One-Dimensional Iodoantimonate(III) and Iodobismuthate(III) Supramolecular Hybrids with Diiodine: Structural Features, Stability and Optical Properties
Abstract
1. Introduction
2. Experimental Part
2.1. Synthesis of 1
2.2. Synthesis of 2
2.3. Synthesis of 3
2.4. Synthesis of 4
2.5. X-ray Diffractometry
2.6. Powder X-ray Diffractometry (PXRD),
2.7. Raman Spectroscopy, Thermogravimetric Analysis, Difuse Reflectance Spectroscopy
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Petrov, A.A.; Fateev, S.A.; Zubavichus, Y.V.; Dorovatovskii, P.V.; Khrustalev, V.N.; Zvereva, I.A.; Petrov, A.V.; Goodilin, E.A.; Tarasov, A.B. Methylammonium Polyiodides: Remarkable Phase Diversity of the Simplest and Low-Melting Alkylammonium Polyiodide System. J. Phys. Chem. Lett. 2019, 10, 5776–5780. [Google Scholar] [CrossRef] [PubMed]
- Frolova, L.A.; Anokhin, D.V.; Piryazev, A.A.; Luchkin, S.Y.; Dremova, N.N.; Troshin, P.A. Exploring the Photovoltaic Performance of All-Inorganic Ag2PbI4/PbI2 Blends. J. Phys. Chem. Lett. 2017, 8, 1651–1656. [Google Scholar] [CrossRef] [PubMed]
- Frolova, L.A.; Gutsev, L.G.; Ramachandran, B.R.; Dremova, N.N.; Aldoshin, S.M.; Troshin, P.A. Exploring CsPbI3–FAI Alloys: Introducing Low-Dimensional Cs2FAPb2I7 Absorber for Efficient and Stable Perovskite Solar Cells. Chem. Eng. J. 2021, 426, 131754. [Google Scholar] [CrossRef]
- Hu, Z.-T.; Shu, X.; Wang, X.; Li, Y.; Xu, R.; Hong, F.; Ma, Z.-Q.; Jiang, Z.-M.; Xu, F. Air-Stable CsPbIBr2 Photodetector via Dual-Ligand-Assisted Solution Strategy. Wuli Xuebao/Acta Phys. Sin. 2022, 71, 116801. [Google Scholar] [CrossRef]
- Udalova, N.N.; Tutantsev, A.S.; Fateev, S.A.; Zharenova, E.A.; Belich, N.A.; Nemygina, E.M.; Ryabova, A.V.; Goodilin, E.A.; Tarasov, A.B. Crystallization Features of MAPbI3 Hybrid Perovskite during the Reaction of PbI2 with Reactive Polyiodide Melts. Russ. J. Inorg. Chem. 2021, 66, 153–162. [Google Scholar] [CrossRef]
- Minbashi, M.; Yazdani, E. Comprehensive Study of Anomalous Hysteresis Behavior in Perovskite-Based Solar Cells. Sci. Rep. 2022, 12, 14916. [Google Scholar] [CrossRef]
- Petrov, A.A.; Fateev, S.A.; Khrustalev, V.N.; Li, Y.; Dorovatovskii, P.V.; Zubavichus, Y.V.; Goodilin, E.A.; Tarasov, A.B. Formamidinium Haloplumbate Intermediates: The Missing Link in a Chain of Hybrid Perovskites Crystallization. Chem. Mater. 2020, 32, 7739–7745. [Google Scholar] [CrossRef]
- Petrov, A.A.; Marchenko, E.I.; Fateev, S.A.; Yumao, L.; Goodilin, E.A.; Tarasov, A.B. Solvate Phases Crystallizing from Hybrid Halide Perovskite Solutions: Chemical Classification and Structural Relations. Mendeleev Commun. 2022, 32, 311–314. [Google Scholar] [CrossRef]
- Fateev, S.A.; Stepanov, N.M.; Petrov, A.A.; Goodilin, E.A.; Tarasov, A.B. Successive Solution–Liquid–Vapor Conversion of Metallic Lead Films for Highly Efficient Perovskite Solar Cells. Russ. J. Inorg. Chem. 2022, 67, 992–996. [Google Scholar] [CrossRef]
- Saliba, M.; Matsui, T.; Seo, J.-Y.; Domanski, K.; Correa-Baena, J.-P.; Nazeeruddin, M.K.; Zakeeruddin, S.M.; Tress, W.; Abate, A.; Hagfeldt, A.; et al. Cesium-Containing Triple Cation Perovskite Solar Cells: Improved Stability, Reproducibility and High Efficiency. Energy Environ. Sci. 2016, 9, 1989–1997. [Google Scholar] [CrossRef]
- Li, H.; Liu, Z.; Chen, Z.; Tan, S.; Zhao, W.; Li, Y.; Shi, J.; Wu, H.; Luo, Y.; Li, D.; et al. Reconfiguring Perovskite Interface via R4NBr Addition Reaction toward Efficient and Stable FAPbI3Based Solar Cells. Sci. China Chem. 2022, 65, 1185–1195. [Google Scholar] [CrossRef]
- Zhu, Z.; Shang, J.; Tang, G.; Wang, Z.; Cui, X.; Jin, J.; Zhou, Y.; Zhang, X.; Zhang, D.; Liu, X.; et al. Vertical Distribution of PbI2 Nanosheets for Robust Air-Processed Perovskite Solar Cells. Chem. Eng. J. 2023, 454, 140163. [Google Scholar] [CrossRef]
- Belich, N.A.; Tychinina, A.S.; Kuznetsov, V.V.; Goodilin, E.A.; Grätzel, M.; Tarasov, A.B. Template Synthesis of Methylammonium Lead Iodide in the Matrix of Anodic Titanium Dioxide via the Direct Conversion of Electrodeposited Elemental Lead. Mendeleev Commun. 2018, 28, 487–489. [Google Scholar] [CrossRef]
- Minns, J.L.; Zajdel, P.; Chernyshov, D.; van Beek, W.; Green, M.A. Structure and Interstitial Iodide Migration in Hybrid Perovskite Methylammonium Lead Iodide. Nat. Commun. 2017, 8, 15152. [Google Scholar] [CrossRef]
- Chen, H.; Ma, Y.; Wang, X.; Yao, G.; Du, Y.; Zhou, J.; Zhu, L.; Zhao, X.; Yang, S.; Liu, X.; et al. Improving the Stability and Efficiency of Inorganic CsPbI2Br Perovskite via Surface Reconstruction Strategy. Chem. Eng. J. 2022, 442, 136242. [Google Scholar] [CrossRef]
- Ganose, A.M.; Savory, C.N.; Scanlon, D.O. Beyond Methylammonium Lead Iodide: Prospects for the Emergent Field of Ns2 Containing Solar Absorbers. Chem. Commun. 2017, 53, 20–44. [Google Scholar] [CrossRef]
- Shestimerova, T.A.; Golubev, N.A.; Yelavik, N.A.; Bykov, M.A.; Grigorieva, A.V.; Wei, Z.; Dikarev, E.V.; Shevelkov, A.V. Role of I2 Molecules and Weak Interactions in Supramolecular Assembling of Pseudo-Three-Dimensional Hybrid Bismuth Polyiodides: Synthesis, Structure, and Optical Properties of Phenylenediammonium Polyiodobismuthate(III). Cryst. Growth Des. 2018, 18, 2572–2578. [Google Scholar] [CrossRef]
- Mezentsev-Cherkes, I.A.; Shestimerova, T.A.; Medved’ko, A.V.; Kalinin, M.A.; Kuznetsov, A.N.; Wei, Z.; Dikarev, E.V.; Vatsadze, S.Z.; Shevelkov, A.V. Synthesis and Supramolecular Organization of the Iodide and Triiodides of a Polycyclic Adamantane-Based Diammonium Cation: The Effects of Hydrogen Bonds and Weak I⋯I Interactions. CrystEngComm 2021, 23, 2384–2395. [Google Scholar] [CrossRef]
- Shestimerova, T.A.; Yelavik, N.A.; Mironov, A.V.; Kuznetsov, A.N.; Bykov, M.A.; Grigorieva, A.V.; Utochnikova, V.V.; Lepnev, L.S.; Shevelkov, A.V. From Isolated Anions to Polymer Structures through Linking with I2: Synthesis, Structure, and Properties of Two Complex Bismuth(III) Iodine Iodides. Inorg. Chem. 2018, 57, 4077–4087. [Google Scholar] [CrossRef]
- Adonin, S.A.; Udalova, L.I.; Abramov, P.A.; Novikov, A.S.; Yushina, I.V.; Korolkov, I.V.; Semitut, E.Y.; Derzhavskaya, T.A.; Stevenson, K.J.; Troshin, P.A.; et al. A Novel Family of Polyiodo-Bromoantimonate(III) Complexes: Cation-Driven Self-Assembly of Photoconductive Metal-Polyhalide Frameworks. Chem. Eur. J. 2018, 24, 14707–14711. [Google Scholar] [CrossRef]
- Novikov, A.V.; Usoltsev, A.N.; Adonin, S.A.; Bardin, A.A.; Samsonenko, D.G.; Shilov, G.V.; Sokolov, M.N.; Stevenson, K.J.; Aldoshin, S.M.; Fedin, V.P.; et al. Tellurium Complex Polyhalides: Narrow Bandgap Photoactive Materials for Electronic Applications. J. Mater. Chem. A 2020, 8, 21988–21992. [Google Scholar] [CrossRef]
- Usoltsev, A.N.; Korobeynikov, N.A.; Novikov, A.S.; Plyusnin, P.E.; Kolesov, B.A.; Fedin, V.P.; Sokolov, M.N.; Adonin, S.A. One-Dimensional Diiodine–Iodobismuthate(III) Hybrids Cat3{[Bi2I9](I2)3}: Syntheses, Stability, and Optical Properties. Inorg. Chem. 2020, 59, 17320–17325. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT—Integrated Space-Group and Crystal-Structure Determination. Acta Crystallogr. Sect. A Found. Adv. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal Structure Refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Hübschle, C.B.; Sheldrick, G.M.; Dittrich, B. ShelXle: A Qt Graphical User Interface for SHELXL. J. Appl. Crystallogr. 2011, 44, 1281–1284. [Google Scholar] [CrossRef]
- Sharutin, V.V.; Yegorova, I.V.; Klepikov, N.N.; Boyarkina, E.A.; Sharutina, O.K. Synthesis and Structure of Bismuth Complexes [Ph3MeP]6+[BiI3Br3]3−[Bi2I6Br3]3−·H2O2, [Ph3EtP]3+[Bi2I9]3−, [Ph3MeP]3+ [Bi3I12]3−, [Ph3(Iso-Pr)P]3+[Bi3I12]3−·2Me2C=O, and [Ph4Bi]3+[Bi5I18]3−. Russ. J. Inorg. Chem. 2009, 54, 52–68. [Google Scholar] [CrossRef]
- Chai, W.-X.; Lin, J.; Song, L.; Qin, L.-S.; Shi, H.-S.; Guo, J.-Y.; Shu, K.-Y. Three Iodometalate Organic-Inorganic Hybrid Materials Based on Methylene Blue Cation: Syntheses, Structures, Properties and DFT Calculations. Solid State Sci. 2012, 14, 1226–1232. [Google Scholar] [CrossRef]
- Kelly, A.W.; Nicholas, A.; Ahern, J.C.; Chan, B.; Patterson, H.H.; Pike, R.D. Alkali Metal Bismuth(III) Chloride Double Salts. J. Alloys Compd. 2016, 670, 337–345. [Google Scholar] [CrossRef]
- Ahern, J.C.; Nicholas, A.D.; Kelly, A.W.; Chan, B.; Pike, R.D.; Patterson, H.H. A Terbium Chlorobismuthate(III) Double Salt: Synthesis, Structure, and Photophysical Properties. Inorg. Chim. Acta 2018, 478, 71–76. [Google Scholar] [CrossRef]
- Adonin, S.A.; Sokolov, M.N.; Fedin, V.P. Polynuclear Halide Complexes of Bi(III): From Structural Diversity to the New Properties. Coord. Chem. Rev. 2016, 312, 1–21. [Google Scholar] [CrossRef]
- Bondi, A. Van Der Waals Volumes and Radii of Metals in Covalent Compounds. J. Phys. Chem. 1966, 70, 3006–3007. [Google Scholar] [CrossRef]
- Hubbard, C.R.; Jacobson, R.A. Molecular Bromine Bridging of SbIII2Br93− Anions and the Crystal Structure of Tetraethylammonium Nonabromodiantimonate(III)-Dibromine. Inorg. Chem. 1972, 11, 2247–2250. [Google Scholar] [CrossRef]
- Adonin, S.A.; Gorokh, I.D.; Samsonenko, D.G.; Sokolov, M.N.; Fedin, V.P. Bi(Iii) Polybromides: A New Chapter in Coordination Chemistry of Bismuth. Chem. Commun. 2016, 52, 5061–5063. [Google Scholar] [CrossRef]
- Adonin, S.A.; Usoltsev, A.N.; Novikov, A.S.; Kolesov, B.A.; Fedin, V.P.; Sokolov, M.N. One- and two- Dimensional iodine-richIodobismuthate(III) complexes: Structure, Optical Properties and features of halogen bonding in the solid state. Inorg. Chem. 2020, 59, 3290–3296. [Google Scholar] [CrossRef]


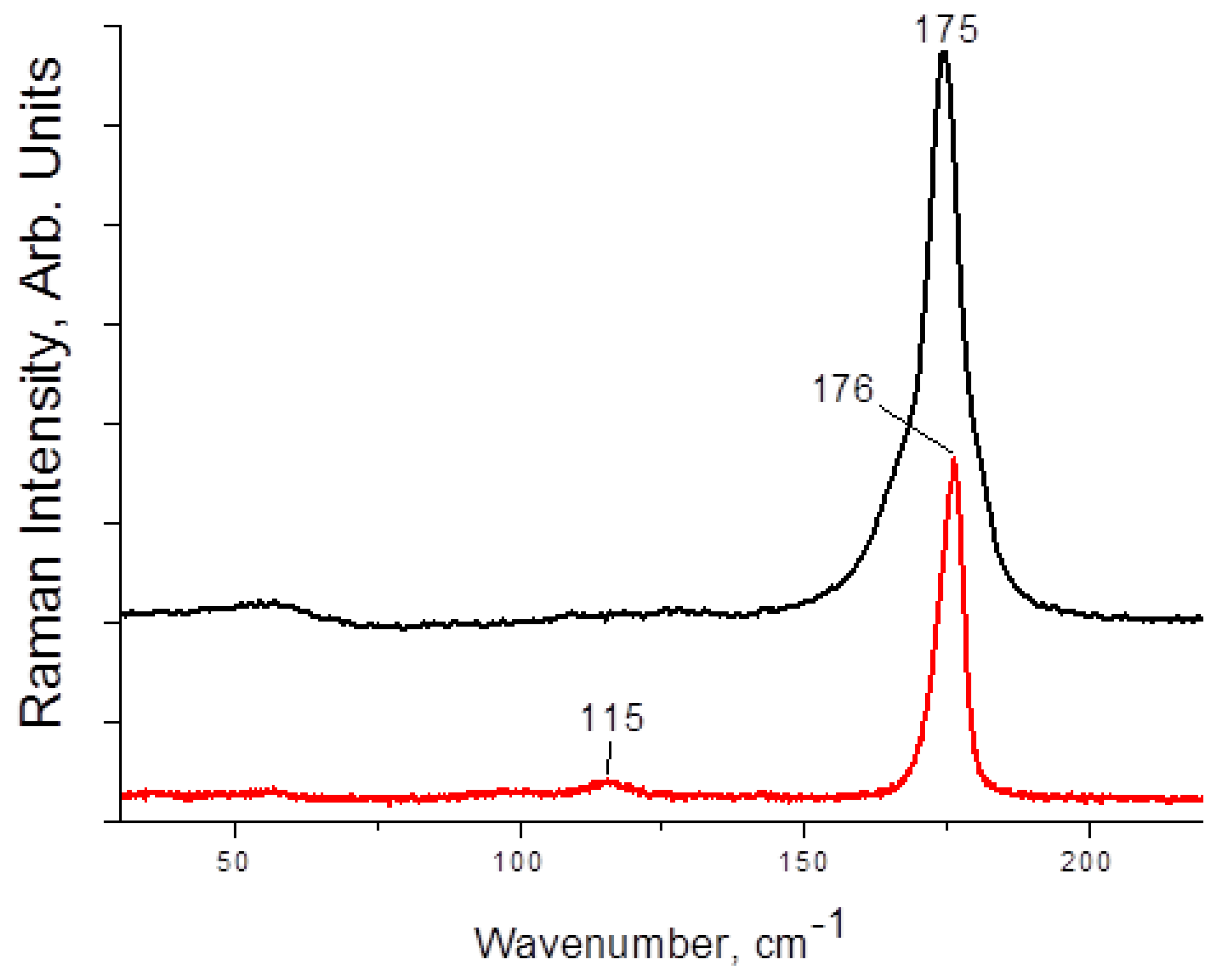
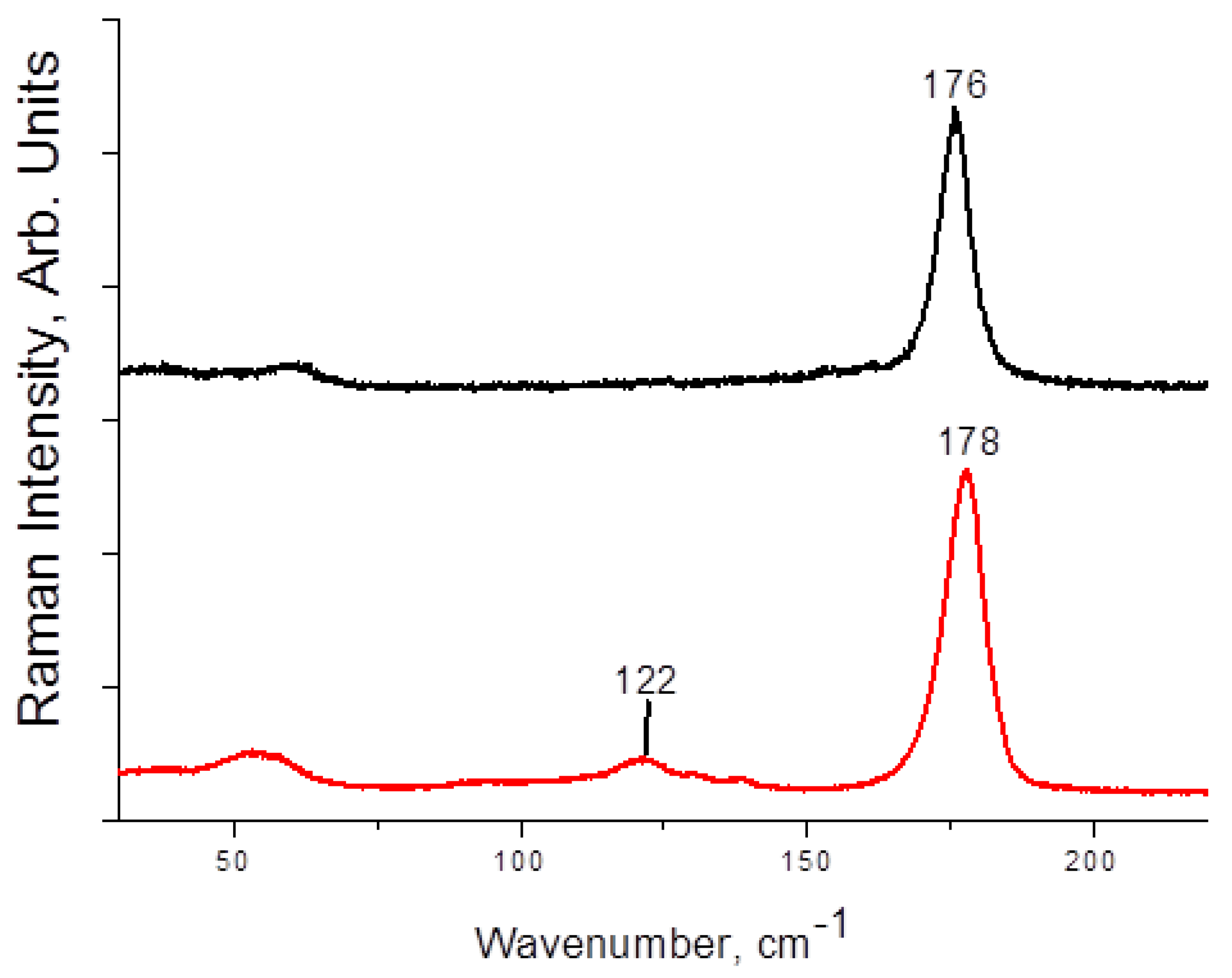

| 1 | 3 | 4 | |
|---|---|---|---|
| M-Iterm | 2.820–2.950 | 2.830–2.935 | 2.909–2.998 |
| M-μ2-I | 3.088–3.329 | 3.154–3.281 | 3.207–3.306 |
| Iterm···II2, Å | 3.259 | 3.415 | 3.410 |
| I-I (in I2), Å | 2.743–2.746 | 2.731 | 2.725 |
| M-Iterm-Ii2,° | 165.67–168.67 | 137.79 | 136.63 |
| Iterm-Ii2-Ii2,° | 175.48–177.55 | 170.20 | 168.26 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Korobeynikov, N.A.; Usoltsev, A.N.; Abramov, P.A.; Sokolov, M.N.; Adonin, S.A. One-Dimensional Iodoantimonate(III) and Iodobismuthate(III) Supramolecular Hybrids with Diiodine: Structural Features, Stability and Optical Properties. Molecules 2022, 27, 8487. https://doi.org/10.3390/molecules27238487
Korobeynikov NA, Usoltsev AN, Abramov PA, Sokolov MN, Adonin SA. One-Dimensional Iodoantimonate(III) and Iodobismuthate(III) Supramolecular Hybrids with Diiodine: Structural Features, Stability and Optical Properties. Molecules. 2022; 27(23):8487. https://doi.org/10.3390/molecules27238487
Chicago/Turabian StyleKorobeynikov, Nikita A., Andrey N. Usoltsev, Pavel A. Abramov, Maxim N. Sokolov, and Sergey A. Adonin. 2022. "One-Dimensional Iodoantimonate(III) and Iodobismuthate(III) Supramolecular Hybrids with Diiodine: Structural Features, Stability and Optical Properties" Molecules 27, no. 23: 8487. https://doi.org/10.3390/molecules27238487
APA StyleKorobeynikov, N. A., Usoltsev, A. N., Abramov, P. A., Sokolov, M. N., & Adonin, S. A. (2022). One-Dimensional Iodoantimonate(III) and Iodobismuthate(III) Supramolecular Hybrids with Diiodine: Structural Features, Stability and Optical Properties. Molecules, 27(23), 8487. https://doi.org/10.3390/molecules27238487








