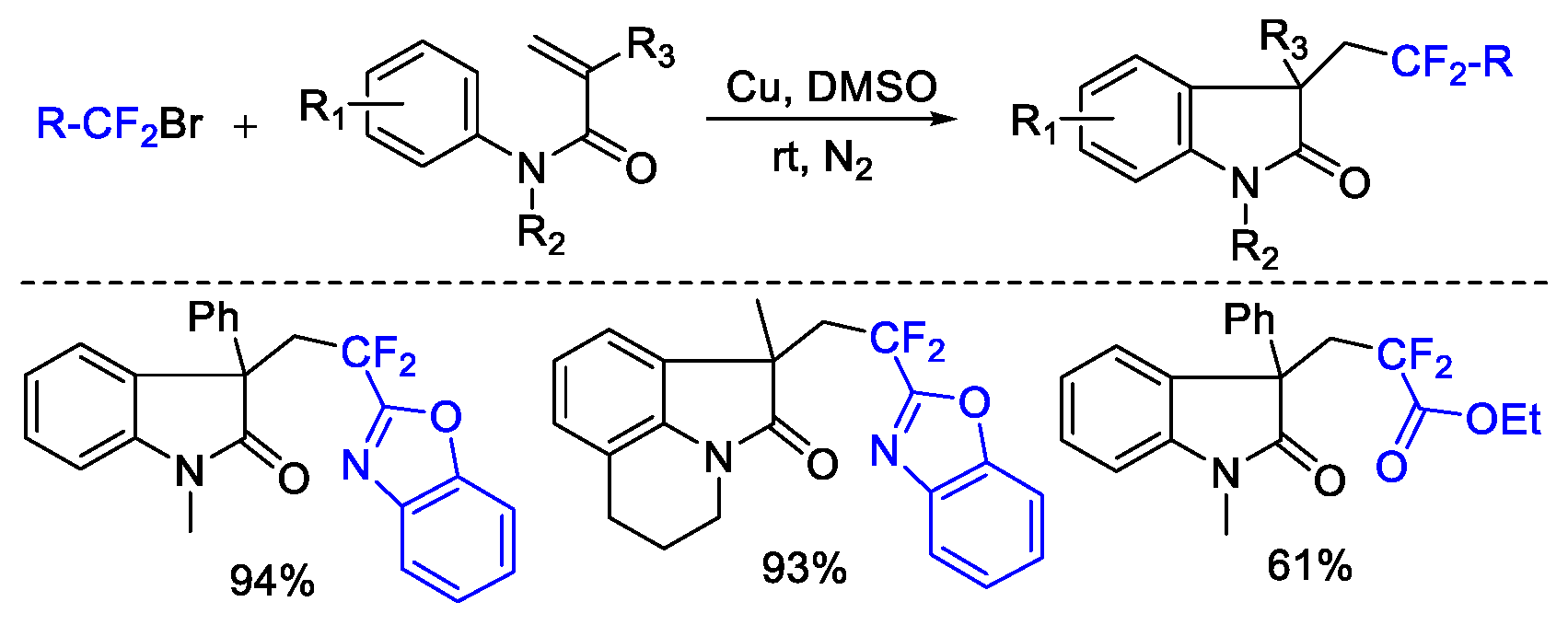
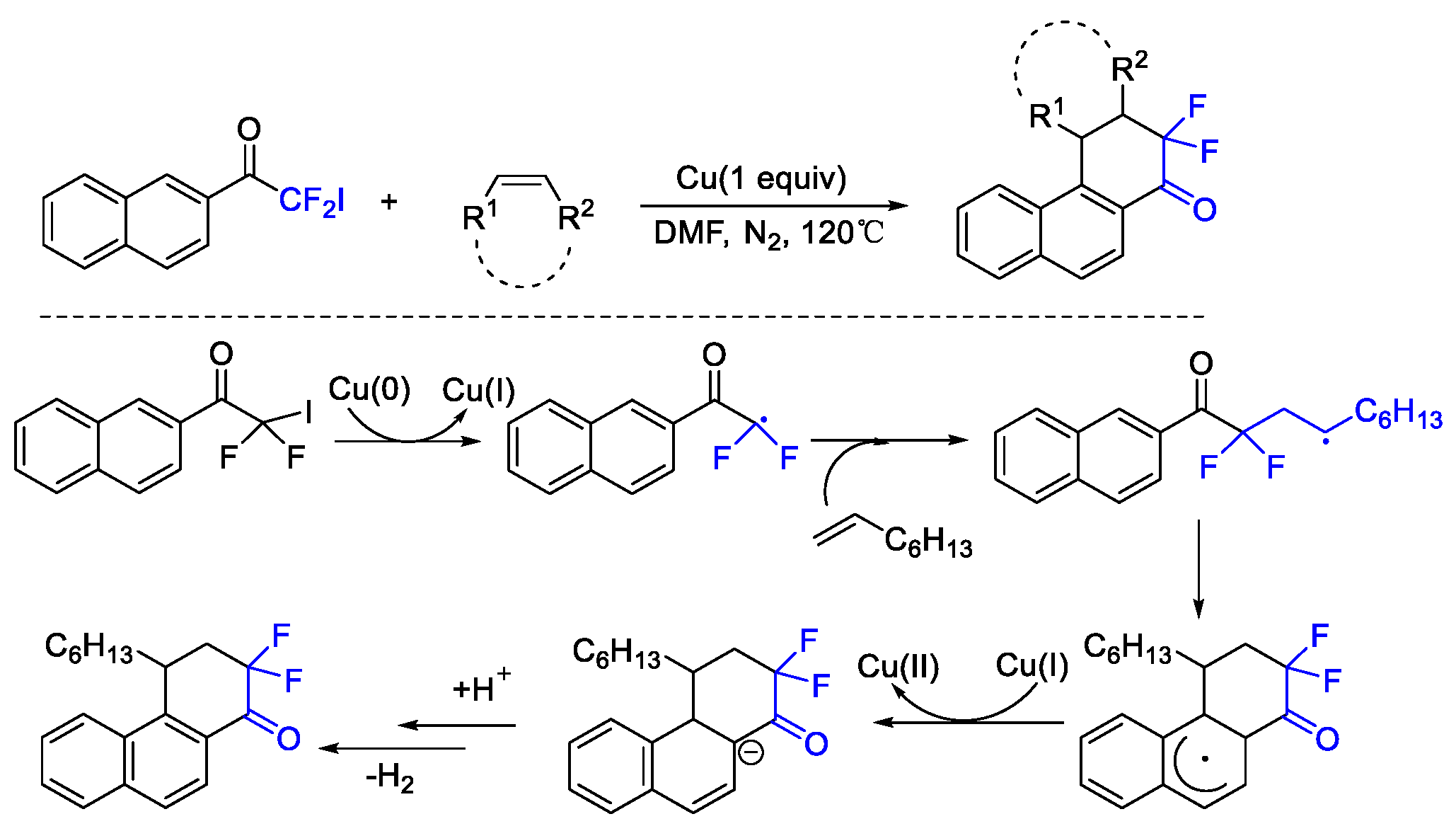
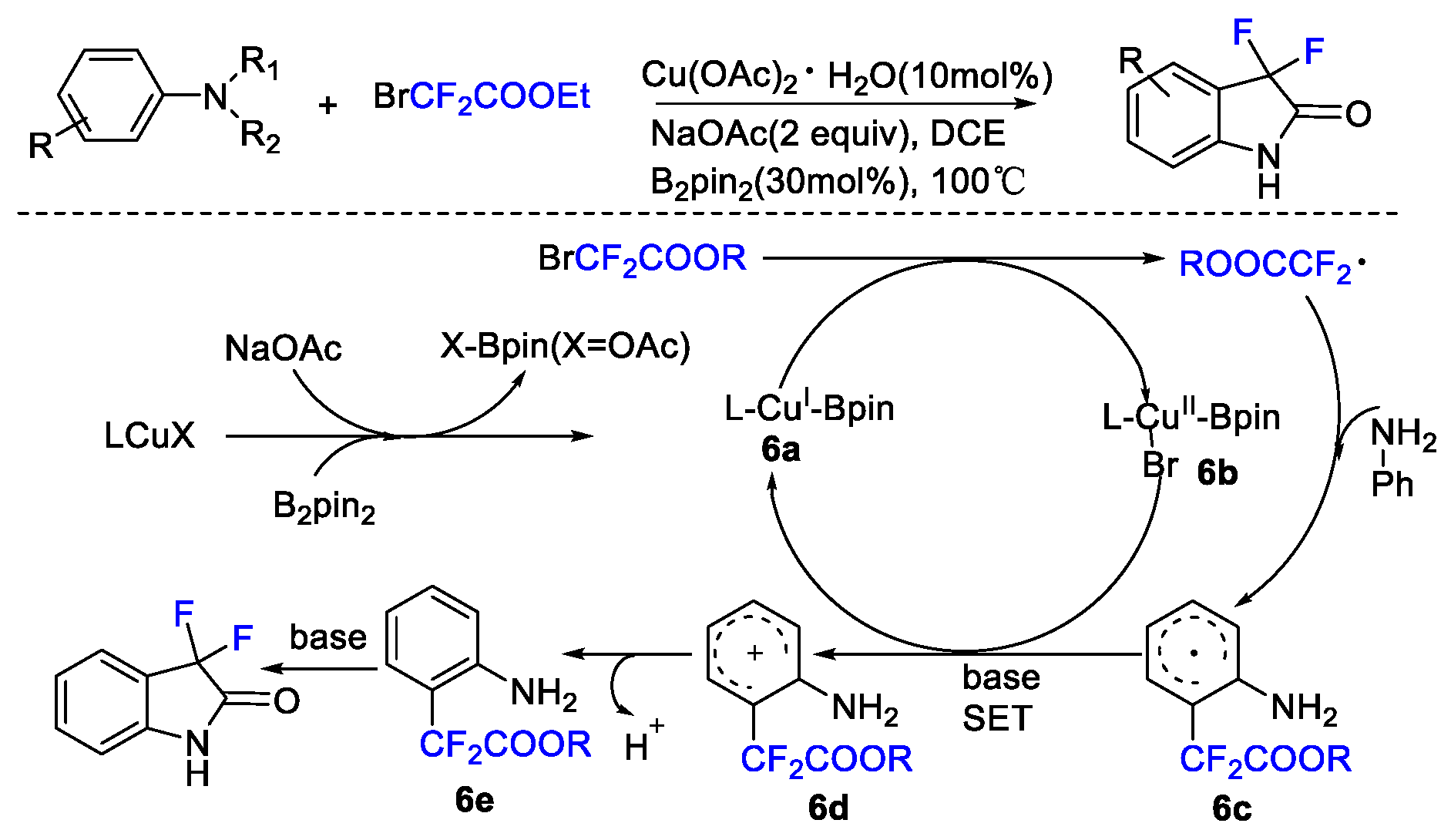
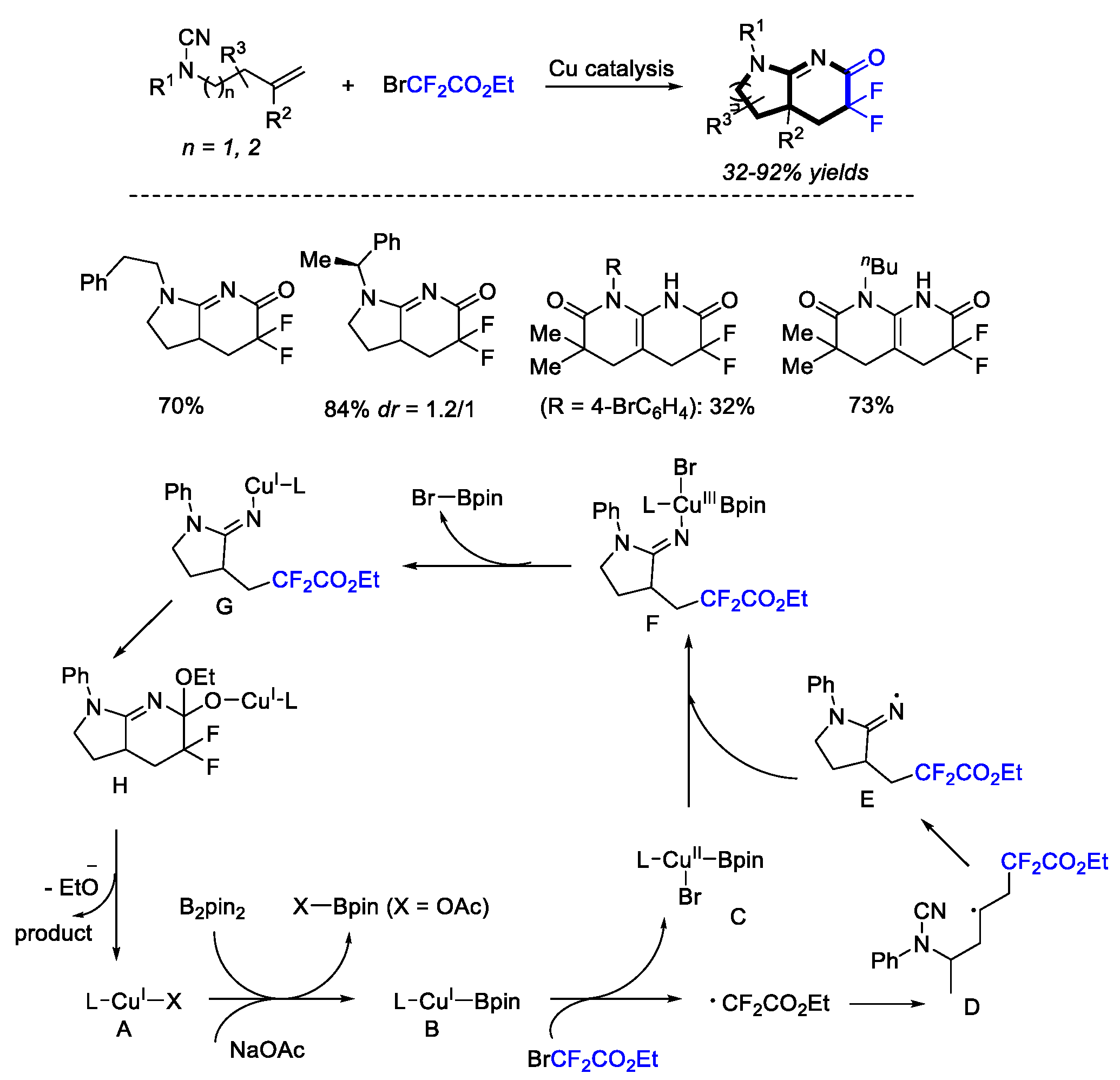
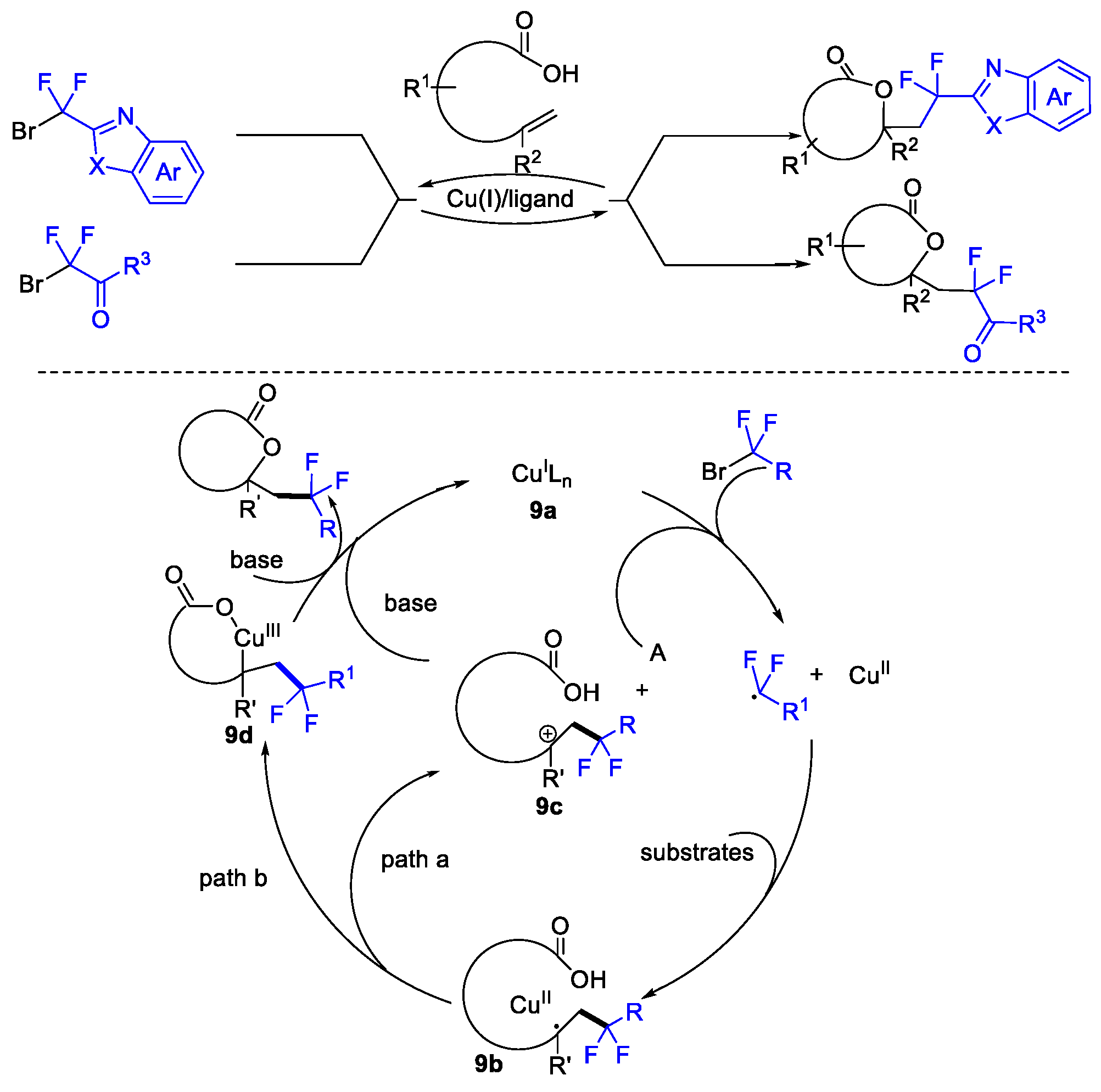
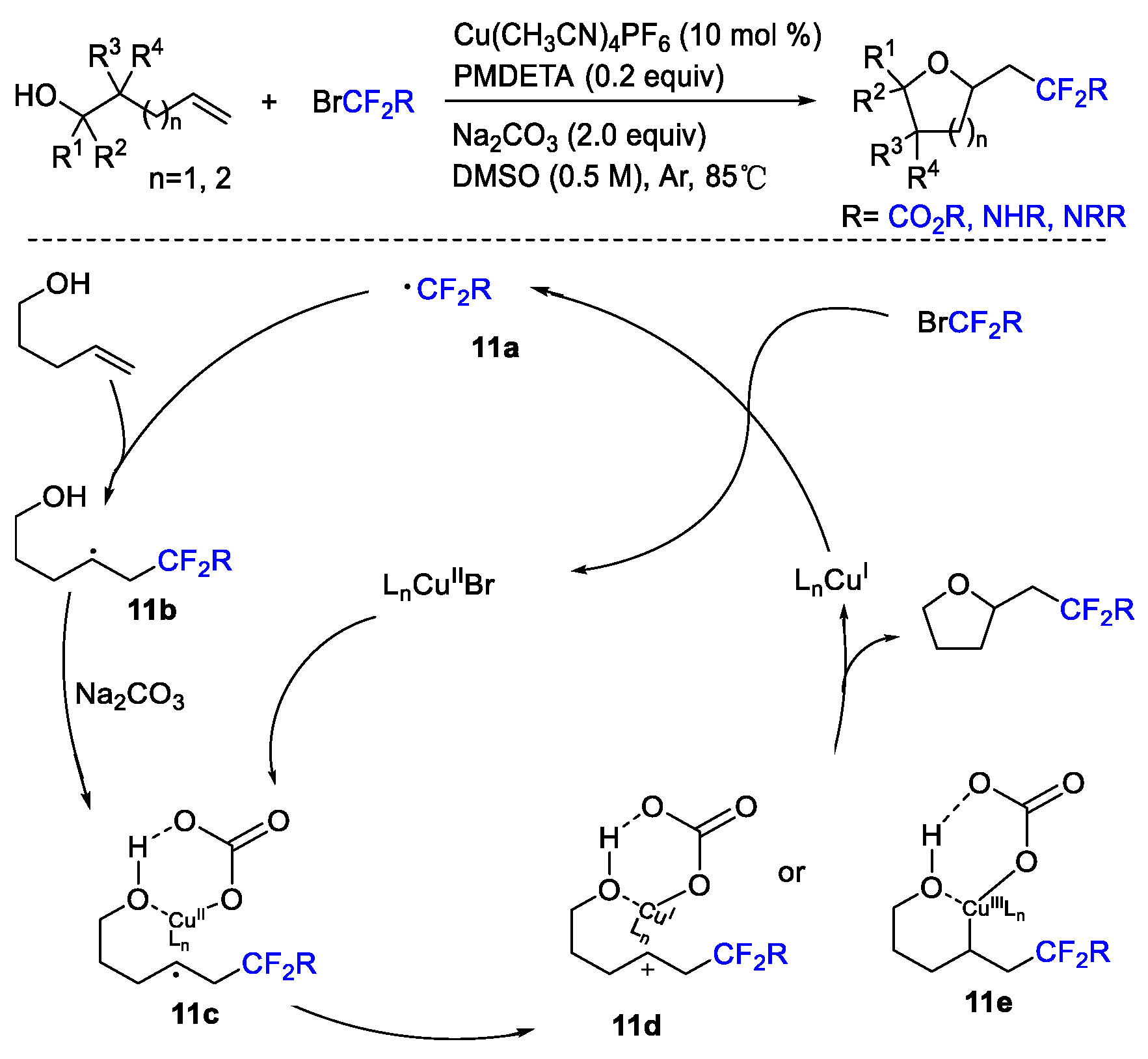
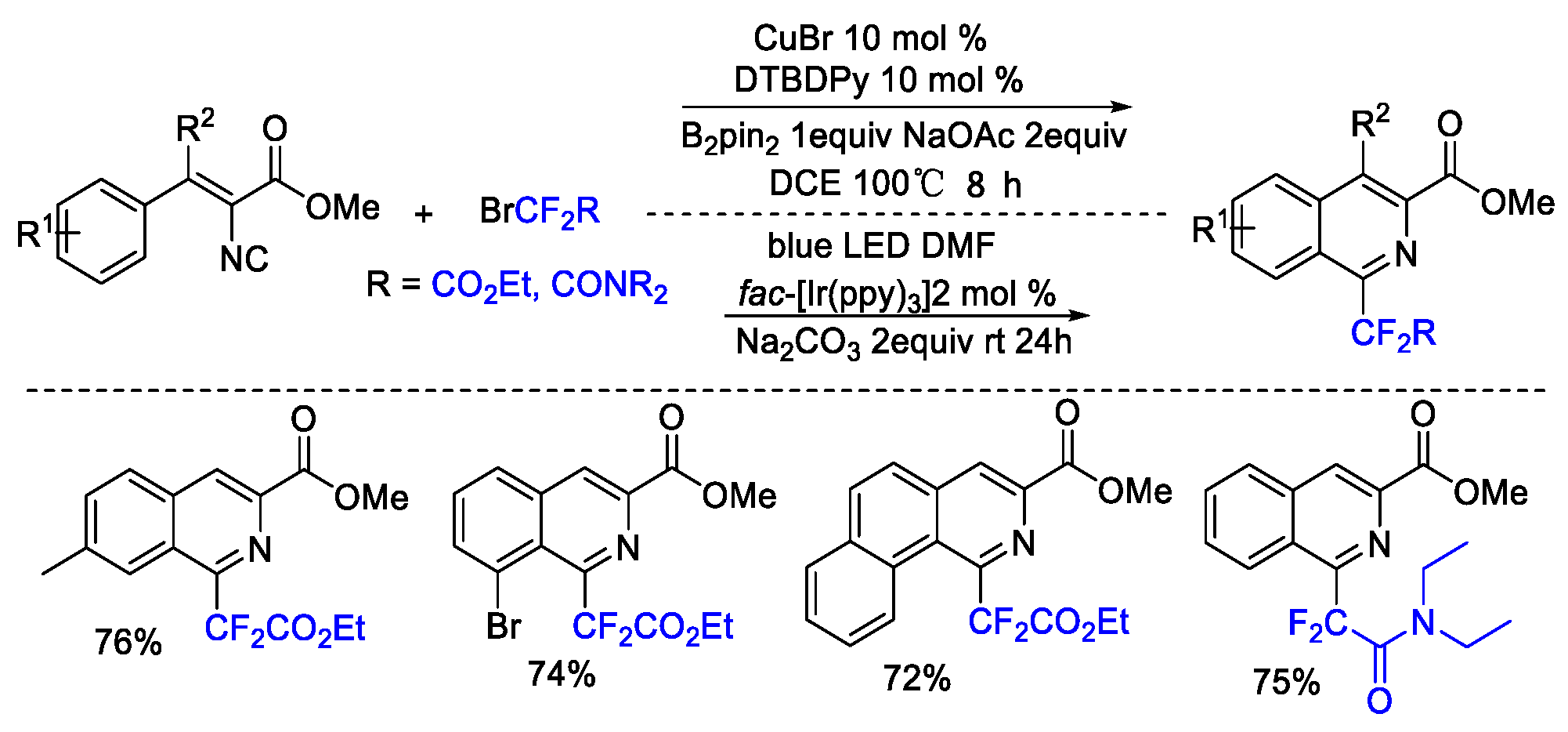
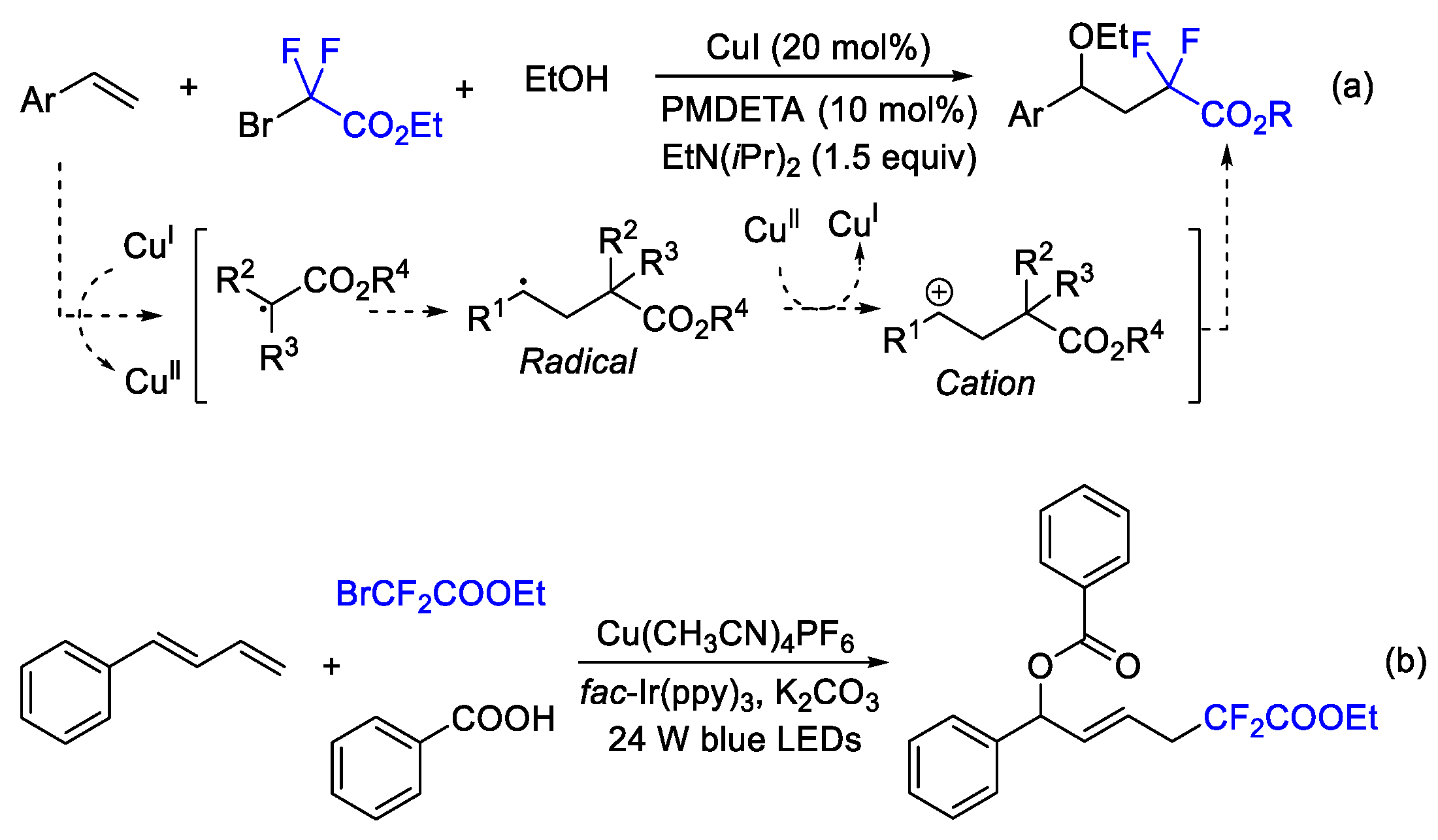
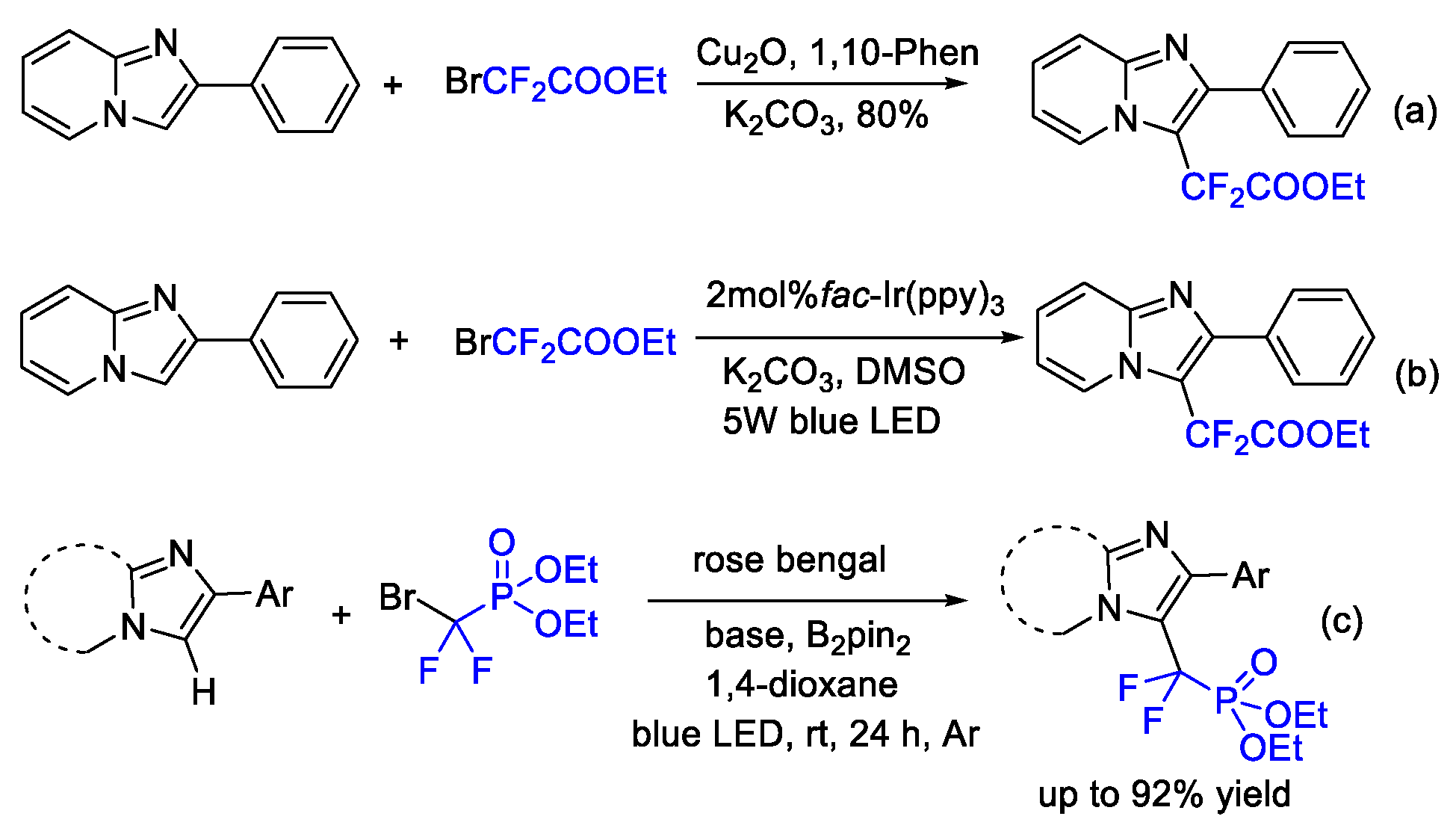
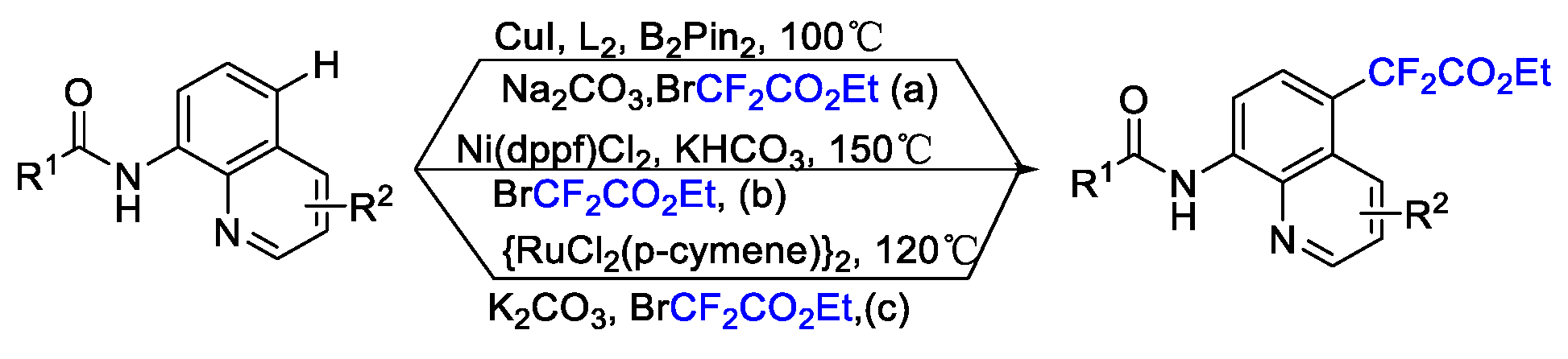
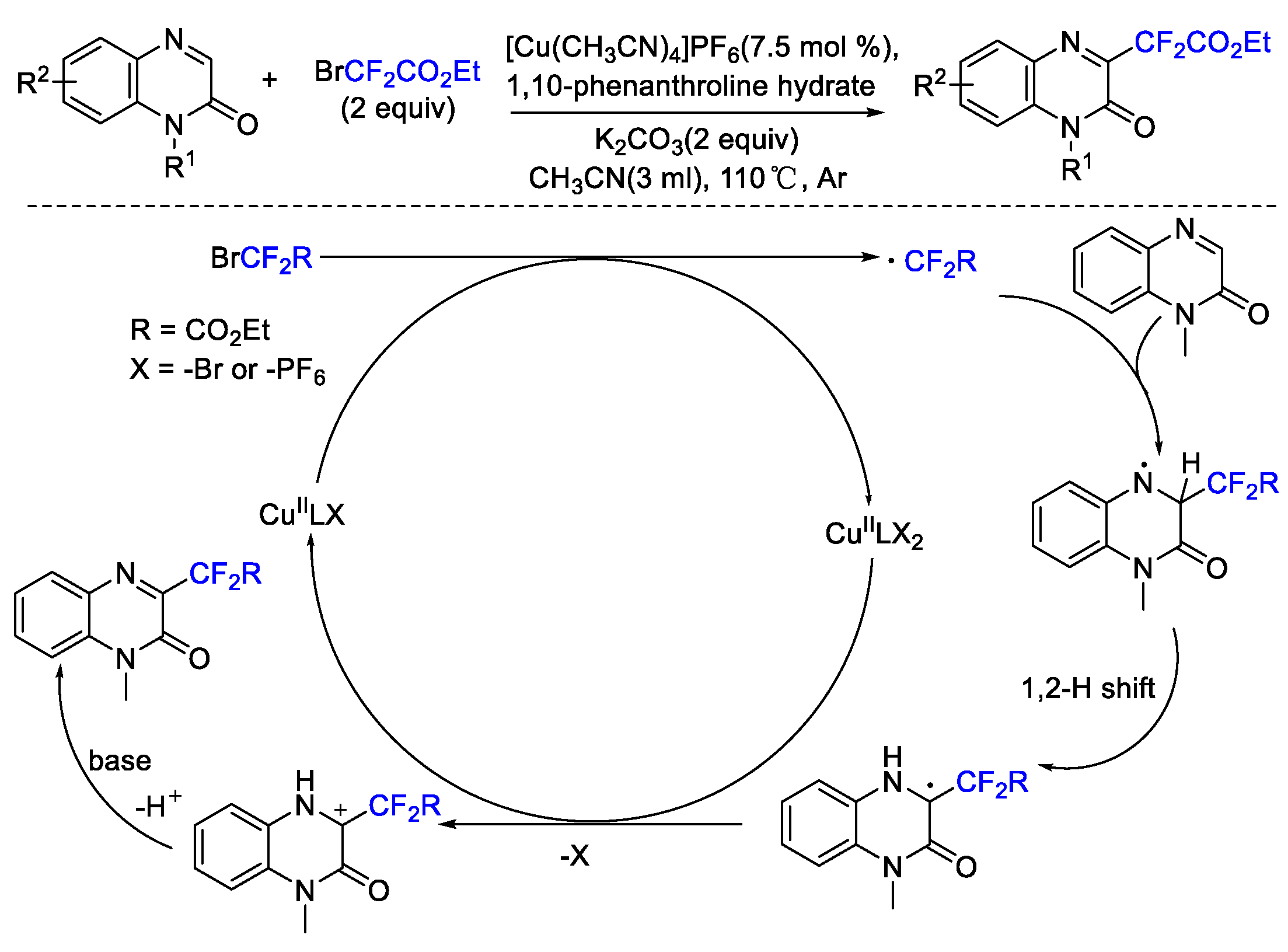
2. Difluoroalkylation–Cyclization Reactions
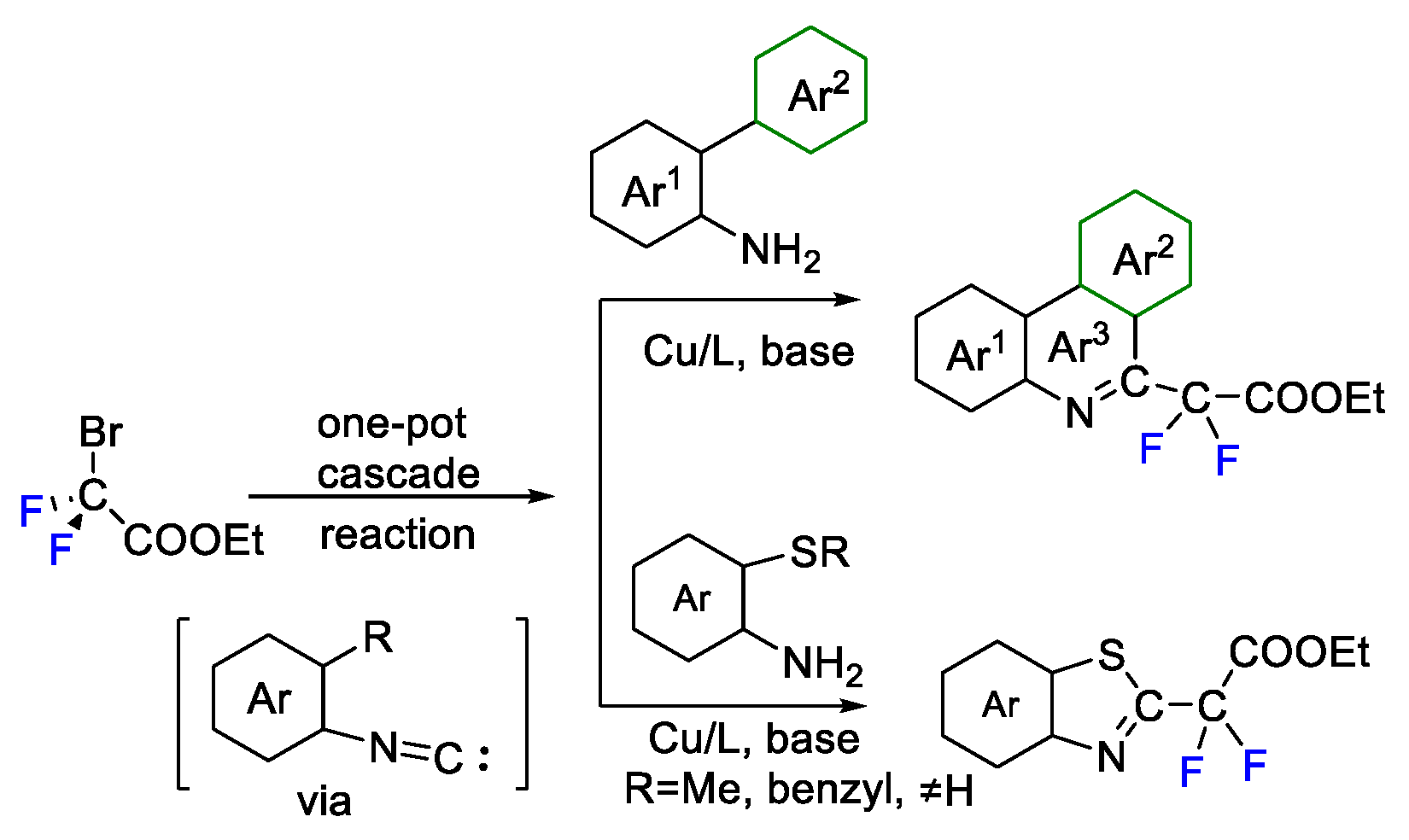
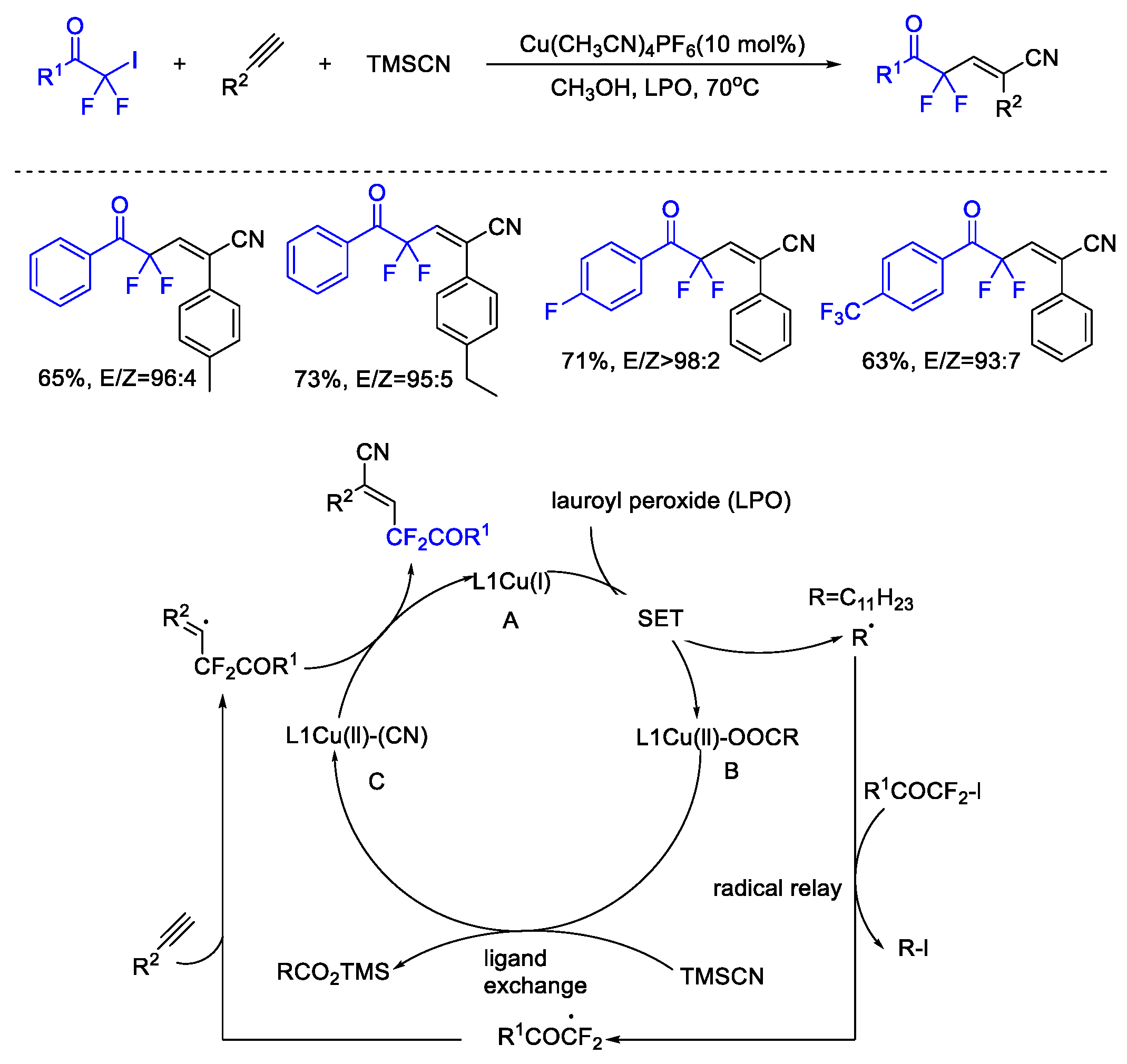
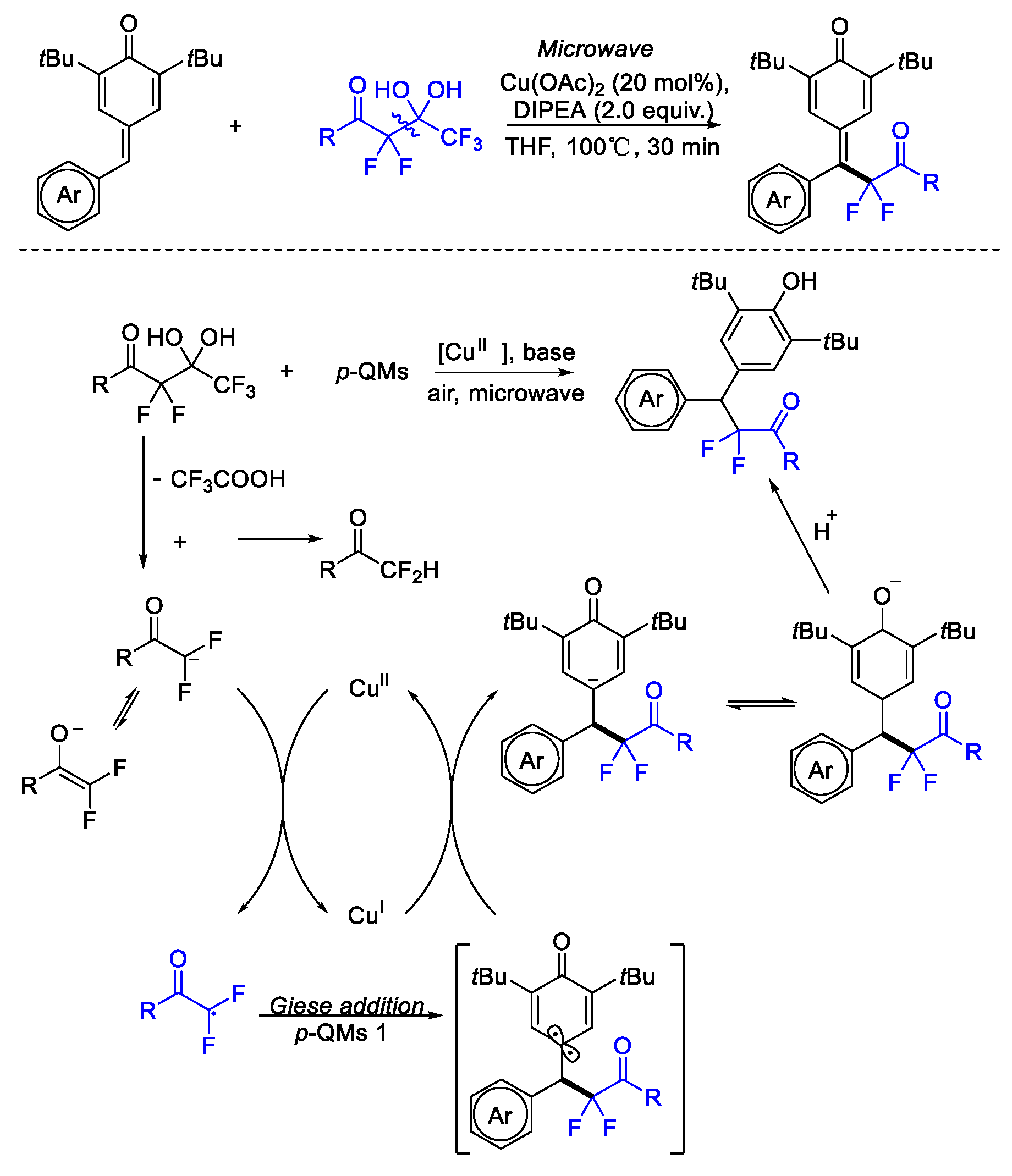
3. Difluoroalkylation–Multicomponent Reactions
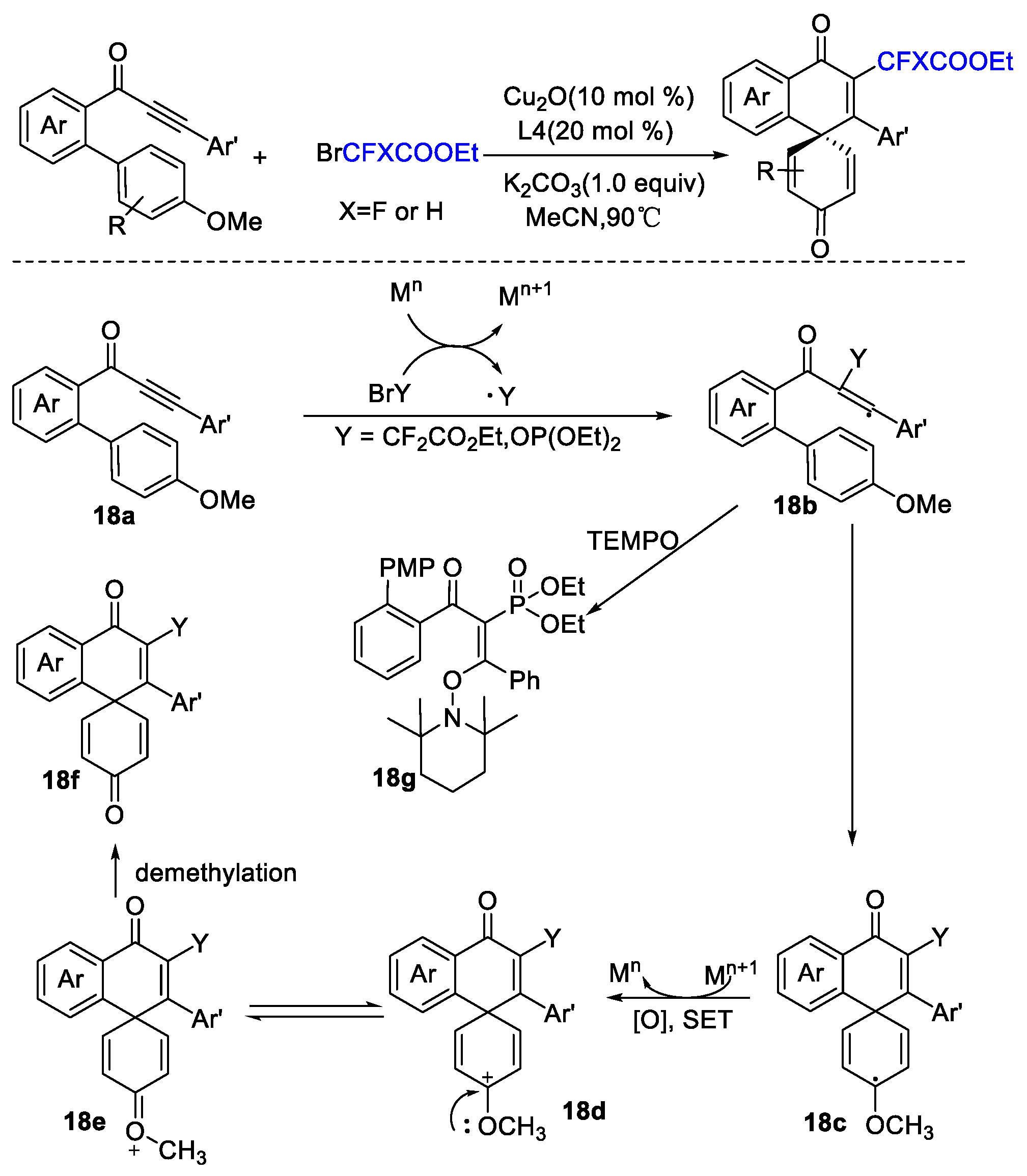
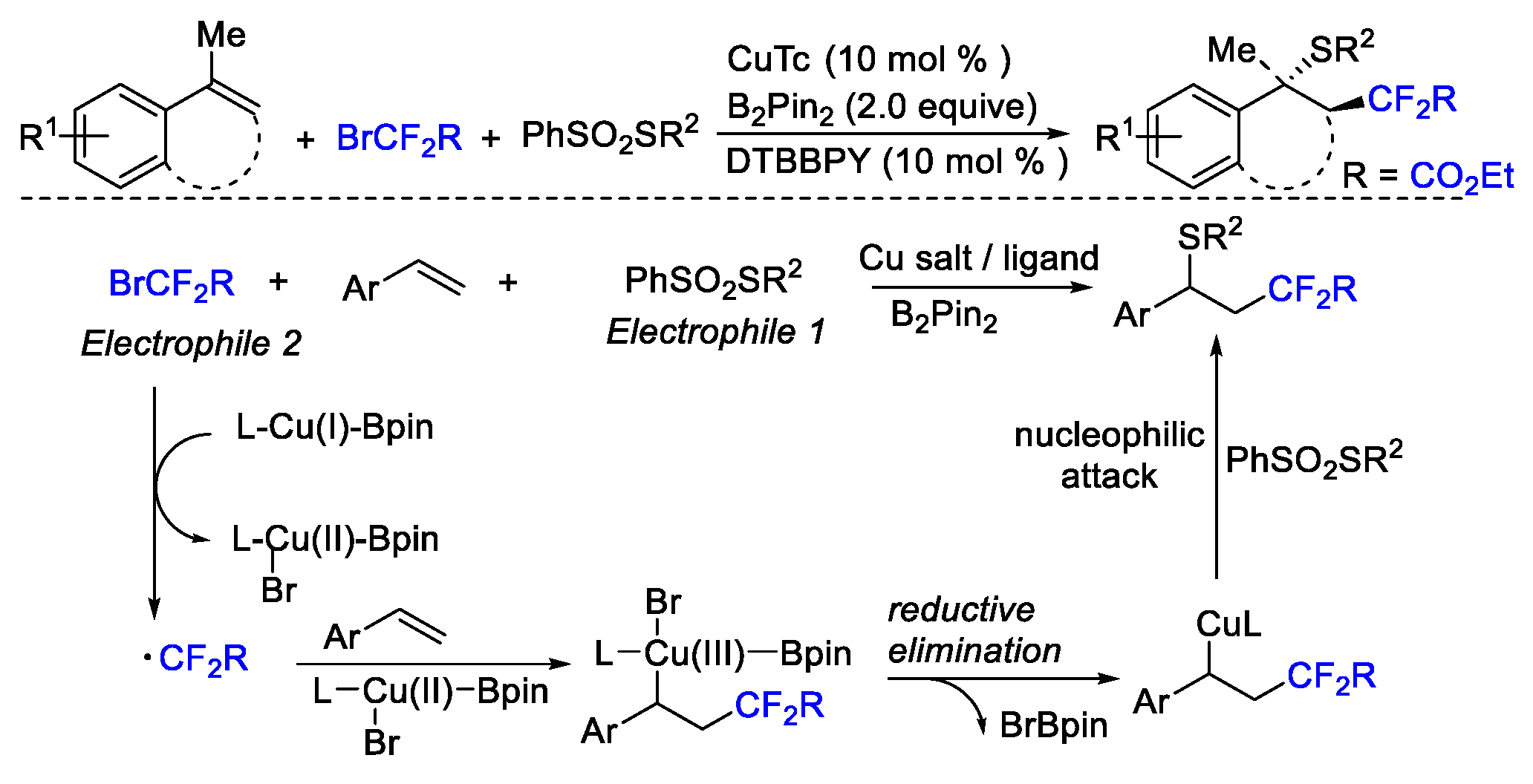
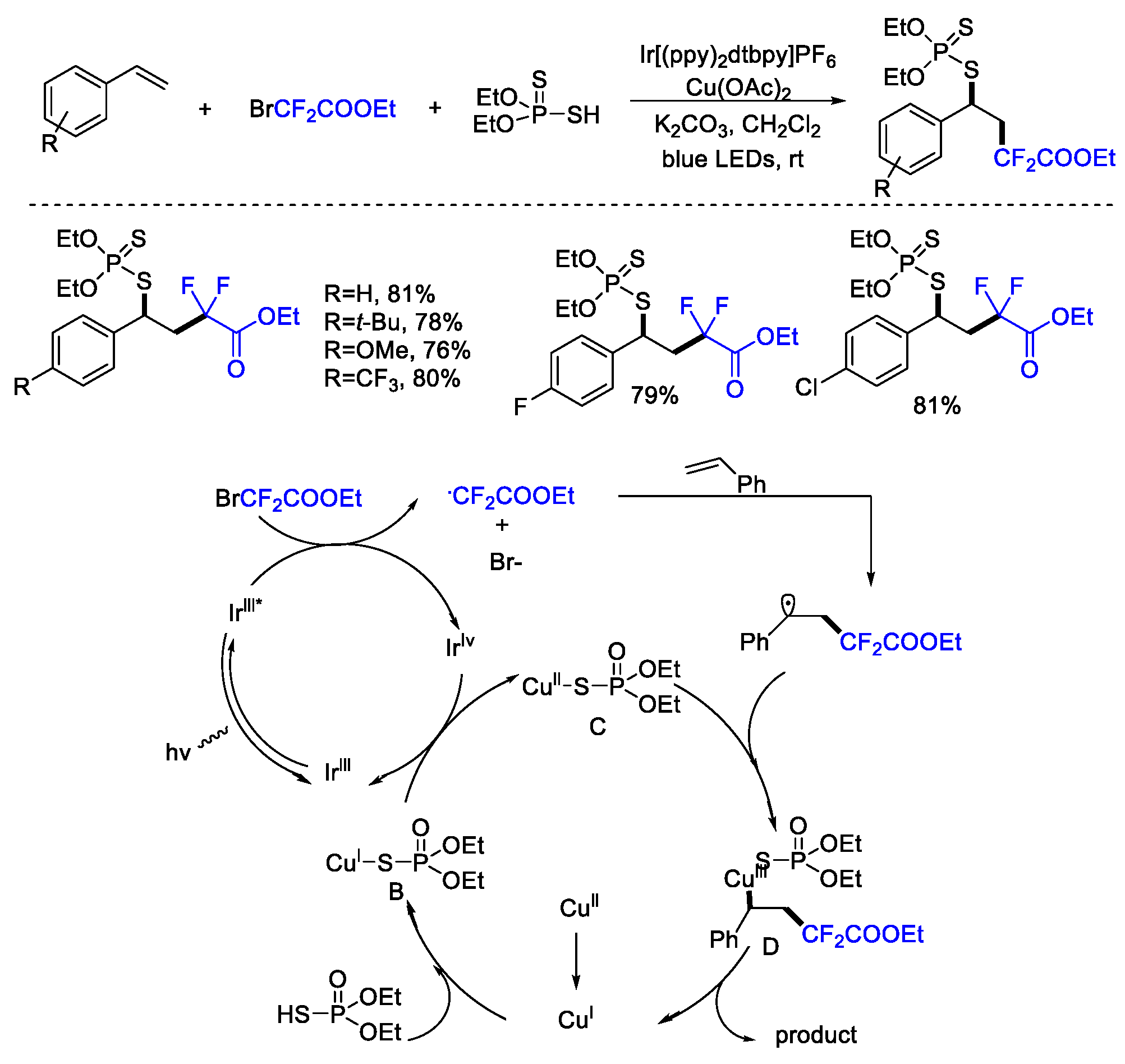
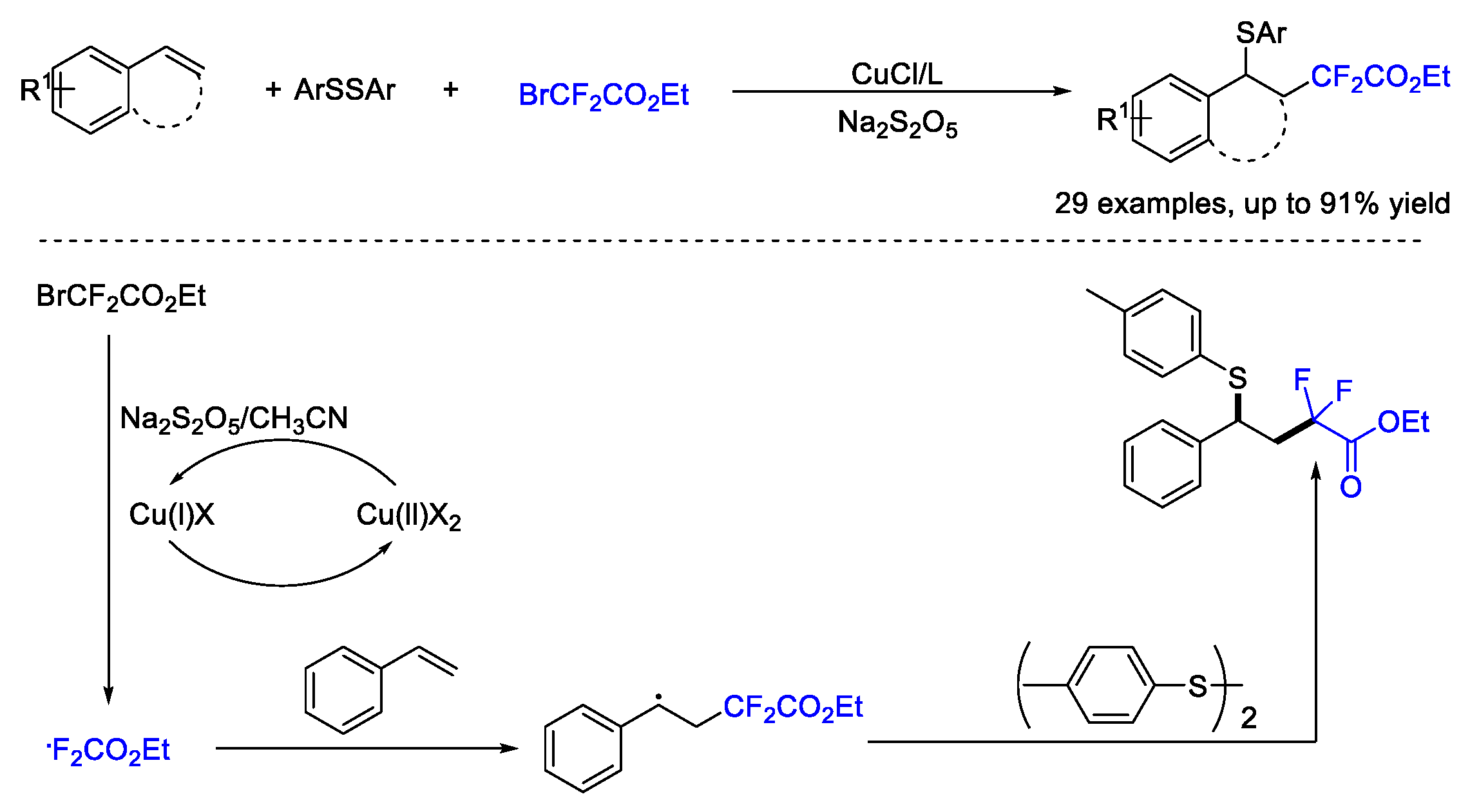
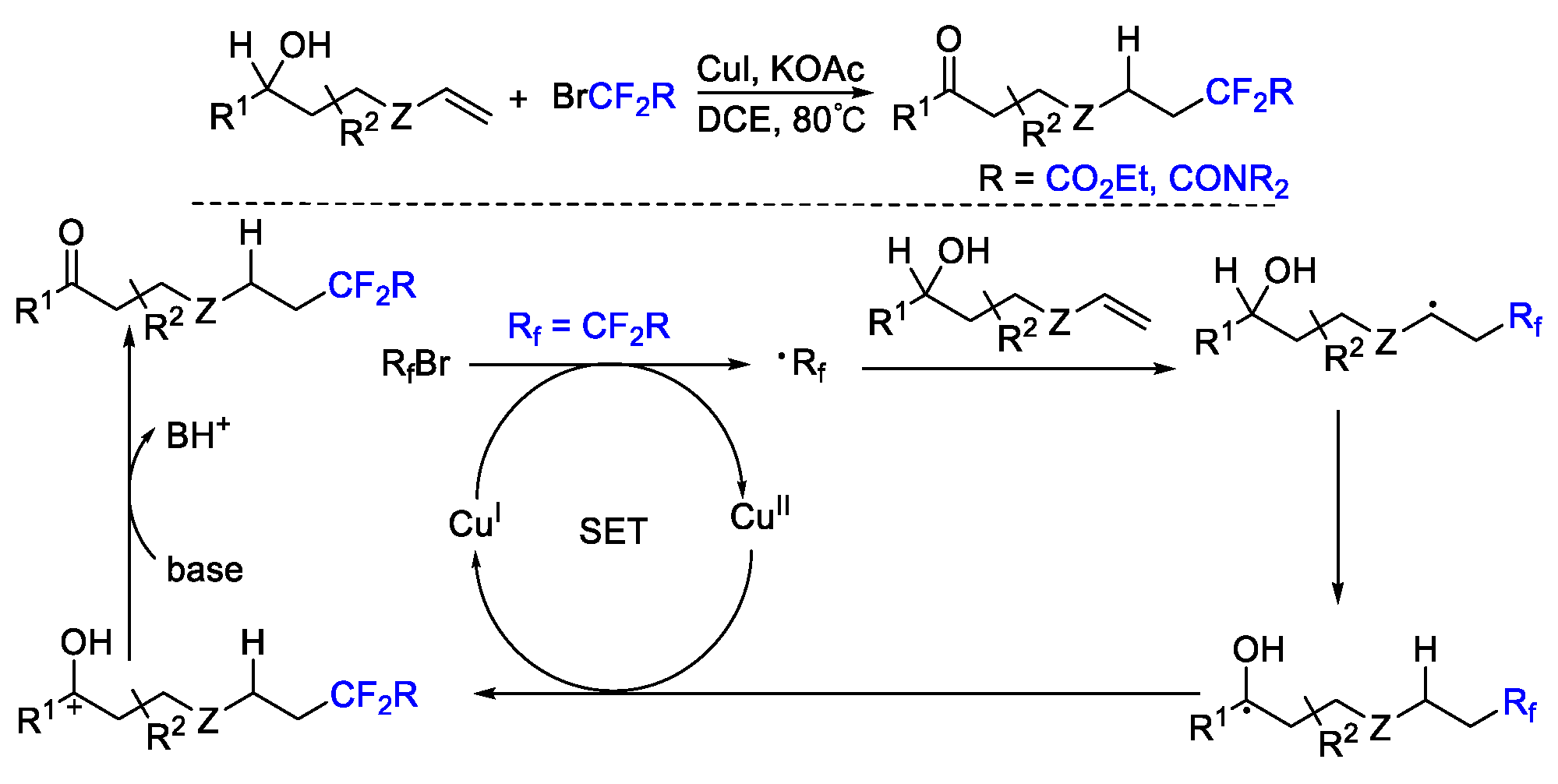
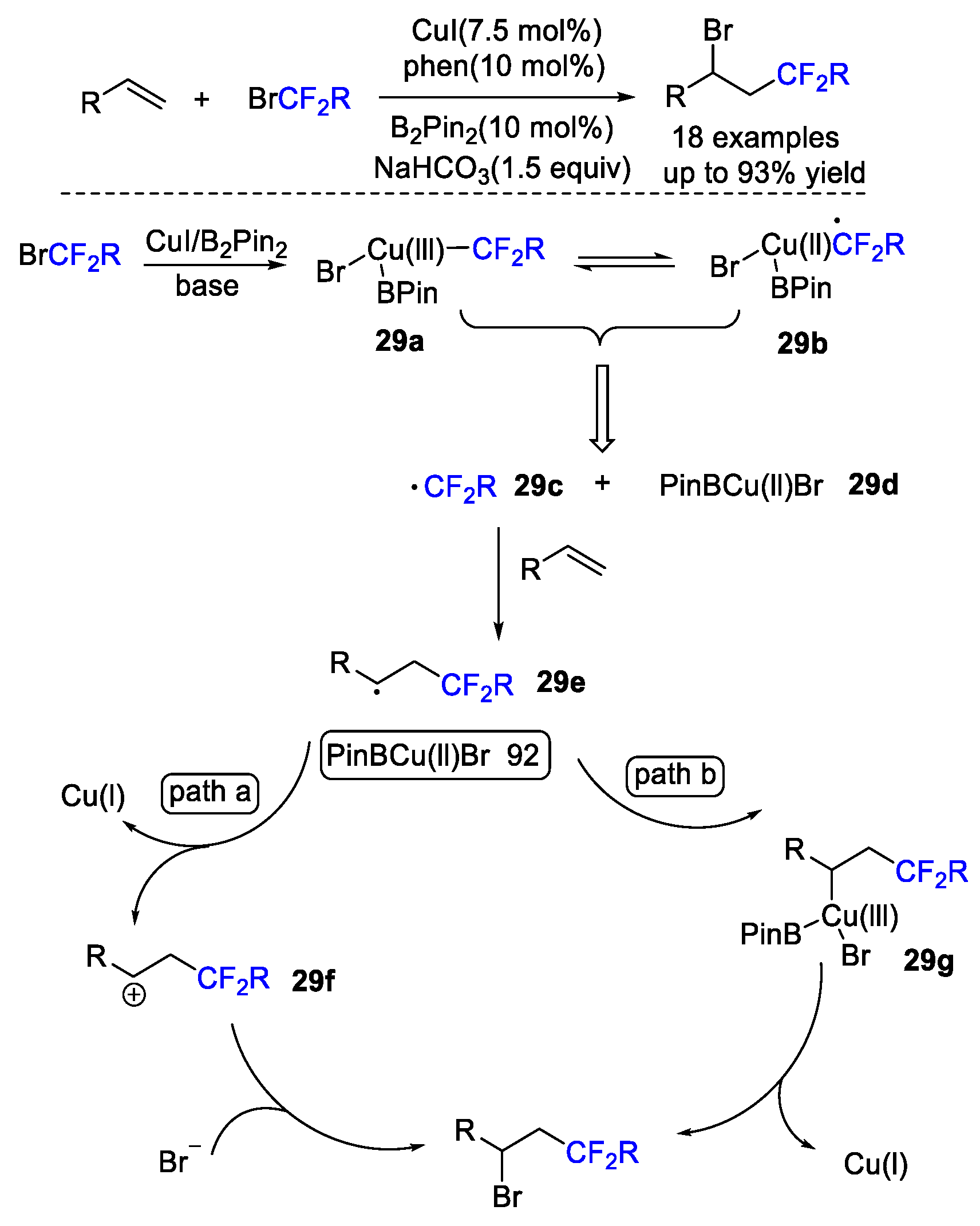
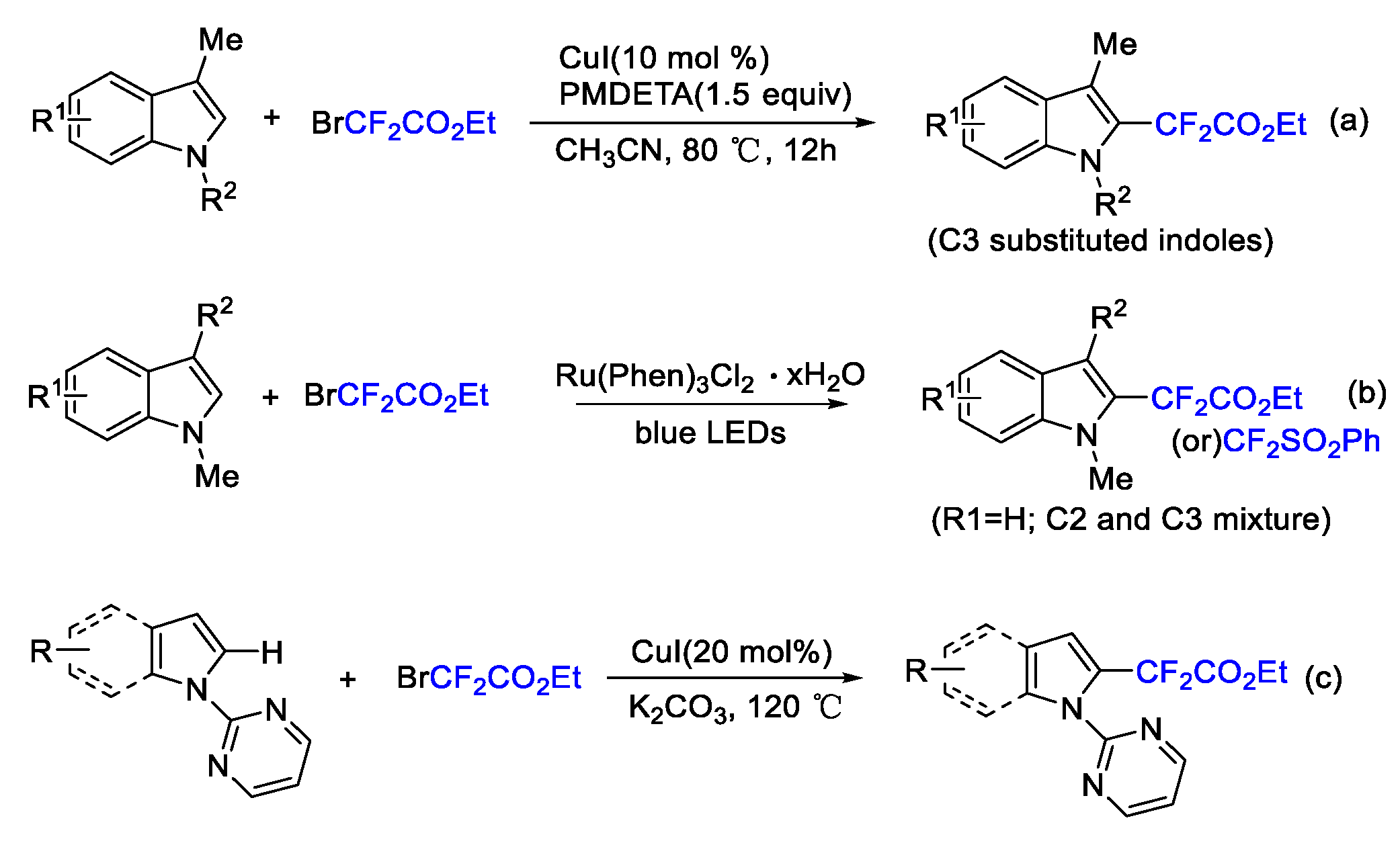
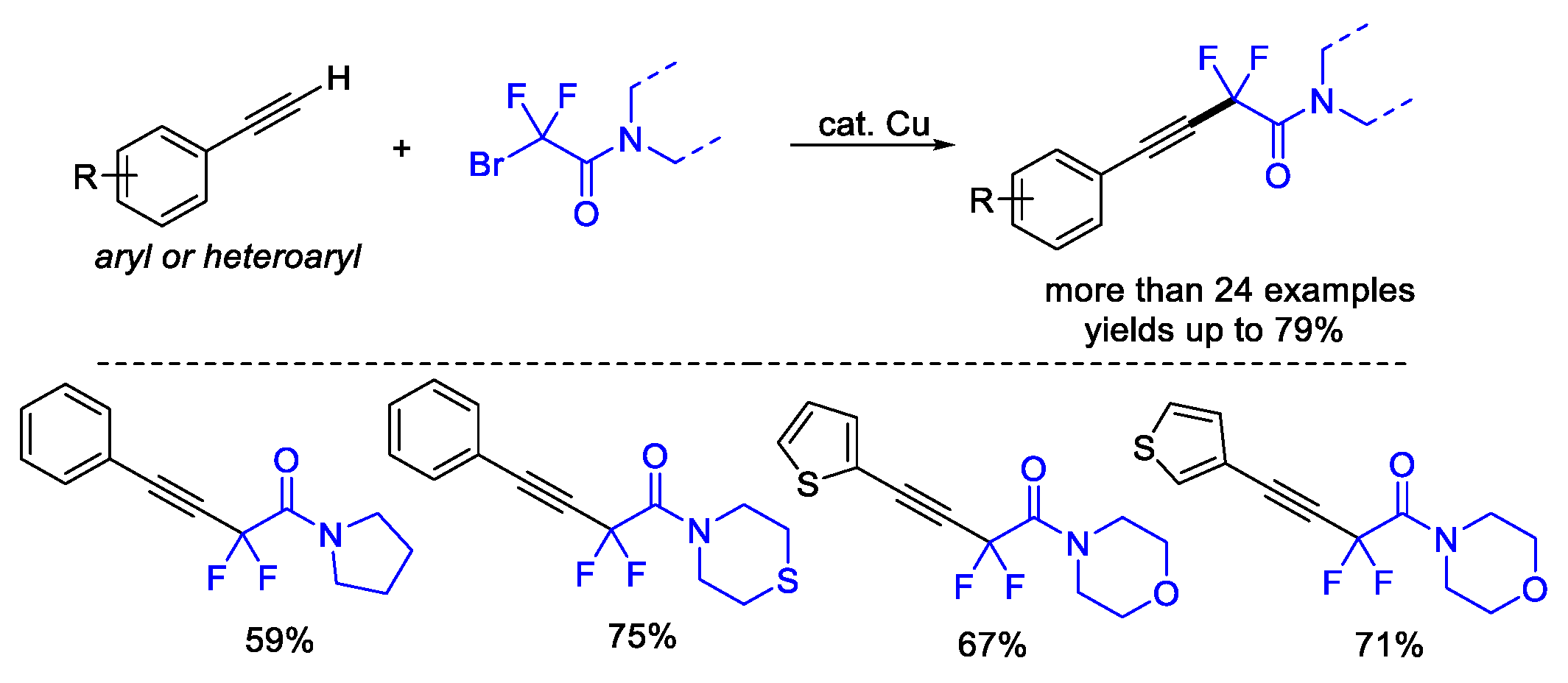
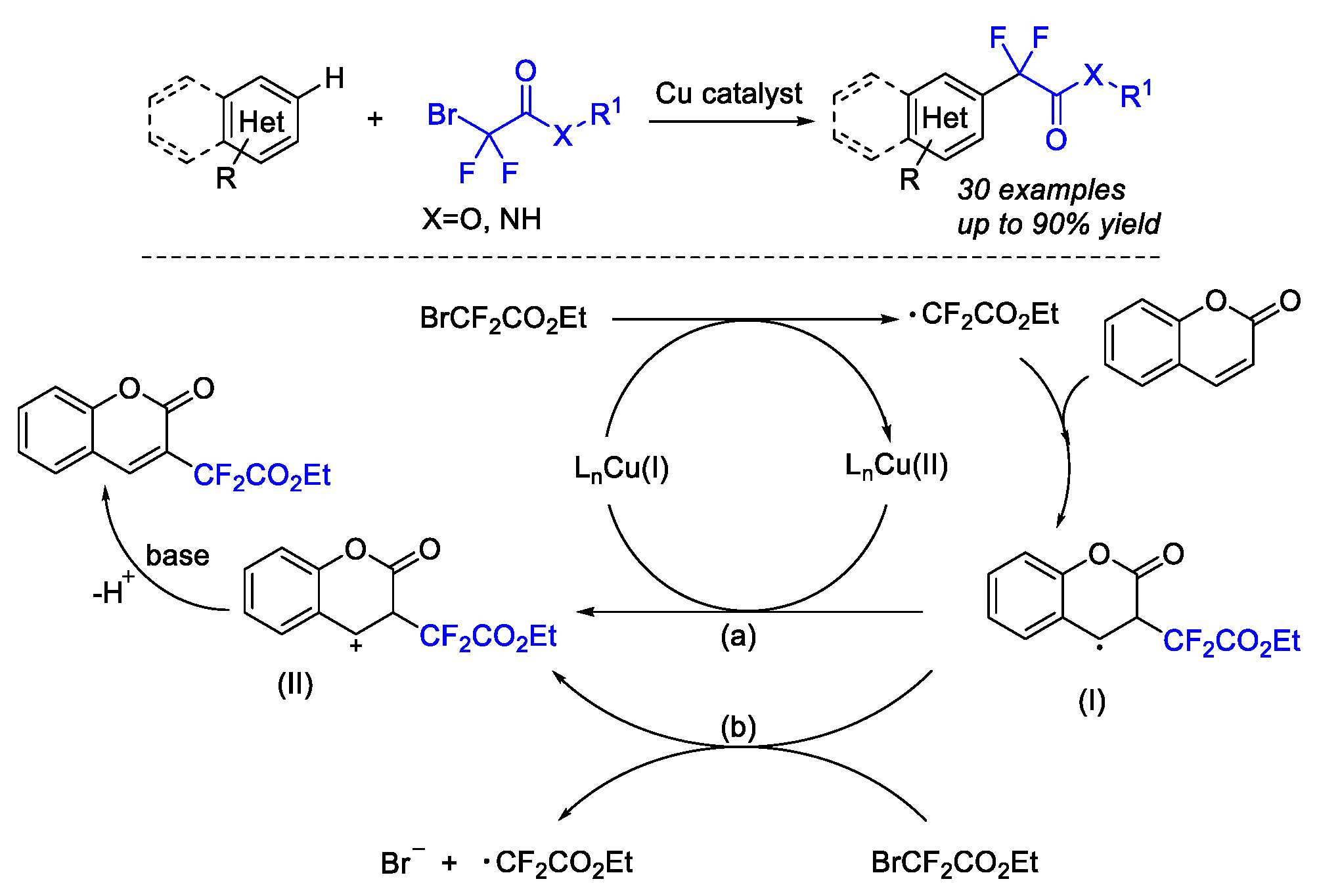
4. Difluoroalkylation–Coupling Reactions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Vulpetti, A.; Dalvit, C. Fluorine local environment: From screening to drug design. Drug Discov. Today 2012, 17, 890–897. [Google Scholar] [CrossRef] [PubMed]
- Dilman, A.D.; Levin, V.V. Difluorocarbene as a Building Block for Consecutive Bond-Forming Reactions. Acc. Chem. Res. 2018, 51, 1272–1280. [Google Scholar] [CrossRef] [PubMed]
- Lasing, T.; Phumee, A.; Siriyasatien, P.; Chitchak, K.; Vanalabhpatana, P.; Mak, K.K.; Hee Ng, C.; Vilaivan, T.; Khotavivattana, T. Synthesis and antileishmanial activity of fluorinated rhodacyanine analogues: The ‘fluorine-walk’ analysis. Bioorg. Med. Chem. 2020, 28, 115187. [Google Scholar] [CrossRef]
- Cech, R.; Zaller, J.G.; Lyssimachou, A.; Clausing, P.; Hertoge, K.; Linhart, C. Pesticide drift mitigation measures appear to reduce contamination of non-agricultural areas, but hazards to humans and the environment remain. Sci. Total Environ. 2023, 854, 158814. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.; Cai, H.; Huang, Q.; Wang, B.; Wang, X.; Luo, Q.; Li, Y.; Zhang, H.; Gong, Q.; Ma, X.; et al. Polymeric dual-modal imaging nanoprobe with two-photon aggregation-induced emission for fluorescence imaging and gadolinium-chelation for magnetic resonance imaging. Bioact. Mater. 2023, 19, 538–549. [Google Scholar] [CrossRef] [PubMed]
- Naz, F.; Arish, M. GPCRs as an emerging host-directed therapeutic target against mycobacterial infection: From notion to reality. Br. J. Pharmacol. 2022, 179, 4899–4909. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.B.; Wang, C.; Cui, M.C.; Du, M.S.; Li, W.W.; Jia, Z.X.; Zhao, Q. Synthesis of Difluoroalkylated Benzofuran, Benzothiophene, and Indole Derivatives via Palladium-Catalyzed Cascade Difluoroalkylation and Arylation of 1,6-Enynes. Org. Lett. 2020, 22, 1149–1154. [Google Scholar] [CrossRef]
- Sun, H.; Jiang, Y.; Yang, Y.-S.; Li, Y.-Y.; Li, L.; Wang, W.-X.; Feng, T.; Li, Z.-H.; Liu, J.-K. Synthesis of difluoromethylated 2-oxindoles and quinoline-2,4-diones via visible light-induced tandem radical cyclization of N-arylacrylamides. Org. Biomol. Chem. 2019, 17, 6629–6638. [Google Scholar] [CrossRef]
- Zhou, J.; Wang, F.; Lin, Z.; Cheng, C.; Zhang, Q.; Li, J. Visible-Light-Induced para-Selective C(sp(2))-H Difluoroalkylation of Diverse (Hetero)aromatic Carbonyls. Org. Lett. 2020, 22, 68–72. [Google Scholar] [CrossRef]
- Taguchi, T.; Kitagawa, O.; Morikawa, T.; Nishiwaki, T.; Uehara, H.; Endo, H.; Kobayashi, Y. Synthesis of 2,2-difluoroesters by iododifluoroacetate-copper with organic halides. Tetrahedron Lett. 1986, 27, 6103–6106. [Google Scholar] [CrossRef]
- Soni, V.; Sharma, D.M.; Punji, B. Nickel-Catalyzed Regioselective C(2)-H Difluoroalkylation of Indoles with Difluoroalkyl Bromides. Chem. Asian J. 2018, 13, 2516–2521. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.J.; Zhang, Y.F.; Hao, J.; Wan, W. Ag-Catalyzed minisci C-H difluoromethylarylation of N-heteroarenes. Org. Biomol. Chem. 2020, 18, 400–404. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, Y.; Qing, F.L. New Strategy for the Construction of Chiral Cyclopropenes: Enantioselective Synthesis of Gem-difluoromethylenated Three-Membered Carbocycles. Chin. J. Org. Chem. 2020, 40, 806–807. [Google Scholar] [CrossRef]
- Feng, Z.; Xiao, Y.L.; Zhang, X.G. Transition-Metal (Cu, Pd, Ni)-Catalyzed Difluoroalkylation via Cross-Coupling with Difluoroalkyl Halides. Acc. Chem. Res. 2018, 51, 2264–2278. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Yan, W.; Paeth, M.; Zacate, S.; Zeng, X. Late-Stage Difluoromethylation of Aliphatic Carboxylic Acids with Copper Catalysis. Synlett 2020, 31, 745–749. [Google Scholar] [CrossRef]
- Tao, X.F.; Sheng, R.; Bao, K.; Wang, Y.X.; Jin, Y.X. Progress of Difluoromethyl Heteroaryl Sulfones as Difluoroalkylation Reagents. Chin. J. Org. Chem. 2019, 39, 2726–2734. [Google Scholar] [CrossRef]
- Wang, W.; Yu, Q.; Zhang, Q.; Li, J.; Hui, F.; Yang, J.; Lü, J. Recent Progress on Difluoromethylation Methods. Chin. J. Org. Chem. 2018, 38, 1569–1585. [Google Scholar] [CrossRef]
- Dong, D.-Q.; Yang, H.; Shi, J.-L.; Si, W.-J.; Wang, Z.-L.; Xu, X.-M. Promising reagents for difluoroalkylation. Org. Chem. Front. 2020, 7, 2538–2575. [Google Scholar] [CrossRef]
- Gui, Q.W.; Teng, F.; Yang, H.; Xun, C.; Huang, W.J.; Lu, Z.Q.; Zhu, M.X.; Ouyang, W.T.; He, W.M. Visible-Light Photosynthesis of CHF2 /CClF2 /CBrF2 -Substituted Ring-fused Quinazolinones in Dimethyl Carbonate. Chem. Asian J. 2022, 17, e202101139. [Google Scholar] [CrossRef]
- Li, X.; Jiang, M.; Zhu, X.; Song, X.; Deng, Q.; Lv, J.; Yang, D. A desulphurization strategy for Sonogashira couplings by visible light/copper catalysis. Org. Chem. Front. 2022, 9, 386–393. [Google Scholar] [CrossRef]
- Cui, W.; Li, Y.; Li, X.; Li, J.; Song, X.; Lv, J.; Jiang, Y.-Y.; Yang, D. Rapid formation of Csp3–Csp3 bonds through copper-catalyzed decarboxylative Csp3–H functionalization. Chin. Chem. Lett. 2023, 34, 107477. [Google Scholar] [CrossRef]
- Chen, X.-J.; Gui, Q.-W.; Yi, R.; Yu, X.; Wu, Z.-L.; Huang, Y.; Cao, Z.; He, W.-M. Copper(i)-catalyzed intermolecular cyanoarylation of alkenes: Convenient access to α-alkylated arylacetonitriles. Org. Biomol. Chem. 2020, 18, 5234–5237. [Google Scholar] [CrossRef] [PubMed]
- Li, G.H.; Dong, D.Q.; Yang, Y.; Yu, X.Y.; Wang, Z.L. Direct Carbamoylation of Quinoline N-oxides with Hydrazinecarboxamides via C-H Bond Activation Catalyzed by Copper Catalyst. Adv. Synth. Catal. 2019, 361, 832–835. [Google Scholar]
- Bhunia, S.; Pawar, G.G.; Kumar, S.V.; Jiang, Y.W.; Ma, D.W. Selected Copper-Based Reactions for C-N, C-O, C-S, and C-C Bond Formation. Angew. Chem. Int. Ed. 2017, 56, 16136–16179. [Google Scholar] [CrossRef] [PubMed]
- Worrell, B.T.; Malik, J.A.; Fokin, V.V. Direct Evidence of a Dinuclear Copper Intermediate in Cu(I)-Catalyzed Azide-Alkyne Cycloadditions. Science 2013, 340, 457–460. [Google Scholar] [CrossRef]
- Gephart, R.T.; Warren, T.H. Copper-Catalyzed sp(3) C-H Amination. Organometallics 2012, 31, 7728–7752. [Google Scholar] [CrossRef]
- Cui, W.; Li, X.; Guo, G.; Song, X.; Lv, J.; Yang, D. Radial Type Ring Opening of Sulfonium Salts with Dichalcogenides by Visible Light and Copper Catalysis. Org. Lett. 2022, 24, 5391–5396. [Google Scholar] [CrossRef] [PubMed]
- Yan, Q.; Cui, W.; Song, X.; Xu, G.; Jiang, M.; Sun, K.; Lv, J.; Yang, D. Sulfonylation of Aryl Halides by Visible Light/Copper Catalysis. Org. Lett. 2021, 23, 3663–3668. [Google Scholar] [CrossRef]
- Zhu, X.; Li, X.; Li, X.; Lv, J.; Sun, K.; Song, X.; Yang, D. Decarboxylative C–H alkylation of heteroarenes by copper catalysis. Org. Chem. Front. 2021, 8, 3128–3136. [Google Scholar] [CrossRef]
- Gan, Z.; Yan, Q.; Li, G.; Li, Q.; Dou, X.; Li, G.-Y.; Yang, D. Copper-Catalyzed Domino Synthesis of Sulfur-Containing Heterocycles Using Carbon Disulfide as a Building Block. Adv. Synth. Catal. 2019, 361, 4558–4567. [Google Scholar] [CrossRef]
- Zhang, T.T.; Chen, B.; Wang, W.; Zhang, Q.; Wang, P.Y.; Wan, W.; Deng, H.M.; Hao, J.; Jiang, H.Z. Copper-Promoted Aryldifluoromethylenation of N-Arylacrylamides to 3-Benzo-diazolyldifluoromethylene-Substituted 2-Oxindoles. Asian J. Org. Chem. 2019, 8, 671–674. [Google Scholar] [CrossRef]
- Ding, F.; Fang, Y.; Jiang, Y.; Lin, K.; Shi, L. Tandem Radical Cyclization for the Construction of Difluoro-Containing Oxindoles and Quinoline-2,4-diones. Chem. Asian J. 2018, 13, 636–640. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Yang, Y.J.; Dong, J.Y.; Zhou, M.D.; Li, L.; Wang, H. Copper/B(2)pin(2)-Catalyzed Difluoroalkylation of Methylenecyclopropanes with Bromodifluorinated Acetates and Acetamides: One-Pot Synthesis of CF2-Containing Dihydronaphthalene Derivatives. J. Org. Chem. 2019, 84, 9937–9945. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Sánchez-Roselló, M.; Aceña, J.L.; del Pozo, C.; Sorochinsky, A.E.; Fustero, S.; Soloshonok, V.A.; Liu, H. Fluorine in Pharmaceutical Industry: Fluorine-Containing Drugs Introduced to the Market in the Last Decade (2001–2011). Chem. Rev. 2014, 114, 2432–2506. [Google Scholar] [CrossRef] [PubMed]
- Anderson, M.O.; Zhang, J.; Liu, Y.; Yao, C.; Phuan, P.-W.; Verkman, A.S. Nanomolar Potency and Metabolically Stable Inhibitors of Kidney Urea Transporter UT-B. J. Med. Chem. 2012, 55, 5942–5950. [Google Scholar] [CrossRef]
- Médebielle, M. Electrochemical addition of chlorodifluoroacetyl aromatic compounds to electron-rich olefinic substrates—A convenient synthesis of gem-difluoro heterocyclic compounds. Tetrahedron Lett. 1995, 36, 2071–2074. [Google Scholar] [CrossRef]
- Qu, C.; Xu, P.; Ma, W.; Cheng, Y.; Zhu, C. A novel visible light mediated radical cyclization of enol lactones: A concise method for fluorinated polycyclic lactone scaffolds. Chem. Commun. 2015, 51, 13508–13510. [Google Scholar] [CrossRef]
- Peng, P.; Huang, G.Z.; Sun, Y.X.; Wang, X.; Wu, J.J.; Wu, F.H. Copper-mediated cascade radical cyclization of olefins with naphthalenyl iododifluoromethyl ketones. Org. Biomol. Chem. 2019, 17, 6426–6431. [Google Scholar] [CrossRef]
- Wang, X.; Liu, J.; Yu, Z.; Guo, M.; Tang, X.; Wang, G. Desulfonylation-Initiated Distal Alkenyl Migration in Copper-Catalyzed Alkenylation of Unactivated Alkenes. Org. Lett. 2018, 20, 6516–6519. [Google Scholar] [CrossRef]
- Ke, M.; Song, Q. Copper/B2pin2-catalyzed C-H difluoroacetylation-cycloamidation of anilines leading to the formation of 3,3-difluoro-2-oxindoles. Chem. Commun. 2017, 53, 2222–2225. [Google Scholar] [CrossRef]
- Cao, A.Z.; Xiao, Y.T.; Wu, Y.C.; Song, R.J.; Xie, Y.X.; Li, J.H. Copper-catalyzed C-H [3 + 2] annulation of N-substituted anilines with alpha-carbonyl alkyl bromides via C(sp(3))-Br/C(sp(2))-H functionalization. Org. Biomol. Chem. 2020, 18, 2170–2174. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Wu, Y.H.; Xi, J.M.; Wei, Z.L.; Liao, W.W. Copper-Catalyzed Difluoroalkylation of Alkene/Nitrile Insertion/Cyclization Tandem Sequences: Construction of Difluorinated Bicyclic Amidines. Org. Lett. 2021, 23, 9591–9596. [Google Scholar] [CrossRef] [PubMed]
- Da, Y.; Han, S.; Du, X.; Liu, S.; Liu, L.; Li, J. Copper(I)-Catalyzed Oxydifluoroalkylation of Alkenes: A Route to Functionalization of Lactones. Org. Lett. 2018, 20, 5149–5152. [Google Scholar] [CrossRef]
- Yuan, F.Y.; Zhou, S.; Yang, Y.Y.; Guo, M.J.; Tang, X.Y.; Wang, G.W. Copper catalyzed one-pot difluoroalkylation and lactonization of unsaturated carboxylic acids. Org. Chem. Front. 2018, 5, 3306–3309. [Google Scholar] [CrossRef]
- Yang, Y.; Yuan, F.; Ren, X.; Wang, G.; Zhao, W.; Tang, X.; Guo, M. Copper-Catalyzed Oxydifluoroalkylation of Hydroxyl-Containing Alkenes. J. Org. Chem. 2019, 84, 4507–4516. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.Y.; Li, M.; Yang, Y.Y.; Guo, M.J.; Tang, X.Y.; Wang, G.W. One-pot Construction of Difluorinated Pyrrolizidine and Indolizidine Scaffolds via Copper-Catalyzed Radical Cascade Annulation. Adv. Synth. Catal. 2018, 360, 2151–2156. [Google Scholar] [CrossRef]
- Liao, J.; Ouyang, L.; Lai, Y.; Luo, R. Photoredox-Catalyzed Oxy-/Aminofluoroalkylative Cyclization of Alkenes. J. Org. Chem. 2020, 85, 5590–5597. [Google Scholar] [CrossRef] [PubMed]
- Fu, W.Q.; Song, Q. Copper-Catalyzed Radical Difluoroalkylation and Redox Annulation of Nitroalkynes for the Construction of C2-Tetrasubstituted Indolin-3-ones. Org. Lett. 2018, 20, 393–396. [Google Scholar] [CrossRef]
- Ding, F.W.; Jiang, Y.Q.; Lin, K.F.; Shi, L. Tandem radical cyclization for the construction of 1-difluoroalkylated isoquinolines via Cu catalyzed and visible light-promoted pathways. Org. Biomol. Chem. 2018, 16, 1812–1815. [Google Scholar] [CrossRef]
- Ma, X.; Mai, S.; Zhou, Y.; Cheng, G.J.; Song, Q. Dual role of ethyl bromodifluoroacetate in the formation of fluorine-containing heteroaromatic compounds. Chem. Commun. 2018, 54, 8960–8963. [Google Scholar] [CrossRef]
- Wang, L.X.; Chen, H.T.; Zhao, W.T.; Wang, G.W. Copper-Catalyzed Oxydifluoroalkylation of beta, gamma-Unsaturated Oximes for the Construction of Isoxazolines with a Difluoroalkyl Side Chain. Synlett 2021, 32, 1029–1033. [Google Scholar] [CrossRef]
- Zhu, S.Q.; Yang, H.B.; Jiang, A.; Zhou, B.; Han, Y.; Yan, C.G.; Shi, Y.C.; Hou, H. Copper-Catalyzed Bromodifluoroacetylative Cyclization of Enynes. J. Org. Chem. 2020, 85, 15667–15675. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, J.; Hu, B.; Ji, M.; Ye, S.; Zhu, G. Synthesis of Difluoromethylated and Phosphorated Spiro[5.5]trienones via Dearomative Spirocyclization of Biaryl Ynones. Org. Lett. 2018, 20, 2988–2992. [Google Scholar] [CrossRef] [PubMed]
- Li, C.W.; Zhao, Y.L.; Zhou, J.X.; Wang, X.; Hou, J.L.; Song, Y.G.; Liu, W.J.; Han, G.F. Synthesis of difluoroalkylated 2-azaspiro 4.5 decane derivatives via copper-catalyzed difluoroalkylation/dearomatization of N-benzylacrylamides. Org. Biomol. Chem. 2020, 18, 8376–8380. [Google Scholar] [CrossRef]
- Lv, X.L.; Wang, C.; Wang, Q.L.; Shu, W. Rapid Synthesis of gamma-Arylated Carbonyls Enabled by the Merge of Copper- and Photocatalytic Radical Relay Alkylarylation of Alkenes. Org. Lett. 2019, 21, 56–59. [Google Scholar] [CrossRef]
- Kong, W.; Yu, C.; An, H.; Song, Q. Copper-Catalyzed Intermolecular Reductive Radical Difluoroalkylation-Thiolation of Aryl Alkenes. Org. Lett. 2018, 20, 4975–4978. [Google Scholar] [CrossRef]
- Xu, R.; Cai, C. Three-component difluoroalkylation-thiolation of alkenes by iron-facilitated visible-light photoredox catalysis. Chem. Commun. 2019, 55, 4383–4386. [Google Scholar] [CrossRef]
- Hagen, K.S.; Reynolds, J.G.; Holm, R.H. Definition of reaction sequences resulting in self-assembly of [Fe4S4(SR)4]2- clusters from simple reactants. J. Am. Chem. Soc. 1981, 103, 4054–4063. [Google Scholar] [CrossRef]
- Xu, R.; Cai, C. Three-component difluoroalkylamination of alkenes mediated by photoredox and iron cooperative catalysis. Org. Biomol. Chem. 2019, 17, 8541–8545. [Google Scholar] [CrossRef]
- Murata, Y.; Shimada, T.; Nishikata, T. Radical and Cation Crossover Reaction System Enables Synthesis of Complex Aliphatic Chains Possessing Functionalized Quaternary Carbons. Bull. Chem. Soc. Jpn. 2019, 92, 1419–1429. [Google Scholar] [CrossRef]
- Bi, M.-H.; Cheng, Y.; Xiao, W.-J.; Chen, J.-R. Visible-Light-Induced Photoredox-Catalyzed Selective 1,4-Difluoroalkylesterification of 1-Aryl-1,3-dienes. Org. Lett. 2022, 24, 7589–7594. [Google Scholar] [CrossRef] [PubMed]
- Lemos, A.; Lemaire, C.; Luxen, A. Progress in Difluoroalkylation of Organic Substrates by Visible Light Photoredox Catalysis. Adv. Synth. Catal. 2019, 361, 1500–1537. [Google Scholar] [CrossRef]
- Liu, Q.; Wu, L.-Z. Recent advances in visible-light-driven organic reactions. Natl. Sci. Rev. 2017, 4, 359–380. [Google Scholar] [CrossRef]
- Chen, Y.; Lu, L.-Q.; Yu, D.-G.; Zhu, C.-J.; Xiao, W.-J. Visible light-driven organic photochemical synthesis in China. Sci. Chin. Chem. 2018, 62, 24–57. [Google Scholar] [CrossRef]
- Gui, Q.W.; Teng, F.; Li, Z.C.; Xiong, Z.Y.; Jin, X.F.; Lin, Y.W.; Cao, Z.; He, W.M. Visible-light-initiated tandem synthesis of difluoromethylated oxindoles in 2-MeTHF under additive-, metal catalyst-, external photosensitizer-free and mild conditions. Chin. Chem. Lett. 2021, 32, 1907–1910. [Google Scholar] [CrossRef]
- He, S.; Chen, X.; Zeng, F.; Lu, P.; Peng, Y.; Qu, L.; Yu, B. Visible-light-promoted oxidative decarboxylation of arylacetic acids in air: Metal-free synthesis of aldehydes and ketones at room temperature. Chin. Chem. Lett. 2020, 31, 1863–1867. [Google Scholar] [CrossRef]
- Sun, Y.Y.; Song, J.C.; Yang, S.H.; Wang, Z.L.; Zhang, E.X.; Han, Q.Q.; Yue, S. Visible light induced radical cascade cyclization of ortho-cyanoarylacrylamides with phosphine oxides for the preparation of phosphorylated quinoline-2,4(1H,3H)-dione. New J. Chem. 2021, 45, 16438–16441. [Google Scholar] [CrossRef]
- Dong, D.; Yue, S.; Wang, Z.; Yang, S.; Han, Q.; Zhang, E.; Qin, Q.; Song, J.; Sun, Y. Recent Progress in Radical Arylation Reaction with Diaryliodonium Salts under Photocatalysis. Chin. J. Org. Chem. 2021, 41, 4651–4660. [Google Scholar]
- Li, G.-H.; Han, Q.-Q.; Sun, Y.-Y.; Chen, D.-M.; Wang, Z.-L.; Xu, X.-M.; Yu, X.-Y. Visible-light induced cascade radical cyclization of sulfinic acids and o-(allyloxy)arylaldehydes towards functionalized chroman-4-ones. Chin. Chem. Lett. 2020, 31, 3255–3258. [Google Scholar] [CrossRef]
- Yang, W.-C.; Chen, C.-Y.; Li, J.-F.; Wang, Z.-L. Radical denitrogenative transformations of polynitrogen heterocycles: Building C–N bonds and beyond. Chin. J. Catal. 2021, 42, 1865–1875. [Google Scholar] [CrossRef]
- Xie, L.-Y.; Liu, Y.-S.; Ding, H.-R.; Gong, S.-F.; Tan, J.-X.; He, J.-Y.; Cao, Z.; He, W.-M. C(sp2)–H/O–H cross-dehydrogenative coupling of quinoxalin-2(1H)-ones with alcohols under visible-light photoredox catalysis. Chin. J. Catal. 2020, 41, 1168–1173. [Google Scholar] [CrossRef]
- He, W.-B.; Gao, L.-Q.; Chen, X.-J.; Wu, Z.-L.; Huang, Y.; Cao, Z.; Xu, X.-H.; He, W.-M. Visible-light-initiated malic acid-promoted cascade coupling/cyclization of aromatic amines and KSCN to 2-aminobenzothiazoles without photocatalyst. Chin. Chem. Lett. 2020, 31, 1895–1898. [Google Scholar] [CrossRef]
- Wang, P.; Zhao, Q.; Xiao, W.; Chen, J. Recent advances in visible-light photoredox-catalyzed nitrogen radical cyclization. Green Synth. Catal. 2020, 1, 42–51. [Google Scholar] [CrossRef]
- Nicewicz, D.A.; MacMillan, D.W.C. Merging photoredox catalysis with organocatalysis: The direct asymmetric alkylation of aldehydes. Science 2008, 322, 77–80. [Google Scholar] [CrossRef]
- Ischay, M.A.; Anzovino, M.E.; Du, J.; Yoon, T.P. Efficient Visible Light Photocatalysis of [2+2] Enone Cycloadditions. J. Am. Chem. Soc. 2008, 130, 12886–12887. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.B.; Li, W.W.; Qu, W.L.; Shu, Z.G.; Tao, Y.J.; Lin, J.M.; Gao, X. Copper and Photocatalytic Radical Relay Enabling Fluoroalkylphosphorothiolation of Alkenes: Modular Synthesis of Fluorine-Containing S-Alkyl Phosphorothioates and Phosphorodithioates. Org. Lett. 2021, 23, 9267–9272. [Google Scholar] [CrossRef]
- Wu, P.J.; Zheng, C.; Wang, X.; Wu, J.J.; Wu, F.H. Copper-Catalyzed Three-Component Reactions of 2-Iodo-2,2-difluoroacetophenones, Alkynes, and Trimethylsilyl Cyanide. Eur. J. Org. Chem. 2021, 2021, 1420–1423. [Google Scholar] [CrossRef]
- Gao, B.; Ni, Y.J.; Liu, X.J.; Jiang, T.; Yan, Q.; Yang, R.T.; Zhang, X.L. Copper-Catalyzed Difluoroalkylation-Thiolation of Alkenes Promoted by Na2S2O5. Eur. J. Org. Chem. 2021, 2021, 1913–1918. [Google Scholar] [CrossRef]
- Zhang, B.S.; Gao, L.Y.; Zhang, Z.; Wen, Y.H.; Liang, Y.M. Three-component difluoroalkylation and trifluoromethylthiolation/trifluoromethylselenolation of pi-bonds. Chem. Commun. 2018, 54, 1185–1188. [Google Scholar] [CrossRef]
- Zhang, J.; Jin, W.; Cheng, C.; Luo, F. Copper-catalyzed remote oxidation of alcohols initiated by radical difluoroalkylation of alkenes: Facile access to difluoroalkylated carbonyl compounds. Org. Biomol. Chem. 2018, 16, 3876–3880. [Google Scholar] [CrossRef]
- Hu, L.; Deng, Q.; Zhou, Y.; Zhang, X.; Xiong, Y. Cu2O-catalyzed phosphonyldifluoromethylation of allylic alcohols through a radical 1,2-aryl migration. Tetrahedron 2020, 76, 130949. [Google Scholar] [CrossRef]
- Li, D.; Mao, T.; Huang, J.; Zhu, Q. Copper-Catalyzed Bromodifluoroacetylation of Alkenes with Ethyl Bromodifluoroacetate. J. Org. Chem. 2018, 83, 10445–10452. [Google Scholar] [CrossRef]
- Feng, X.R.; Wang, X.Y.; Chen, H.T.; Tang, X.Y.; Guo, M.J.; Zhao, W.T.; Wang, G.W. Copper-mediated regioselective hydrodifluoroalkylation of alkynes. Org. Biomol. Chem. 2018, 16, 2841–2845. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, S.; Liu, J.; Zhu, D.; Guo, M.; Tang, X.; Wang, G. Copper-Catalyzed C–H Difluoroalkylations and Perfluoroalkylations of Alkenes and (Hetero)arenes. Org. Lett. 2017, 19, 4187–4190. [Google Scholar] [CrossRef]
- Lin, Q.; Chu, L.; Qing, F.-L. Direct Introduction of Ethoxycarbonyldifluoromethyl-Group to Heteroarenes with Ethyl Bromodifluoroacetate via Visible-Light Photocatalysis. Chin. J. Chem. 2013, 31, 885–891. [Google Scholar] [CrossRef]
- Yan, S.Y.; Zhang, Z.Z.; Liu, Y.H.; Liao, G.; Li, P.X.; Shi, B.F. Copper-Catalyzed C-H Ethoxycarbonyldifluoromethylation of Indoles and Pyrroles. Asian J. Org. Chem. 2018, 7, 1319–1322. [Google Scholar] [CrossRef]
- Chen, X.; Xu, W.; Wang, K.; Mo, M.; Zhang, W.; Du, L.; Yuan, X.; Xu, Y.; Wang, Y.; Shen, J. Discovery of a Novel Series of Imidazo[1,2-a]pyrimidine Derivatives as Potent and Orally Bioavailable Lipoprotein-Associated Phospholipase A2 Inhibitors. J. Med. Chem. 2015, 58, 8529–8541. [Google Scholar] [CrossRef] [PubMed]
- Cecile, E.-G.; Alain, G. Recent Progress in the Pharmacology of Imidazo[1,2-a]pyridines. Mini-Rev. Med. Chem. 2007, 7, 888–899. [Google Scholar]
- Mishra, S.; Mondal, P.; Ghosh, M.; Mondal, S.; Hajra, A. Copper-catalyzed C–H ethoxycarbonyldifluoromethylation of imidazoheterocycles. Org. Biomol. Chem. 2016, 14, 1432–1436. [Google Scholar] [CrossRef]
- Yin, G.; Zhu, M.; Fu, W. Visible-light-induced photocatalytic difluoroacetylation of imidazopyridines via direct and regioselective C H functionalization. J. Fluorine Chem. 2017, 199, 14–19. [Google Scholar] [CrossRef]
- Singsardar, M.; Mondal, S.; Laru, S.; Hajra, A. Organophotoredox-Catalyzed C(sp(2))-H Difluoromethylenephosphonation of Imidazoheterocycles. Org. Lett. 2019, 21, 5606–5610. [Google Scholar] [CrossRef]
- Han, S.; Liang, A.; Ren, X.; Gao, X.; Li, J.; Zou, D.; Wu, Y.; Wu, Y. Copper-catalyzed remote CH ethoxycarbonyldifluoromethylation of 8-aminoquinolines with bis(pinacolato)diboron as reductant. Tetrahedron Lett. 2017, 58, 4859–4863. [Google Scholar] [CrossRef]
- Chen, H.; Li, P.H.; Wang, M.; Wang, L. Nickel-Catalyzed Site-Selective C-H Bond Difluoroalkylation of 8-Aminoquinolines on the C5-Position. Org. Lett. 2016, 18, 4794–4797. [Google Scholar] [CrossRef]
- Chen, C.P.; Zeng, R.S.; Zhang, J.Y.; Zhao, Y.S. Ruthenium-Catalyzed Difluoroalkylation of 8-Aminoquinoline Amides at the C5-Position. Eur. J. Org. Chem. 2017, 2017, 6947–6950. [Google Scholar] [CrossRef]
- Han, Q.-Q.; Chen, D.-M.; Wang, Z.-L.; Sun, Y.-Y.; Yang, S.-H.; Song, J.-C.; Dong, D.-Q. Hypervalent iodine mediated C-H amination of quinoxalinones with heteroaromatic amines under metal-free conditions. Chin. Chem. Lett. 2021, 32, 2559–2561. [Google Scholar] [CrossRef]
- Wang, L.; Liu, H.; Li, F.; Zhao, J.; Zhang, H.Y.; Zhang, Y. Copper–Catalyzed C3−H Difluoroacetylation of Quinoxalinones with Ethyl Bromodifluoroacetate. Adv. Synth. Catal. 2019, 361, 2354–2359. [Google Scholar] [CrossRef]
- Dai, J.L.; Lei, W.L.; Liu, Q. Visible-Light-Driven Difluoroalkylation of Aromatics Catalyzed by Copper. Acta Chim. Sin. 2019, 77, 911–915. [Google Scholar] [CrossRef]
- Prieto, A.; Melot, R.; Bouyssi, D.; Monteiro, N. C–H Difluoroalkylation of Aldehyde Hydrazones with Functionalized Difluoromethyl Bromides under Copper Catalysis. ACS Catal. 2016, 6, 1093–1096. [Google Scholar] [CrossRef]
- Prieto, A.; Melot, R.; Bouyssi, D.; Monteiro, N. Palladium-Catalyzed C(sp2)−H Alkylation of Aldehyde-Derived Hydrazones with Functionalized Difluoromethyl Bromides. Angew. Chem. Int. Ed. 2016, 55, 1885–1889. [Google Scholar] [CrossRef]
- Xu, P.; Wang, G.; Zhu, Y.; Li, W.; Cheng, Y.; Li, S.; Zhu, C. Visible-Light Photoredox-Catalyzed C−H Difluoroalkylation of Hydrazones through an Aminyl Radical/Polar Mechanism. Angew. Chem. Int. Ed. 2016, 55, 2939–2943. [Google Scholar] [CrossRef]
- Xie, J.; Zhang, T.; Chen, F.; Mehrkens, N.; Rominger, F.; Rudolph, M.; Hashmi, A.S. Gold-Catalyzed Highly Selective Photoredox C(sp(2) )-H Difluoroalkylation and Perfluoroalkylation of Hydrazones. Angew. Chem. Int. Ed. 2016, 55, 2934–2938. [Google Scholar] [CrossRef] [PubMed]
- Ke, M.; Song, Q. Copper-Catalyzed C(sp(2))-H Difluoroalkylation of Aldehyde Derived Hydrazones with Diboron as Reductant. J. Org. Chem. 2016, 81, 3654–3664. [Google Scholar] [CrossRef]
- Levitre, G.; Granados, A.; Cabrera-Afonso, M.J.; Molander, G.A. Synthesis of ?-Fluorinated Areneacetates through Photoredox/Copper Dual Catalysis. Org. Lett. 2022, 24, 3194–3198. [Google Scholar] [CrossRef] [PubMed]
- Hu, G.Q.; Yao, L.W.; Gui, S.S.; Geng, C.; Zhang, W.Y.; Liu, J.H.; Zhao, B. Copper-Catalyzed Cross-Coupling of Arylacetylenes with Bromodifluoroacetamides. Synlett 2022, 33, 594–598. [Google Scholar]
- Rao, M.; Wei, Z.W.; Yuan, Y.F.; Cheng, J.J. Copper-Catalyzed C-H Difluoroalkylation of Coumarins with Fluoroalkyl Bromides. Chemcatchem 2020, 12, 5256–5260. [Google Scholar] [CrossRef]
- Qu, C.H.; Song, G.T.; Tang, D.Y.; Shao, J.W.; Li, H.Y.; Xu, Z.G.; Chen, Z.Z. Microwave-Assisted Copper Catalysis of alpha-Difluorinated gem-Diol toward Difluoroalkyl Radical for Hydrodifluoroalkylation of para-Quinone Methides. J. Org. Chem. 2020, 85, 12785–12796. [Google Scholar] [CrossRef]
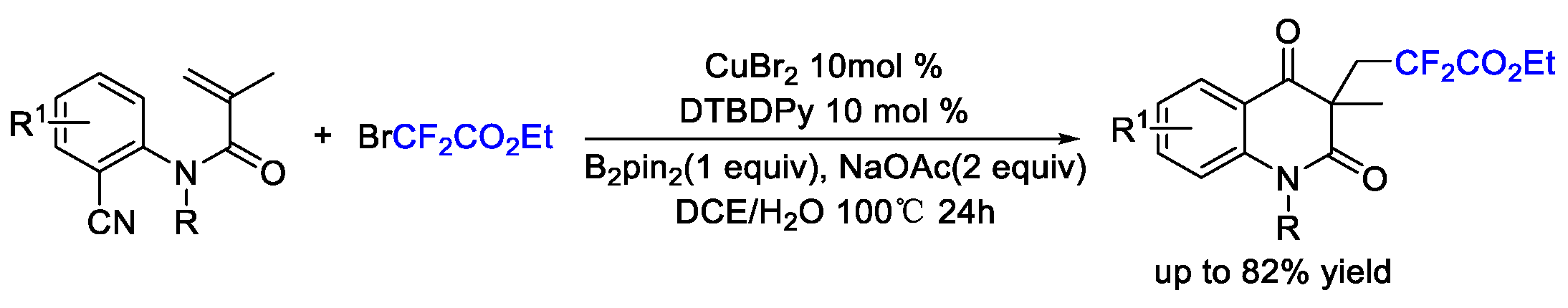


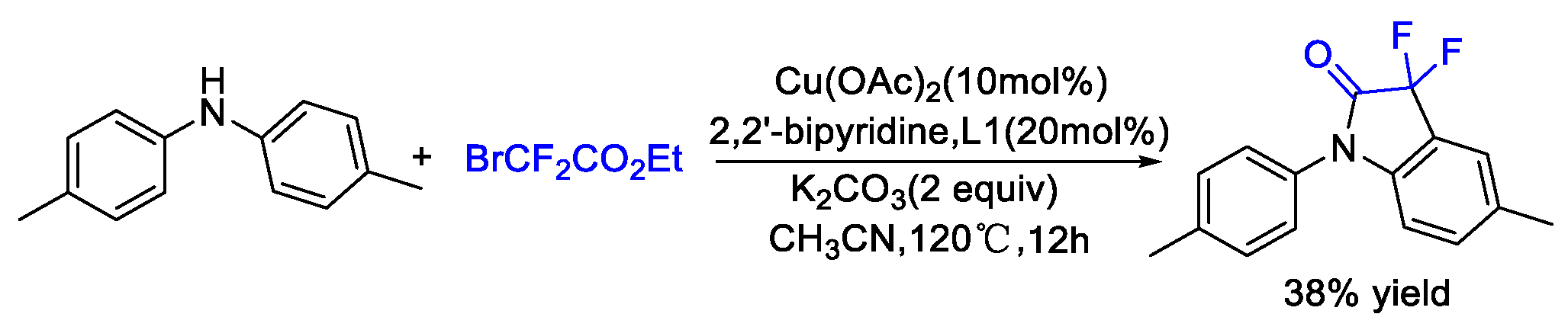

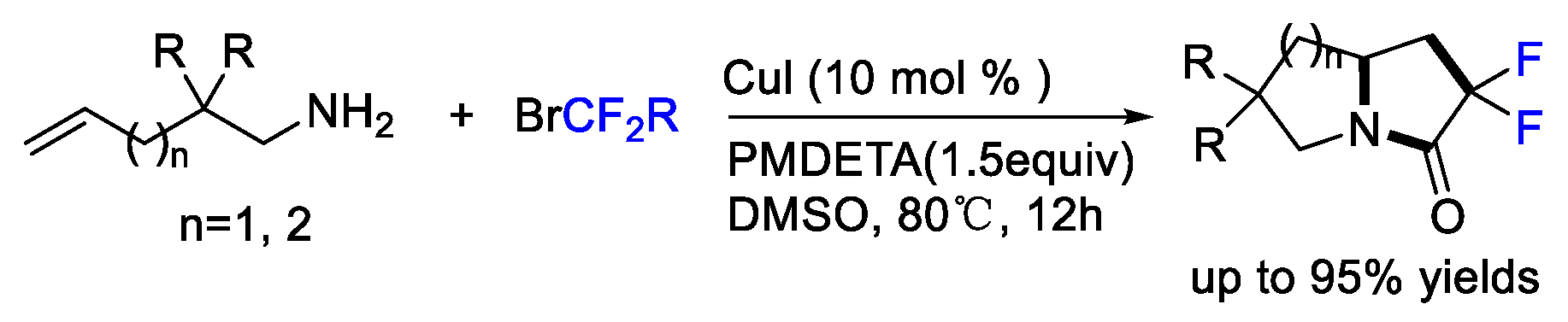
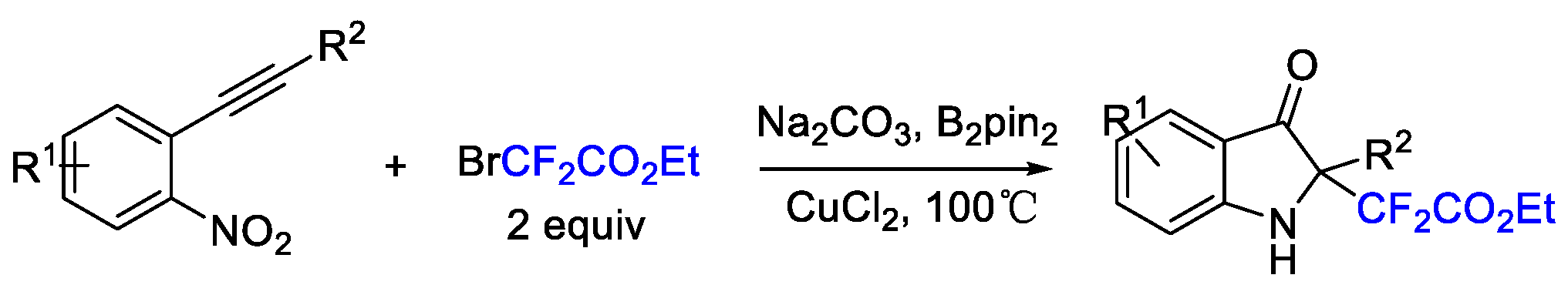





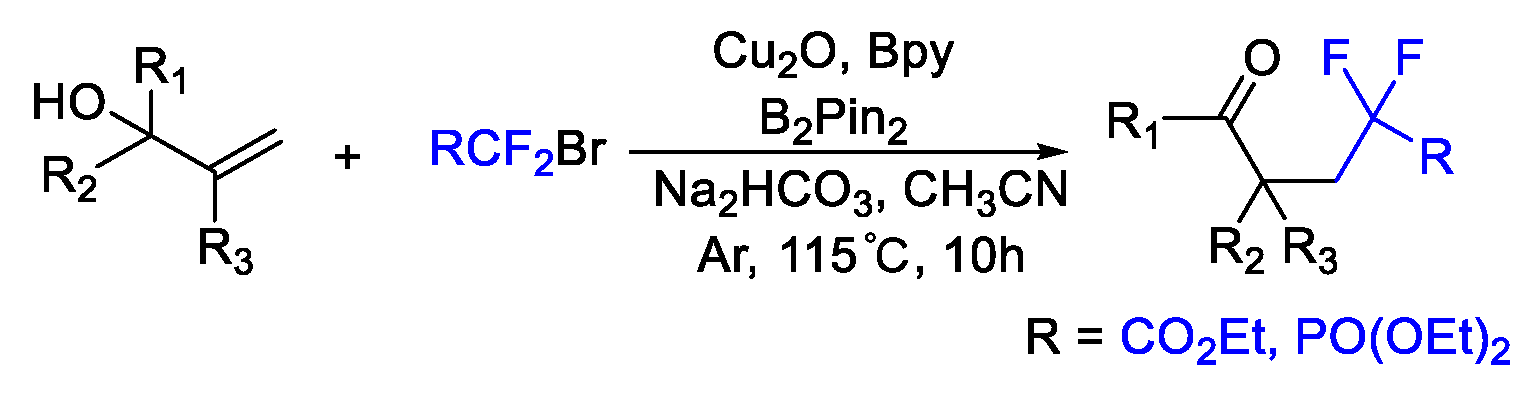


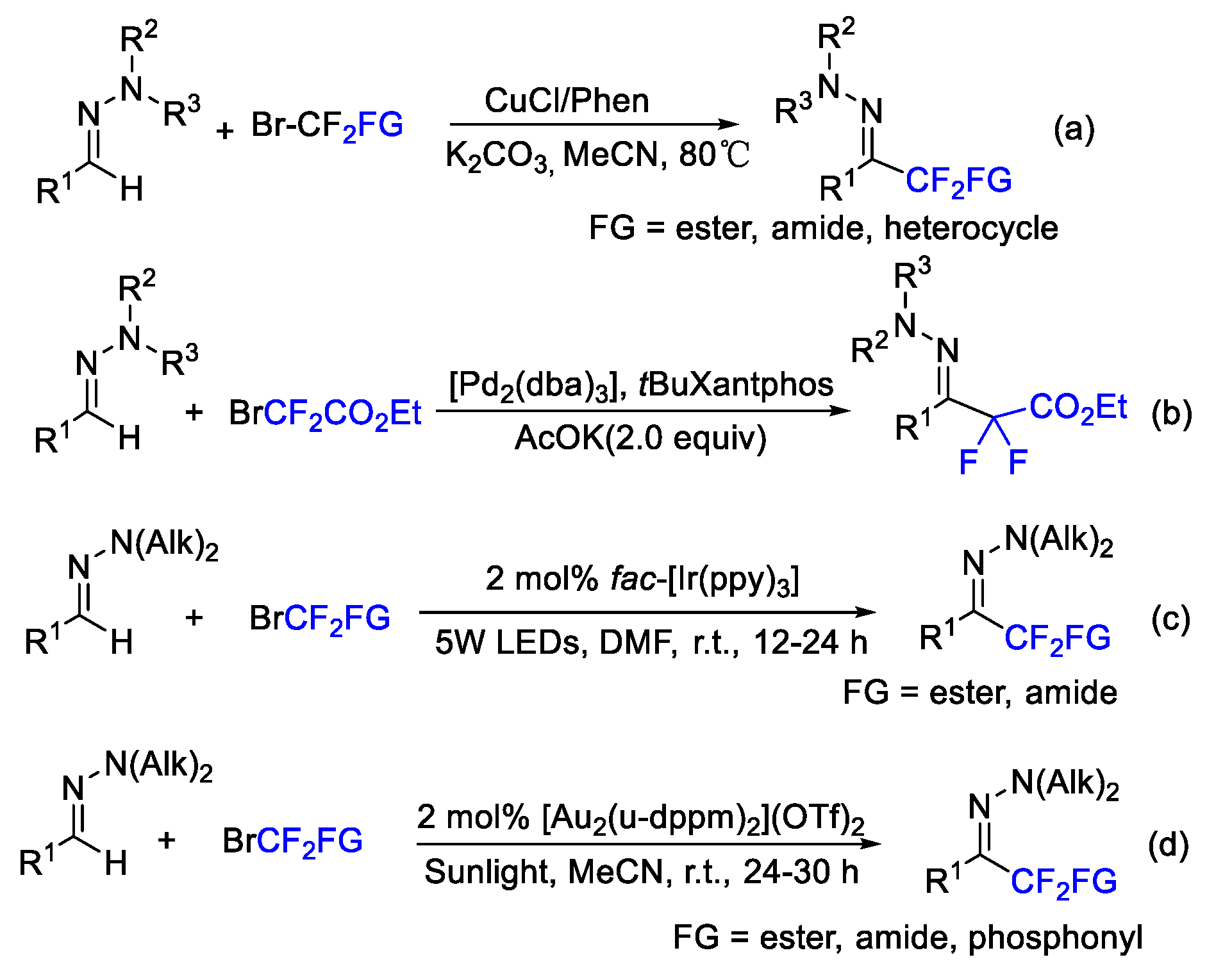


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, D.-Q.; Yang, S.-H.; Wu, P.; Wang, J.-Z.; Min, L.-H.; Yang, H.; Zhou, M.-Y.; Wei, Z.-H.; Ding, C.-Z.; Wang, Y.-L.; et al. Copper-Catalyzed Difluoroalkylation Reaction. Molecules 2022, 27, 8461. https://doi.org/10.3390/molecules27238461
Dong D-Q, Yang S-H, Wu P, Wang J-Z, Min L-H, Yang H, Zhou M-Y, Wei Z-H, Ding C-Z, Wang Y-L, et al. Copper-Catalyzed Difluoroalkylation Reaction. Molecules. 2022; 27(23):8461. https://doi.org/10.3390/molecules27238461
Chicago/Turabian StyleDong, Dao-Qing, Shao-Hui Yang, Pei Wu, Jin-Zhi Wang, Ling-Hao Min, Hao Yang, Meng-Yu Zhou, Ze-Hui Wei, Cai-Zhen Ding, Yan-Li Wang, and et al. 2022. "Copper-Catalyzed Difluoroalkylation Reaction" Molecules 27, no. 23: 8461. https://doi.org/10.3390/molecules27238461
APA StyleDong, D.-Q., Yang, S.-H., Wu, P., Wang, J.-Z., Min, L.-H., Yang, H., Zhou, M.-Y., Wei, Z.-H., Ding, C.-Z., Wang, Y.-L., Gao, J.-H., Wang, S.-J., & Wang, Z.-L. (2022). Copper-Catalyzed Difluoroalkylation Reaction. Molecules, 27(23), 8461. https://doi.org/10.3390/molecules27238461




