Abstract
Two isostructural pairs of supramolecular iodoantimonate(III) and iodobismuthate(III) complexes with I2 units “trapped” in solid state via halogen bonding—Cat3[[M2I9](I2)} (Cat = tetramethylammonium and 1-methylpyridinium, M = Sb(III) and Bi(III)) were prepared. For all compounds, values of optical band gaps were determined, together with thermal stability; the complexes were additionally characterized by Raman spectroscopy.
1. Introduction
Within the past decades, there has been an ongoing growth of interest on halide complexes of p-block elements. The motivation of research in this field is mostly explained by the fact that iodometalates (in particular, iodoplumbates(II) [1,2,3,4]) can be used as components of solar cells and/or photodetectors—so-called perovskite photovoltaics constituted an area that has developed extremely rapidly [5,6,7,8,9,10,11,12].
One of the problems in this area is the fact that “true” isotropic 3D halometalates (with all covalent bonds), ideally suitable for the use as light absorbers in photovoltaic devices, are extremely rare: those are formed by either Sn(II) or Pb(II) with a very limited number of small organic (such as methylammonium [13,14]) or inorganic (such as cesium [3,15]) cations. This fact encourages the search for alternative candidates in this role. Halometalates of Sb, Bi and other p-elements have been extensively tested [16], mostly revealing low-to-moderate efficiencies. This is generally explained by the less favorable molecular structure of these compounds (those are 0D, 1D or, rarely, 2D).
There are different approaches towards the overcoming of this obstacle. Recently, Shevelkov et al., presented the first supramolecular iodobismuthate(III) complexes containing diiodine units [17,18,19]. In such hybrids, I2 is “captured” in solid state, forming halogen bonding with iodometalate anions and acting therefore as “linker”. Such structures feature therefore higher dimensionality of halometalate framework which is, as mentioned above, desirable for the enhancement of photovoltaic properties. It is also notable that these hybrids usually feature lower optical band gaps than “pure iodobismuthates” (<1.7 eV) and, at the same time, can be thermally stable enough to be used in solar cells.
Performing research in the field of polyhalogen-halometalates since the last years, we confirmed that representatives of this class indeed can be suitable for photovoltaic applications (mostly as photodetectors) [20,21]. Our work with polyiodo-iosobismuthates(III) resulted in the discovery of new structural types, including 1D and 2D supramolecular frameworks [22]. However, the number of these compounds is yet small. Moreover, those are virtually unknown for the closest neighbor of Bi(III)—antimony, which also readily forms halide complexes. On this reason, we decided to try preparing the pairs of polyiodo-iodometalates of Sb(III) and Bi(III) in order to compare their structural features (suggesting that those can be isostructural), as well as physical properties.
Hereby, we present four new supramolecular halometalates: (Me4N)3{[M2I9](I2)} (M = Sb (1), Bi (2)) and (1-MePy)3{[M2I9](I2)} (M = Sb (3), Bi (4)). Those were characterized by X-ray diffractometry, Raman and diffuse reflectance spectroscopy, as well as thermogravimetric analysis (TGA).
2. Experimental Part
The chemicals were purchased from commercial sources and used without additional purification. Solvents were purified according to the standard procedures. The 1-methylpyridinium iodide was prepared by reaction of pyridine and methyl iodide (1:1.05) following the standard method of alkylation of substituted pyridines. In all cases, concentrate HI was used.
2.1. Synthesis of 1
We dissolved 58 mg (0.2 mmol) of Sb2O3, 121 mg (0.3 mmol) of Me4NI and 51 mg (0.2 mmol) of I2 in 4.5 mL of HI (70 °C, stirring, 30 min). Resulting solution was slowly cooled to r.t., resulting in formation of dark crystals within 1 d. Yield 79%. For C12H36N3Sb2I11 calcd, %: C, 7.74; H, 1.95; N, 2.26; found, %: C, 7.91; H, 2.04; N, 2.38.
2.2. Synthesis of 2
The procedure was the same as for 1, using Bi2O3 (93 mg, 0.2 mmol) instead of Sb2O3. Yield 82%. For C12H36N3Bi2I11 calcd, %: C, 7.07; H, 1.78, N, 2.06; found, %: C, 7.16, H, 1.89; N, 2.20.
2.3. Synthesis of 3
29 mg (0.1 mmol) of Sb2O3, 67 mg (0.3 mmol) of 1-MePyI and 25 mg (0.1 mmol) of I2 were dissolved in 3.5 mL of HI (70 °C, stirring, 30 min). Resulting solution was slowly cooled to r.t. Partial evaporation of the solvent results in formation of dark crystals. Yield 64%. For C18H24N3Sb2I11 calcd, %: C, 11.25; H, 1.26; N, 2.19; found, %: C, 11.31; H, 1.32; N, 2.29.
2.4. Synthesis of 4
The procedure was the same as for 3, using Bi2O3 (47 mg, 0.1 mmol) instead of Sb2O3. Yield 79%. For C18H24N3Bi2I11 calcd, %: C, 10.30; H, 1.15, N, 2.00; found, %: C, 10.41, H, 1.27; N, 2.14.
2.5. X-ray Diffractometry
Crystallographic data and refinement details for 1–4 are given in Table S1 (Supplementary Materials). For 1, 2 and 4, the data were collected on a Bruker D8 Venture diffractometer with a CMOS PHOTON III detector and IµS 3.0 source (Mo Kα radiation, λ = 0.71073 Å) at 150 K. The φ- and ω-scan techniques were employed. Absorption correction was applied by SADABS (Bruker Apex3 software suite: Apex3, SADABS-2016/2 and SAINT, version 2018.7-2; Bruker AXS Inc.: Madison, WI, USA, 2017). For 3, the data were collected on a New Xcalibur (Agilent Technologies) diffractometer with MoKα radiation (λ = 0.71073) by doing φ scans of narrow (0.5°) frames at 150 K. Absorption correction was done empirically using SCALE3 ABSPACK (CrysAlisPro, Agilent Technologies, Version 1.171.37.35 (release 13 August 2014 CrysAlis171.NET). Structures were solved by SHELXT [23] and refined by full-matrix least-squares treatment against |F|2 in anisotropic approximation with SHELX 2014/7 [24] in ShelXle program [25].
CCDC 2214619-2214621 contain the supplementary crystallographic data for 1, 3 and 4 correspondingly. These data can be obtained free of charge via http://www.ccdc.cam.ac.uk/conts/retrieving.html, or from the Cambridge Crystallographic Data Centre, 12 Union Road, Cambridge CB2 1EZ, UK; fax: (+44) 1223-336-033; or e-mail: deposit@ccdc.cam.ac.uk.
2.6. Powder X-ray Diffractometry (PXRD),
XRD analysis of polycrystals was performed on Shimadzu XRD-7000 diffractometer (CuK-alpha radiation, Ni—filter, linear One Sight detector, 0.0143° 2θ step, 2s per step). Plotting of PXRD patterns and data treatment was performed using X’Pert Plus software (see Supplementary Materials).
2.7. Raman Spectroscopy, Thermogravimetric Analysis, Difuse Reflectance Spectroscopy
Details are given in Supplementary Materials.
3. Results and Discussion
There is a general method for preparation of polyhalogen-halometalates. There are three components: (1) metal source (oxide, oxohalide or halide), which is converted into halometalate by addition of either hydrohalic acid or other source of halide ligand, (2) halide salt of certain cation and (3) dihalogen. Depending on the precursors, synthesis can be conducted in acidic aqueous solutions or in organic solvents, such as acetonitrile. Preparation of 1–4 follows this paradigm as well, with no specific details (see Experimental Section). The yields can be regarded as good.
The main feature of the pairs 1–2 and 3–4 is that each of them is isostructural. In all cases, the halometalate building blocks are binuclear [Sb2I9]3− or [Bi2I9]3− anions consisting of two {MI6} octahedra connected via shared face. This type of anion is very common in structural chemistry of Group 15 halometalates [26,27,28,29,30]. The M-Iterm and M-μ2-I bond lengths in 1–4 are given in Table 1.

Table 1.
Selected geometric parameters in 1–4.
In the crystal structure of 1, the [Sb2I9]3− units have orientational disorder over two closed positions with 0.97/0.03 occupancies. All TMA+ cations are disordered over two different orientations with different occupancies. In the case of complex 2, the quality of crystalline material was too low to extract enough data for well-solved crystal structure. Complex 2 has practically the same unit cell parameters as complex 1. Therefore, the use of structural atomic coordinates from 1 in the refinement of 2 gave the structural model with R c.a. 12%.
The main structural feature of 1–4 is the presence of diiodine units which, as follows from analysis of interatomic distances (<3.98 Å, which is the sum of Bondi’s van der Waals radii [31]), likely interact with terminal iodide ligands of [M2I9]3− anions. The geometric parameters of M-I···I and I-I···I fragments are given in Table 1.
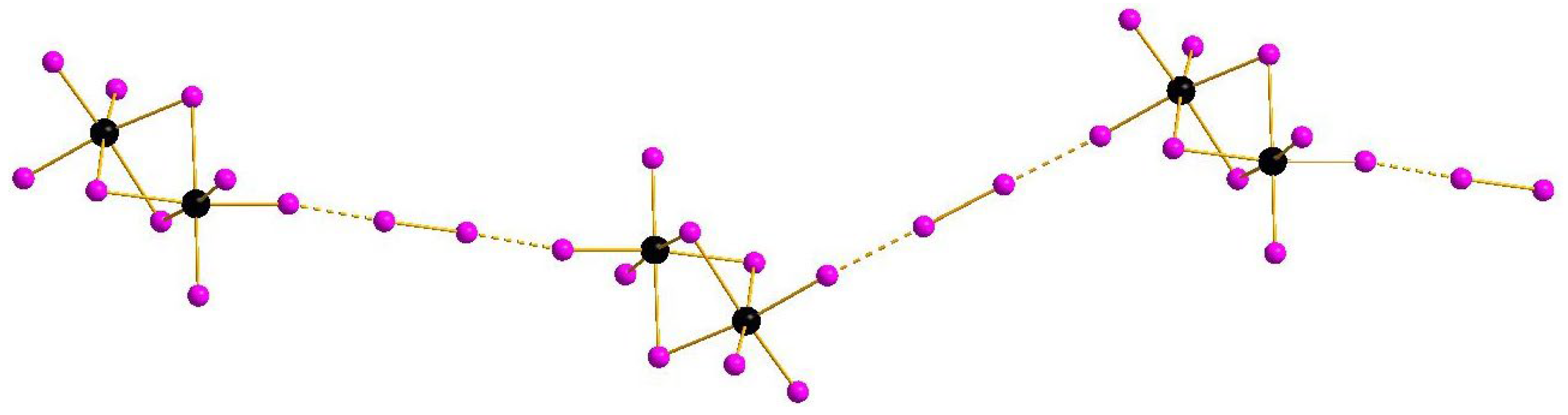
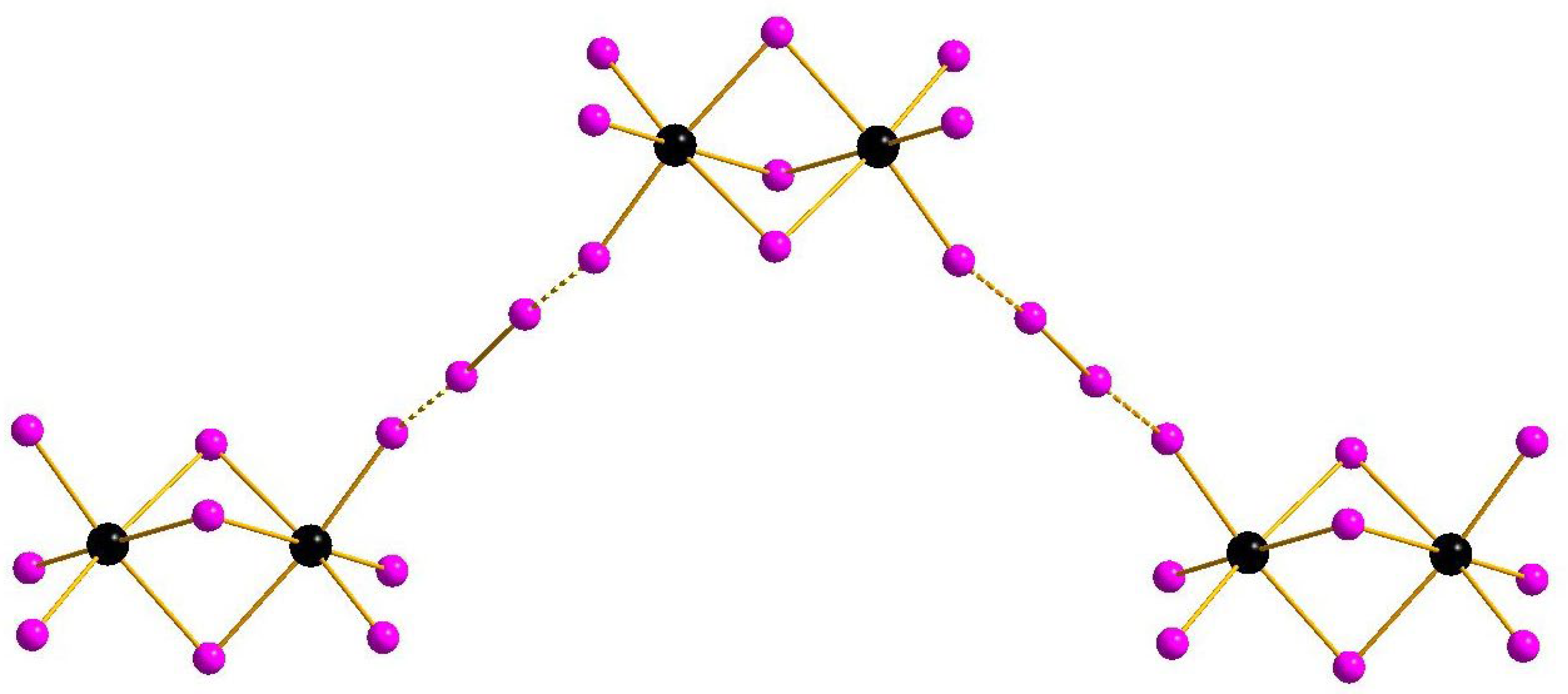
Complexes of general formula {[M2X9](X2)} were known earlier for M = Sb [32] and Bi [33], demonstrating isomerism due to diverse systems of halogen···halogen interactions. This feature appears also in the pairs 1–2 and 3–4: mutual orientation of [M2I9]3− and I2 building blocks vary, resulting in assembly of 1D supramolecular chains of different shape (Figure 1 and Figure 2). The crystal packings for the pairs 1–2 and 3–4 are given in Supplementary Materials.
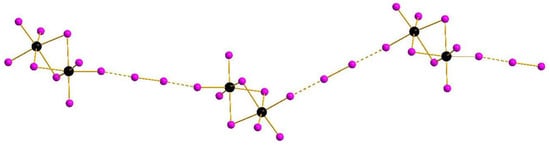
Figure 1.
{[M2I9](I2)} supramolecular chains in the structures of 1 and 2. Here and below: Sb or Bi black, I purple, non-covalent interactions dashed.
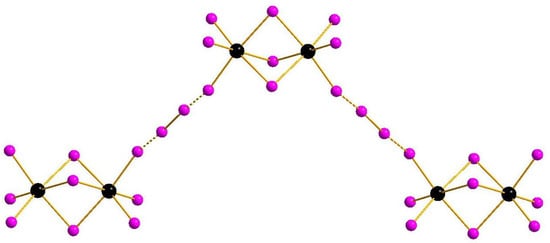
Figure 2.
{[M2I9](I2)} supramolecular chains in the structures of 3 and 4.
Powder X-ray diffractometry data indicate that 1–4 are precipitated as pure single phases (see Supplementary Materials, Figures S1–S4). This fact makes possible in-depth investigation of physical properties of these compounds.
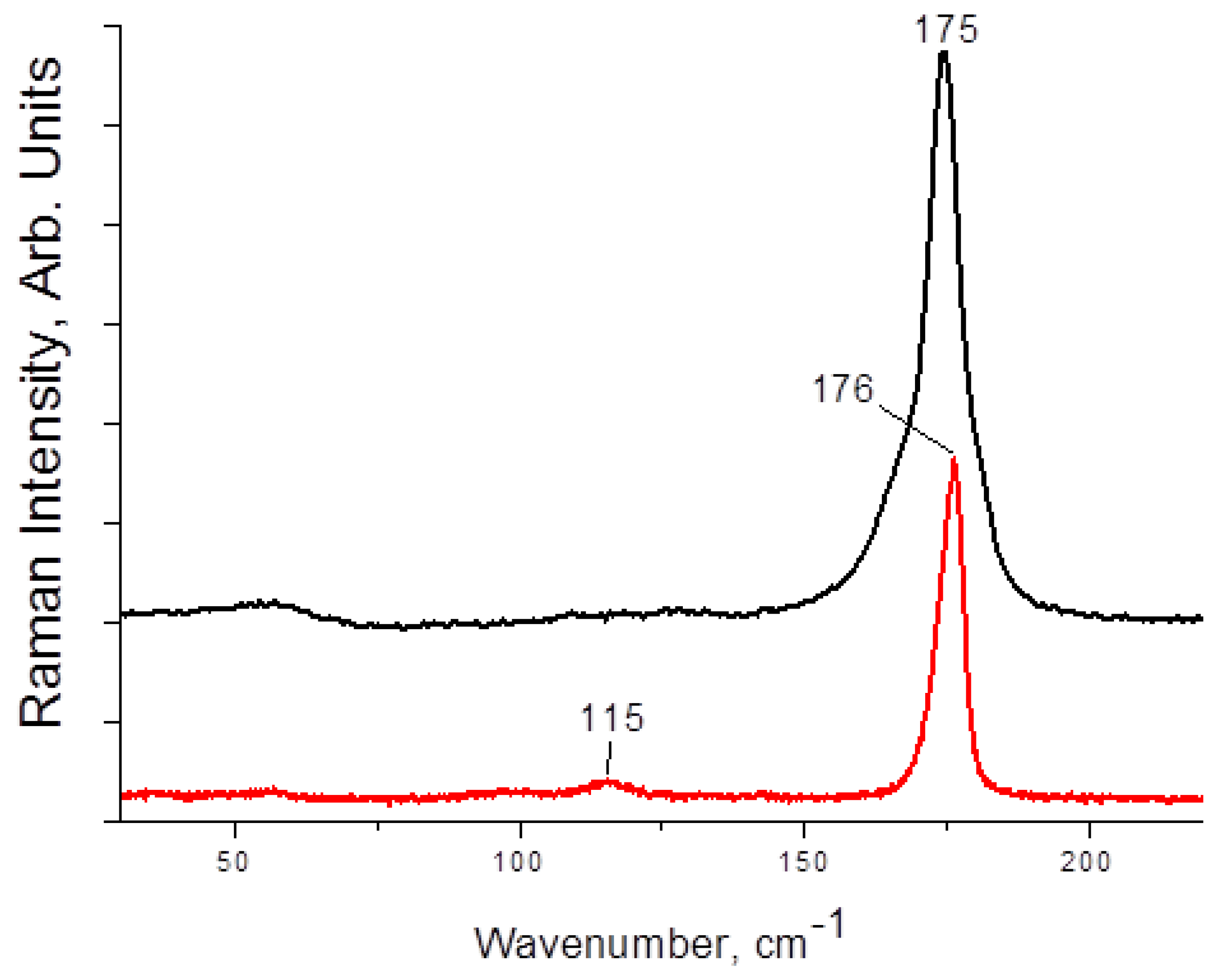
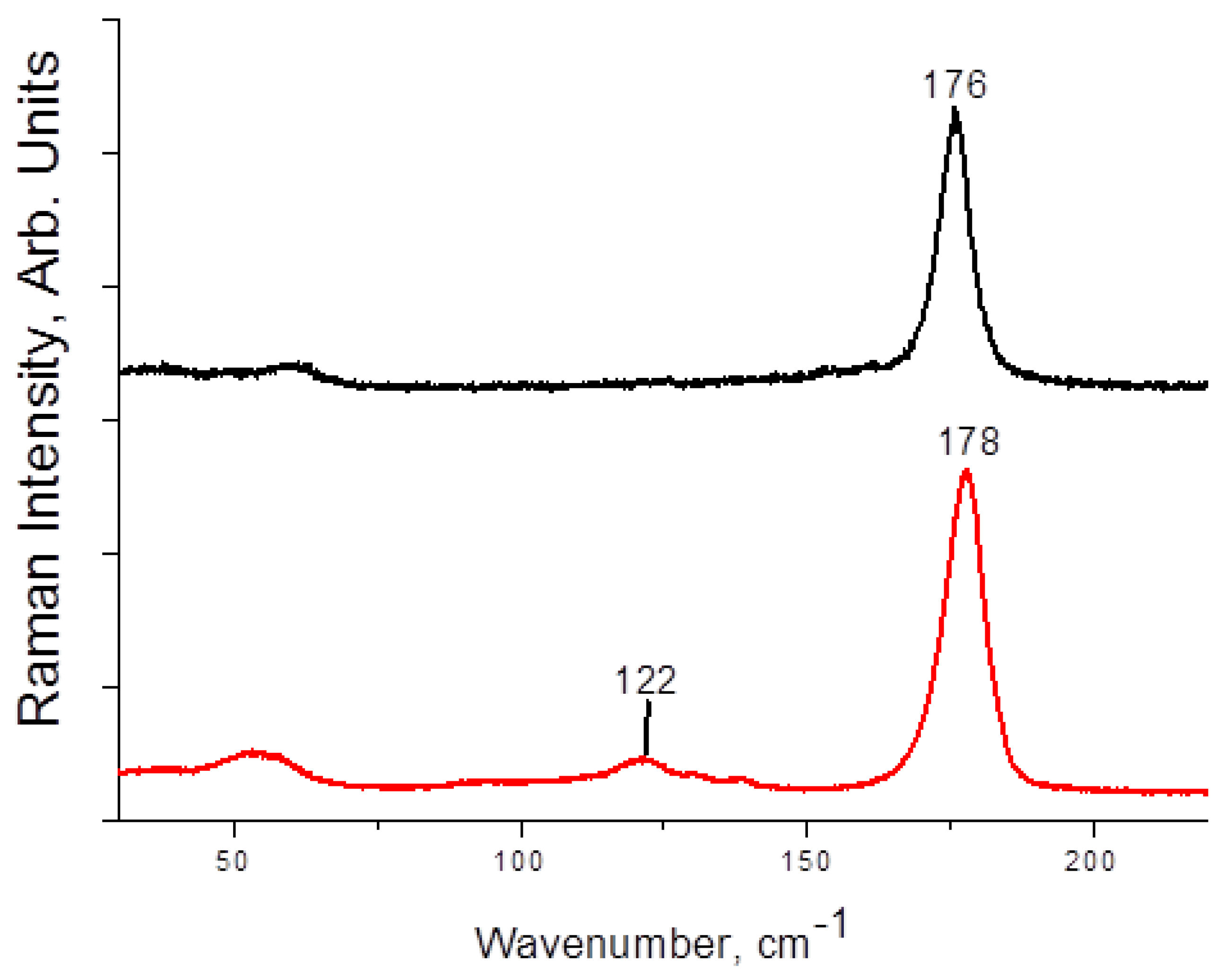
Raman spectra of 1–4 are presented on Figure 3 and Figure 4. The main and most characteristic spectral feature appears at 175–178 cm−1; it corresponds to the diiodine fragment. Interestingly, this band is not metal-sensitive (the differences are minor), and it is not strongly affected by the variations in Iterm···II2 distances (Table 1). This fact agrees well with the Raman data obtained by us earlier for other diiodine-iodobismuthates(III): the range of shifts for incorporated I2 is rather narrow (170–175 cm−1 [22,34]). The bands at <150 cm−1 are very weak (also similar to the earlier results [22]); those correspond to different modes of {MI6} octahedra.
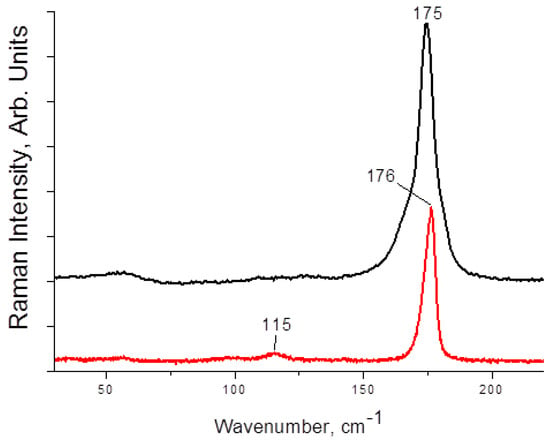
Figure 3.
Raman spectrum of 1 (black) and 2 (red).
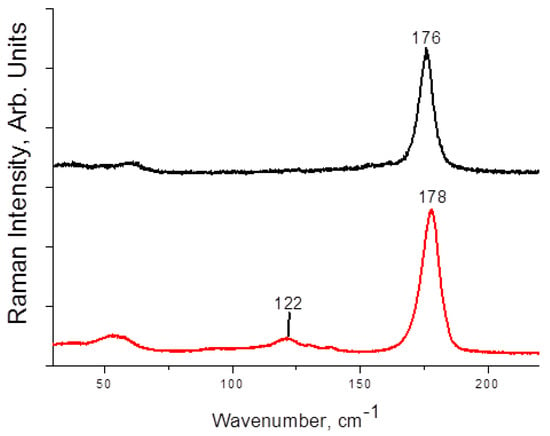
Figure 4.
Raman spectrum of 3 (black) and 4 (red).
Overall, these observations confirm that the use of Raman spectroscopy can shed light on the nature of polyhalide units incorporated into the polyhalogen-halometalate framework or just indicate their presence (in express analysis of the outcomes of such syntheses).
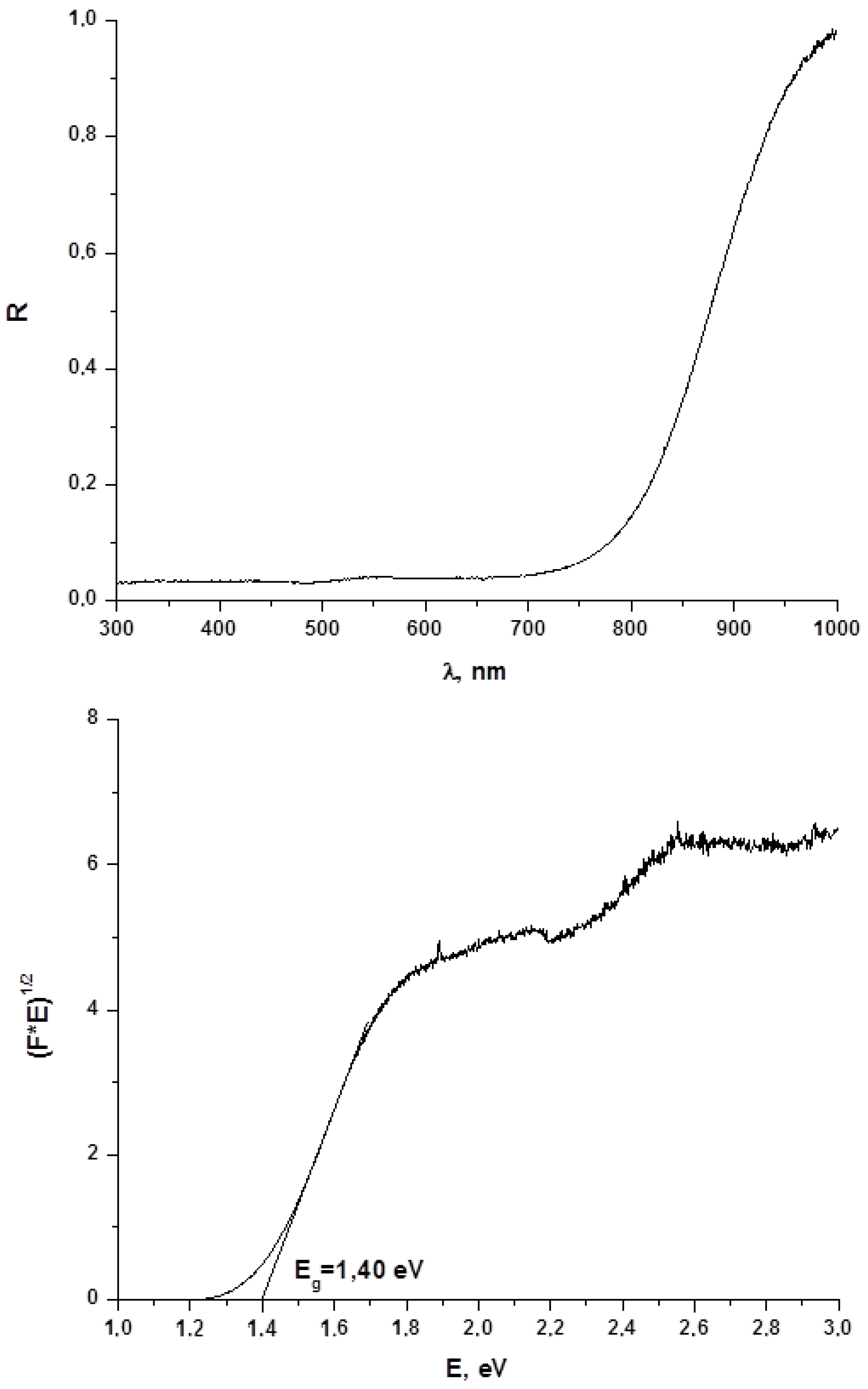
Diffuse reflectance spectroscopy is a powerful method for evaluation of optical band gap values of both bulk phases and thin films, and this parameter is crucial for estimation of whether obtained materials are promising for further use in photovoltaics. On this reason, we characterized 1–4 by this technique. Results are demonstrated on Figure 5 (for 1) and in Supplementary Materials (for 2–4, respectively; Figures S5–S10). As follows from these data, the Eg values for 1–4 are 1.40, 1.45, 1.42 and 1.49 eV, respectively, being lower than one for BiI3 (≈ 1.7 eV [16]). On one hand, these data agree well with those obtained for other representatives of this class of compounds [22], on another, those clearly demonstrate that optical properties of 1–4 make these compounds suitable for further photovoltaic tests.
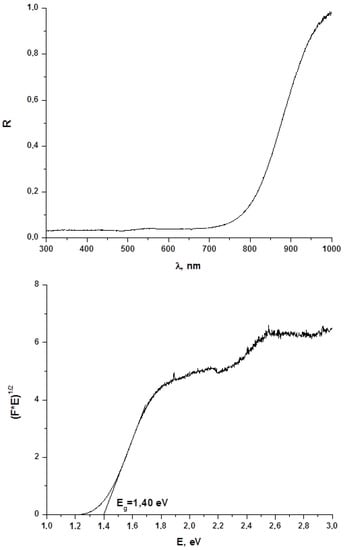
Figure 5.
Diffuse reflectance spectrum and determination of optical band gap for 1.
Another very important parameter is thermal stability of halometalate hybrids. For its estimation, we used thermogravimetric analysis (TGA). As follows from these data (see Supplementary Materials, Figures S11–S14), antimony- and bismuth-containing complexes demonstrate different behavior. For Sb(III) derivatives, the loss of incorporated I2 is gradual, and it begins almost simultaneously with the start of heating. For iodobismuthates(III), this stage of thermal decomposition has a narrower temperature range (Figures S12 and S14), and it can be stated that 2 and 4 are stable at least up to 80 and 110 °C, respectively. These facts allow us suggesting that 4 is the most suitable candidate for possible photovoltaic studies.
4. Conclusions
We expanded the series of diiodine-halometalates of Group 15 elements (antimony and bismuth) by two isostructural pairs belonging to two different isomers of {[M2I9](I2)}3n− type. Although all prepared complexes feature optical band gaps favorable for photovoltaic applications, only one compound (4) demonstrated higher thermal stability making such tests practically reasonable. We believe, however, that the studies of polyiodo-iodometalates in terms of photovoltaics will continue within the upcoming years.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules27238487/s1, XRD, PXRD and TGA data, UV-Vis spectra, Raman spectroscopy details.
Author Contributions
Conceptualization, S.A.A. and M.N.S.; methodology, S.A.A.; validation, P.A.A. and A.N.U.; formal analysis, S.A.A., A.N.U. and P.A.A.; investigation, N.A.K. and P.A.A.; resources, S.A.A.; data curation, M.N.S. and A.N.U.; writing—original draft preparation, S.A.A., A.N.U., N.A.K. and P.A.A.; writing—review and editing, M.N.S.; visualization, N.A.K., A.N.U. and S.A.A.; supervision, M.N.S. and S.A.A.; project administration, S.A.A.; funding acquisition, S.A.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Russian Science Foundation, Grant No. 18-73-10040, and partially supported by Ministry of Science and Higher Education of the Russian Federation (structural characterization of the samples, 121031700313-8).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Petrov, A.A.; Fateev, S.A.; Zubavichus, Y.V.; Dorovatovskii, P.V.; Khrustalev, V.N.; Zvereva, I.A.; Petrov, A.V.; Goodilin, E.A.; Tarasov, A.B. Methylammonium Polyiodides: Remarkable Phase Diversity of the Simplest and Low-Melting Alkylammonium Polyiodide System. J. Phys. Chem. Lett. 2019, 10, 5776–5780. [Google Scholar] [CrossRef] [PubMed]
- Frolova, L.A.; Anokhin, D.V.; Piryazev, A.A.; Luchkin, S.Y.; Dremova, N.N.; Troshin, P.A. Exploring the Photovoltaic Performance of All-Inorganic Ag2PbI4/PbI2 Blends. J. Phys. Chem. Lett. 2017, 8, 1651–1656. [Google Scholar] [CrossRef] [PubMed]
- Frolova, L.A.; Gutsev, L.G.; Ramachandran, B.R.; Dremova, N.N.; Aldoshin, S.M.; Troshin, P.A. Exploring CsPbI3–FAI Alloys: Introducing Low-Dimensional Cs2FAPb2I7 Absorber for Efficient and Stable Perovskite Solar Cells. Chem. Eng. J. 2021, 426, 131754. [Google Scholar] [CrossRef]
- Hu, Z.-T.; Shu, X.; Wang, X.; Li, Y.; Xu, R.; Hong, F.; Ma, Z.-Q.; Jiang, Z.-M.; Xu, F. Air-Stable CsPbIBr2 Photodetector via Dual-Ligand-Assisted Solution Strategy. Wuli Xuebao/Acta Phys. Sin. 2022, 71, 116801. [Google Scholar] [CrossRef]
- Udalova, N.N.; Tutantsev, A.S.; Fateev, S.A.; Zharenova, E.A.; Belich, N.A.; Nemygina, E.M.; Ryabova, A.V.; Goodilin, E.A.; Tarasov, A.B. Crystallization Features of MAPbI3 Hybrid Perovskite during the Reaction of PbI2 with Reactive Polyiodide Melts. Russ. J. Inorg. Chem. 2021, 66, 153–162. [Google Scholar] [CrossRef]
- Minbashi, M.; Yazdani, E. Comprehensive Study of Anomalous Hysteresis Behavior in Perovskite-Based Solar Cells. Sci. Rep. 2022, 12, 14916. [Google Scholar] [CrossRef]
- Petrov, A.A.; Fateev, S.A.; Khrustalev, V.N.; Li, Y.; Dorovatovskii, P.V.; Zubavichus, Y.V.; Goodilin, E.A.; Tarasov, A.B. Formamidinium Haloplumbate Intermediates: The Missing Link in a Chain of Hybrid Perovskites Crystallization. Chem. Mater. 2020, 32, 7739–7745. [Google Scholar] [CrossRef]
- Petrov, A.A.; Marchenko, E.I.; Fateev, S.A.; Yumao, L.; Goodilin, E.A.; Tarasov, A.B. Solvate Phases Crystallizing from Hybrid Halide Perovskite Solutions: Chemical Classification and Structural Relations. Mendeleev Commun. 2022, 32, 311–314. [Google Scholar] [CrossRef]
- Fateev, S.A.; Stepanov, N.M.; Petrov, A.A.; Goodilin, E.A.; Tarasov, A.B. Successive Solution–Liquid–Vapor Conversion of Metallic Lead Films for Highly Efficient Perovskite Solar Cells. Russ. J. Inorg. Chem. 2022, 67, 992–996. [Google Scholar] [CrossRef]
- Saliba, M.; Matsui, T.; Seo, J.-Y.; Domanski, K.; Correa-Baena, J.-P.; Nazeeruddin, M.K.; Zakeeruddin, S.M.; Tress, W.; Abate, A.; Hagfeldt, A.; et al. Cesium-Containing Triple Cation Perovskite Solar Cells: Improved Stability, Reproducibility and High Efficiency. Energy Environ. Sci. 2016, 9, 1989–1997. [Google Scholar] [CrossRef]
- Li, H.; Liu, Z.; Chen, Z.; Tan, S.; Zhao, W.; Li, Y.; Shi, J.; Wu, H.; Luo, Y.; Li, D.; et al. Reconfiguring Perovskite Interface via R4NBr Addition Reaction toward Efficient and Stable FAPbI3Based Solar Cells. Sci. China Chem. 2022, 65, 1185–1195. [Google Scholar] [CrossRef]
- Zhu, Z.; Shang, J.; Tang, G.; Wang, Z.; Cui, X.; Jin, J.; Zhou, Y.; Zhang, X.; Zhang, D.; Liu, X.; et al. Vertical Distribution of PbI2 Nanosheets for Robust Air-Processed Perovskite Solar Cells. Chem. Eng. J. 2023, 454, 140163. [Google Scholar] [CrossRef]
- Belich, N.A.; Tychinina, A.S.; Kuznetsov, V.V.; Goodilin, E.A.; Grätzel, M.; Tarasov, A.B. Template Synthesis of Methylammonium Lead Iodide in the Matrix of Anodic Titanium Dioxide via the Direct Conversion of Electrodeposited Elemental Lead. Mendeleev Commun. 2018, 28, 487–489. [Google Scholar] [CrossRef]
- Minns, J.L.; Zajdel, P.; Chernyshov, D.; van Beek, W.; Green, M.A. Structure and Interstitial Iodide Migration in Hybrid Perovskite Methylammonium Lead Iodide. Nat. Commun. 2017, 8, 15152. [Google Scholar] [CrossRef]
- Chen, H.; Ma, Y.; Wang, X.; Yao, G.; Du, Y.; Zhou, J.; Zhu, L.; Zhao, X.; Yang, S.; Liu, X.; et al. Improving the Stability and Efficiency of Inorganic CsPbI2Br Perovskite via Surface Reconstruction Strategy. Chem. Eng. J. 2022, 442, 136242. [Google Scholar] [CrossRef]
- Ganose, A.M.; Savory, C.N.; Scanlon, D.O. Beyond Methylammonium Lead Iodide: Prospects for the Emergent Field of Ns2 Containing Solar Absorbers. Chem. Commun. 2017, 53, 20–44. [Google Scholar] [CrossRef]
- Shestimerova, T.A.; Golubev, N.A.; Yelavik, N.A.; Bykov, M.A.; Grigorieva, A.V.; Wei, Z.; Dikarev, E.V.; Shevelkov, A.V. Role of I2 Molecules and Weak Interactions in Supramolecular Assembling of Pseudo-Three-Dimensional Hybrid Bismuth Polyiodides: Synthesis, Structure, and Optical Properties of Phenylenediammonium Polyiodobismuthate(III). Cryst. Growth Des. 2018, 18, 2572–2578. [Google Scholar] [CrossRef]
- Mezentsev-Cherkes, I.A.; Shestimerova, T.A.; Medved’ko, A.V.; Kalinin, M.A.; Kuznetsov, A.N.; Wei, Z.; Dikarev, E.V.; Vatsadze, S.Z.; Shevelkov, A.V. Synthesis and Supramolecular Organization of the Iodide and Triiodides of a Polycyclic Adamantane-Based Diammonium Cation: The Effects of Hydrogen Bonds and Weak I⋯I Interactions. CrystEngComm 2021, 23, 2384–2395. [Google Scholar] [CrossRef]
- Shestimerova, T.A.; Yelavik, N.A.; Mironov, A.V.; Kuznetsov, A.N.; Bykov, M.A.; Grigorieva, A.V.; Utochnikova, V.V.; Lepnev, L.S.; Shevelkov, A.V. From Isolated Anions to Polymer Structures through Linking with I2: Synthesis, Structure, and Properties of Two Complex Bismuth(III) Iodine Iodides. Inorg. Chem. 2018, 57, 4077–4087. [Google Scholar] [CrossRef]
- Adonin, S.A.; Udalova, L.I.; Abramov, P.A.; Novikov, A.S.; Yushina, I.V.; Korolkov, I.V.; Semitut, E.Y.; Derzhavskaya, T.A.; Stevenson, K.J.; Troshin, P.A.; et al. A Novel Family of Polyiodo-Bromoantimonate(III) Complexes: Cation-Driven Self-Assembly of Photoconductive Metal-Polyhalide Frameworks. Chem. Eur. J. 2018, 24, 14707–14711. [Google Scholar] [CrossRef]
- Novikov, A.V.; Usoltsev, A.N.; Adonin, S.A.; Bardin, A.A.; Samsonenko, D.G.; Shilov, G.V.; Sokolov, M.N.; Stevenson, K.J.; Aldoshin, S.M.; Fedin, V.P.; et al. Tellurium Complex Polyhalides: Narrow Bandgap Photoactive Materials for Electronic Applications. J. Mater. Chem. A 2020, 8, 21988–21992. [Google Scholar] [CrossRef]
- Usoltsev, A.N.; Korobeynikov, N.A.; Novikov, A.S.; Plyusnin, P.E.; Kolesov, B.A.; Fedin, V.P.; Sokolov, M.N.; Adonin, S.A. One-Dimensional Diiodine–Iodobismuthate(III) Hybrids Cat3{[Bi2I9](I2)3}: Syntheses, Stability, and Optical Properties. Inorg. Chem. 2020, 59, 17320–17325. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT—Integrated Space-Group and Crystal-Structure Determination. Acta Crystallogr. Sect. A Found. Adv. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal Structure Refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Hübschle, C.B.; Sheldrick, G.M.; Dittrich, B. ShelXle: A Qt Graphical User Interface for SHELXL. J. Appl. Crystallogr. 2011, 44, 1281–1284. [Google Scholar] [CrossRef]
- Sharutin, V.V.; Yegorova, I.V.; Klepikov, N.N.; Boyarkina, E.A.; Sharutina, O.K. Synthesis and Structure of Bismuth Complexes [Ph3MeP]6+[BiI3Br3]3−[Bi2I6Br3]3−·H2O2, [Ph3EtP]3+[Bi2I9]3−, [Ph3MeP]3+ [Bi3I12]3−, [Ph3(Iso-Pr)P]3+[Bi3I12]3−·2Me2C=O, and [Ph4Bi]3+[Bi5I18]3−. Russ. J. Inorg. Chem. 2009, 54, 52–68. [Google Scholar] [CrossRef]
- Chai, W.-X.; Lin, J.; Song, L.; Qin, L.-S.; Shi, H.-S.; Guo, J.-Y.; Shu, K.-Y. Three Iodometalate Organic-Inorganic Hybrid Materials Based on Methylene Blue Cation: Syntheses, Structures, Properties and DFT Calculations. Solid State Sci. 2012, 14, 1226–1232. [Google Scholar] [CrossRef]
- Kelly, A.W.; Nicholas, A.; Ahern, J.C.; Chan, B.; Patterson, H.H.; Pike, R.D. Alkali Metal Bismuth(III) Chloride Double Salts. J. Alloys Compd. 2016, 670, 337–345. [Google Scholar] [CrossRef]
- Ahern, J.C.; Nicholas, A.D.; Kelly, A.W.; Chan, B.; Pike, R.D.; Patterson, H.H. A Terbium Chlorobismuthate(III) Double Salt: Synthesis, Structure, and Photophysical Properties. Inorg. Chim. Acta 2018, 478, 71–76. [Google Scholar] [CrossRef]
- Adonin, S.A.; Sokolov, M.N.; Fedin, V.P. Polynuclear Halide Complexes of Bi(III): From Structural Diversity to the New Properties. Coord. Chem. Rev. 2016, 312, 1–21. [Google Scholar] [CrossRef]
- Bondi, A. Van Der Waals Volumes and Radii of Metals in Covalent Compounds. J. Phys. Chem. 1966, 70, 3006–3007. [Google Scholar] [CrossRef]
- Hubbard, C.R.; Jacobson, R.A. Molecular Bromine Bridging of SbIII2Br93− Anions and the Crystal Structure of Tetraethylammonium Nonabromodiantimonate(III)-Dibromine. Inorg. Chem. 1972, 11, 2247–2250. [Google Scholar] [CrossRef]
- Adonin, S.A.; Gorokh, I.D.; Samsonenko, D.G.; Sokolov, M.N.; Fedin, V.P. Bi(Iii) Polybromides: A New Chapter in Coordination Chemistry of Bismuth. Chem. Commun. 2016, 52, 5061–5063. [Google Scholar] [CrossRef]
- Adonin, S.A.; Usoltsev, A.N.; Novikov, A.S.; Kolesov, B.A.; Fedin, V.P.; Sokolov, M.N. One- and two- Dimensional iodine-richIodobismuthate(III) complexes: Structure, Optical Properties and features of halogen bonding in the solid state. Inorg. Chem. 2020, 59, 3290–3296. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).