Cholinesterases Inhibition, Anticancer and Antioxidant Activity of Novel Benzoxazole and Naphthoxazole Analogs
Abstract
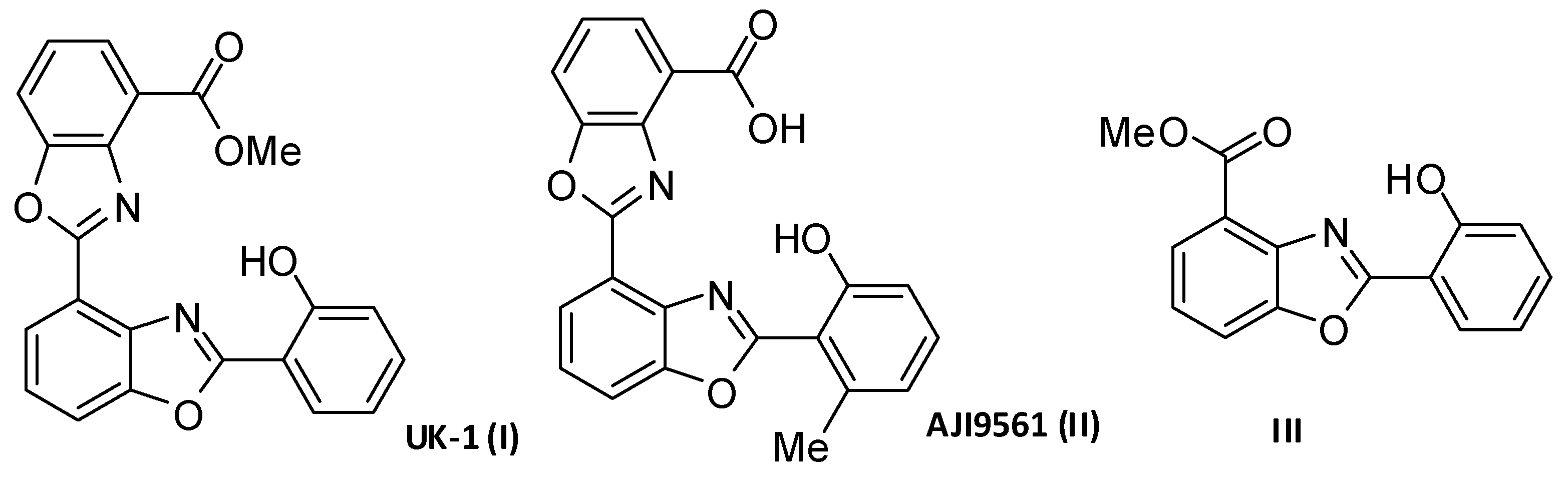
1. Introduction
2. Results
2.1. Chemistry
2.2. Biological Evaluation
3. Discussion
4. Materials and Methods
4.1. Chemistry
4.1.1. General
4.1.2. Synthesis of the Compounds
4.1.3. Chromatography
4.1.4. Computational Methods
4.2. Biological Assays
4.2.1. SRB Cytotoxicity Assay
4.2.2. Cytotoxicity Assessment
Cell Culture and Treatments
LDH Assay
4.2.3. In Vitro AChE and Bche Inhibition Assay
4.2.4. Kinetic Study of AChE Inhibition Assay
4.2.5. DPPH Free Radical Scavenging Activity
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Rodriguez, A.D.; Ramirez, C.; Rodriguez, I.I.; Gonzalez, E. Novel antimycobacterial benzoxazole alkaloids, from the west Indian Sea whip Pseudopterogorgia elisabethae. Org. Lett. 1999, 1, 527–530. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, P.; Tripathi, P.N.; Sharma, P.; Rai, S.N.; Singh, S.; Srivastava, R.K.; Shankar, S.; Shrivastava, S.K. Design and development of some phenyl benzoxazole derivatives as a potent acetylcholinesterase inhibitor with antioxidant property to enhance learning and memory. Eur. J. Med. Chem. 2019, 163, 116–135. [Google Scholar] [CrossRef] [PubMed]
- Luo, B.; Li, D.; Zhang, A.-L.; Gao, J.-M. Synthesis, Antifungal Activities and Molecular Docking Studies of Benzoxazole and Benzothiazole Derivatives. Molecules 2018, 23, 2457. [Google Scholar] [CrossRef] [PubMed]
- Susithra, E.; Rajkumar, S.; Pansare, S.K.W.; Praveena, S.; Arun, P.P.S.; Chekkara, R.; Kiran, G. Design, synthesis, antimicrobial and anticancer activity of some novel benzoxazole-isatin conjugates. Biointerface Res. Appl. Chem. 2022, 12, 2392–2403. [Google Scholar] [CrossRef]
- Gualtiere, F.; Brody, G.; Fieldsteel, A.H.; Skinner, W.A. Antiviral Agents .1. Benzothiazole and Benzoxazole Analogs of 2-(Alpha-Hydroxybenzyl)Benzimidazole. J. Med. Chem. 1971, 14, 546–549. [Google Scholar]
- Xiang, P.; Zhou, T.; Wang, L.; Sun, C.-Y.; Hu, J.; Zhao, Y.-L.; Yang, L. Novel Benzothiazole, Benzimidazole and Benzoxazole Derivatives as Potential Antitumor Agents: Synthesis and Preliminary In Vitro Biological Evaluation. Molecules 2012, 17, 873–883. [Google Scholar] [CrossRef] [PubMed]
- Afzal, O.; Altamimi, A.; Shahroz, M.; Sharma, H.; Riadi, Y.; Hassan, Q. Analgesic and Anticancer Activity of Benzoxazole Clubbed 2-Pyrrolidinones as Novel Inhibitors of Monoacylglycerol Lipase. Molecules 2021, 26, 2389. [Google Scholar] [CrossRef] [PubMed]
- McKee, M.L.; Kerwin, S.M. Synthesis, metal ion binding, and biological evaluation of new anticancer 2-(2′-hydroxyphenyl)benzoxazole analogs of UK-1. Bioorganic Med. Chem. 2008, 16, 1775–1783. [Google Scholar] [CrossRef] [PubMed]
- Sato, S.; Kajiura, T.; Noguchi, M.; Takehana, K.; Kobayashi, T.; Tsuji, T. AJI9561, a New Cytotoxic Benzoxazole Derivative Produced by Streptomyces sp. J. Antibiot. 2001, 54, 102–104. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.-T.; Hsei, I.-J.; Chen, C. Synthesis and anticancer evaluation of bis(benzimidazoles), bis(benzoxazoles), and benzothiazoles. Bioorganic Med. Chem. 2006, 14, 6106–6119. [Google Scholar] [CrossRef]
- Kumar, D.; Jacob, M.R.; Reynolds, M.B.; Kerwin, S.M. Synthesis and evaluation of anticancer benzoxazoles and benzimidazoles related to UK-1. Bioorganic Med. Chem. 2002, 10, 3997–4004. [Google Scholar] [CrossRef]
- Khajondetchairit, P.; Phuangsawai, O.; Suphakun, P.; Rattanabunyong, S.; Choowongkomon, K.; Gleeson, M.P. Design, synthesis, and evaluation of the anticancer activity of 2-amino-aryl-7-aryl-benzoxazole compounds. Chem. Biol. Drug Des. 2017, 90, 987–994. [Google Scholar] [CrossRef]
- Ghoshal, T.; Patel, T.M. Anticancer activity of benzoxazole derivative (2015 onwards): A review. Futur. J. Pharm. Sci. 2020, 6, 94. [Google Scholar] [CrossRef]
- Osmaniye, D.; Çelikateş, B.K.; Sağlık, B.N.; Levent, S.; Çevik, U.A.; Çavuşoğlu, B.K.; Ilgın, S.; Özkay, Y.; Kaplancıklı, Z.A. Synthesis of some new benzoxazole derivatives and investigation of their anticancer activities. Eur. J. Med. Chem. 2021, 210, 112979. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.B.; Maghami, N.; Goodlin, V.L.; Smith, P.J. Critical structural motif for the catalytic inhibition of human topoisomerase II by UK-1 and analogs. Bioorganic Med. Chem. Lett. 2004, 14, 3221–3226. [Google Scholar] [CrossRef] [PubMed]
- An, Y.; Lee, E.; Yu, Y.; Yun, J.; Lee, M.Y.; Kang, J.S.; Kim, W.-Y.; Jeon, R. Design and synthesis of novel benzoxazole analogs as Aurora B kinase inhibitors. Bioorganic Med. Chem. Lett. 2016, 26, 3067–3072. [Google Scholar] [CrossRef]
- Pissot-Soldermann, C.; Gerspacher, M.; Furet, P.; Gaul, C.; Holzer, P.; McCarthy, C.; Radimerski, T.; Regnier, C.H.; Baffert, F.; Drueckes, P.; et al. Discovery and SAR of potent, orally available 2,8-diaryl-quinoxalines as a new class of JAK2 inhibitors. Bioorganic Med. Chem. Lett. 2010, 20, 2609–2613. [Google Scholar] [CrossRef] [PubMed]
- Abdelgawad, M.A.; Bakr, R.; Omar, H. Design, synthesis and biological evaluation of some novel benzothiazole/benzoxazole and/or benzimidazole derivatives incorporating a pyrazole scaffold as antiproliferative agents. Bioorganic Chem. 2017, 74, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Temiz-Arpaci, O.; Arisoy, M.; Sac, D.; Doganc, F.; Tasci, M.; Senol, F.S.; Orhan, I.E. Biological evaluation and docking studies of some benzoxazole derivatives as inhibitors of acetylcholinesterase and butyrylcholinesterase. Z. Nat. C 2016, 71, 409–413. [Google Scholar] [CrossRef]
- Celik, I.; Erol, M.; Arpaci, O.T.; Senol, F.S.; Orhan, I.E. Evaluation of Activity of Some 2,5-Disubstituted Benzoxazole Derivatives against Acetylcholinesterase, Butyrylcholinesterase and Tyrosinase: ADME Prediction, DFT and Comparative Molecular Docking Studies. Polycycl. Aromat. Compd. 2022, 42, 412–423. [Google Scholar] [CrossRef]
- Shrivastava, S.K.; Sinha, S.K.; Srivastava, P.; Tripathi, P.N.; Sharma, P.; Tripathi, M.K.; Tripathi, A.; Choubey, P.K.; Waiker, D.K.; Aggarwal, L.M.; et al. Design and development of novel p-aminobenzoic acid derivatives as potential cholinesterase inhibitors for the treatment of Alzheimer’s disease. Bioorganic Chem. 2019, 82, 211–223. [Google Scholar] [CrossRef] [PubMed]
- Pouramiri, B.; Moghimi, S.; Mahdavi, M.; Nadri, H.; Moradi, A.; Tavakolinejad-Kermani, E.; Firoozpour, L.; Asadipour, A.; Foroumadi, A. Synthesis and anticholinesterase activity of new substituted benzo[d ]oxazole-based derivatives. Chem. Biol. Drug Des. 2017, 89, 783–789. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.-R.; Ren, S.-T.; Wang, L.; Wang, Y.-X.; Liu, S.-H.; Liu, W.-W.; Shi, D.-H.; Cao, Z.-L. Synthesis and anticholinesterase activities of novel glycosyl benzoxazole derivatives. J. Chem. Res. 2020, 44, 363–366. [Google Scholar] [CrossRef]
- Oglah, M.K.; Mustafa, Y.F. Curcumin analogs: Synthesis and biological activities. Med. Chem. Res. 2020, 29, 479–486. [Google Scholar] [CrossRef]
- Sharma, K. Cholinesterase inhibitors as Alzheimer’s therapeutics (Review). Mol. Med. Rep. 2019, 20, 1479–1487. [Google Scholar] [CrossRef] [PubMed]
- Hussain, H.; Ahmad, S.; Shah, S.W.A.; Ullah, A.; Ali, N.; Almehmadi, M.; Ahmad, M.; Khalil, A.A.K.; Jamal, S.B.; Ahmad, H.; et al. Attenuation of Scopolamine-Induced Amnesia via Cholinergic Modulation in Mice by Synthetic Curcumin Analogs. Molecules 2022, 27, 2468. [Google Scholar] [CrossRef] [PubMed]
- Hussain, H.; Ahmad, S.; Shah, S.W.A.; Ghias, M.; Ullah, A.; Rahman, S.U.; Kamal, Z.; Khan, F.A.; Khan, N.M.; Muhammad, J.; et al. Neuroprotective Potential of Synthetic Mono-Carbonyl Curcumin Analogs Assessed by Molecular Docking Studies. Molecules 2021, 26, 7168. [Google Scholar] [CrossRef] [PubMed]
- Okereke, O.I.; Meadows, M.-E. More Evidence of an Inverse Association Between Cancer and Alzheimer Disease. JAMA Netw. Open 2019, 2, e196167. [Google Scholar] [CrossRef] [PubMed]
- Jończyk, J.; Godyń, J.; Stawarska, E.; Morak-Młodawska, B.; Jeleń, M.; Pluta, K.; Malawska, B. Dual Action of Dipyridothiazine and Quinobenzothiazine Derivatives—Anticancer and Cholinesterase-Inhibiting Activity. Molecules 2020, 25, 2604. [Google Scholar] [CrossRef]
- Wang, X.-Z.; Jiang, G.-B.; Xie, Y.-Y.; Liu, Y.-J. Synthesis, molecular structure, DNA interaction and antioxidant activity of novel naphthoxazole compound. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 118, 448–453. [Google Scholar] [CrossRef] [PubMed]
- Tseng, C.-H.; Lin, C.-K.; Chen, Y.-L.; Tseng, C.-K.; Lee, J.-Y.; Lee, J.-C. Discovery of naphtho[1,2-d]oxazole derivatives as potential anti-HCV agents through inducing heme oxygenase-1 expression. Eur. J. Med. Chem. 2018, 143, 970–982. [Google Scholar] [CrossRef] [PubMed]
- Pottorf, R.S.; Chadha, N.K.; Katkevics, M.; Ozola, V.; Suna, E.; Ghane, H.; Regberg, T.; Player, M.R. Parallel synthesis of benzoxazoles via microwave-assisted dielectric heating. Tetrahedron Lett. 2003, 44, 175–178. [Google Scholar] [CrossRef]
- Evindar, G.; Batey, R.A. Parallel Synthesis of a Library of Benzoxazoles and Benzothiazoles Using Ligand-Accelerated Copper-Catalyzed Cyclizations of ortho-Halobenzanilides. J. Org. Chem. 2006, 71, 1802–1808. [Google Scholar] [CrossRef]
- Matysiak, J.; Niewiadomy, A. Application of Sulfinyl bis(2,4-dihydroxythiobenzoyl) in the Synthesis of N-Substituted 2-Amino-5-(2,4-dihydroxyphenyl)-1,3,4-thiadiazoles. Synth. Commun. 2006, 36, 1621–1630. [Google Scholar] [CrossRef]
- Roldán-Peña, J.M.; Alejandre-Ramos, D.; López, Ó.; Maya, I.; Lagunes, I.; Padrón, J.M.; Peña-Altamira, L.E.; Bartolini, M.; Monti, B.; Bolognesi, M.L.; et al. New tacrine dimers with antioxidant linkers as dual drugs: Anti-Alzheimer’s and antiproliferative agents. Eur. J. Med. Chem. 2017, 138, 761–773. [Google Scholar] [CrossRef] [PubMed]
- Karpińka, M.M.; Matysiak, J.; Niewiadomy, A. Synthesis of novel 4-(1H-benzimidazol-2-yl)benzene-1,3-diols and their cytotoxic activity against human cancer cell lines. Arch. Pharmacal Res. 2011, 34, 1639–1647. [Google Scholar] [CrossRef]
- Matysiak, J.; Opolski, A. Synthesis and antiproliferative activity of N-substituted 2-amino-5-(2,4-dihydroxyphenyl)-1,3,4-thiadiazoles. Bioorganic Med. Chem. 2006, 14, 4483–4489. [Google Scholar] [CrossRef] [PubMed]
- Rzeski, W.; Matysiak, J.; Kandefer-Szerszeń, M. Anticancer, neuroprotective activities and computational studies of 2-amino-1,3,4-thiadiazole based compound. Bioorganic Med. Chem. 2007, 15, 3201–3207. [Google Scholar] [CrossRef] [PubMed]
- Skrzypek, A.; Matysiak, J.; Karpińska, M.; Czarnecka, K.; Kręcisz, P.; Stary, D.; Kukułowicz, J.; Paw, B.; Bajda, M.; Szymański, P.; et al. Biological evaluation and molecular docking of novel 1,3,4-thiadiazole-resorcinol conjugates as multifunctional cholinesterases inhibitors. Bioorganic Chem. 2021, 107, 104617. [Google Scholar] [CrossRef]
- Skrzypek, A.; Matysiak, J.; Karpińska, M.M.; Niewiadomy, A. Synthesis and anticholinesterase activities of novel 1,3,4-thiadiazole based compounds. J. Enzym. Inhib. Med. Chem. 2013, 28, 816–823. [Google Scholar] [CrossRef] [PubMed]
- Matysiak, J.; Skrzypek, A.; Karpińska, M.; Czarnecka, K.; Szymański, P.; Bajda, M.; Niewiadomy, A. Biological Evaluation, Molecular Docking, and SAR Studies of Novel 2-(2,4-Dihydroxyphenyl)-1H- Benzimidazole Analogues. Biomolecules 2019, 9, 870. [Google Scholar] [CrossRef] [PubMed]
- Skrzypek, A.; Matysiak, J.; Niewiadomy, A.; Bajda, M.; Szymański, P. Synthesis and biological evaluation of 1,3,4-thiadiazole analogues as novel AChE and BuChE inhibitors. Eur. J. Med. Chem. 2013, 62, 311–319. [Google Scholar] [CrossRef] [PubMed]
- Burk, D.; Lineweaver, H.; Horner, C.K. The Specific Influence of Acidity on the Mechanism of Nitrogen Fixation by Azotobacter. J. Bacteriol. 1934, 27, 325–340. [Google Scholar] [CrossRef] [PubMed]
- Cornish-Bowden, A. Fundamentals of Enzyme Kinetics, 3rd ed.; Portland Press Ltd.: London, UK, 2004; pp. 422–428. [Google Scholar]
- Chen, Z.; Bertin, R.; Froldi, G. EC50 estimation of antioxidant activity in DPPH assay using several statistical programs. Food Chem. 2013, 138, 414–420. [Google Scholar] [CrossRef]
- Valko, K. Measurements of lipophilicity and acid/base character using HPLC methods. Pharm. Profiling Drug Discov. Lead Selection. 2004, 1, 127–182. [Google Scholar]
- Hollósy, F.; Valkó, K.; Hersey, A.; Nunhuck, S.; Kéri, G.; Bevan, C. Estimation of Volume of Distribution in Humans from High Throughput HPLC-Based Measurements of Human Serum Albumin Binding and Immobilized Artificial Membrane Partitioning. J. Med. Chem. 2006, 49, 6958–6971. [Google Scholar] [CrossRef] [PubMed]
- Matysiak, J.; Niewiadomy, A.; Macik-Niewiadomy, G.; Kornillowicz, T. Dependence of fungistatic activity of 2,4-dihydroxythiobenzanilideson the structure and lipophilic nature of the compounds. Eur. J. Med. Chem. 2000, 35, 393–404. [Google Scholar] [CrossRef]
- Janicka, M.; Kwietniewski, L.; Matysiak, J. A new method for estimating log k(w) values and solute biological activity. J. Planar Chromatogr.-Mod. TLC 2000, 13, 285–289. [Google Scholar]
- Ghose, A.K.; Crippen, G.M. Atomic physicochemical parameters for three-dimensional-structure-directed quantitative structure-activity relationships. 2. Modeling dispersive and hydrophobic interactions. J. Chem. Inf. Comput. Sci. 1987, 27, 21–35. [Google Scholar] [CrossRef] [PubMed]
- Oprea, T.; Gottfries, J.; Sherbukhin, V.; Svensson, P.; Kühler, T.C. Chemical information management in drug discovery: Optimizing the computational and combinatorial chemistry interfaces. J. Mol. Graph. Model. 2000, 18, 512–524. [Google Scholar] [CrossRef] [PubMed]
- Giacobini, E. Cholinesterase inhibitors: New roles and therapeutic alternatives. Pharmacol. Res. 2004, 50, 433–440. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, W.; Li, L.; Perry, G.; Lee, H.-G.; Zhu, X. Oxidative stress and mitochondrial dysfunction in Alzheimer’s disease. Biochim. Biophys. BBA Mol. Basis Dis. 2014, 1842, 1240–1247. [Google Scholar] [CrossRef] [PubMed]
- Karpińska, M.M.; Matysiak, J.; Niewiadomy, A.; Wietrzyk, J.; Kłopotowska, D. Synthesis and biological activity of novel 4- and 6-(1-alkyl/aryl-1H-benzimidazol-2-yl)benzene-1,3-diols. Monatsh. Chem. 2012, 143, 269–276. [Google Scholar] [CrossRef]
- Niewiadomy, A.; Matysiak, J.; Karpińska, M.M. Synthesis and Anticancer Activity of New 2-Aryl-4H-3,1-benzothiazines. Arch. der Pharm. 2011, 344, 224–230. [Google Scholar] [CrossRef] [PubMed]
- Soczewiński, E.; Wachtmeister, C.A. The relation between the composition of certain ternary two-phase solvent systems and RM values. J. Chromatogr. A 1962, 7, 311–320. [Google Scholar] [CrossRef]
- ChemDraw Ultra 10.0.; CambridgeSoftCorporation: Cambridge, MA, USA, 2006.
- MedChem Designer(TM) 3.0.0.30; Simulations Plus, Inc.: Lancaster, CA, USA; pp. 2011–2014.
- Orellana, E.A.; Kasinski, A.L. Sulforhodamine B (SRB) Assay in Cell Culture to Investigate Cell Proliferation. Bio. Protoc. 2016, 6, e1984. [Google Scholar] [CrossRef] [PubMed]
- Nevozhay, D.N. Cheburator software for automatically calculating drug inhibitory concentrations from In Vitro screening assays. PLoS ONE 2014, 9, 106186–106196. [Google Scholar] [CrossRef] [PubMed]
- Wróblewska-Łuczka, P.; Cabaj, J.; Bąk, W.; Bargieł, J.; Grabarska, A.; Góralczyk, A.; Łuszczki, J.J. Additive Interactions between Betulinic Acid and Two Taxanes in In Vitro Tests against Four Human Malignant Melanoma Cell Lines. Int. J. Mol. Sci. 2022, 23, 9641. [Google Scholar] [CrossRef]
- Narkhede, H.I.; Dhake, A.S.; Surana, A.R. Synthesis and screening of thiosemicarbazide-dithiocarbamate conjugates for antioxidant and anticancer activities. Bioorganic Chem. 2022, 124, 105832. [Google Scholar] [CrossRef]




| Compound | AChE 1 IC50 [μM] | BChE 2 IC50 [μM] | Selectivity for AChE 3 | IC50 (DPPH) 4 [µM] |
|---|---|---|---|---|
| 3 | 0.113 ± 0.011 * | 1.132 ± 0.016 * | 10.017 | 0.286 ± 0.122 ** |
| 6 | 0.831 ± 0.014 | 0.328 ± 0.027 | 0.394 | 0.861 ± 0.071 |
| 7 | 0.183 ± 0.031 | 0.667 ± 0.004 | 3.644 | 0.887 ± 0.064 |
| 8 | 0.058 ± 0.010 | 0.981 ± 0.015 | 18.166 | 0.452 ± 0.031 |
| 9 | 0.516 ± 0.009 | 1.017 ± 0.032 | 1.971 | 0.214 ± 0.024 |
| 10 | 0.694 ± 0.011 | 0.887 ± 0.041 | 1.278 | 0.633 ± 0.146 |
| donepezil | 0.017 ± 0.002 | 1.159 ± 0.016 | 68.176 | - |
| quercetin | - | - | - | 0.032 ± 0.001 |
| Concentration [nM] | KM [nM] | Vmax [A/min] |
|---|---|---|
| 75 | 0.254 | 0.021 |
| 50 | 0.212 | 0.023 |
| 25 | 0.117 | 0.031 |
| 0 | 0.072 | 0.033 |
| Compound | Cell Line/IC50 1/[µM] | |||
|---|---|---|---|---|
| HCV 29T | A549 | T47D | SW 707 | |
| 1 | 33.93 ± 4.77 | - 2 | - 2 | - 2 |
| 2 | 8.34 ± 1.53 | 6.81 ± 2.23 | 5.28 ± 0.16 | 4.38 ± 1.57 |
| 3 | 6.43 ± 1.12 | 4.28 ± 0.55 | 3.37 ± 1.52 | 5.47 ± 2.16 |
| 4 | 4.64 ± 0.93 | 3.71 ± 0.40 | 3.20 ± 0.84 | 3.36 ± 0.84 |
| 5 | 3.54 ± 1.05 | 3.08 ± 0.26 | 4.11 ± 1.27 | 2.13 ± 0.49 |
| 6 | 7.54 ± 3.15 | 10.48 ± 5.61 | 6.61 ± 2.48 | 6.04 ± 0.89 |
| 7 | 23.33 ± 4.07 | - 2 | - 2 | - 2 |
| 8 | 2.50 ± 1.88 | 4.17 ± 0.19 | 2.86 ± 0.18 | 3.35 ± 0.16 |
| 9 | 3.72 ± 0.36 | 4.94 ± 0.69 | 7.28 ± 2.50 | 8.58 ± 3.40 |
| 10 | 2.24 ± 0.83 | 2.71 ± 0.12 | 2.18 ± 1.05 | 2.89 ± 0.34 |
| cisplatin | 2.40 ± 0.32 | 3.07 ± 0.45 | 4.01 ± 1.58 | 3.65 ± 0.76 |
| Compound | -S | log kw | r2 | n | Mlog P | log P 1 | Clog P |
|---|---|---|---|---|---|---|---|
| 1 | 4.358 | 3.273 | 0.994 | 7 | 1.778 | 2.26 | 2.1600 |
| 2 | 4.64 | 3.301 | 0.998 | 7 | 1.522 | 2.27 | 2.15 |
| 3 | 5.325 | 2.92 | 0.999 | 7 | 1.337 | 2.34 | 1.98 |
| 4 | 5.002 | 3.411 | 0.988 | 7 | 1.586 | 2.74 | 2.46 |
| 5 | 5.855 | 3.774 | 0.996 | 6 | 1.829 | 3.32 | 2.88 |
| 6 | 5.477 | 4.223 | 0.987 | 6 | 1.586 | 2.86 | 2.54 |
| 7 | 4.811 | 2.800 | 0.990 | 6 | 0.577 | 0.77 | 1.59 |
| 8 | 4.263 | 3.936 | 0.988 | 7 | 2.641 | 3.43 | 3.15 |
| 9 | 5.395 | 4.901 | 0.996 | 6 | 3.111 | 4.41 | 4.06 |
| 10 | 4.996 | 4.518 | 0.994 | 6 | 3.146 | 3.95 | 3.71 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Skrzypek, A.; Karpińska, M.; Juszczak, M.; Grabarska, A.; Wietrzyk, J.; Krajewska-Kułak, E.; Studziński, M.; Paszko, T.; Matysiak, J. Cholinesterases Inhibition, Anticancer and Antioxidant Activity of Novel Benzoxazole and Naphthoxazole Analogs. Molecules 2022, 27, 8511. https://doi.org/10.3390/molecules27238511
Skrzypek A, Karpińska M, Juszczak M, Grabarska A, Wietrzyk J, Krajewska-Kułak E, Studziński M, Paszko T, Matysiak J. Cholinesterases Inhibition, Anticancer and Antioxidant Activity of Novel Benzoxazole and Naphthoxazole Analogs. Molecules. 2022; 27(23):8511. https://doi.org/10.3390/molecules27238511
Chicago/Turabian StyleSkrzypek, Alicja, Monika Karpińska, Małgorzata Juszczak, Aneta Grabarska, Joanna Wietrzyk, Elżbieta Krajewska-Kułak, Marek Studziński, Tadeusz Paszko, and Joanna Matysiak. 2022. "Cholinesterases Inhibition, Anticancer and Antioxidant Activity of Novel Benzoxazole and Naphthoxazole Analogs" Molecules 27, no. 23: 8511. https://doi.org/10.3390/molecules27238511
APA StyleSkrzypek, A., Karpińska, M., Juszczak, M., Grabarska, A., Wietrzyk, J., Krajewska-Kułak, E., Studziński, M., Paszko, T., & Matysiak, J. (2022). Cholinesterases Inhibition, Anticancer and Antioxidant Activity of Novel Benzoxazole and Naphthoxazole Analogs. Molecules, 27(23), 8511. https://doi.org/10.3390/molecules27238511








