Quinoxalinones as A Novel Inhibitor Scaffold for EGFR (L858R/T790M/C797S) Tyrosine Kinase: Molecular Docking, Biological Evaluations, and Computational Insights
Abstract
1. Introduction
2. Results and Discussion
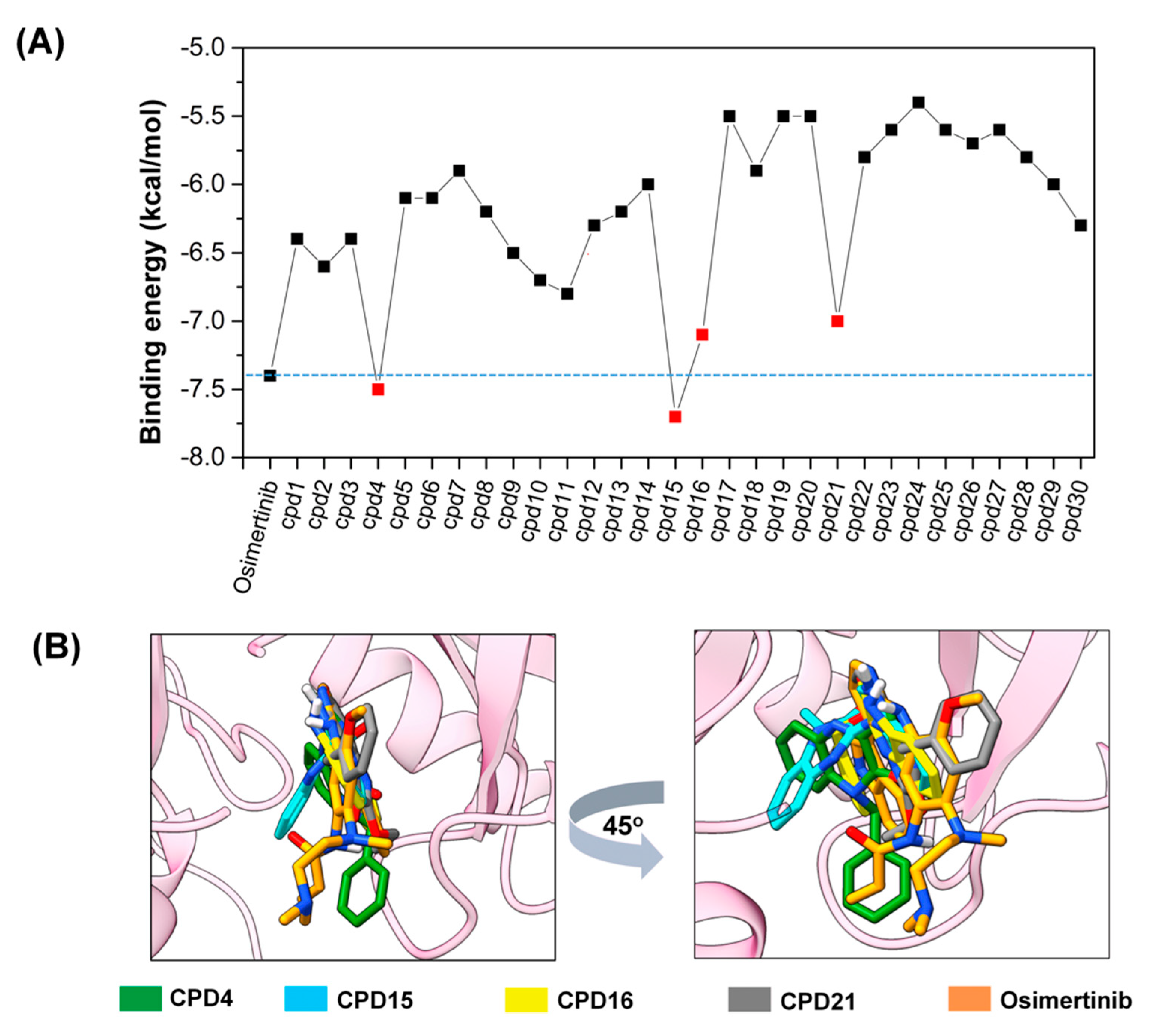
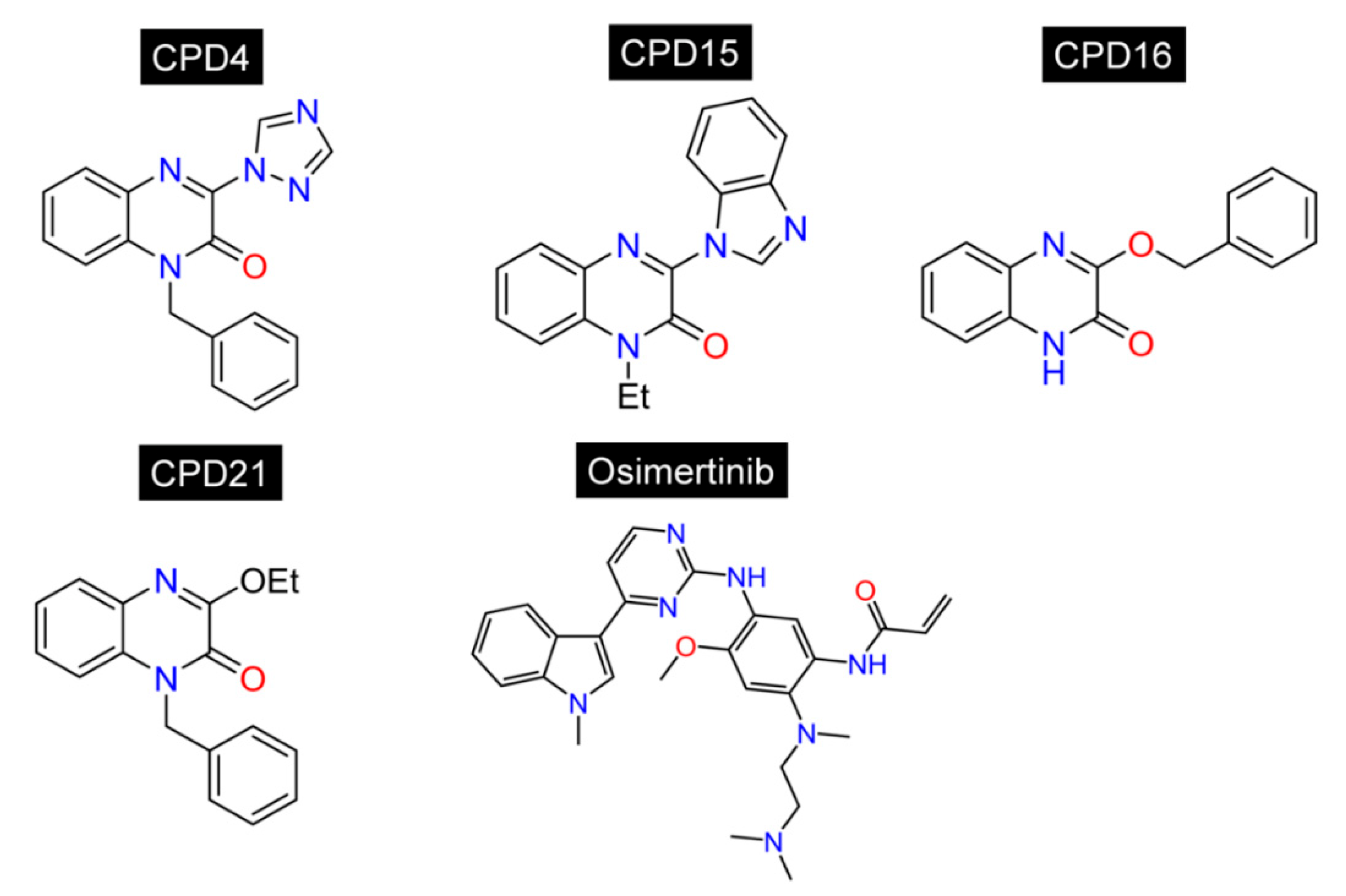
2.1. Docking-Based Virtual Screening
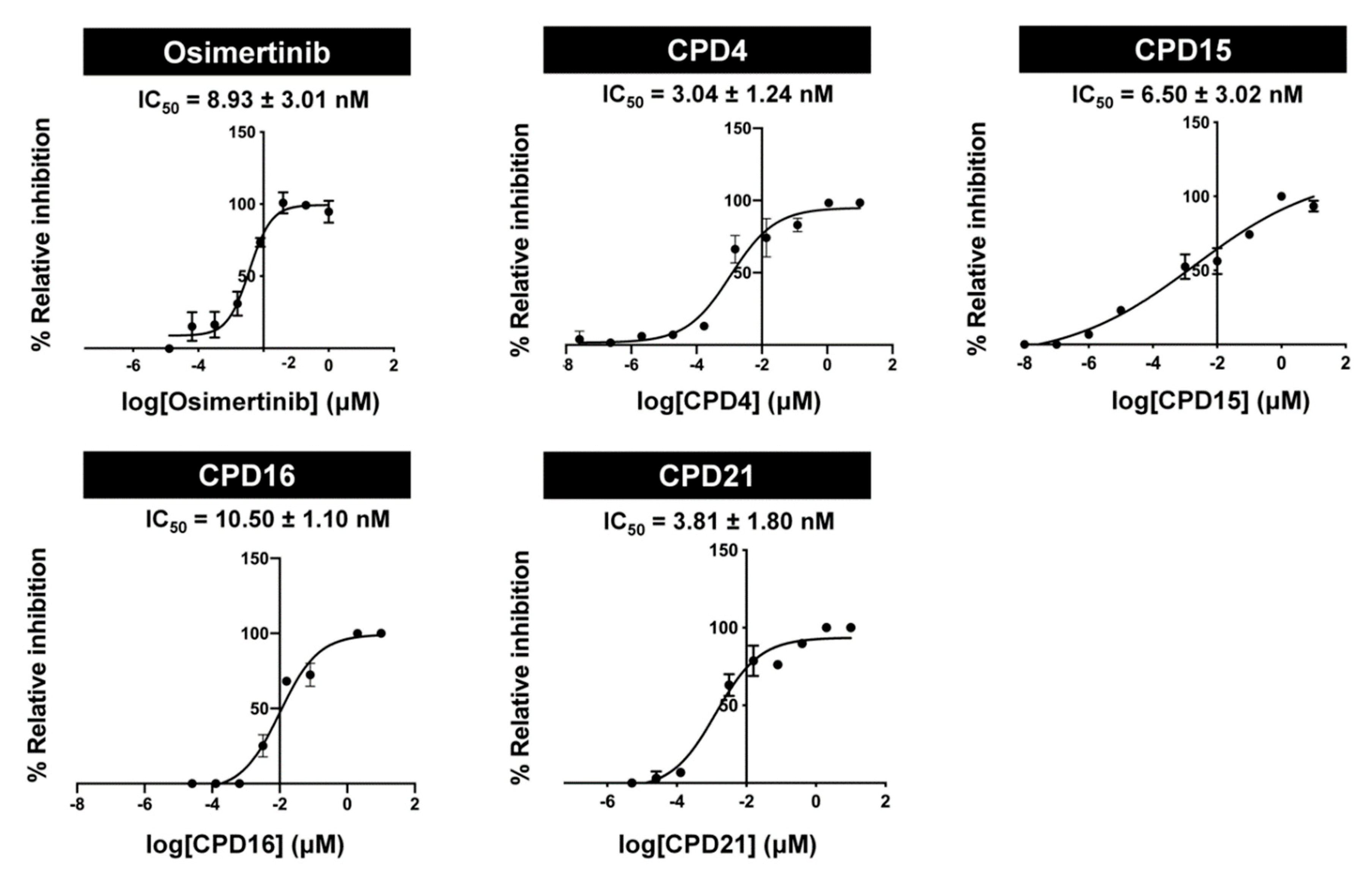
2.2. In Vitro Assay of EGFR (L858R/T790M/C797S) Inhibition
2.3. Drug-Likeness
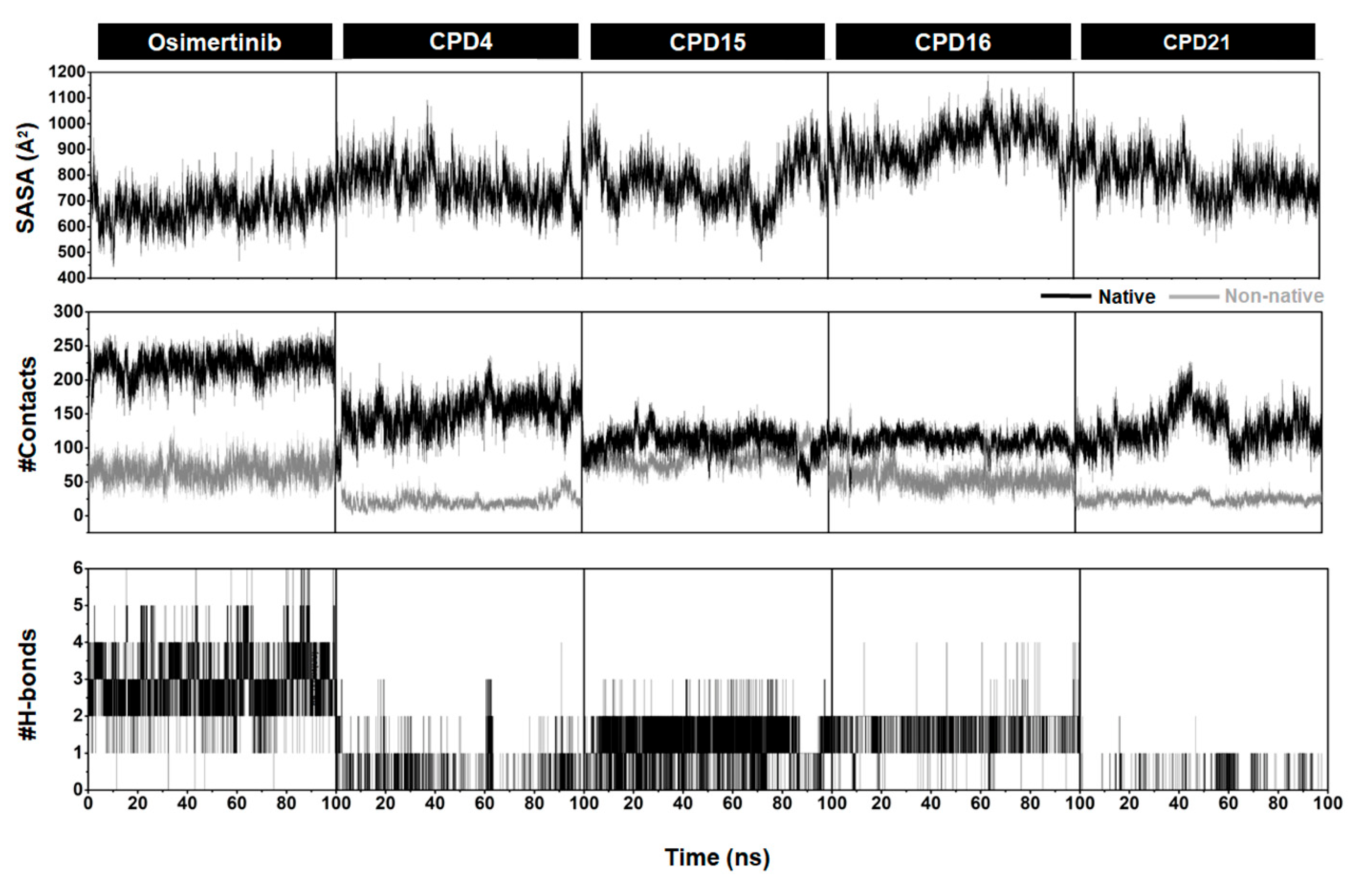
2.4. SASA, Number of H-Bonds, and Contact Atoms
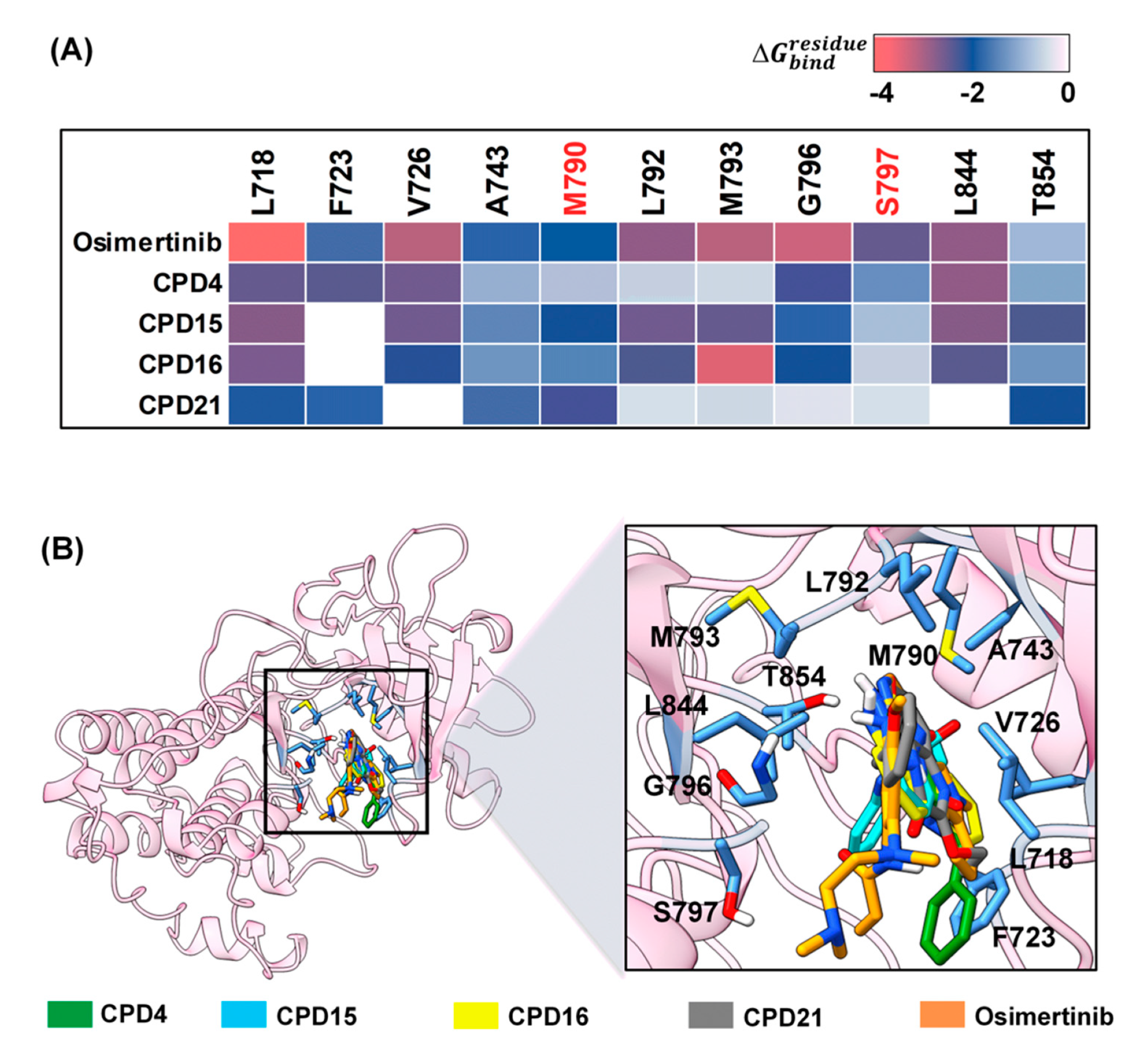
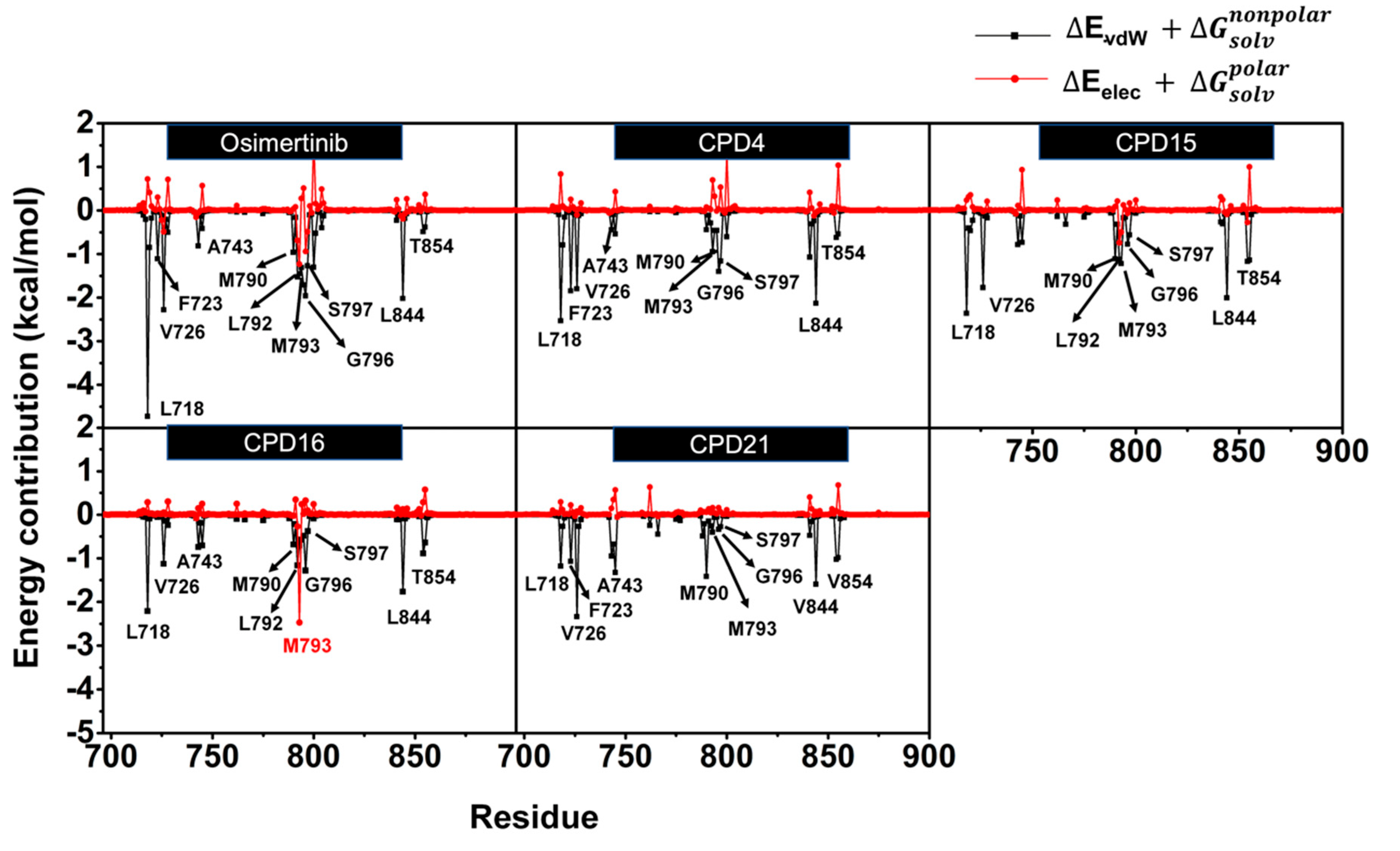
2.5. Hot-Spot Residues
2.6. Toxicity Prediction
2.7. Effect of CPD Derivatives on the Cell Viability
3. Materials and Methods
3.1. Docking-Based Virtual Screening
3.2. EGFR Tyrosine Kinase Inhibition
3.3. Molecular Dynamic (MD) Simulations
3.4. Pharmacokinetic Properties Prediction
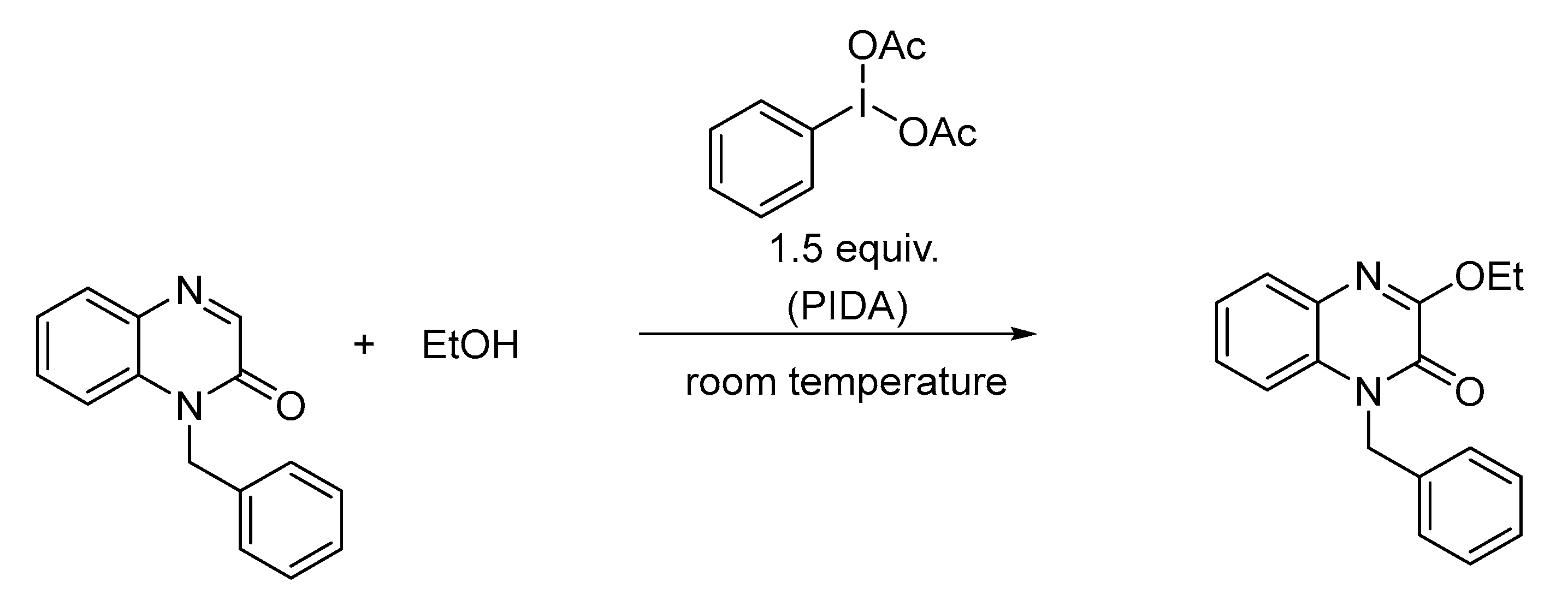
3.5. Synthesis and Characterization of CPD21
3.6. Toxicity Prediction
3.7. Cell Lines, Culture, and Cytotoxicity Assay
3.8. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Sequist, L.V.; Lynch, T.J. EGFR Tyrosine Kinase Inhibitors in Lung Cancer: An Evolving Story. Annu. Rev. Med. 2008, 59, 429–442. [Google Scholar] [CrossRef] [PubMed]
- George Priya Doss, C.; Rajith, B.; Chakraborty, C.; NagaSundaram, N.; Ali, S.K.; Zhu, H. Structural signature of the G719S-T790M double mutation in the EGFR kinase domain and its response to inhibitors. Sci. Rep. 2014, 4, 5868. [Google Scholar] [CrossRef]
- Ciardiello, F.; Tortora, G. EGFR antagonists in cancer treatment. N. Engl. J. Med. 2008, 358, 1160–1174. [Google Scholar] [CrossRef] [PubMed]
- Chong, C.R.; Jänne, P.A. The quest to overcome resistance to EGFR-targeted therapies in cancer. Nat. Med. 2013, 19, 1389–1400. [Google Scholar] [CrossRef] [PubMed]
- Sigismund, S.; Avanzato, D.; Lanzetti, L. Emerging functions of the EGFR in cancer. Mol. Oncol. 2018, 12, 3–20. [Google Scholar] [CrossRef] [PubMed]
- Bethune, G.; Bethune, D.; Ridgway, N.; Xu, Z. Epidermal growth factor receptor (EGFR) in lung cancer: An overview and update. J. Thorac. Dis. 2010, 2, 48–51. [Google Scholar]
- Salomon, D.S.; Brandt, R.; Ciardiello, F.; Normanno, N. Epidermal growth factor-related peptides and their receptors in human malignancies. Crit. Rev. Oncol./Hematol. 1995, 19, 183–232. [Google Scholar] [CrossRef]
- Gupta, R.; Dastane, A.M.; Forozan, F.; Riley-Portuguez, A.; Chung, F.; Lopategui, J.; Marchevsky, A.M. Evaluation of EGFR abnormalities in patients with pulmonary adenocarcinoma: The need to test neoplasms with more than one method. Mod. Pathol. 2009, 22, 128–133. [Google Scholar] [CrossRef]
- Huang, L.; Fu, L. Mechanisms of resistance to EGFR tyrosine kinase inhibitors. Acta Pharm. Sin. B 2015, 5, 390–401. [Google Scholar] [CrossRef]
- Pérez-Soler, R.; Chachoua, A.; Hammond, L.A.; Rowinsky, E.K.; Huberman, M.; Karp, D.; Rigas, J.; Clark, G.M.; Santabárbara, P.; Bonomi, P. Determinants of tumor response and survival with erlotinib in patients with non—Small-cell lung cancer. J. Clin. Oncol. 2004, 22, 3238–3247. [Google Scholar] [CrossRef]
- Mitsudomi, T.; Morita, S.; Yatabe, Y.; Negoro, S.; Okamoto, I.; Tsurutani, J.; Seto, T.; Satouchi, M.; Tada, H.; Hirashima, T. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): An open label, randomised phase 3 trial. Lancet Oncol. 2010, 11, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Mok, T.S.; Wu, Y.-L.; Thongprasert, S.; Yang, C.-H.; Chu, D.-T.; Saijo, N.; Sunpaweravong, P.; Han, B.; Margono, B.; Ichinose, Y. Gefitinib or carboplatin–paclitaxel in pulmonary adenocarcinoma. N. Engl. J. Med. 2009, 361, 947–957. [Google Scholar] [CrossRef]
- Maemondo, M.; Inoue, A.; Kobayashi, K.; Sugawara, S.; Oizumi, S.; Isobe, H.; Gemma, A.; Harada, M.; Yoshizawa, H.; Kinoshita, I. Gefitinib or chemotherapy for non–small-cell lung cancer with mutated EGFR. N. Engl. J. Med. 2010, 362, 2380–2388. [Google Scholar] [CrossRef]
- Kunimasa, K.; Sugimoto, N.; Tamiya, M.; Inoue, T.; Kawamura, T.; Kanzaki, R.; Okami, J.; Nishino, K. Dacomitinib overcomes afatinib-refractory carcinomatous meningitis in a lung cancer patient harbouring EGFR Ex.19 deletion and G724S mutation; a case report. Investig. New Drugs 2022, 40, 1137–1140. [Google Scholar] [CrossRef] [PubMed]
- Wecker, H.; Waller, C.F. Afatinib. In Small Molecules in Oncology; Springer: Cham, Switzerland, 2018; pp. 199–215. [Google Scholar]
- Dungo, R.T.; Keating, G.M. Afatinib: First global approval. Drugs 2013, 73, 1503–1515. [Google Scholar] [CrossRef] [PubMed]
- Sakuma, Y.; Yamazaki, Y.; Nakamura, Y.; Yoshihara, M.; Matsukuma, S.; Nakayama, H.; Yokose, T.; Kameda, Y.; Koizume, S.; Miyagi, Y. WZ4002, a third-generation EGFR inhibitor, can overcome anoikis resistance in EGFR-mutant lung adenocarcinomas more efficiently than Src inhibitors. Lab. Investig. 2012, 92, 371–383. [Google Scholar] [CrossRef]
- Walter, A.O.; Sjin, R.T.T.; Haringsma, H.J.; Ohashi, K.; Sun, J.; Lee, K.; Dubrovskiy, A.; Labenski, M.; Zhu, Z.; Wang, Z. Discovery of a Mutant-Selective Covalent Inhibitor of EGFR that Overcomes T790M-Mediated Resistance in NSCLCDevelopment of Covalent EGFRT790M Inhibitor in NSCLC. Cancer Discov. 2013, 3, 1404–1415. [Google Scholar] [CrossRef]
- Planchard, D.; Brown, K.H.; Kim, D.W.; Kim, S.W.; Ohe, Y.; Felip, E.; Leese, P.; Cantarini, M.; Vishwanathan, K.; Jänne, P.A.; et al. Osimertinib Western and Asian clinical pharmacokinetics in patients and healthy volunteers: Implications for formulation, dose, and dosing frequency in pivotal clinical studies. Cancer Chemother. Pharmacol. 2016, 77, 767–776. [Google Scholar] [CrossRef]
- Eberlein, C.A.; Stetson, D.; Markovets, A.A.; Al-Kadhimi, K.J.; Lai, Z.; Fisher, P.R.; Meador, C.B.; Spitzler, P.; Ichihara, E.; Ross, S.J. Acquired Resistance to the Mutant-Selective EGFR Inhibitor AZD9291 Is Associated with Increased Dependence on RAS Signaling in Preclinical ModelsRAS Pathway Activation and Resistance to EGFR TKIs. Cancer Res. 2015, 75, 2489–2500. [Google Scholar] [CrossRef]
- Ricciuti, B.; Baglivo, S.; Paglialunga, L.; De Giglio, A.; Bellezza, G.; Chiari, R.; Crinò, L.; Metro, G. Osimertinib in patients with advanced epidermal growth factor receptor T790M mutation-positive non-small cell lung cancer: Rationale, evidence and place in therapy. Ther. Adv. Med. Oncol. 2017, 9, 387–404. [Google Scholar] [CrossRef]
- Zhao, J.; Zou, M.; Lv, J.; Han, Y.; Wang, G.; Wang, G. Effective treatment of pulmonary adenocarcinoma harboring triple EGFR mutations of L858R, T790M, and cis-C797S by osimertinib, bevacizumab, and brigatinib combination therapy: A case report. OncoTargets Ther. 2018, 11, 5545–5550. [Google Scholar] [CrossRef]
- Günther, M.; Juchum, M.; Kelter, G.; Fiebig, H.; Laufer, S. Lung Cancer: EGFR Inhibitors with Low Nanomolar Activity against a Therapy-Resistant L858R/T790M/C797S Mutant. Angew. Chem. Int. Ed. 2016, 55, 10890–10894. [Google Scholar] [CrossRef] [PubMed]
- Günther, M.; Lategahn, J.; Juchum, M.; Döring, E.; Keul, M.; Engel, J.; Tumbrink, H.L.; Rauh, D.; Laufer, S. Trisubstituted Pyridinylimidazoles as Potent Inhibitors of the Clinically Resistant L858R/T790M/C797S EGFR Mutant: Targeting of Both Hydrophobic Regions and the Phosphate Binding Site. J. Med. Chem. 2017, 60, 5613–5637. [Google Scholar] [CrossRef]
- Lei, H.; Fan, S.; Zhang, H.; Liu, Y.-J.; Hei, Y.-Y.; Zhang, J.-J.; Zheng, A.Q.; Xin, M.; Zhang, S.-Q. Discovery of novel 9-heterocyclyl substituted 9H-purines as L858R/T790M/C797S mutant EGFR tyrosine kinase inhibitors. Eur. J. Med. Chem. 2020, 186, 111888. [Google Scholar] [CrossRef] [PubMed]
- Karnik, K.S.; Sarkate, A.P.; Tiwari, S.V.; Azad, R.; Burra, P.V.L.S.; Wakte, P.S. Computational and Synthetic approach with Biological Evaluation of Substituted Quinoline derivatives as small molecule L858R/T790M/C797S triple mutant EGFR inhibitors targeting resistance in Non-Small Cell Lung Cancer (NSCLC). Bioorganic Chem. 2021, 107, 104612. [Google Scholar] [CrossRef]
- Shen, J.; Zhang, T.; Zhu, S.-J.; Sun, M.; Tong, L.; Lai, M.; Zhang, R.; Xu, W.; Wu, R.; Ding, J. Structure-based design of 5-methylpyrimidopyridone derivatives as new wild-type sparing inhibitors of the epidermal growth factor receptor triple mutant (EGFRL858R/T790M/C797S). J. Med. Chem. 2019, 62, 7302–7308. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, J.; Shen, Y.; Wang, H.-Y.; Duan, W.-M.; Zhao, H.-Y.; Hei, Y.-Y.; Xin, M.; Cao, Y.-X.; Zhang, S.-Q. Discovery of 2, 4, 6-trisubstitued pyrido [3, 4-d] pyrimidine derivatives as new EGFR-TKIs. Eur. J. Med. Chem. 2018, 148, 221–237. [Google Scholar]
- Yang, J.; Wang, L.-J.; Liu, J.-J.; Zhong, L.; Zheng, R.-L.; Xu, Y.; Ji, P.; Zhang, C.-H.; Wang, W.-J.; Lin, X.-D. Structural optimization and structure–activity relationships of N 2-(4-(4-Methylpiperazin-1-yl) phenyl)-N 8-phenyl-9 H-purine-2, 8-diamine derivatives, a new class of reversible kinase inhibitors targeting both EGFR-activating and resistance mutations. J. Med. Chem. 2012, 55, 10685–10699. [Google Scholar] [CrossRef]
- Ferlenghi, F.; Scalvini, L.; Vacondio, F.; Castelli, R.; Bozza, N.; Marseglia, G.; Rivara, S.; Lodola, A.; La Monica, S.; Minari, R. A sulfonyl fluoride derivative inhibits EGFRL858R/T790M/C797S by covalent modification of the catalytic lysine. Eur. J. Med. Chem. 2021, 225, 113786. [Google Scholar]
- Mahalapbutr, P.; Leechaisit, R.; Thongnum, A.; Todsaporn, D.; Prachayasittikul, V.; Rungrotmongkol, T.; Prachayasittikul, S.; Ruchirawat, S.; Prachayasittikul, V.; Pingaew, R. Discovery of Anilino-1,4-naphthoquinones as Potent EGFR Tyrosine Kinase Inhibitors: Synthesis, Biological Evaluation, and Comprehensive Molecular Modeling. ACS Omega 2022, 7, 17881–17893. [Google Scholar] [CrossRef]
- Jiang, X.; Wu, K.; Bai, R.; Zhang, P.; Zhang, Y. Functionalized quinoxalinones as privileged structures with broad-ranging pharmacological activities. Eur. J. Med. Chem. 2022, 229, 114085. [Google Scholar] [CrossRef] [PubMed]
- An, Z.; Aksoy, O.; Zheng, T.; Fan, Q.W.; Weiss, W.A. Epidermal growth factor receptor and EGFRvIII in glioblastoma: Signaling pathways and targeted therapies. Oncogene 2018, 37, 1561–1575. [Google Scholar] [CrossRef] [PubMed]
- Koebel, M.R.; Schmadeke, G.; Posner, R.G.; Sirimulla, S. AutoDock VinaXB: Implementation of XBSF, new empirical halogen bond scoring function, into AutoDock Vina. J. Cheminformatics 2016, 8, 27. [Google Scholar] [CrossRef] [PubMed]
- Suriya, U.; Mahalapbutr, P.; Rungrotmongkol, T. Integration of In Silico Strategies for Drug Repositioning towards P38α Mitogen-Activated Protein Kinase (MAPK) at the Allosteric Site. Pharmaceutics 2022, 14, 1461. [Google Scholar] [PubMed]
- Todsaporn, D.; Mahalapbutr, P.; Poo-arporn, R.P.; Choowongkomon, K.; Rungrotmongkol, T. Structural dynamics and kinase inhibitory activity of three generations of tyrosine kinase inhibitors against wild-type, L858R/T790M, and L858R/T790M/C797S forms of EGFR. Comput. Biol. Med. 2022, 147, 105787. [Google Scholar] [CrossRef] [PubMed]
- Fabbro, D.; Cowan-Jacob, S.W.; Moebitz, H. Ten things you should know about protein kinases: IUPHAR R eview 14. Br. J. Pharmacol. 2015, 172, 2675–2700. [Google Scholar] [CrossRef]
- Kashima, K.; Kawauchi, H.; Tanimura, H.; Tachibana, Y.; Chiba, T.; Torizawa, T.; Sakamoto, H. CH7233163 Overcomes Osimertinib-Resistant EGFR-Del19/T790M/C797S Mutation. Mol. Cancer Ther. 2020, 19, 2288–2297. [Google Scholar] [CrossRef]
- Waterhouse, A.; Bertoni, M.; Bienert, S.; Studer, G.; Tauriello, G.; Gumienny, R.; Heer, F.T.; de Beer, T.A.P.; Rempfer, C.; Bordoli, L.; et al. SWISS-MODEL: Homology modelling of protein structures and complexes. Nucleic Acids Res. 2018, 46, W296–W303. [Google Scholar] [CrossRef]
- Laskowski, R.A.; MacArthur, M.W.; Moss, D.S.; Thornton, J.M. PROCHECK: A program to check the stereochemical quality of protein structures. J. Appl. Crystallogr. 1993, 26, 283–291. [Google Scholar] [CrossRef]
- Wimonsong, W.; Yotphan, S. PIDA-induced oxidative C–N bond coupling of quinoxalinones and azoles. Tetrahedron 2021, 81, 131919. [Google Scholar] [CrossRef]
- Frisch, M.J.W.C. Gaussian 09 Revision D. 01; Sian Inc.: Wallingford, CT, USA, 2009; Volume 112. [Google Scholar]
- Sangpheak, K.; Tabtimmai, L.; Seetaha, S.; Rungnim, C.; Chavasiri, W.; Wolschann, P.; Choowongkomon, K.; Rungrotmongkol, T. Biological Evaluation and Molecular Dynamics Simulation of Chalcone Derivatives as Epidermal Growth Factor-Tyrosine Kinase Inhibitors. Molecules 2019, 24, 1092. [Google Scholar] [CrossRef] [PubMed]
- Zegzouti, H.; Zdanovskaia, M.; Hsiao, K.; Goueli, S.A. ADP-Glo: A Bioluminescent and Homogeneous ADP Monitoring Assay for Kinases. ASSAY Drug Dev. Technol. 2009, 7, 560–572. [Google Scholar] [CrossRef] [PubMed]
- Sanachai, K.; Aiebchun, T.; Mahalapbutr, P.; Seetaha, S.; Tabtimmai, L.; Maitarad, P.; Xenikakis, I.; Geronikaki, A.; Choowongkomon, K.; Rungrotmongkol, T. Discovery of novel JAK2 and EGFR inhibitors from a series of thiazole-based chalcone derivatives. RSC Med. Chem. 2021, 12, 430–438. [Google Scholar] [CrossRef] [PubMed]
- Thirunavukkarasu, M.K.; Suriya, U.; Rungrotmongkol, T.; Karuppasamy, R. In Silico Screening of Available Drugs Targeting Non-Small Cell Lung Cancer Targets: A Drug Repurposing Approach. Pharmaceutics 2022, 14, 59. [Google Scholar] [CrossRef] [PubMed]
- Verma, K.; Mahalapbutr, P.; Suriya, U.; Somboon, T.; Aiebchun, T.; Shi, L.; Maitarad, P.; Rungrotmongkol, T. In Silico Screening of DNA Gyrase B Potent Flavonoids for the Treatment of Clostridium difficile Infection from PhytoHub Database. Braz. Arch. Biol. Technol. 2021, 64, e21200402. [Google Scholar] [CrossRef]
- Wang, J.; Wolf, R.M.; Caldwell, J.W.; Kollman, P.A.; Case, D.A. Development and testing of a general amber force field. J. Comput. Chem. 2004, 25, 1157–1174. [Google Scholar] [CrossRef]
- Jorgensen, W.L.; Chandrasekhar, J.; Madura, J.D.; Impey, R.W.; Klein, M.L. Comparison of simple potential functions for simulating liquid water. J. Chem. Phys. 1983, 79, 926–935. [Google Scholar] [CrossRef]
- Soe, H.M.H.; Chamni, S.; Mahalapbutr, P.; Kongtaworn, N.; Rungrotmongkol, T.; Jansook, P. The investigation of binary and ternary sulfobutylether-β-cyclodextrin inclusion complexes with asiaticoside in solution and in solid state. Carbohydr. Res. 2020, 498, 108190. [Google Scholar] [CrossRef]
- Klaewkla, M.; Charoenwongpaiboon, T.; Mahalapbutr, P. Molecular basis of the new COVID-19 target neuropilin-1 in complex with SARS-CoV-2 S1 C-end rule peptide and small-molecule antagonists. J. Mol. Liq. 2021, 335, 116537. [Google Scholar] [CrossRef]
- Darden, T.; York, D.; Pedersen, L. Particle mesh Ewald: An N⋅ log (N) method for Ewald sums in large systems. J. Chem. Phys. 1993, 98, 10089–10092. [Google Scholar] [CrossRef]
- Ryckaert, J.-P.; Ciccotti, G.; Berendsen, H.J.C. Numerical integration of the cartesian equations of motion of a system with constraints: Molecular dynamics of n-alkanes. J. Comput. Phys. 1977, 23, 327–341. [Google Scholar] [CrossRef]
- Uberuaga, B.P.; Anghel, M.; Voter, A.F. Synchronization of trajectories in canonical molecular-dynamics simulations: Observation, explanation, and exploitation. J. Chem. Phys. 2004, 120, 6363–6374. [Google Scholar] [CrossRef] [PubMed]
- Berendsen, H.J.C.; Postma, J.P.M.; Van Gunsteren, W.F.; DiNola, A.; Haak, J.R. Molecular dynamics with coupling to an external bath. J. Chem. Phys. 1984, 81, 3684–3690. [Google Scholar] [CrossRef]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef]
- Sander, T.; Freyss, J.; von Korff, M.; Rufener, C. DataWarrior: An Open-Source Program For Chemistry Aware Data Visualization And Analysis. J. Chem. Inf. Model. 2015, 55, 460–473. [Google Scholar] [CrossRef]
- Banerjee, P.; Eckert, A.O.; Schrey, A.K.; Preissner, R. ProTox-II: A webserver for the prediction of toxicity of chemicals. Nucleic Acids Res. 2018, 46, W257–W263. [Google Scholar] [CrossRef]

| Lipinski’s Rule of Five | |||||||
|---|---|---|---|---|---|---|---|
| Compounds | M.W. (Da) | HBD | HBA | Rotatable Bonds | PSA (Å2) | logP | Drug-Likeness |
| CPD4 | 303.32 | 0 | 4 | 3 | 65.60 | 2.45 | Yes |
| CPD15 | 290.32 | 0 | 3 | 2 | 52.71 | 2.83 | Yes |
| CPD16 | 252.27 | 1 | 3 | 3 | 54.98 | 2.32 | Yes |
| CPD21 | 280.32 | 0 | 3 | 4 | 44.12 | 3.00 | Yes |
| Osimertinib | 499.61 | 2 | 5 | 10 | 90.78 | 3.36 | Yes |
| Features | Compounds | ||||
|---|---|---|---|---|---|
| Osimertinib | CPD4 | CPD15 | CPD16 | CPD21 | |
| LD50 (mg/kg) | 100 | 1000 | 1000 | 800 | 1000 |
| Toxicity class | 3 | 4 | 4 | 4 | 4 |
| Mutagenicity | High | None | None | None | None |
| Tumorigenicity | Low | None | None | None | None |
| Irritant | Low | None | None | None | None |
| Reproductive effect | Low | Low | None | None | None |
| Compounds | IC50 (μM) | |
|---|---|---|
| H1975 | Vero | |
| CPD4 | 3.47 ± 2.20 | >20 |
| CPD15 | 31.25 ± 3.40 | >50 |
| CPD16 | 79.43 ± 4.35 | >100 |
| CPD21 | 44.67 ± 2.34 | >100 |
| Osimertinib | 18.33 ± 2.02 | >100 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suriya, U.; Mahalapbutr, P.; Wimonsong, W.; Yotphan, S.; Choowongkomon, K.; Rungrotmongkol, T. Quinoxalinones as A Novel Inhibitor Scaffold for EGFR (L858R/T790M/C797S) Tyrosine Kinase: Molecular Docking, Biological Evaluations, and Computational Insights. Molecules 2022, 27, 8901. https://doi.org/10.3390/molecules27248901
Suriya U, Mahalapbutr P, Wimonsong W, Yotphan S, Choowongkomon K, Rungrotmongkol T. Quinoxalinones as A Novel Inhibitor Scaffold for EGFR (L858R/T790M/C797S) Tyrosine Kinase: Molecular Docking, Biological Evaluations, and Computational Insights. Molecules. 2022; 27(24):8901. https://doi.org/10.3390/molecules27248901
Chicago/Turabian StyleSuriya, Utid, Panupong Mahalapbutr, Watchara Wimonsong, Sirilata Yotphan, Kiattawee Choowongkomon, and Thanyada Rungrotmongkol. 2022. "Quinoxalinones as A Novel Inhibitor Scaffold for EGFR (L858R/T790M/C797S) Tyrosine Kinase: Molecular Docking, Biological Evaluations, and Computational Insights" Molecules 27, no. 24: 8901. https://doi.org/10.3390/molecules27248901
APA StyleSuriya, U., Mahalapbutr, P., Wimonsong, W., Yotphan, S., Choowongkomon, K., & Rungrotmongkol, T. (2022). Quinoxalinones as A Novel Inhibitor Scaffold for EGFR (L858R/T790M/C797S) Tyrosine Kinase: Molecular Docking, Biological Evaluations, and Computational Insights. Molecules, 27(24), 8901. https://doi.org/10.3390/molecules27248901








