Abstract
Four new heterometallic complexes combining [MII(H2dapsc)]2+ cations with the chelating H2dapsc {2,6-diacetylpyridine-bis(semicarbazone)} Schiff base ligand and [Cr(CN)6]3− anion were synthesized: {[MII(H2dapsc)]CrIII(CN)6K(H2O)2.5(EtOH)0.5}n·1.2n(H2O), M = Mn (1) and Co (2), {[Mn(H2dapsc)]2Cr(CN)6(H2O)2}Cl·H2O (3) and {[Co(H2dapsc)]2Cr(CN)6(H2O)2}Cl·2EtOH·3H2O (4). In all the compounds, M(II) centers are seven-coordinated by N3O2 atoms of H2dapsc in the equatorial plane and N or O atoms of two apical –CN/water ligands. Crystals 1 and 2 are isostructural and contain infinite negatively charged chains of alternating [MII(H2dapsc)]2+ and [CrIII(CN)6]3− units linked by CN-bridges. Compounds 3 and 4 consist of centrosymmetric positively charged trimers in which two [MII(H2dapsc)]2+ cations are bound through one [CrIII(CN)6]3− anion. All structures are regulated by π-stacking of coplanar H2dapsc moieties as well as by an extensive net of hydrogen bonding. Adjacent chains in 1 and 2 interact also by coordination bonds via a pair of K+ ions. The compounds containing MnII (1, 3) and CoII (2, 4) show a significant difference in magnetic properties. The ac magnetic measurements revealed that complexes 1 and 3 behave as a spin glass and a field-induced single-molecule magnet, respectively, while 2 and 4 do not exhibit slow magnetic relaxation in zero and non-zero dc fields. The relationship between magnetic properties and non-covalent interactions in the structures 1–4 was traced.
1. Introduction
Molecular nanomagnets, the so-called single-molecular magnets (SMMs), single-chain magnets (SCMs) and single-ion magnets (SIMs) are attracting much attention due to their unique magnetic properties, such as superparamagnetism, relaxation, blocking and quantum tunneling magnetization [1,2,3], as well as the prospects for their application in information technology, spintronics and quantum calculations [4,5,6]. However, at present, the practical use of molecular nanomagnets is difficult, since they have low critical magnetization blocking temperatures (TB), below which their unique magnetic properties appear. Although, in recent times, SIMs, mononuclear metallocene complexes of Dy, with blocking temperatures close to liquid nitrogen temperature have been synthesized [7,8], their application is unlikely since these compounds are extremely air-sensitive and have short magnetization relaxation times. For instance, for SIM ([(CpiPr5)Dy(Cp*)]+ with TB = 80 K, long relaxation times (τ > 100 s) occur only below ~30 K [8]. In 2021, Tang et al. reported an air-stable hexagonal bipyramidal Dy(III) SMM, [Dy(LN6)(Ph3SiO)2][PF6], which displayed a record anisotropy barrier (Ueff) exceeding 1800 K (TB = 20 K) and the longest relaxation time approaching 2500 s at 2.0 K for all known air-stable SIMs [9]. However, it is difficult to expect that sufficiently long magnetization relaxation times can be achieved with mononuclear complexes. Recently, a new concept for the design of advanced molecular nanomagnets has been proposed based on the use of low-spin (S = 1/2) pentagonal-bipyramidal complexes of 4d3 and 5d3 metals as building blocks for the synthesis of M(4d/5d)–M(3d) exchange-coupled pairs with Ising-type exchange interactions [10].
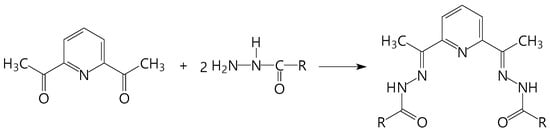
Increasing the magnetization reversal barriers (Ueff) and blocking temperatures (TB∞Ueff) for molecular nanomagnets are associated, on the one hand, with using the metal ions that have large uniaxial magnetic anisotropy (4d, 5d, 4f and some 3d elements) [3,11,12,13] and, on the other hand, with coordination environment of the metal centers [11,12,13,14,15,16,17,18,19,20,21]. The coordination geometry around the metal essentially affects its local magnetic anisotropy [14,17,18,19,20,21]. Experimental and theoretical studies of seven-coordinated pentagonal-bipyramidal (PBP) complexes show that PBP geometry of a coordination polyhedron contributes to an increase in magnetic anisotropy of the metal center [14,22,23,24,25,26,27,28]. In this regard, of considerable interest is the family of acyclic pentadentate (N3O2) ligands (Scheme 1), which are widely used for the targeted synthesis of PBP complexes of 3d, and more recently, 4d, 5d and 4f metals [10,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48]. Their PBP geometry results from the pentagonal coordination around the metal ion of nearly planar N3O2 ligand in the equatorial plane and two apical labile ligands (usually solvent molecules and/or various simple anions). Moreover, depending on the synthesis conditions, these ligands can be in neutral, monoanionic or dianionic forms, which leads to a wide variety of these complexes. The study of the magnetic properties of mononuclear PBP complexes of this family showed that some of them (FeII, CoII, Dy and Er) are field-induced SIMs [22,23,40,43,45,46,47,48]. It should be noted that the Co PBP complexes have a planar magnetic anisotropy (+D), in contrast to similar complexes of Fe(II) and Ni(II), which have an axial Ising-type anisotropy (–D) [23,24,26,46,47,49].
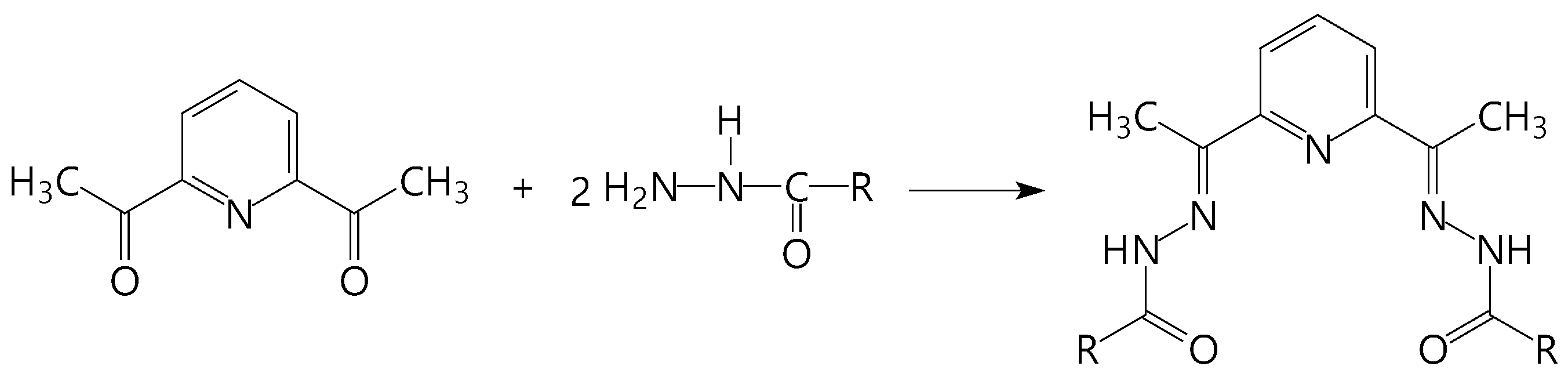
Scheme 1.
Some diacetylpyridine-based acyclic pentadentate (N3O2) ligands: R = NH2 (H2dapsc), 2-OHC6H4 (H4daps); 4-OCH3C6H4 (H2dapmbh), C6H5 (H2dapbh), C6H5-C6H4 (Biph), CH3CHOH (l-daplh).
The presence of labile ligands in the axial positions of the PBP complexes makes them attractive building blocks for the construction of low-dimensional heteronuclear compounds by replacing these ligands with bridging cyanometallates or the PBP complexes with cyanide apical groups [17,23,24,37,49,50,51,52,53,54,55,56]. Up to date, tri-, penta-, and decanuclear SMMs and coordination polymeric SCMs have been obtained along the way.
Earlier, we studied the reactions of [Mn(H2dapsc)Cl2]·H2O (dapsc=2,6-diacetylpyridine bis(semicarbazone), Scheme 1) with K3[Mn(CN)6] and K3[Fe(CN)6] and obtained the chain polymeric complexes {[Mn(H2dapsc)][Mn(CN)6][K(H2O)2.75(MeOH)0.5]}n·0.5n(H2O) and {[Mn(H2dapsc)][Fe(CN)6][K(H2O)3.5]}n·1.5nH2O, which revealed single-chain magnet (SCM) behavior [50,52].
In this work, we investigated the reactions of complexes [Mn(H2dapsc)Cl2]·H2O and [Co(H2dapsc)Cl(H2O)]Cl·H2O with K3[Cr(CN)6] and (Ph4P)3[Cr(CN)6]. As a result, four cyano-bridged heterometallic complexes were synthesized: {[M(H2dapsc)]Cr(CN)6K(H2O)2.5(EtOH)0.5}n·1.2n(H2O), M = Mn (1) and Co (2); and {[Mn(H2dapsc)]2Cr(CN)6(H2O)2}Cl·H2O (3) and {[Co(H2dapsc)]2Cr(CN)6(H2O)2}Cl·2EtOH·3H2O (4). Their crystal structures and magnetic properties have been studied. Herein, we present these results.
2. Results and Discussion
2.1. Synthesis and Crystal Structure
The reactions of [Mn(H2dapsc)Cl2]·H2O (5) and [Co(H2dapsc)ClH2O]Cl·2H2O (6) with K3[Cr(CN)6] and (Ph4P)3Cr(CN)6 were studied. The interaction of 5 and 6 with K3[Cr(CN)6] in ethanol/water led to the formation of the chain polymeric complexes 1 and 2, respectively. The use of (Ph4P)3Cr(CN)3 instead of K3[Cr(CN)6] gave linear trinuclear complexes 3 and 4, see Materials and Methods.
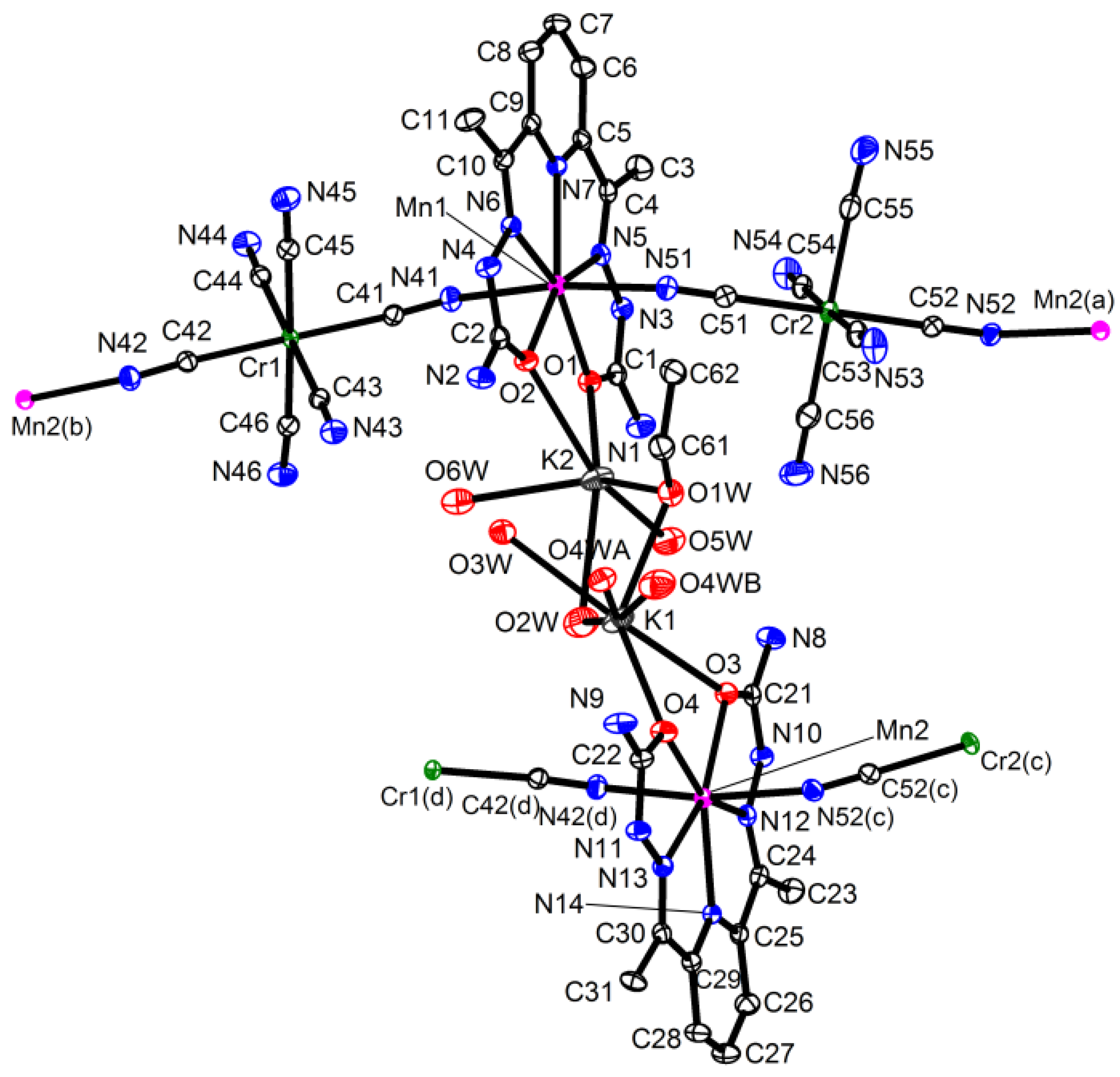
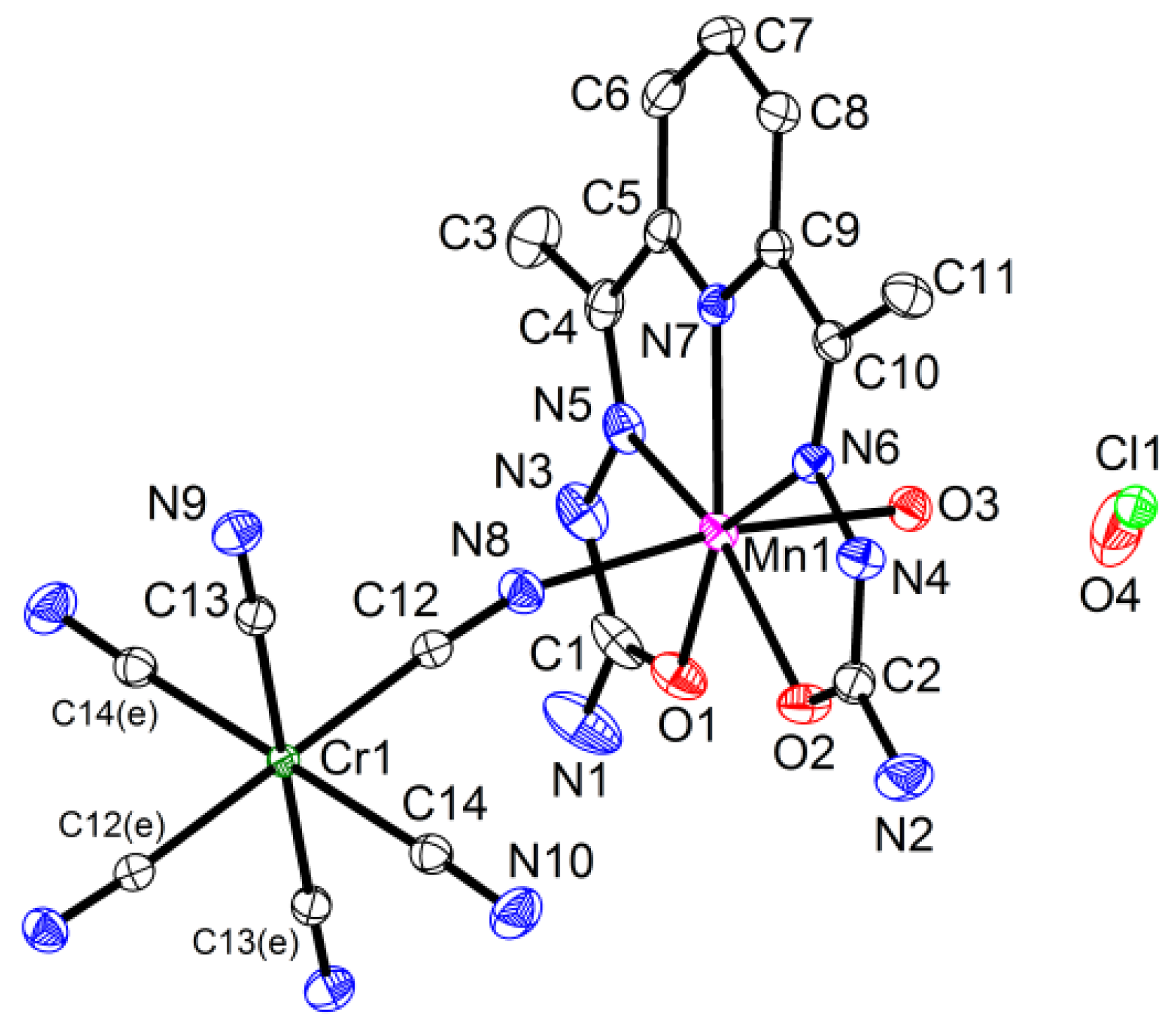
{[M(H2dapsc)]Cr(CN)6K(H2O)2.5(EtOH)0.5}n·1.2n(H2O), M = Mn (1) or Co (2). The complexes 1 and 2 are isostructural and crystallize in the monoclinic P21 space group. The asymmetric unit includes two [MII(H2dapsc)], two [Cr(CN)6] moieties, two K+ ions, one EtOH solvent and eight water molecules, all in general positions. Two water molecules (O8w, O9w) have 0.7 occupancy; one water (O4w) is disordered between two sites with an occupancy ratio of 0.8/0.2 in 1 and 0.6/0.4 in 2. An ORTEP drawing of 1 is shown in Figure 1, and the key bond distances and angles in 1 and 2 are listed in Table A1 of the Appendix A section.
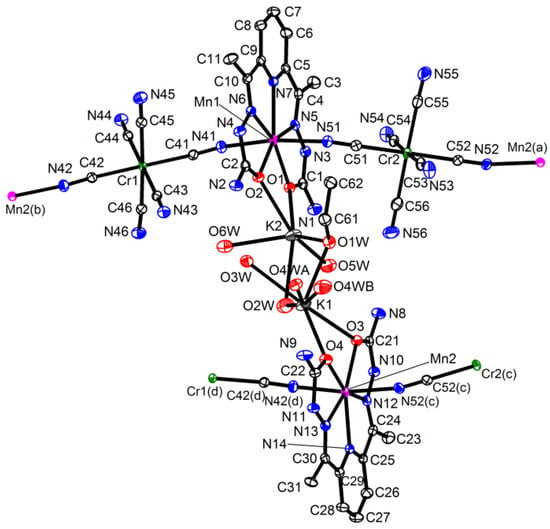
Figure 1.
Asymmetric unit in 1 with atom numbering scheme (ORTEP drawing with 50% probability ellipsoids, solvate water molecules are omitted for clarity). Colored atoms: Cr (green), K (grey), Mn (magenta), N (blue), O (red). Symmetry codes: (a) (−x, y − 0.5, −z), (b) (1 − x, y − 0.5, 1 − z), (c) (−x, y + 0.5, −z) and (d) (1 − x, y + 0.5, 1 − z).
The crystal structure contains infinite negatively charged chains of alternating cationic [MII(H2dapsc)]2+ and anionic [CrIII(CN)6]3− units running along [1 0 1] (Figure 2). The metal centers along the chain are connected through the CN-linkage. The chain is bent due to non-linear M(II)-N-C angles (150–152° in 1 and 155–161° in 2), whereas the Cr-C-N angles are closer to 180° (173–176° in 1 and 170–173° in 2, Table A1). The M(II) ions have a pentagonal bipyramidal coordination geometry and are surrounded by two O and three N atoms from H2dapsc in the equatorial plane and two axial N atoms from CN-bridges. The M-O,N bond lengths in the [MII(H2dapsc)] moieties are shorter in the Co(II) compound 2 compared with the Mn(II) compound 1, the maximal difference ~0.1 Å being observed for M-N bonds (Table A1). Both independent [MII(H2dapsc)] fragments in 1 and 2 are almost flat: the dihedral angle between the two semicarbazone planes of the H2dapsc ligand defined by seven non-metallic atoms of two pentagonal cycles [for example, O1, C1, N3, N5, C4, C5, N7 and O2, C2, N4, N6, C10, C9, N7] is 1.48(4)° for Mn(1), 1.22(4)° for Mn(2), 3.05(5)° for Co(1) and 2.58(4)° for Co(2). The Cr-CCN distances in the anionic units are in the range of 2.06-2.08 Å.
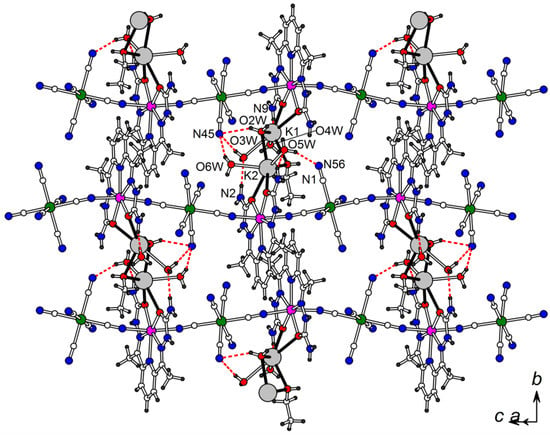
Figure 2.
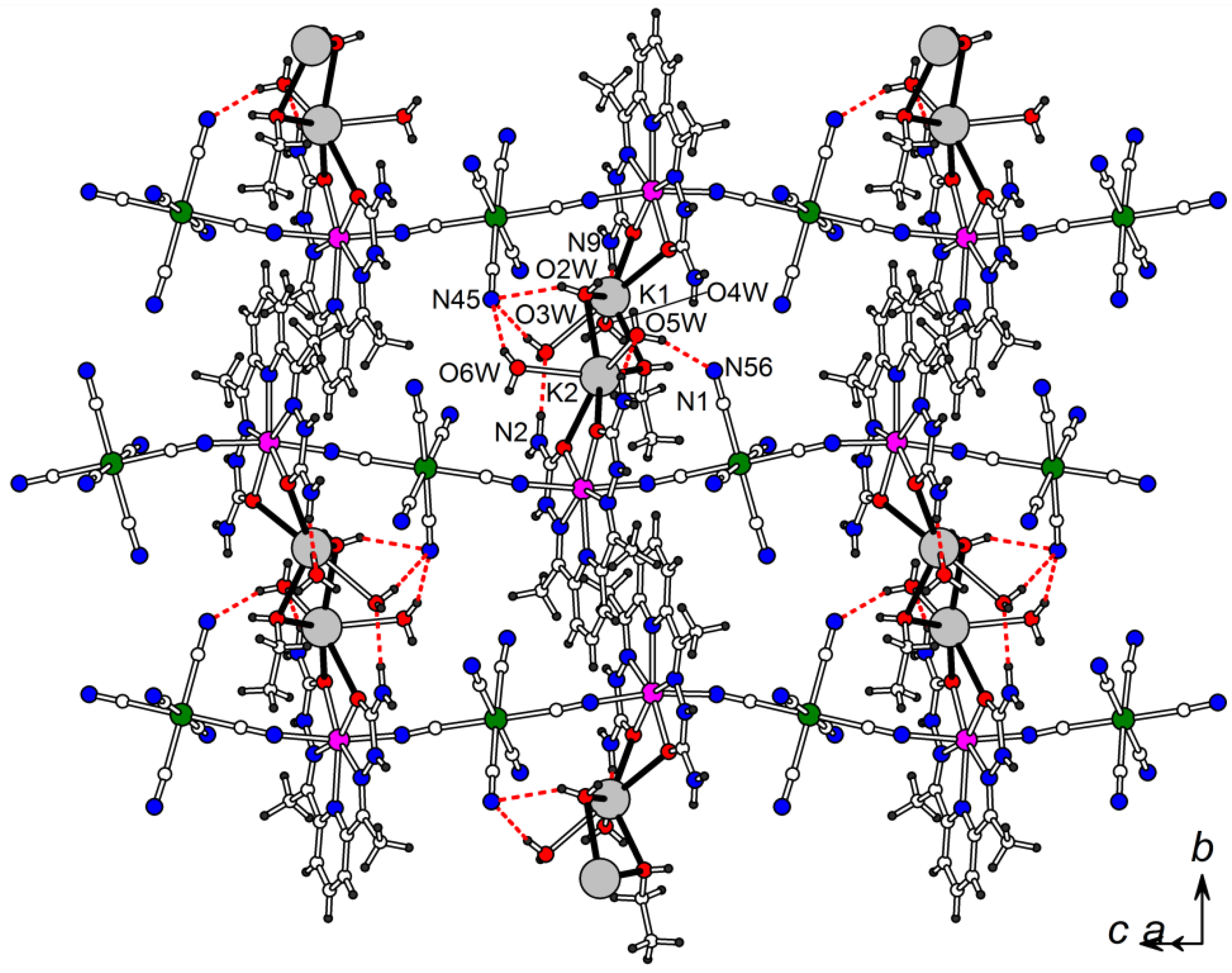
Infinite CN-bridged {[MII(H2dapsc)]2+[CrIII(CN)6]3−}n chains in 1 interacting by π-π stacking of H2dapsc ligands and coordination bonds via K+/O in the (1 0 –1) plane. Interchain coordination bonds via pairs of K+ cations are shown by black bonds. Hydrogen bonds are shown by red dashed lines (see Tables S1 and S2 for hydrogen bond geometry in 1 and 2, respectively).
The compounds 1 and 2 include K+ ions compensating for the negative charge of the {[MII(H2dapsc)]2+[CrIII(CN)6]3−}n chain. K+ is coordinated with oxygen atoms of MII(H2dapsc) at the K-O distances of 2.713(2)–2.783(2) Å in 1 and 2.721(3)–2.752(3) Å in 2. The ethanol molecule and five of the eight independent water molecules O(2w)-O(6w) also belong to the coordination sphere of K+ ions; the corresponding K-O distances are 2.73–2.84 Å. Two K+ cations linked to [M(H2dapsc)] from adjacent chains are at the K-K distance of 4.154(1) and 4.165(1) Å in 1 and 2, respectively, and bridged together through two oxygen atoms from one water and one ethanol molecule. The coordination bonds via the pairs of K+ ions join adjacent infinite chains in the (1 0 –1) plane (Figure 2). Additionally, the chains are fixed together by non-covalent interactions: by π-π stacking of the nearest H2dapsc ligands as well as by hydrogen bonds N−HH2dapsc···Owater, O−Hwater···Nanion in the (1 0 –1) plane and N−HH2dapsc...Nanion in the (0 1 0) plane (Figure 2 and Figure S1 in the Supplementary Materials, hydrogen bond geometry is given in Tables S1 and S2).
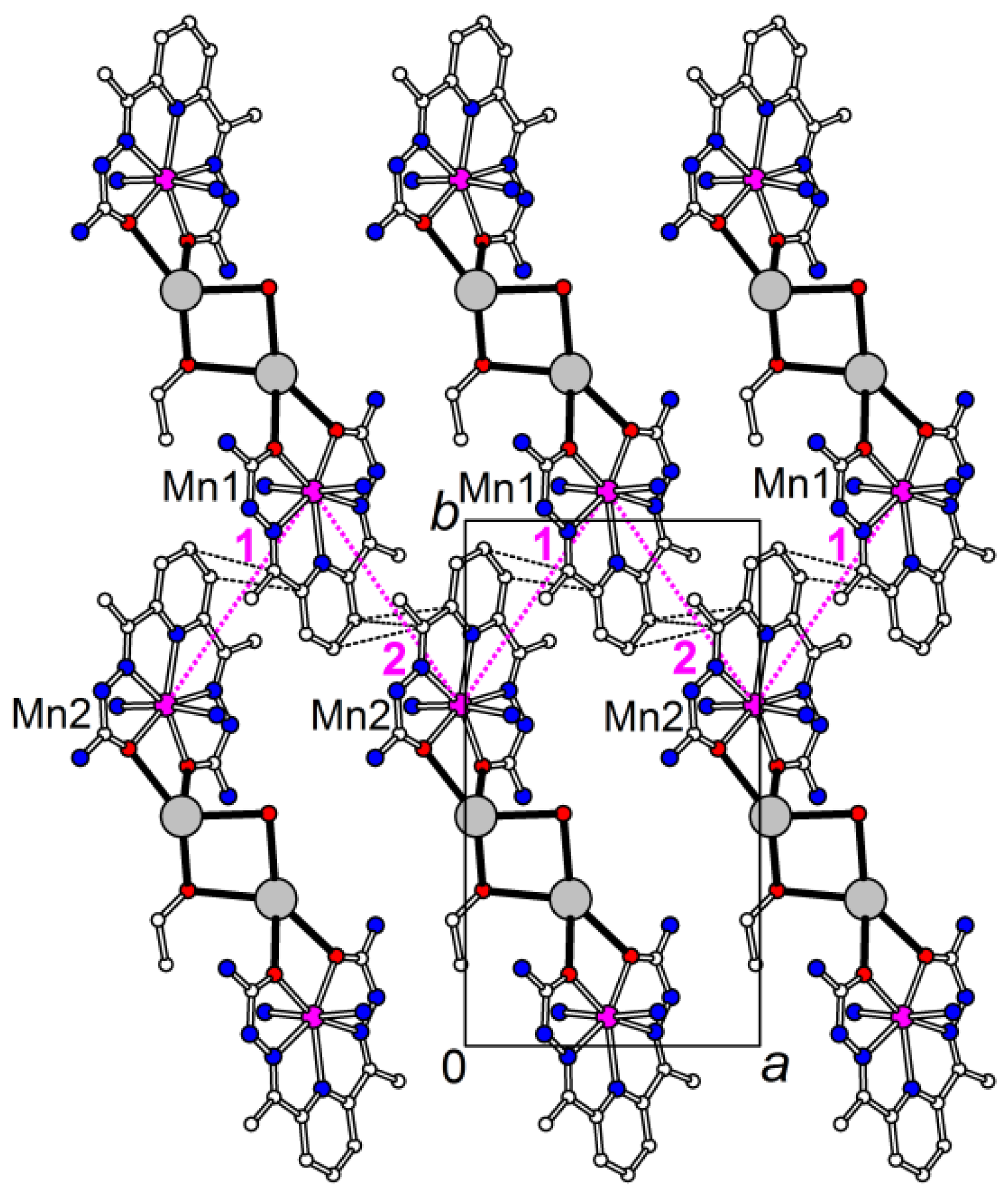
The MnII-CrIII distances along the chain are 5.2292(5), 5.2404(5) for Mn(1) and 5.2190(5), 5.2340(6) Å for Mn(2). The CoII-CrIII chain is more compact, the corresponding CoII-CrIII distances are 5.1579(7), 5.1703(7) for Co(1) and 5.1570(7), 5.1903(7) Å for Co(2). The shortest interchain intermetallic distances are found between the MII(1) and MII(2) centers connected via π-π stacking of dapsc ligands: 8.2341(6) and 8.2357(6) Å in the MnII structure and 8.0793(7) and 8.1262(7) Å in the CoII structure. The π-stacked coplanar [M(II)(H2dapsc)] units in both the 1 and 2 structures form infinite zigzag chains along the a-direction with almost uniform M(II)-M(II) separations (Figure 3).
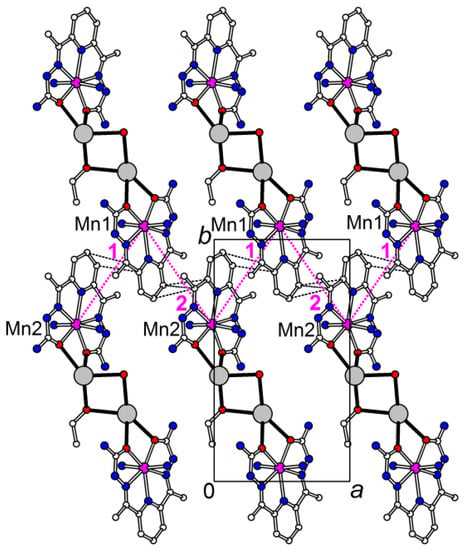
Figure 3.
Part of the structure 1 displaying zigzag infinite chain of π-stacked coplanar [Mn(H2dapsc)] units; the [Cr(CN)6] anions, H atoms and most of water molecules are omitted for clarity. The Mn-Mn distances are 8.2341(6) and 8.2357(6) Å for 1 and 2 interactions, respectively (magenta dotted lines). Shortened C…C contacts (<3.6 Å) between H2dapsc fragments are shown by black dashed lines.
The complexes 1 and 2 are isostructural to the other chain complex {[Mn(H2dapsc)][Mn(CN)6][K(H2O)2.75(MeOH)0.5]}n·0.5n(H2O) (7) [50]. The main difference between them is another solvent composition and the presence of disorder in the K+ site in 7. The chain complex {[Mn(H2dapsc)][Fe(CN)6][K(H2O)3.5]}n·1.5nH2O (8) [52] also has similar unit cell parameters but higher symmetry (P21/n) because the pair of K+ ions in 8 are bridged by two water molecules related by inversion symmetry instead of water/ethanol in 1 and 2 or water/methanol in 7.
{[Mn(H2dapsc)]2Cr(CN)6(H2O)2}Cl·H2O (3). Complex 3 crystallizes in the monoclinic P21/c space group. The asymmetric unit includes half of the formula unit with Cr(1) atom in the inversion center; a half-occupied Cl− anion and solvate water are mixed in the close sites. An ORTEP drawing of 3 is shown in Figure 4, and the key bond distances and angles are listed in Table A1.
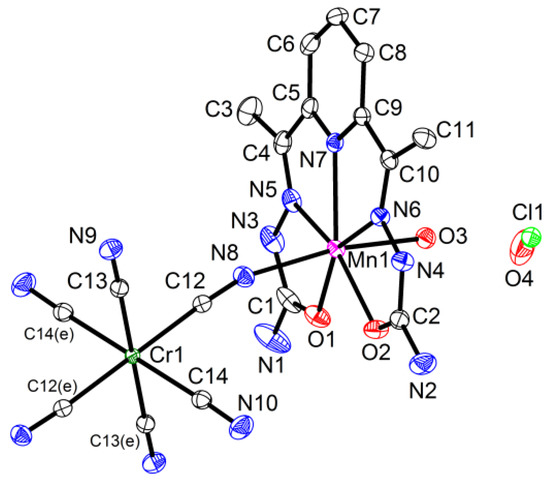
Figure 4.
Asymmetric unit in 3 with atom numbering scheme (ORTEP drawing with 50% probability ellipsoids). Colored atoms: Cl (light green), Cr (green), Mn (magenta), N (blue), O (red). Symmetry code: (e) (1 − x, −y, −z).
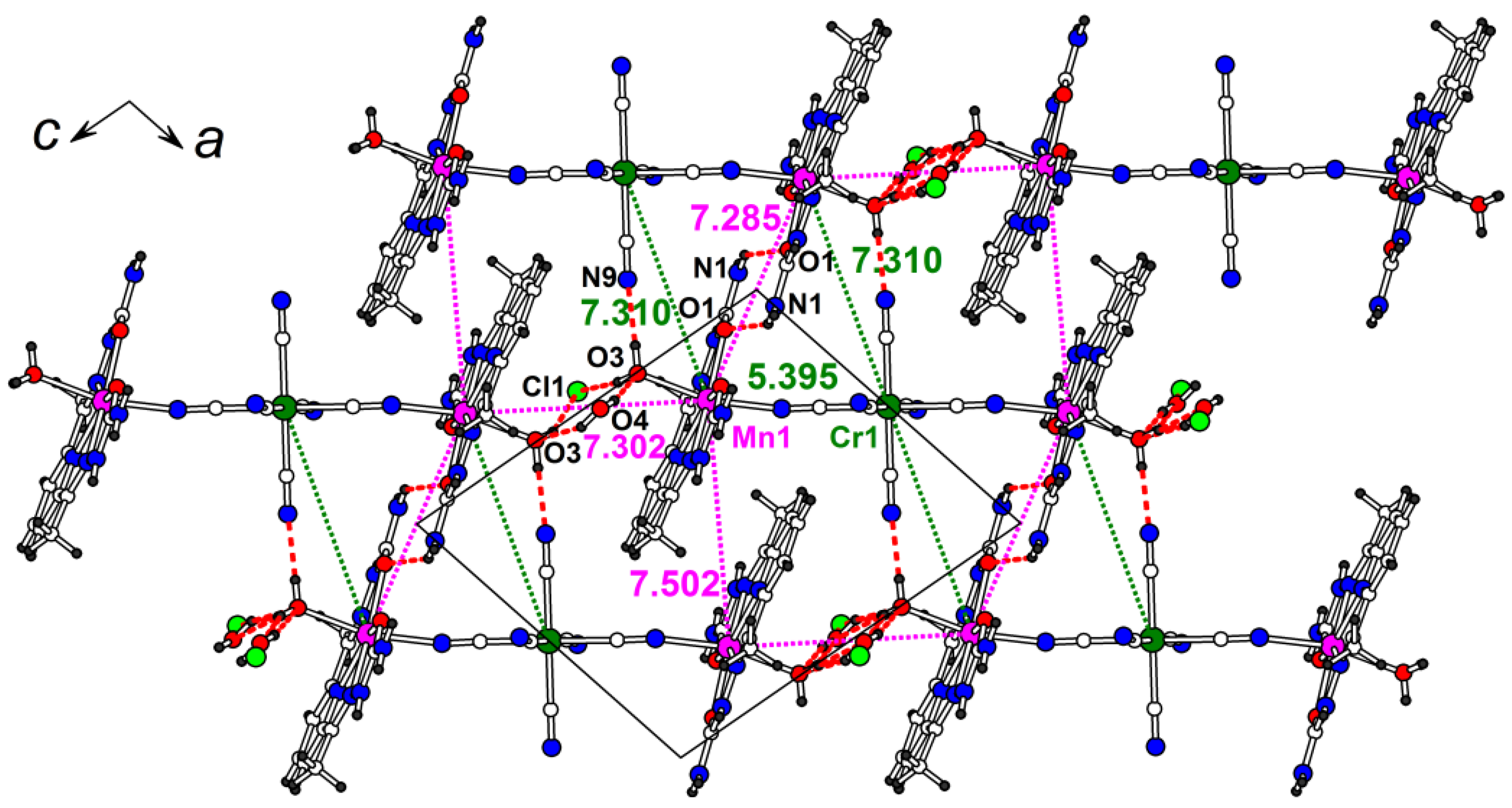
Structure 3 is built from centrosymmetric positively charged trimers {H2O-[Mn(H2dapsc)]2+-[Cr(CN)6]3−-[Mn(H2dapsc)]2+-H2O}+ elongated along [1 0 –1] (Figure 5). Mn(II) and Cr(III) ions are linked through the trans-CN ligands of [Cr(CN)6]; the Mn-N-C angle is 164.7(1)°, the Mn-Cr distance is 5.3952(3) Å. The Mn(II) center has a distorted pentagonal bipyramidal environment of two oxygen and three nitrogen atoms of the equatorial H2dapsc ligand and axial N, O atoms from CN-bridge and terminal H2O ligand. The H2dapsc ligand is flattened: the dihedral angle between two semicarbazone planes defined by seven non-metallic atoms of two pentagonal cycles of H2dapsc (as for the structure 1) is 4.55(4)°.
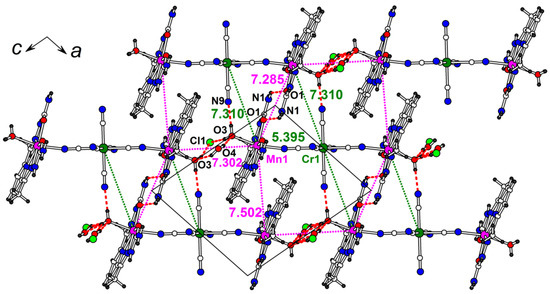
Figure 5.
The ac layer in the structure 3. Intermetallic distances (in Å) are shown (magenta color for Mn…Mn, green color for Mn…Cr). Hydrogen bonds are shown by red dashed lines (see Table S3 for hydrogen bond geometry).
The non-covalent intermolecular interactions are well represented in the structure 3. The trimers are connected with each other and with the anion/water site by hydrogen bonding (red dashed lines in Figure 5 and Table S3 for details). The shortest intermetallic distances are found in the ac plane. The adjacent [Mn(H2dapsc)] units with the smallest Mn-Mn distance, 7.2853(5) Å, interact through a pair of strong N-H…O bonds formed between -NH2 function and oxygen atoms of H2dapsc ligands. The nearby Mn-Cr distance is comparable (7.3100(4) Å) and the additional O-HH2O…Nanion hydrogen bond is formed. The terminal water molecules linked to Mn are hydrogen bonded to the similar water terminal of another trimer via the Cl−/H2O pair, the Mn…Mn distance in this interaction is 7.3022(5) Å. The π-π stacking in pairs of Mn(H2dapsc) moieties from adjacent trimers is also observed (Mn-Mn is 7.5018(5) Å, Figure 5).
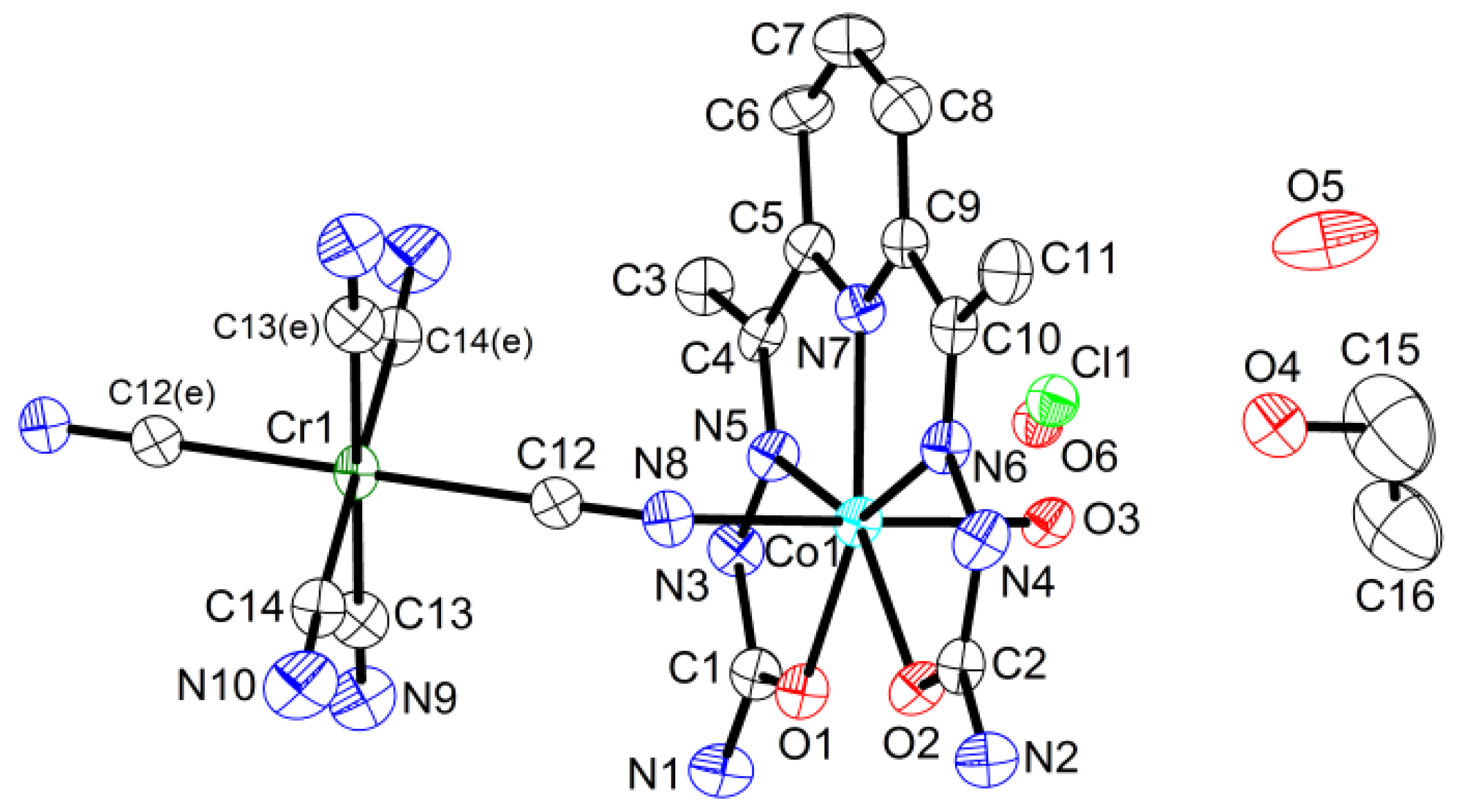
{[Co(H2dapsc)]2Cr(CN)6(H2O)2}Cl·2EtOH·3H2O (4). Complex 4 crystallizes in the triclinic P space group. The asymmetric unit includes half of the formula unit with the Cr(1) atom in the inversion center; the half-occupied Cl− and water are mixed in the close sites. An ORTEP drawing of 4 is shown in Figure 6, and the key bond distances and angles are listed in Table A1.
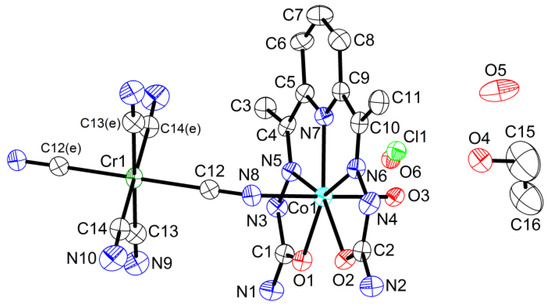
Figure 6.
Asymmetric unit in 4 with atom numbering scheme (ORTEP drawing with 50% probability ellipsoids). Colored atoms: Cl (light green), Co (cyan), Cr (green), N (blue), O (red). Symmetry code: (e) (1 − x, −y, −z).
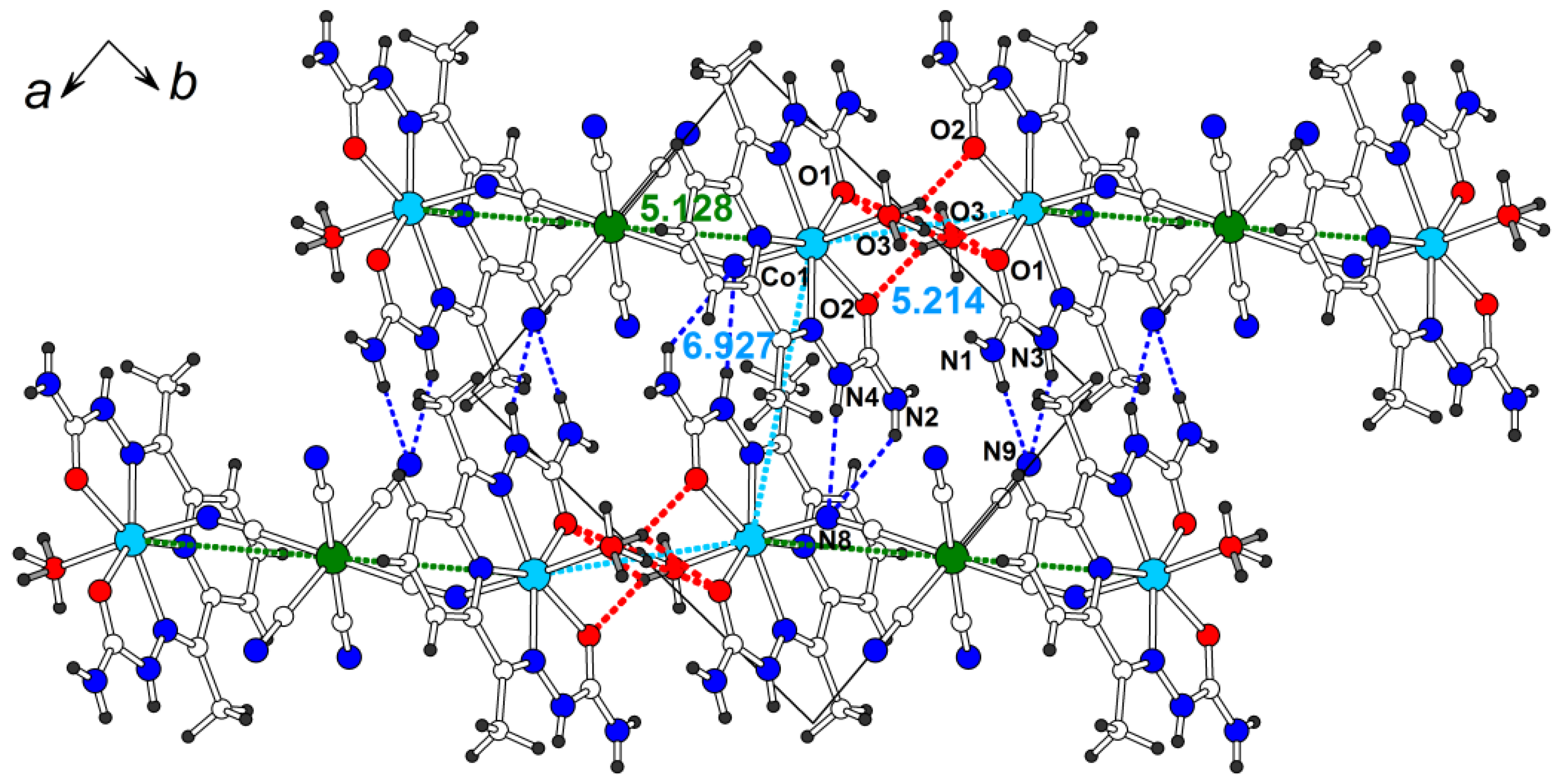
Structure 4 contains centrosymmetric positively charged trimers like structure 3, although these compounds are not isostructural. The Co-Cr-Co trimer in 4 is more compact than the Mn-Cr-Mn trimer in 3 due to the smaller ionic radius of Co(II) (0.72 Å) in comparison with Mn(II) (0.82 Å) and the stronger bending of the Co chain (the Co-N-C angle is 151.3(4)°). The Co-Cr distance in the trimer is 5.1281(9) Å. The dihedral angle between the two semicarbazone planes in the H2dapsc ligand is 1.2(1)°.
The shortest intermetallic distances are observed for the non-covalently interacting trimers in the ab plane (Figure 7). A pair of strong O-HH2O…OH2dapsc hydrogen bonds (red dashed lines in Figure 7 and Table S4 for details) are observed between adjacent trimers along the [1 −1 0] direction whose terminal water molecules are close to each other. The Co-Co distance in this contact, 5.214(1) Å, is very short and only slightly larger than the Co-Cr distance inside the trimer. For this reason, it is possible to consider the trimers interacting in this way as an infinite chain of the [-Co(H2dapsc)-(2H2O)-Co(H2dapsc)-Cr(CN)6-]∞ composition. The Co-Co distance between two [Co(H2dapsc)] moieties interacting by π-stacking is 6.927(1) Å (Figure 7). The N−HH2dapsc...Nanion hydrogen bonds are also formed in the ab plane (blue dashed lines in Figure 7). The presence of mixed H2O/Cl− position causes a disorder in the hydrogen atoms attached to the water and ethanol molecules.
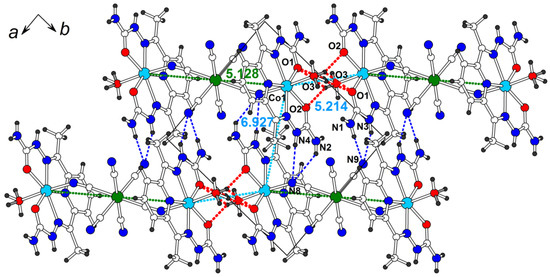
Figure 7.
The ab layer in the structure 4 (solvate water and ethanol are omitted for clarity). Intermetallic distances (in Å) are shown (cyan color for Co…Co, green color for Co…Cr). Hydrogen O-H…O/N-H…N bonds are shown by red/blue dashed lines (see Table S4 for hydrogen bond geometry).
2.2. Magnetic Properties
2.2.1. Static (dc) Magnetic Properties
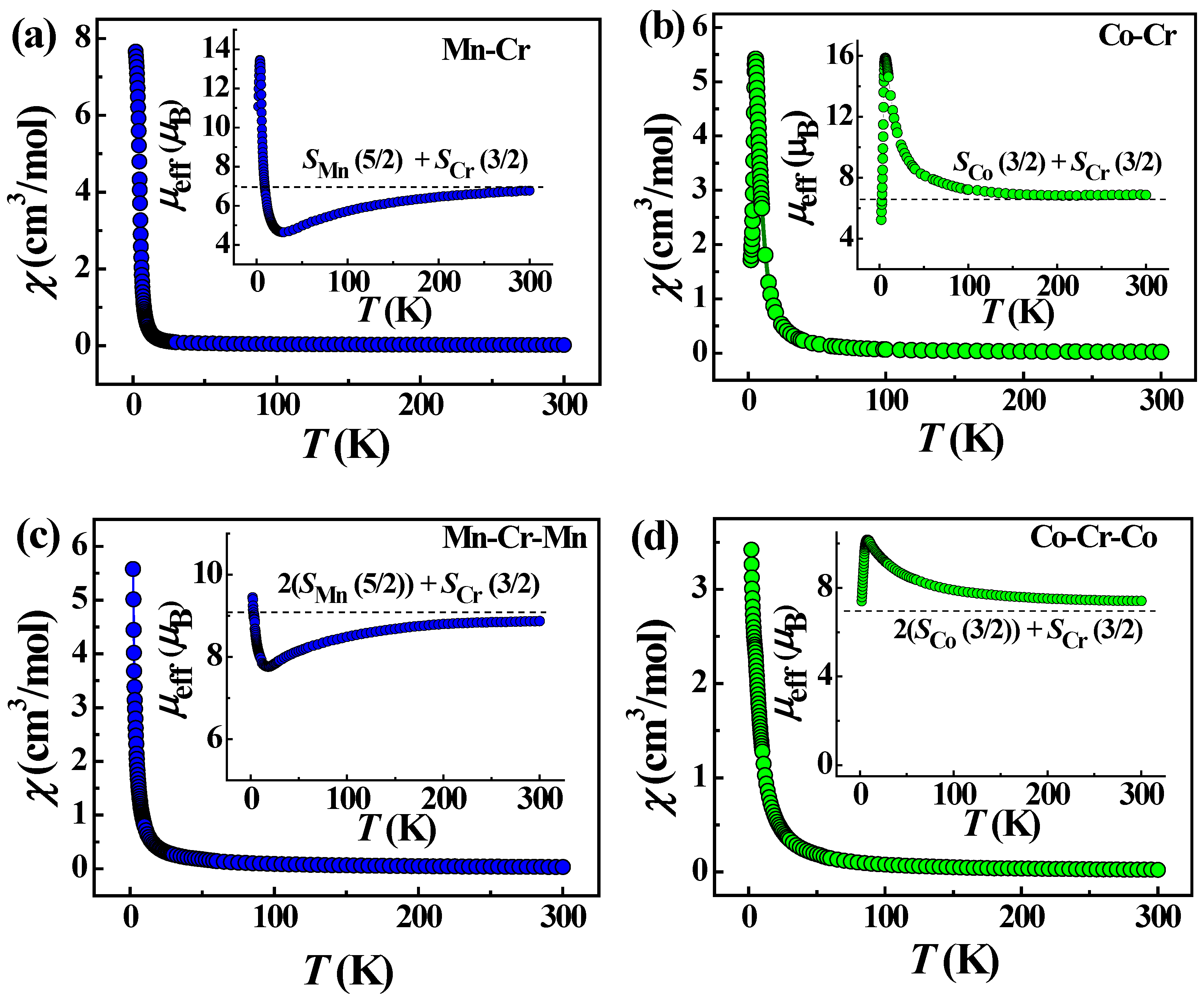
Variable-temperature magnetic measurements were performed on polycrystalline samples of complexes 1–4 in the range of 1.8–300 K under a dc field of 1000 Oe. The plots of temperature dependencies of the magnetic susceptibility (χM) and effective magnetic moment (μeff) for the isostructural chain complexes 1 and 2 are depicted in Figure 8a,b.
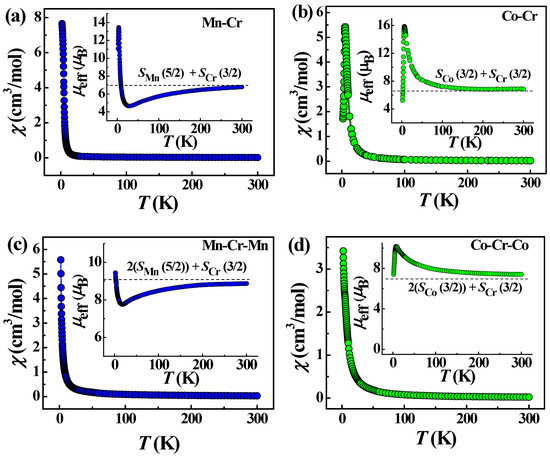
Figure 8.
Temperature dependencies of magnetic susceptibility χmol and effective magnetic moment μeff (insets) for 1 (a), 2 (b), 3 (c) and 4 (d). The blue and green colors of the symbols distinguish the data for Mn- and Co-based complexes, respectively. The dashed lines on the insets show the estimations of effective magnetic moment for a unit cell with the Co, Cr and Mn spins, indicated on the legends.
The μeff value at 300 K for 1 is close to the expected value (7.06 μB) for non-interacting Mn(II) and Cr(III) ions with S = 5/2 (g = 2.0) and 3/2 (g = 2.0), respectively. For 1, at cooling, the μeff value gradually decreases down to 50 K, and then, it decreases rapidly to the minimum value at ∼20 K. Upon further lowering the temperature, the μeff increases abruptly to reach a maximum at 3 K, followed by a slight drop to 1.8 K. The latter feature is probably attributed to the presence of antiferromagnetic (AF) coupling between the chains through the hydrogen bonding network, π−π stacking and the interchain coordination bonds via solvent molecules and K+ ions. The general behavior of the μeff with temperature suggests AF exchange interactions between Mn(II) and Cr(III) spin carriers within the chains, resulting in a ferrimagnetic spin arrangement along the chains. The Curie–Weiss fit of the magnetic susceptibility data above 75 K gave a Weiss constant of −75.0 K (Figure S2, Table S5). The large negative Weiss constant confirms the dominance of AF interactions in the chains of complex 1. The rapid growth of μeff below 20 K indicates a ferrimagnetic ordering in the chains of 1.
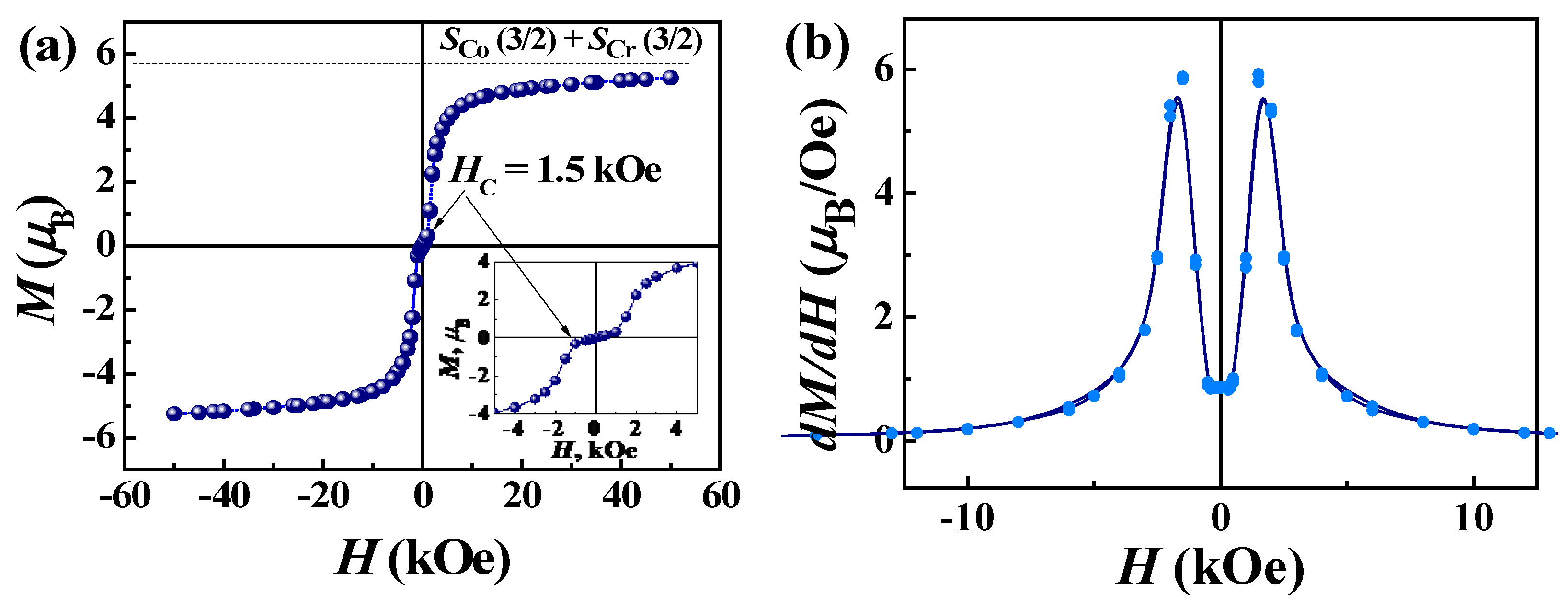
The magnetic properties of complex 2 differ significantly from those of 1. The μeff value at room temperature is higher than the spin-only value (5.47 μB) for one Co(II) of S = 3/2 (g = 2.0) and one Cr(III) of S = 3/2 (g = 2.0) due to the orbital contributions of Co(II) ions, Figure 8b. As the temperature decreases, the effective magnetic moment does not decrease, as in the case of 1, but, on the contrary, increases to a sharp maximum around 6.0 K, indicating an intrachain ferromagnetic coupling between the Co(II) and Cr(III) ions, which is expected for pseudolinear Co(II)-NC-Cr(III) bridges [49,51,57,58]. The Weiss constant is positive and amounts to 7.0 K (Figure S2, Table S5), which confirms the presence of ferromagnetic interactions in the chains of complex 2. X-ray diffraction analysis of 1 and 2 showed that the Co-Cr chains are more compact; the Co-Cr distances are ~0.07 Å shorter than the Mn-Cr distances. Below 6 K, the value of the effective magnetic moment for 2 shows a sharp and deep drop to 1.8 K, which is probably due to the presence of intrinsic magnetic anisotropy of the Co(II) centers [59] and/or antiferromagnetic ordering associated with coupling between chains through non-covalent interactions. The χM vs. T behavior also exhibits a maximum (Figure 8b), suggesting antiferromagnetic ordering. The interchain AF interactions in structure 2 are much stronger than in 1. The shortest interchain distance between Co(1) and Co(2) centers linked through π-π-stacking of H2dapsc ligands is 0.15 Å shorter than the Mn(1)–Mn(2) distance in 1. The study of the field-dependent magnetization at 2 K indicates a metamagnetic-like transition from an AF state to a state of spontaneous magnetization under the field Hc = 1.5 kOe, Figure 9a. The dM/dH(H) dependence exhibits distinct peaks in the fields 1.5 kOe and −1/5 kOe, Figure 9b. The metamagnetic nature of the transition is also evidenced by the character of the magnetization curves M(T) and the curves χmol(T) recorded at different magnetic fields (Figure S3) as well as the data of ac magnetic susceptibility. The field dependence of the real part (χ’) of ac susceptibility shows a maximum of χ’ at a field of 1.5 kOe (Figure S4). The application of an external constant magnetic field leads to the suppression of interchain AF coupling and causes a metamagnetic transition. Such transitions have been observed in other heterospin as well as homospin compounds with interchain AF ordering [52,60,61,62,63,64,65,66,67].
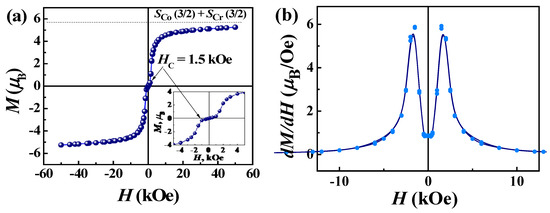
Figure 9.
M(H) loop (a) and its first derivative (b) at 2 K for complex 2.
Unlike 1 and 2, the trinuclear complexes 3 and 4 are not isostructural. Their magnetic properties differ markedly and, in some respects, are close to those of the corresponding chain complexes. The temperature dependencies of the magnetic susceptibility (χM) and effective magnetic moment (μeff) for the Mn2Cr trimer 3 are depicted in Figure 8c. The μeff value at 300 K is close to the expected value (9.2 μB) for two non-interacting Mn(II) and one Cr(III) ions. Upon cooling from room temperature, the μeff value decreases to approximately 16 K, as in the case of complex 1, indicating antiferromagnetic interaction between the metallic centers, as was observed in the similar trimer [68]. On further cooling, the μeff increases to 1.8 K. Unlike 1, this increase is not accompanied by a subsequent decrease in μeff associated with AF interactions between the chains in structure 1. Theoretical analysis of the antiferromagnetic interactions between the MnII(H2dapsc) units belonging to the neighboring chains, which contact through π-π stacking of planar H2dapsc ligands and a system of hydrogen bonds in the complex {[Mn(H2dapsc)][Fe(CN)6][K(H2O)3.5]}n·1.5nH2O, showed that contacts through π-π stacking play a decisive role in these superexchange AF interactions [52]. In the structure 1, along the a-axis, there are infinite chains of ...-Mn1-Mn2-Mn1-Mn2-... through π-π stacking of the H2dapsc ligands with distances between metals of 8.2341(6) and 8.2357(6) Å (Figure 3), whereas in 3, such contacts are present only in pairs (Figure 5). Probably for this reason, interchain AF interactions in 3 are very weak compared to those in 1.
Temperature behavior of μeff for the Co2Cr trimer (4) (Figure 8d) is close to that for the chain complex (-Cr-CN-Co-NC-)n (2), Figure 8b. Upon cooling, the μeff value increases, reaching a maximum near 7.8 K, which indicates a ferromagnetic exchange between the metal centers in the trimer, and then decreases. The Weiss constant is positive and equal to 27.0 K (Figure S2, Table S5). It is possible that the strong ferromagnetic interaction in 4 is due to the fact that trimers in its structure are joined into infinite chains [-Co(H2dapsc)-(2H2O)-Co(H2dapsc)-Cr(CN)6-]n through the pairs of strong intertrimer hydrogen bonds O-HH2O…OH2daps (Figure 7 and Table S4). The metal–metal separations along the formed chain are rather uniform: the Co-Cr distance inside the trimer is 5.128 Å, whereas the Co-Co distance between the trimers is only slightly longer, 5.214 Å. The drop of μeff to 1.8 K is not as sharp and deep as in the case of complex 2 (Figure 8b,d). This is probably due to the weaker interchain AF interactions in the Co-Co pairs coupled via π-π stacking of the H2dapsc ligands in complex 4 (Figure 7) compared to such interactions in infinite Co-Co chains of π-stacked complexes in 2 (similar to the Mn-Mn chains in 1 shown in Figure 3).
2.2.2. Dynamic (ac) Magnetic Properties
In order to examine possible SCM or SMM properties of the complexes 1, 2 and 3, 4, respectively, the in-phase χ’ and out-of-phase χ″ components of ac magnetic susceptibility were measured in the frequency range of 0.1 Hz–1000 Hz at temperatures 1.8 K–5.0 K in a zero and in non-zero magnetic field. Chain (2) and trimeric (4) complexes, in which the Co(II) and Cr(III) metal centers are linked by cyanide bridges, did not show a frequency dependence of χ″ in zero and non-zero static magnetic fields, thus precluding the single chain or single molecule magnetic behavior for 2 and 4, respectively (Figures S5–S7). This result agrees with the statement made in [23] that seven-coordinated Co(II) PBP complexes with planar anisotropy are not suitable as building blocks for the creation of exchange-coupled polynuclear ensembles demonstrating slow magnetization relaxation, in contrast, for example, to PBP complexes of Fe(II) and Ni(II) with Ising anisotropy.
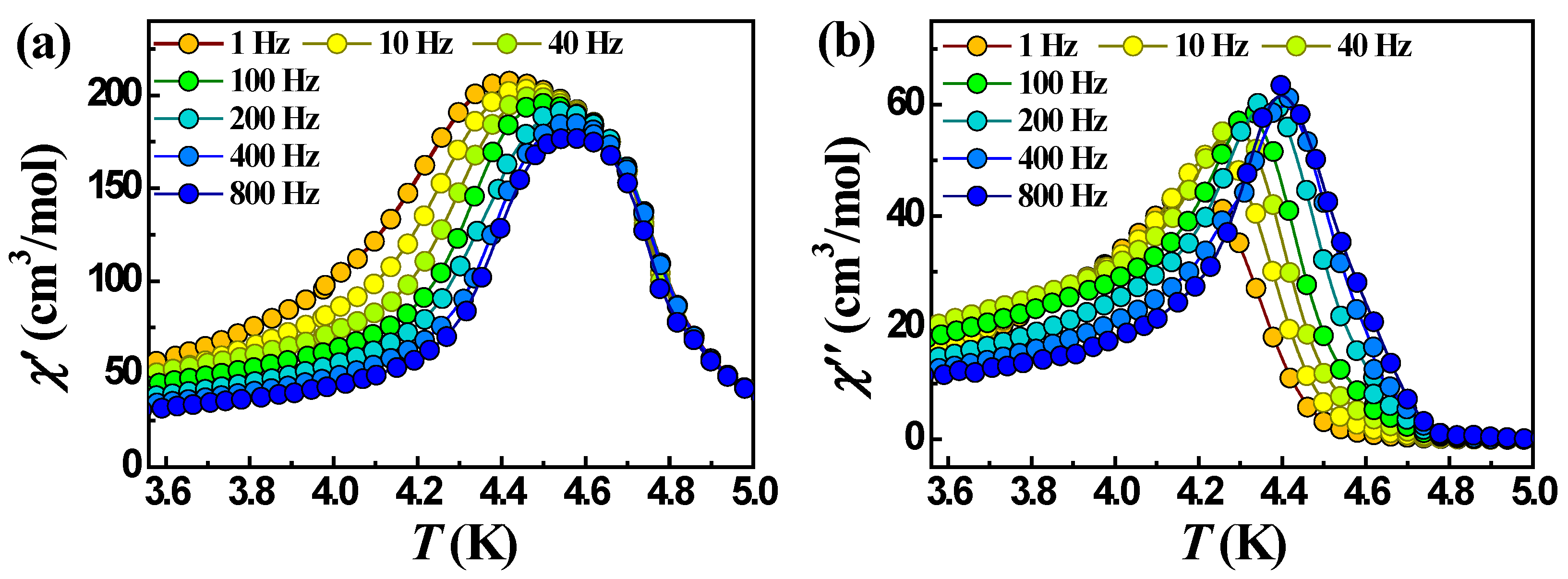
Unlike 2 and 4, complexes 1 and 3 which contain Mn(II) instead of Co(II) exhibit frequency-dependent signals χ’ and χ″. The plots of temperature and frequency dependencies of χ’ and χ″ for 1 in a zero dc field are shown in Figure 10 and Figure S8. Below 4.7 K, an out-of-phase signal reveals a frequency-dependent maximum that indicates a slow relaxation of magnetization. However, the relative change of the temperature of the χ″ maximum depending on the frequency of the oscillating field, expressed by the so-called Mydosh parameter ϕ = (ΔTp/Tp)/Δ(logf) [69], turned out to be 0.023, which is in the range of values characteristic of spin glass (0.004 > ϕ < 0.08) and not superparamagnets for which the Mydosh parameter is usually an order of magnitude larger (>0.1) [70,71]. The relaxation time (τ) was fitted to the Arrhenius equation, τ = τ0exp(Ueff/kBT), where τ = 1/2πν, to allow the estimation of a pre-exponential factor, τ0 = 6.5·10−55 s, and the effective barrier to the relaxation of magnetization, Ueff = 512 K (Figure S9). These values are outside the normal range for typical SCMs (τ0 usually >10−13 s) and indicate that 1 shows typical spin-glass behavior. Similar behavior was found in other 1D chain complexes [72,73,74,75,76]. Antiferromagnetic interactions between different structural units can lead to randomness (disorder, defects) and frustration, which are responsible for the spin-glass system [73,74,76,77]. In 1, the antiferromagnetic interaction between the chains, transferred through π-π stacking and hydrogen bonds, disrupts the ferrimagnetic ordering in the chains and leads to the disorder of the electron spin system, which causes the spin-glass behavior of complex 1. Moreover, the loss of the coordination solvent (EtOH), which is involved in the formation of hydrogen bonds in 1, may contribute to the formation of the spin-glass state, as was observed in the works [73,77].
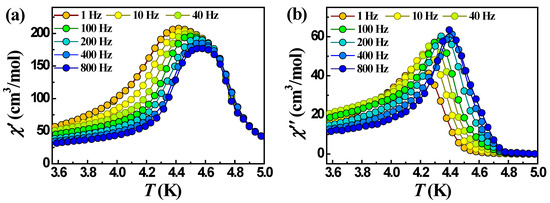
Figure 10.
Temperature dependencies of χ’ (a) and χ″ (b) at different frequencies for complex 1 in a zero dc field.
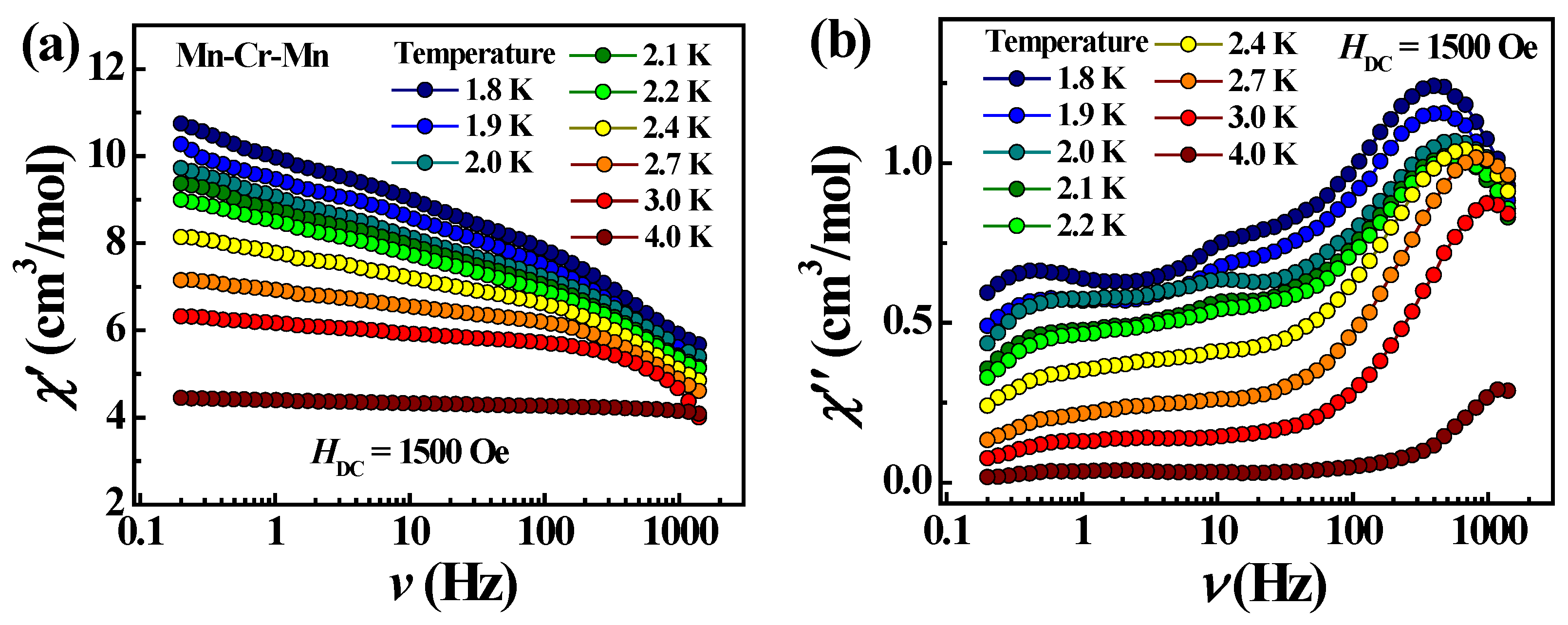
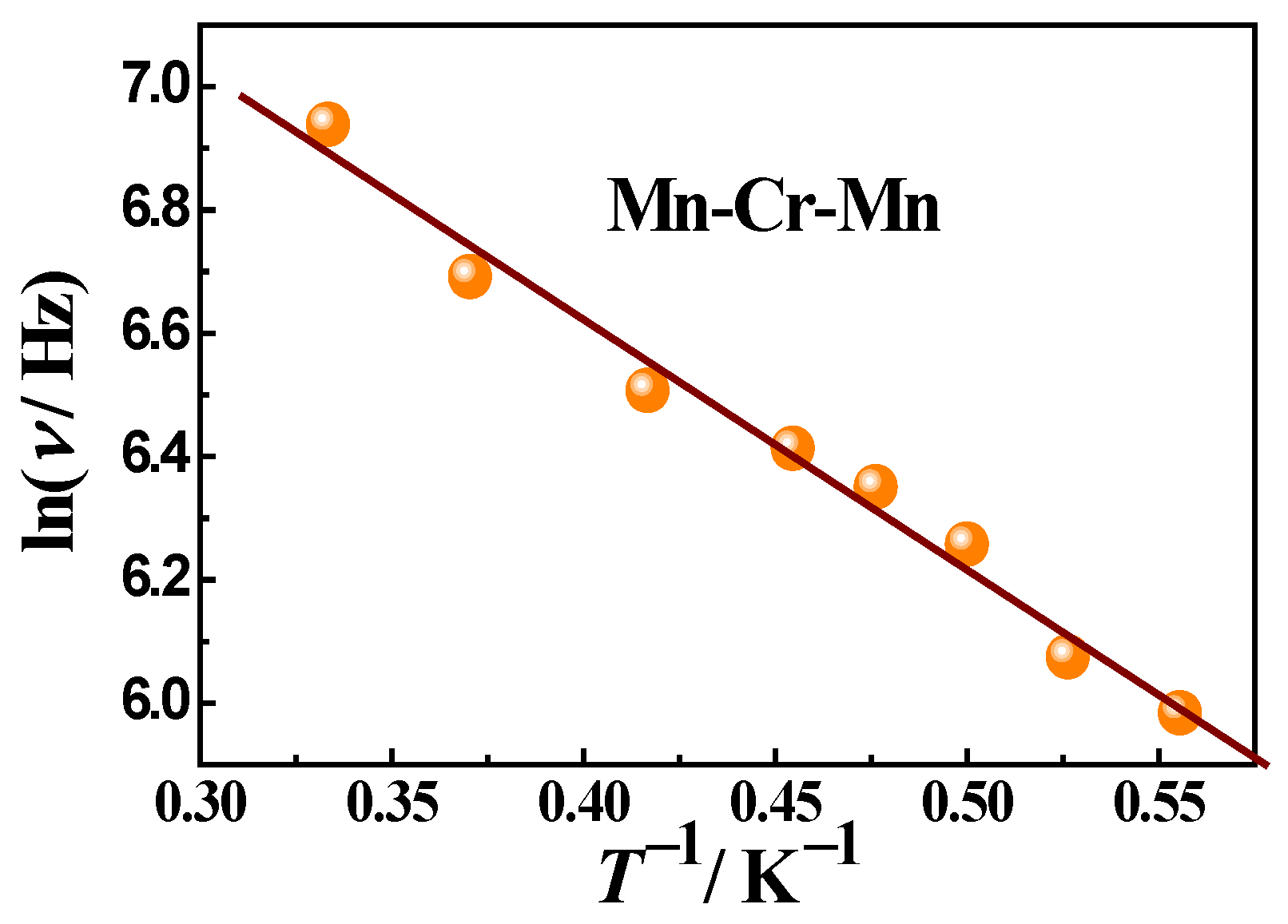
In the case of trimeric complex 3, the studies of ac susceptibility showed frequency-dependent signals of χ″ when applying a dc field of 1500 Oe (Figure 11), indicating a slow relaxation of the magnetization. The pronounced χ″ maxima appear one after another in the frequency range above 100 Hz and shift towards higher frequencies with increasing temperature, which is typical for SMMs. The Mydosh parameter is 0.9. The temperature dependence of the χ″ peak frequency follows an Arrhenius law with an effective energy barrier Ueff = 4.1 K and a pre-exponential factor τ0 being 4.2·10−6 s, suggesting a thermally activated relaxation (Figure 12). Thus, complex 3 is a field-induced SMM.
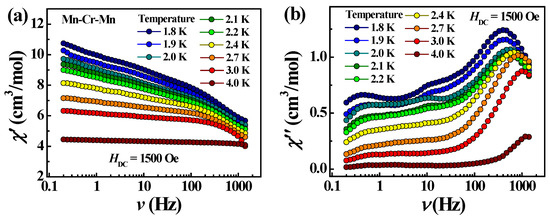
Figure 11.
Frequency dependencies of χ’ (a) and χ″ (b) at different temperatures for complex 3 in 1500 Oe dc field.
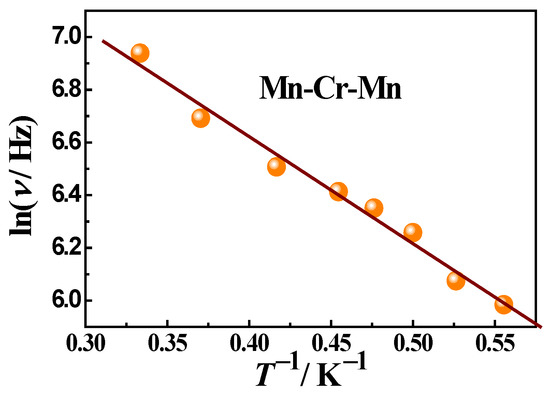
Figure 12.
The dependence of lnτ vs. 1/T for 3 in 1500 Oe dc field and its approximation using the Arrhenius formula (solid line). The points were obtained from the frequency dependencies χ″(ν) at different temperatures (Figure 11, main text). Approximation parameters: activation energy Ueff = 4.1 K, a pre-exponential factor τ0 = 4.2·10−6 s.
3. Materials and Methods
All chemicals and solvents were reagent grade and used without further purification. The starting compounds [Mn(H2dapsc)Cl2]·H2O [78] and [Co(H2dapsc)Cl(H2O)]Cl·2H2O [31] were prepared according to the literature procedures. The (PPh4)3[Cr(CN)6]·2H2O was synthesized by metathesis in water using K3[Cr(CN)6] and PPh4Cl.
The C, H, N, O elemental analyses were carried out with a Vario Micro Cube analyzing device. The infrared spectra were measured on solid samples using a VERTEX 70v (Bruker) spectrometer in the range of 4000–500 cm−1. The thermogravimetric analysis (TGA) was performed in an argon atmosphere with a heating rate of 5.0 °C min−1 using a NETZSCH STA 409 C Luxx thermal analyzer. X-ray powder diffraction spectra were recorded using a Siemens D500 powder diffractometer with a linear detector at room temperature (CuKα1-radiation, λ = 1.5406 Å, step = 0.02°, single crystal sample holder). The dc and ac magnetic susceptibility of powder samples of complexes 1–4 were measured using a Quantum Design MPMS-5 SQUID magnetometer. The experimental data were corrected for the sample holder and for the diamagnetic contribution calculated from Pascal constants.
3.1. Synthesis
3.1.1. {[Mn(H2dapsc)]CrIII(CN)6K(H2O)2.5(EtOH)0.5}n·1.2n(H2O) (1)
The yellow crystals of 1 were obtained by slow diffusion of K3[Cr(CN)6] solution (30 mg; 0.092 mmol in 3 mL H2O) through frit with a pore diameter of 10–20 microns into [Mn(H2dapsc)Cl2]·H2O solution (39 mg; 0.092 mmol) in 10 mL of an ethanol-water (2:1) mixture for two weeks at 8–10 °C. The resulting crystalline precipitate was filtered, washed with ethanol and dried in vacuum. After drying in vacuum, the crystals lost 0.5 molecule of C2H5OH. Yield: 47 mg (80%). Anal. calc. (%) for C17H22.4N13O5.7CrKMn: C, 31.60; H, 3.49; N, 28.18; O, 14.11. Found (%): C, 31.2; H, 3.5; N, 27.9; O, 14.2. This stoichiometry corresponds to the dried sample of 1 as confirmed by TGA analysis (Figure S10). Characteristic IR data (cm−1): ν(C≡N) 2152, 2130; ν(C=N) 1663 (imine); ν(Cr-C) 458 (Figure S11).
The thermogram of the dried crystalline sample (Figure S10) demonstrates a mass loss of 7.72% in the temperature range 50–150 °C with an endothermic peak at 111.6 °C which corresponds to the loss of 2.8 molecules H2O. In the mass spectrum recorded in the gas phase, the peaks are observed at m/z = 18 and m/z = 17 from H2O molecules, while peaks from ethanol molecules are not observed. The decomposition of the complex starts above 200 °C with DSC peak at 280.9 °C and is accompanied by the release of CN- (m/z = 26), OH- (m/z = 17) and CH3− (m/z = 15) fragments.
3.1.2. {[Co(H2dapsc)]CrIII(CN)6K(H2O)2.5(EtOH)0.5}n·1.2n(H2O) (2)
The light orange crystals of 2 were obtained at room temperature by slow diffusion of the starting reagents: [Co(H2dapsc)ClH2O]Cl·2H2O (46.1 mg; 0.1 mmol) in the mixture of 5 mL ethanol and 2 mL water and K3[Cr(CN)6] (32.5 mg; 0.1 mmol) in 5 mL H2O into 10 mL of an ethanol-water (2:1) medium. After 5–7 days, the resulting crystals were filtered, washed with ethanol and dried in vacuum. Yield: 35 mg (~54%). Elemental analysis showed that upon drying the crystals lost 0.5 molecule of C2H5OH, which was additionally confirmed by the TGA of dried samples (Figure S12). Anal. calc. (%) for C17H22.4N13O5.7CoCrK: C, 31.41; H, 3.47; N, 28.01; O, 14.03. Found (%): C, 31.5; H, 3.5; N, 27.9; O, 14.2. Characteristic IR data (cm−1): ν(C≡N) 2160, 2129; ν(C=N) 1670 (imine); ν(Cr-C) 456 (Figure S13).
The thermal analysis of complex 2 after drying in vacuum showed that a mass loss begins at ~50 °C with an endothermic peak at 112.7 °C and reaches 9.5% at 150 °C, that corresponds to the loss of 3.5 molecules H2O (Figure S12). In the mass spectrum of the gas phase, the peaks are observed at m/z = 18 and m/z = 17 from H2O molecules. The peaks from ethanol molecules are absent. The second weight-loss step appears above 200 °C with the release of fragments of the decaying complex.
For the single crystal X-ray diffraction analysis, crystals of 1, 2 were kept in contact with mother liquid to prevent an ethanol loss. After drying, the complexes preserve crystallinity. X-ray powder diffraction pattern of the dried sample of 2 shows a coincidence with the simulated pattern calculated from the single crystal data (Figure S14).
3.1.3. {[Mn(H2dapsc)]2Cr(CN)6(H2O)2}Cl·H2O (3)
The crystals of 3 were obtained by slow diffusion of (PPh4)3[Cr(CN)6]·2H2O solution (77 mg; 0.06 mmol) in 3 mL methanol into 12 mL methanol-water (3:1) solution of [Mn(H2dapsc)Cl2]·H2O (50 mg; 0.12 mmol) at 8–10 °C in the course of 5-7 days. The resulting crystals were filtered, washed with methanol and dried in vacuum. Yield: 38 mg (65%). Anal. calc. (%) for C28H36N20O7ClCrMn2: C, 34.95; H, 3.77; N, 29.12; Cl, 3.68. Found (%): C, 35.2; H, 3.8; N, 29.0; Cl, 3.5. Characteristic IR data (cm−1): ν(C≡N) 2142, 2131; ν(C=N) 1660 (imine); ν(Cr-C) 448 (Figure S15).
3.1.4. {[Co(H2dapsc)]2Cr(CN)6(H2O)2}Cl·2EtOH·3H2O (4)
The crystals of 4 were obtained in an H-shaped tube. One compartment of the H-tube was filled with solution of [Co(H2dapsc)ClH2O]Cl·2H2O (28 mg; 0.06 mmol) in 3 mL of ethanol and 1.5 mL of H2O. The second compartment was filled with solution of (PPh4)3[Cr(CN)6]·2H2O (38 mg; 0.03 mmol) in 4 mL of ethanol. The tube was filled with ethanol and left in a refrigerator. After three weeks, the resulting orange crystals were filtered, washed with ethanol twice and dried in vacuum. Yield: 14 mg (~45%). Elemental analysis showed that crystals 4 lost the crystallized ethanol. Anal. calc. (%) for C28H40N20O9ClCo2Cr: C, 33.42; H, 4.01; N, 27.84; Cl, 3.52. Found (%): C, 33.3; H, 4.1; N, 27.5; Cl, 3.5. Characteristic IR data (cm−1): ν(C≡N) 2166, 2123; ν(C=N) 1668 (imine); ν(Cr-C) 450 (Figure S16).
3.2. Crystal Structure Determination
X-ray single crystal diffraction data were collected at low temperatures on an Oxford Diffraction Gemini-R CCD diffractometer equipped with an Oxford cryostream cooler [λ(MoKα) = 0.71073 Å, graphite monochromator, ω-scans]. Single crystals of the complexes 1–4 were taken from the mother liquid using a nylon loop with oil and immediately transferred into the cold nitrogen stream of the diffractometer. Data reduction with empirical absorption correction of experimental intensities (Scale3AbsPack program) was made with the CrysAlisPro software [79].
The structures were solved by a direct method and refined by a full-matrix least squares method using SHELX-2016 program [80]. All non-hydrogen atoms were refined anisotropically. The positions of H-atoms were refined in a riding model with isotropic displacement parameters depending on the Ueq of the connected atom. The hydrogen atoms in water molecules were found from the difference Fourier map. All N-H and O-H bond distances were refined, with additional geometrical restraints (SADI/DFIX) applied in some cases. Acentric structures 1 and 2 were refined as two-component inversion twins, with refined twin fractions of 0.50(1) and 0.48(1), respectively. Main crystal and experimental data for 1–4 are listed in Table 1.

Table 1.
Crystal data and structural refinement parameters for the complexes 1–4.
4. Conclusions
Four heterometallic cyano-bridged complexes combining [MII(H2dapsc)]2+ cations and [Cr(CN)6]3− anions were synthesized: {[M(H2dapsc)]Cr(CN)6K(H2O)2.5(EtOH)0.5}n·1.2n(H2O), M = Mn (1) and Co (2); {[Mn(H2dapsc)]2Cr(CN)6(H2O)2}Cl·H2O (3) and {[Co(H2dapsc)]2Cr(CN)6(H2O)2}Cl·2EtOH·3H2O (4). The single crystal X-ray diffraction study showed that the complexes contain magnetic M(II) centers in the pentagonal bipyramidal coordination incorporated in the chain (in 1, 2) or trimeric (in 3, 4) structure by linking through [Cr(CN)6] bridging units. The pentadentate H2dapsc ligands in all four compounds keep the protons in both hydrazine –NH moieties and retain a neutral state. Structural analysis revealed the important role of intermolecular non-covalent interactions such as hydrogen bonding and π…π stacking in the stabilization of crystal packing. In the chain compounds 1 and 2, neighbor chains are linked additionally by coordination bonds via pairs of potassium cations and oxygen atoms from water/ethanol molecules. Pairs of compounds 1, 3 and 2, 4 containing Mn(II) and Co(II), respectively, differ significantly in their dc and ac magnetic properties. In contrast to 1, in compound 2, a field-induced metamagnetic transition with a critical field of 1500 Oe was found. Complexes 1 and 3 exhibit a frequency-dependent ac magnetic susceptibility due to the spin-glass behavior in the chain complex 1 and the field-induced single molecule magnetism in the trimeric complex 3. At the same time, 2 and 4 did not show a frequency dependence of χ″ signals of ac susceptibility in the zero and non-zero dc field, thus precluding the single chain or single molecule magnetic behavior for 2 and 4, respectively. This result implies that seven-coordinated Co(II) PBP complexes with planar anisotropy are not suitable as building units for the design of advanced molecular nanomagnets based on the M(4d/5d)–M(3d) exchange-coupled pairs with Ising-type exchange interactions [10,81]. At the same time, Mn(II) PBP complexes with high spin (S = 5/2) can be used for these purposes.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules27238518/s1, Cif and CheckCif files; Figure S1: Interchain hydrogen bonds in the (0 1 0) plane of the structure 1; Figure S2: Temperature dependencies of inverted magnetic susceptibility for 1–4; Figure S3: Temperature dependencies of the magnetization (a) and dc magnetic susceptibility (b) for 2 at different magnetic fields; Figure S4: Magnetic dc field dependencies of real (χ’) and imaginary (χ″) parts of ac magnetic susceptibility at 2.0 K for complex 2; Figure S5: Frequency dependencies of ac magnetic susceptibility at 2.0, 2.5 and 3.0 K for complex 2 in a zero dc field; Figure S6: Frequency dependencies of ac magnetic susceptibility at 2.0–3.5 K temperature range for complex 2 under 1.5 kOe dc field; Figure S7: Frequency dependencies of ac magnetic susceptibility at 1.8–5.0 K temperature range for complex 4 under 1.5 kOe dc field; Figure S8: Frequency dependencies of χ’ and χ″ at different temperatures for complex 1 in a zero dc field; Figure S9: The dependence of ln(τ) vs. T−1 for 1 in a zero dc field; Figure S10: TG-DSC curves and mass spectra for complex 1 after drying in vacuum; Figure S11: IR (a) and Raman (b) spectra of the complex 1; Figure S12: TG-DSC curves and mass spectra for complex 2 after drying in vacuum; Figure S13: IR spectrum of the complex 2; Figure S14: Powder diffraction pattern for a dried sample of 2; Figure S15: IR spectrum of the complex 3; Figure S16: IR spectrum of the complex 4; Tables S1, S2, S3, S4: Hydrogen bond geometry in 1, 2, 3, 4, respectively; Table S5: Weiss temperatures in the complexes 1–4.
Author Contributions
Conceptualization, E.B.Y.; synthesis and characterization, V.D.S.; X-ray crystallography, formal analysis, L.V.Z. and S.V.S.; magnetometry experiment, formal analysis, A.D.T.; visualization, L.V.Z. and A.D.T.; writing—original draft preparation, E.B.Y. and L.V.Z.; writing—review and editing, E.B.Y. and L.V.Z.; supervision, project administration, E.B.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Russian Science Foundation, grant number 18-13-00264.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in the present article and the Supplementary Materials section.
Acknowledgments
This work was performed using the equipment of the Research Centre, FRC PCP MC RAS, https://icp.ac.ru/en/ (accessed on 2 February 2018). The structural study was partially supported by the Ministry of Science and Higher Education within the State assignment for ISSP RAS.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A

Table A1.
Selected bond lengths (Å) and angles (°) in 1–4.
Table A1.
Selected bond lengths (Å) and angles (°) in 1–4.
| 1 | |||
|---|---|---|---|
| Mn(1)-O(1) | 2.244(2) | Mn(2)-O(3) | 2.272(2) |
| Mn(1)-O(2) | 2.255(2) | Mn(2)-O(4) | 2.218(2) |
| Mn(1)-N(5) | 2.291(2) | Mn(2)-N(12) | 2.274(2) |
| Mn(1)-N(6) | 2.311(2) | Mn(2)-N(13) | 2.310(2) |
| Mn(1)-N(7) | 2.306(3) | Mn(2)-N(14) | 2.282(3) |
| Mn(1)-N(41) | 2.210(3) | Mn(2)-N(42) d | 2.227(3) |
| Mn(1)-N(51) | 2.221(2) | Mn(2)-N(52) c | 2.214(3) |
| Cr(1)-CCN | 2.063(3)–2.080(3) | Cr(2)-CCN | 2.070(3)–2.076(3) |
| O(1)-Mn(1)-O(2) | 84.21(7) | O(3)-Mn(2)-O(4) | 83.08(7) |
| O(1)-Mn(1)-N(5) | 70.68(8) | O(3)-Mn(2)-N(12) | 70.41(8) |
| O(2)-Mn(1)-N(6) | 69.53(8) | O(4)-Mn(2)-N(13) | 69.76(8) |
| N(5)-Mn(1)-N(7) | 68.14(9) | N(12)-Mn(2)-N(14) | 68.77(9) |
| N(6)-Mn(1)-N(7) | 67.46(8) | N(13)-Mn(2)-N(14) | 67.98(9) |
| N(41)-Mn(1)-N(51) | 171.0(1) | N(42)d-Mn(2)-N(52) c | 170.0(1) |
| Mn(1)-N(41)-C(41) | 152.3(2) | Mn(2)-N(42)d-C(42) d | 151.8(2) |
| Mn(1)-N(51)-C(51) | 151.4(2) | Mn(2)-N(52)c-C(52)c | 149.6(2) |
| Cr(1)-C(41)-N(41) | 173.3(3) | Cr(1)-C(42)-N(42) | 173.1(3) |
| Cr(2)-C(51)-N(51) | 175.2(2) | Cr(2)-C(52)-N(52) | 175.9(2) |
| 2 | |||
| Co(1)-O(1) | 2.208(2) | Co(2)-O(3) | 2.243(2) |
| Co(1)-O(2) | 2.225(2) | Co(2)-O(4) | 2.189(2) |
| Co(1)-N(5) | 2.204(3) | Co(2)-N(12) | 2.185(3) |
| Co(1)-N(6) | 2.220(3) | Co(2)-N(13) | 2.218(3) |
| Co(1)-N(7) | 2.205(3) | Co(2)-N(14) | 2.178(3) |
| Co(1)-N(41) | 2.091(3) | Co(2)-N(42)d | 2.097(3) |
| Co(1)-N(51) | 2.099(3) | Co(2)-N(52)c | 2.098(3) |
| Cr(1)-CCN | 2.068(3)–2.081(4) | Cr(2)-CCN | 2.063(4)–2.079(4) |
| O(1)-Co(1)-O(2) | 77.5(1) | O(3)-Co(2)-O(4) | 76.4(1) |
| O(1)-Co(1)-N(5) | 71.9(1) | O(3)-Co(2)-N(12) | 71.6(1) |
| O(2)-Co(1)-N(6) | 70.8(1) | O(4)-Co(2)-N(13) | 71.2(1) |
| N(5)-Co(1)-N(7) | 70.3(1) | N(12)-Co(2)-N(14) | 70.8(1) |
| N(6)-Co(1)-N(7) | 69.5(1) | N(13)-Co(2)-N(14) | 70.0(1) |
| N(41)-Co(1)-N(51) | 170.2(1) | N(42)d-Co(2)-N(52) c | 170.6(1) |
| Co(1)-N(41)-C(41) | 158.3(3) | Co(2)-N(42)d-C(42) d | 160.9(3) |
| Co(1)-N(51)-C(51) | 155.2(3) | Co(2)-N(52)c-C(52)c | 154.9(3) |
| Cr(1)-C(41)-N(41) | 171.1(3) | Cr(1)-C(42)-N(42) | 170.4(3) |
| Cr(2)-C(51)-N(51) | 173.4(3) | Cr(2)-C(52)-N(52) | 173.3(3) |
| 3 | 4 | ||
| Mn(1)-O(1) | 2.205(1) | Co(1)-O(1) | 2.157(4) |
| Mn(1)-O(2) | 2.272(1) | Co(1)-O(2) | 2.168(3) |
| Mn(1)-N(5) | 2.301(1) | Co(1)-N(5) | 2.182(4) |
| Mn(1)-N(6) | 2.285(1) | Co(1)-N(6) | 2.181(4) |
| Mn(1)-N(7) | 2.323(1) | Co(1)-N(7) | 2.171(4) |
| Mn(1)-N(8) | 2.226(1) | Co(1)-N(8) | 2.106(4) |
| Mn(1)-O(3) | 2.265(1) | Co(1)-O(3) | 2.164(3) |
| Cr(1)-CCN | 2.069(1)–2.087(1) | Cr(1)-CCN | 2.058(5)–2.072(6) |
| O(1)-Mn(1)-O(2) | 85.12(4) | O(1)-Co(1)-O(2) | 74.8(1) |
| O(1)-Mn(1)-N(5) | 70.34(4) | O(1)-Co(1)-N(5) | 71.6(1) |
| O(2)-Mn(1)-N(6) | 69.26(4) | O(2)-Co(1)-N(6) | 72.3(1) |
| N(5)-Mn(1)-N(7) | 67.21(4) | N(5)-Co(1)-N(7) | 70.9(2) |
| N(6)-Mn(1)-N(7) | 67.75(4) | N(6)-Co(1)-N(7) | 70.5(1) |
| N(8)-Mn(1)-O(3) | 165.80(4) | N(8)-Co(1)-O(3) | 176.4(1) |
| Mn(1)-N(8)-C(12) | 164.7(1) | Co(1)-N(8)-C(12) | 151.3(4) |
| Cr(1)-C(12)-N(8) | 174.1(1) | Cr(1)-C(12)-N(8) | 176.9(4) |
Symmetry codes: c (−x, y + 0.5, −z), d (1 − x, y + 0.5, 1 − z).
References
- Gatteschi, D.; Sessoli, R.; Villain, J. Molecular Nanomagnets; Oxford University Press: Oxford, UK, 2006. [Google Scholar]
- Benelli, C.; Gatteschi, D. Introduction to Molecular Magnetism; Wiley-VCH Verlag & Co. KGaA: Weinheim, Germany, 2015. [Google Scholar]
- Dey, A.; Kalita, P.; Chandrasekhar, V. Lanthanide(III)-based single-ion magnets. ACS Omega 2018, 3, 9462–9475. [Google Scholar] [CrossRef] [PubMed]
- Bogani, L.; Wernsdorfer, W. Molecular spintronics using single-molecule magnets. Nat. Mater. 2008, 7, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Aromí, G.; Aguilà, D.; Gamez, P.; Luis, F.; Roubeau, O. Design of magnetic coordination complexes for quantum computing. Chem. Soc. Rev. 2012, 41, 537–546. [Google Scholar] [CrossRef]
- Bartolomé, S.J.; Luis, F.; Fernández, J.F. (Eds.) Molecular Magnets: Physics and Applications; Springer: New York, NY, USA, 2014. [Google Scholar]
- Goodwin, C.P.; Ortu, F.; Reta, D.; Chilton, N.F.; Mills, D.P. Molecular magnetic hysteresis at 60 kelvin in dysprosocenium. Nature 2017, 548, 439–445. [Google Scholar] [CrossRef] [PubMed]
- Guo, F.-S.; Day, B.M.; Chen, Y.-C.; Tong, M.-L.; Mansikkamäki, A.; Layfield, R.A. Magnetic hysteresis up to 80 kelvin in a dysprosium metallocene single-molecule magnet. Science 2018, 362, 1400–1403. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Zhao, C.; Feng, T.; Liu, X.; Ying, X.; Li, X.-L.; Zhang, Y.-Q.; Tang, J. Air-stable chiral single-molecule magnets with record anisotropy barrier exceeding 1800 K. J. Am. Chem. Soc. 2021, 143, 10077–10082. [Google Scholar] [CrossRef]
- Mironov, V.; Bazhenova, T.; Manakin, Y.; Yagubskii, E. Pentagonal-bipyramidal 4d and 5d complexes with unquenched orbital angular momentum as a unique platform for advanced single-molecule magnets: Current state and perspectives. Dalton Trans. 2022, in press. [Google Scholar]
- Craig, G.A.; Murrie, M. 3d single-ion magnets. Chem. Soc. Rev. 2015, 44, 2135–2147. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-Y.; Avendaño, C.; Dunbar, K.R. Molecular magnetic materials based on 4d and 5d transition metals. Chem. Soc. Rev. 2011, 40, 3213–3238 and references therein. [Google Scholar] [CrossRef]
- Layfield, R.A.; Murugesu, M. Lanthanides and Actinides in Molecular Magnetism; Wiley-VCH Verlag & Co. KGaA: Weinheim, Germany, 2015. [Google Scholar]
- Bar, A.K.; Pichon, C.; Sutter, J.-P. Magnetic anisotropy in two- to eight-coordinated transition-metal complexes: Recent developments in molecular magnetism. Coord. Chem. Rev. 2016, 308, 346–380. [Google Scholar] [CrossRef]
- Boča, R. Zero-field splitting in metal complexes. Coord. Chem. Rev. 2004, 248, 757–815. [Google Scholar] [CrossRef]
- Rebilly, J.-N.; Charron, G.; Rivière, E.; Guillot, R.; Barra, A.-L.; Serrano, M.D.; van Slageren, J.; Mallah, T. Large magnetic anisotropy in pentacoordinate NiII complexes. Chem. Eur. J. 2008, 14, 1169–1177. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-Z.; Wang, B.-W.; Sato, O.; Gao, S. First Fe(II)-based cyano-bridged single molecule magnet [CrIIIFeII] with a large anisotropy. Chem. Commun. 2010, 46, 6959–6961. [Google Scholar] [CrossRef] [PubMed]
- Rinehart, J.D.; Long, J.R. Exploiting single-ion anisotropy in the design of f-element single-molecule magnets. Chem. Sci. 2011, 2, 2078–2085. [Google Scholar] [CrossRef]
- Gómes-Coca, S.; Cremades, E.; Aliaga-Alcaldeand, N.; Ruiz, E. Mononuclear single-molecule magnets: Tailoring the magnetic anisotropy of first-row transition-metal complexes. J. Am. Chem. Soc. 2013, 135, 7010–7018. [Google Scholar] [CrossRef]
- Meng, Y.-S.; Jiang, S.D.; Wang, B.-W.; Gao, S. Understanding the magnetic anisotropy toward single-ion magnets. Acc. Chem. Res. 2016, 49, 2381–2389. [Google Scholar] [CrossRef]
- Saberand, M.R.; Dunbar, K.R. Ligands effects on the magnetic anisotropy of tetrahedral cobalt complexes. Chem. Commun. 2014, 50, 12266–12269. [Google Scholar]
- Huang, X.-C.; Zhou, C.; Shao, D.; Wang, X.-Y. Field-induced slow magnetic relaxation in cobalt(II) compounds with pentagonal bipyramid geometry. Inorg. Chem. 2014, 53, 12671–12673. [Google Scholar] [CrossRef]
- Bar, A.K.; Gogoi, N.; Pichon, C.; Goli, D.P.; Thlijeni, M.; Duhayon, C.; Suaud, N.; Guihéry, N.; Barra, A.L.; Ramasesha, S.; et al. Pentagonal bipyramid FeII complexes: Robust Ising-spin units towards heteropolynuclear nanomagnets. Chem. Eur. J. 2017, 23, 4380–4396. [Google Scholar] [CrossRef]
- Gogoi, N.; Thlijeni, M.; Duhayon, C.; Sutter, J.-P. Heptacoordinated nickel(II) as an Ising-type anisotropic building unit: Illustration with a pentanuclear [(NiL)3{W(CN)8}2] complex. Inorg. Chem. 2013, 52, 2283–2285. [Google Scholar] [CrossRef]
- Chen, Y.-C.; Liu, J.-L.; Ungur, L.; Liu, J.; Li, Q.-W.; Wang, L.-F.; Ni, Z.-P.; Chibotaru, L.F.; Chen, X.-M.; Tong, M.-L. Symmetry-supported magnetic blocking at 20 K in pentagonal bipyramidal Dy(III) single-ion magnets. J. Am. Chem. Soc. 2016, 138, 2829–2837. [Google Scholar] [CrossRef] [PubMed]
- Ruamps, R.; Batchelor, L.J.; Maurice, R.; Gogoi, N.; Jiménez-Lozano, P.; Guihéry, N.; de Graaf, C.; Barra, A.L.; Sutter, J.-P.; Mallah, T. Origin of the magnetic anisotropy in heptacoordinate NiII and CoII complexes. Chem. Eur. J. 2013, 19, 950–956. [Google Scholar] [CrossRef] [PubMed]
- Gavey, E.L.; Beldjoudi, Y.; Rawson, J.M.; Stamatatosand, T.C.; Pilkington, M. Slow relaxation in the first penta-aza Dy(iii) macrocyclic complex. Chem. Commun. 2014, 50, 3741–3743. [Google Scholar] [CrossRef]
- Unger, L.; Chibotaru, L.F. Strategies toward high-temperature lantanide-based single-molecule magnets. Inorg. Chem. 2016, 55, 10043–10056. [Google Scholar] [CrossRef] [PubMed]
- Palenik, G.J.; Wester, D.W.; Rychlewska, U.; Palenik, R.C. Pentagonal-bipyramidal complexes. Synthesis and crystal structures of diaqua [2,6-diacetylpyridine bis(semicarbazone)]chromium(III) hydroxide dinitrate hydrate and dichloro[2,6-diacetylpyridine bis(semicarbazone)]iron(III) chloride dihydrate. Inorg. Chem. 1976, 15, 1814–1819. [Google Scholar] [CrossRef]
- Palenik, G.J.; Wester, D.W. Pentagonal-bipyramidal complexes. Crystal and molecular structures of chloroaqua(2,6-diacetylpyridine bis(semicarbazone))manganese(II), -iron(II), -cobalt(II), and -zinc(II) chloride dihydrates. Inorg. Chem. 1978, 17, 864–870. [Google Scholar] [CrossRef]
- Giordano, T.J.; Palenik, G.J.; Palenik, R.C.; Sullivan, D.A. Pentagonal-bipyramidal complexes. Synthesis and characterization of aqua(nitrato)[2,6-diacetylpyridine bis(benzoyl hydrazone)]cobalt(II) nitrate and diaqua[2,6-diacetylpyridine bis(benzoyl hydrazone)]nickel(II) nitrate dihydrate. Inorg. Chem. 1979, 18, 2445–2450. [Google Scholar] [CrossRef]
- Gerloch, M.; Morgenstern-Badarau, I. Magnetic and spectral properties of chloroaqua[2,6-diacetylpyridinebis(semicarbazone)]iron(II) and diaqua[2,6-diacetylpyridinebis(semicarbazone)]nickel(II): Ligand fields and bonding in pentagonal-bipyramidal complexes. Inorg. Chem. 1979, 18, 3225–3229. [Google Scholar] [CrossRef]
- Lorenzini, C.; Pelizzi, C.; Pelizzi, G.; Predieri, G. Investigation into aroylhydrazones as chelating agents. Part 3. Synthesis and spectroscopic characterization of complexes of MnII, CoII, NiII, CuII, and ZnII with 2,6-diacetylpyridine bis(benzoylhydrazone) and X-ray structure of aquachloro[2,6-diacetylpyridine bis(benzoylhydrazone)]manganese(II) chloride. J. Chem. Soc. Dalton Trans. 1983, 721–727. [Google Scholar] [CrossRef]
- Ianelli, S.; Pelizzi, C.; Pelizzi, G.; Tarasconi, P. Heptacoordination in MnII, NiII, and CuII complexes of 2,6-diacetylpyridine bis(acetylhydrazone). J. Chem. Crystallogr. 1996, 26, 195–201. [Google Scholar] [CrossRef]
- Carcelli, M.; Ianelli, S.; Pelagatti, P.; Pelizzi, G. Structural characterization of a new ligand mode of 2,6-diacetylpyridine bis(semicarbazone), H2daps. Inorg. Chim. Acta 1999, 292, 121–126. [Google Scholar] [CrossRef]
- Ivanović-Burmazović, I.; Andjelković, K. Transition metal complexes with bis(hydrazone) ligands of 2,6-diacetylpyridine. Hepta-coordination of 3d metals. Adv. Inorg. Chem. 2004, 55, 315–360. [Google Scholar]
- Pichon, C.; Elrez, B.; Béreau, V.; Duhayon, C.; Sutter, J.-P. From heptacoordinated CrIII complexes with cyanide or isothiocyanate apical groups to 1D heterometallic assemblages with all-pentagonal-bipyramid coordination geometries. Eur. J. Inorg. Chem. 2018, 2018, 340–348. [Google Scholar] [CrossRef]
- Bazhenova, T.A.; Zorina, L.V.; Simonov, S.V.; Mironov, V.S.; Maximova, O.V.; Spillecke, L.; Koo, C.; Klingeler, R.; Manakin, Y.V.; Vasiliev, A.N.; et al. The first pentagonal-bipyramidal vanadium(III) complexes with a Schiff-base N3O2 pentadentate ligand: Synthesis, structure and magnetic properties. Dalton Trans. 2020, 49, 15287–15298. [Google Scholar] [CrossRef]
- Bazhenova, T.A.; Zorina, L.V.; Simonov, S.V.; Manakin, Y.V.; Kornev, A.B.; Lyssenko, K.A.; Mironov, V.S.; Gilmutdinov, I.F.; Yagubskii, E.B. A novel family of hepta-coordinated Cr(III) complexes with a planar pentadentate N3O2 Schiff base ligand: Synthesis, structure and magnetism. Inorg. Chim. Acta 2021, 522, 120358. [Google Scholar] [CrossRef]
- Bar, A.K.; Kalita, P.; Sutter, J.-P.; Chandrasekhar, V. Pentagonal-bipyramid Ln(III) complexes exhibiting single-ion-magnet behavior: A rational synthetic approach for a rigid equatorial plane. Inorg. Chem. 2018, 57, 2398–2401. [Google Scholar] [CrossRef]
- Mironov, V.S.; Bazhenova, T.A.; Manakin, Y.V.; Lyssenko, K.A.; Talantsev, A.D.; Yagubskii, E.B. A new Mo(IV) complex with the pentadentate (N3O2) Schiff-base ligand: The first non-cyanide pentagonal-bipyramidal paramagnetic 4d complex. Dalton Trans. 2017, 46, 14083–14087. [Google Scholar] [CrossRef]
- Manakin, Y.V.; Mironov, V.S.; Bazhenova, T.A.; Lyssenko, K.A.; Gilmutdinov, I.F.; Bikbaev, K.S.; Masitov, A.A.; Yagubskii, E.B. (Et4N)[MoIII(DAPBH)Cl2], the first pentagonal-bipyramidal Mo(III) complex with a N3O2-type Schiff-base ligand: Manifestation of unquenched orbital momentum and Ising-type magnetic anisotropy. Chem. Commun. 2018, 54, 10084–10087. [Google Scholar] [CrossRef]
- Bazhenova, T.A.; Kopotkov, V.A.; Korchagin, D.V.; Manakin, Y.V.; Zorina, L.V.; Simonov, S.V.; Yakushev, I.A.; Mironov, V.S.; Vasiliev, A.N.; Maximova, O.V.; et al. A series of novel pentagonal-bipyramidal erbium(III) complexes with acyclic chelating N3O2 Schiff-base ligands: Synthesis, structure, and magnetism. Molecules 2021, 26, 6908. [Google Scholar] [CrossRef]
- Spillecke, L.; Koo, C.; Maximova, O.; Mironov, V.S.; Kopotkov, V.A.; Korchagin, D.V.; Vasiliev, A.N.; Yagubskii, E.B.; Klingeler, R. Magnetic behavior of the novel pentagonal-bipyramidal erbium(III) complex (Et3NH)[Er(H2DAPS)Cl2]: High-frequency EPR study and crystal-field analysis. Dalton Trans. 2021, 50, 18143–18154. [Google Scholar] [CrossRef]
- Kalita, P.; Ahmed, N.; Bar, A.K.; Dey, S.; Jana, A.; Rajaraman, G.; Sutter, J.-P.; Chandrasekhar, V. Pentagonal bipyramidal Ln(III) complexes containing an axial phosphine oxide ligand: Field-induced single-ion magnetism behavior of the Dy(III) analogues. Inorg. Chem. 2020, 59, 6603–6612. [Google Scholar] [CrossRef]
- Mondal, A.K.; Mondal, A.; Dey, B.; Konar, S. Influence of the coordination environment on easy-plane magnetic anisotropy of pentagonal bipyramidal cobalt(II) complexes. Inorg. Chem. 2018, 57, 9999–10008. [Google Scholar] [CrossRef] [PubMed]
- Kopotkov, V.A.; Korchagin, D.V.; Sasnovskaya, V.D.; Gilmutdinov, I.F.; Yagubskii, E.B. A series of field-induced single-ion magnets based on the seven-coordinate Co(II) complexes with pentadentate (N3O2) H2dapsc ligand. Magnetochemistry 2019, 5, 58. [Google Scholar] [CrossRef]
- Mondal, A.K.; Mondal, A.; Konar, S. Slow magnetic relaxation in a one-dimensional coordination polymer constructed from hepta-coordinate cobalt(II) nodes. Magnetochemistry 2020, 6, 45. [Google Scholar] [CrossRef]
- Batchelor, L.J.; Sangalli, M.; Guillot, R.; Guihéry, N.; Maurice, R.; Tuna, F.; Mallah, T. Pentanuclear cyanide-bridged complexes based on highly anisotropic CoII seven-coordinate building blocks: Synthesis, structure, and magnetic behavior. Inorg. Chem. 2011, 50, 12045–12052. [Google Scholar] [CrossRef]
- Sasnovskaya, V.D.; Kopotkov, V.A.; Talantsev, A.D.; Morgunov, R.B.; Yagubskii, E.B.; Simonov, S.V.; Zorina, L.V.; Mironov, V.S. Synthesis, structure, and magnetic properties of 1D {[MnIII(CN)6][MnII(dapsc)]}n coordination polymers: Origin of unconventional single-chain magnet behavior. Inorg. Chem. 2017, 56, 8926–8943. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.-H.; Li, Z.-Y.; Yamashita, M.; Bu, X.-H. Recent progress on cyano-bridged transition-metal-based single-molecule magnets and single-chain magnets. Coord. Chem. Rev. 2021, 428, 213617. [Google Scholar] [CrossRef]
- Zorina, L.V.; Simonov, S.V.; Sasnovskaya, V.D.; Talantsev, A.D.; Morgunov, R.B.; Mironov, V.S.; Yagubskii, E.B. Slow magnetic relaxation, antiferromagnetic ordering, and metamagnetism in MnII(H2dapsc)-FeIII(CN)6 chain complex with highly anisotropic Fe-CN-Mn spin coupling. Chem. Eur. J. 2019, 25, 14583–14597. [Google Scholar] [CrossRef] [PubMed]
- Dey, M.; Sarma, B.; Gogoi, N. Cyano bridged heterometallic Mn(II)-Fe(III) aggregates: Synthesis, structure and magnetic properties. Inorg. Chim. Acta 2018, 469, 20–24. [Google Scholar] [CrossRef]
- Pichon, C.; Suaud, N.; Duhayon, C.; Guihéry, N.; Sutter, J.-P. Cyano-bridged Fe(II)−Cr(III) single-chain magnet based on pentagonal bipyramid units: On the added value of aligned axial anisotropy. J. Am. Chem. Soc. 2018, 140, 7698–7704. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, A.; Pichon, C.; Sutter, J.-P.; Pamu, D.; Sarma, B.; Mudoia, P.P.; Gogoi, N. Accessing water processable cyanido bridged chiral heterobimetallic Co(II)–Fe(III) one dimensional network. Chem. Commun. 2021, 57, 207. [Google Scholar] [CrossRef]
- Bretosh, K.; Béreau, V.; Duhayon, C.; Pichon, C.; Sutter, J.-P. A ferromagnetic Ni(II)–Cr(III) single-chain magnet based on pentagonal bipyramidal building units. Inorg. Chem. Front. 2020, 7, 1503–1511. [Google Scholar] [CrossRef]
- Mallah, T.; Auberger, C.; Verdaguer, M.; Veillet, P. A heptanuclear CrIIINiII6 complex with a low-lying S = 15/2 ground state. Chem. Commun. 1995, 61–62. [Google Scholar] [CrossRef]
- Zhang, Y.-Z.; Sato, O. A cyano-bridged CrIIICoII ferromagnet with a chiral nanotubular structure constituted of interlocked single and double helices. Inorg. Chem. 2010, 49, 1271–1273. [Google Scholar] [CrossRef] [PubMed]
- Mabbs, F.; Machin, D. Magnetism and Transition Metal Complexes, 2nd ed.; Dover Publications Inc.: New York, NY, USA, 2008; pp. 1–206. [Google Scholar]
- Zheng, Y.-Z.; Xue, W.; Tong, M.-L.; Chen, X.-M.; Grandjean, F.; Long, G.J. A two-dimensional iron(II) carboxylate linear chain polymer that exhibits a metamagnetic spin-canted antiferromagnetic to single-chain magnetic transition. Inorg. Chem. 2008, 47, 4077–4087. [Google Scholar] [CrossRef] [PubMed]
- Wöhlert, S.; Boeckmann, J.; Wriedt, M.; Näther, C. Coexistence of metamagnetism and slow relaxation of the magnetization in a cobalt thiocyanate 2D coordination network. Angew. Chem. Int. Ed. 2011, 50, 6920–6923. [Google Scholar] [CrossRef]
- Wöhlert, S.; Tomkowicz, Z.; Rams, M.; Ebbinghaus, S.G.; Fink, L.; Schmidt, M.U.; Näther, C. Influence of the co-ligand on the magnetic and relaxation properties of layered cobalt(II) thiocyanato coordination polymers. Inorg. Chem. 2014, 53, 8298–8310. [Google Scholar] [CrossRef]
- Ni, W.-W.; Ni, Z.-H.; Cui, A.-L.; Liang, X.; Kou, H.-Z. Cyanide-bridged Mn(III)−Fe(III) bimetallic complexes based on the pentacyano(1-methylimidazole)ferrate(III) building block: structure and magnetic characterizations. Inorg. Chem. 2007, 46, 22–33. [Google Scholar] [CrossRef]
- Li, X.-B.; Ma, Y.; Gao, E.-Q. Random Co(II)–Ni(II) ferromagnetic chains showing coexistent antiferromagnetism, metamagnetism, and single-chain magnetism. Inorg. Chem. 2018, 57, 7446–7454. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Zhang, Y.; Jiao, C.; Zhao, L.; Hu, J.; Wang, J.; Liu, T. Coexistence of metamagnetism and single chain magnet behavior in a FeIII2CoII layer compound. Sci. China Chem. 2016, 59, 735–739. [Google Scholar] [CrossRef]
- Nakabayashi, K.; Chorazy, S.; Takahashi, D.; Kinoshita, T.; Sieklucka, B.; Ohkoshi, S.-I. Cesium cyano-bridged CoII−MV (M = Mo and W) layered frameworks exhibiting high thermal durability and metamagnetism. Cryst. Growth Des. 2014, 14, 6093–6100. [Google Scholar] [CrossRef]
- Wen, H.-R.; Tang, Y.-Z.; Liu, C.-M.; Chen, J.-L.; Yu, C.-L. One-dimensional homochiral cyano-bridged heterometallic chain coordination polymers with metamagnetic or ferroelectric properties. Inorg. Chem. 2009, 48, 10177–10185. [Google Scholar] [CrossRef]
- Bonadio, F.; Senna, M.-C.; Ensling, J.; Sieber, A.; Neels, A.; Stoeckli-Evans, H.; Decurtins, S. Cyano-bridged structures based on [MnII(N3O2-macrocycle)]2+: a synthetic, structural, and magnetic study. Inorg. Chem. 2005, 44, 969–978. [Google Scholar] [CrossRef] [PubMed]
- Mydosh, J.A. Spin Glasses: An Experimental Introduction; Taylor & Francis: Washington, DC, USA, 1993. [Google Scholar]
- Binder, K.; Young, A.P. Spin glasses: Experimental facts, theoretical concepts, and open questions. Rev. Mod. Phys. 1986, 58, 801–976. [Google Scholar] [CrossRef]
- Rebilly, J.-N.; Mallah, T. Synthesis of single-molecule magnets using metallocyanates. Struct. Bonding 2006, 122, 103–131. [Google Scholar]
- Kim, J.I.; Kwak, H.Y.; Yoon, J.H.; Ryu, D.W.; Yoo, I.Y.; Yang, N.; Cho, B.K.; Park, J.G.; Lee, H.; Hong, C.S. Cyanide-bridged FeIII−MnIII bimetallic complexes with dimeric and chain structures constructed from a newly made mer-Fe tricyanide: Structures and magnetic properties. Inorg. Chem. 2009, 48, 2956–2966. [Google Scholar] [CrossRef]
- Liu, X.; Cen, P.; Li, H.; Ke, H.; Zhang, S.; Wei, Q.; Xie, G.; Chen, S.; Gao, S. Solvent-induced syntheses, crystal structures, magnetic properties, and single-crystal-to-single-crystal tansformation of azido-Cu(II) coordination polymers with 2-naphthoic acid as co-ligand. Inorg. Chem. 2014, 53, 8088–8097. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, Y.-J.; Hu, J.-X.; Jiao, C.-Q.; Wang, J.-L.; Duan, C.-Y.; Liu, T. Coexistence of the single chain magnet and spin-glass behavior in a cyano-bridged {FeIII2FeII} chain. Inorg. Chem. Commun. 2016, 66, 55–58. [Google Scholar] [CrossRef]
- Yao, M.-X.; Zheng, Q.; Cai, X.-M.; Li, Y.-Z.; Song, Y.; Zuo, J.-L. Chiral cyanide-bridged CrIII−MnIII heterobimetallic chains based on [(Tp)Cr(CN)3]−: Synthesis, structures, and magnetic properties. Inorg. Chem. 2012, 51, 2140–2149. [Google Scholar] [CrossRef]
- Zhao, L.; Duan, R.; Zhuang, P.-F.; Zheng, H.; Jiao, C.-Q.; Wang, J.-L.; He, C.; Liu, T. 12-Metal 36-membered ring based WV–CoII layers showing spin-glass behavior. Dalton. Trans. 2015, 44, 12613–12617. [Google Scholar] [CrossRef]
- Ghosh, A.K.; Shatruk, M.; Bertolasi, V.; Pramanik, K.; Ray, D. Self-assembled tetra- and pentanuclear nickel(II) aggregates from phenoxido-based ligand bound {Ni2} fragments: Carboxylate bridge controlled structures. Inorg. Chem. 2013, 52, 13894–13903. [Google Scholar] [CrossRef] [PubMed]
- Kaur, P.; Jyoti; Robinson, W.T.; Singh, K. Tetracyanoquinodimethane derivatives of pentagonal bipyramidal complexes of manganese(II), iron(II), nickel(II) and copper(II) with 2,6-diacetylpyridinebis(semicarbazone): Single crystal structure of dichloro [2,6-diacetylpyridinebis (semicarbazone)] manganese(II)monohydrate. J. Coord. Chem. 2002, 55, 281–285. [Google Scholar]
- Rigaku Oxford Diffraction Ltd. CrysAlisPro, Version 1.171.38; Rigaku Oxford Diffraction Ltd.: Oxford, UK, 2015.
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. Sect. C 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Manakin, Y.V.; Mironov, V.S.; Bazhenova, T.A.; Yakushev, I.A.; Gilmutdinov, I.F.; Simonov, S.V.; Yagubskii, E.B. (Et4N)[WIII(DAPBH)(CN)2], the first pentagonal-bipyramidal W(III) complex with unquenched orbital angular momentum: A novel Ising-type magnetic building block for single-molecule magnets. Chem. Comm. 2022; in press. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).