Recent Advances in Excimer-Based Fluorescence Probes for Biological Applications
Abstract
:1. Introduction
2. Design Strategies for Excimer-Based Fluorescent Probes
3. Fluorescent Probes and Their Biological Applications
3.1. Pyrene-Based Probes
3.2. Perylene-Based Probes
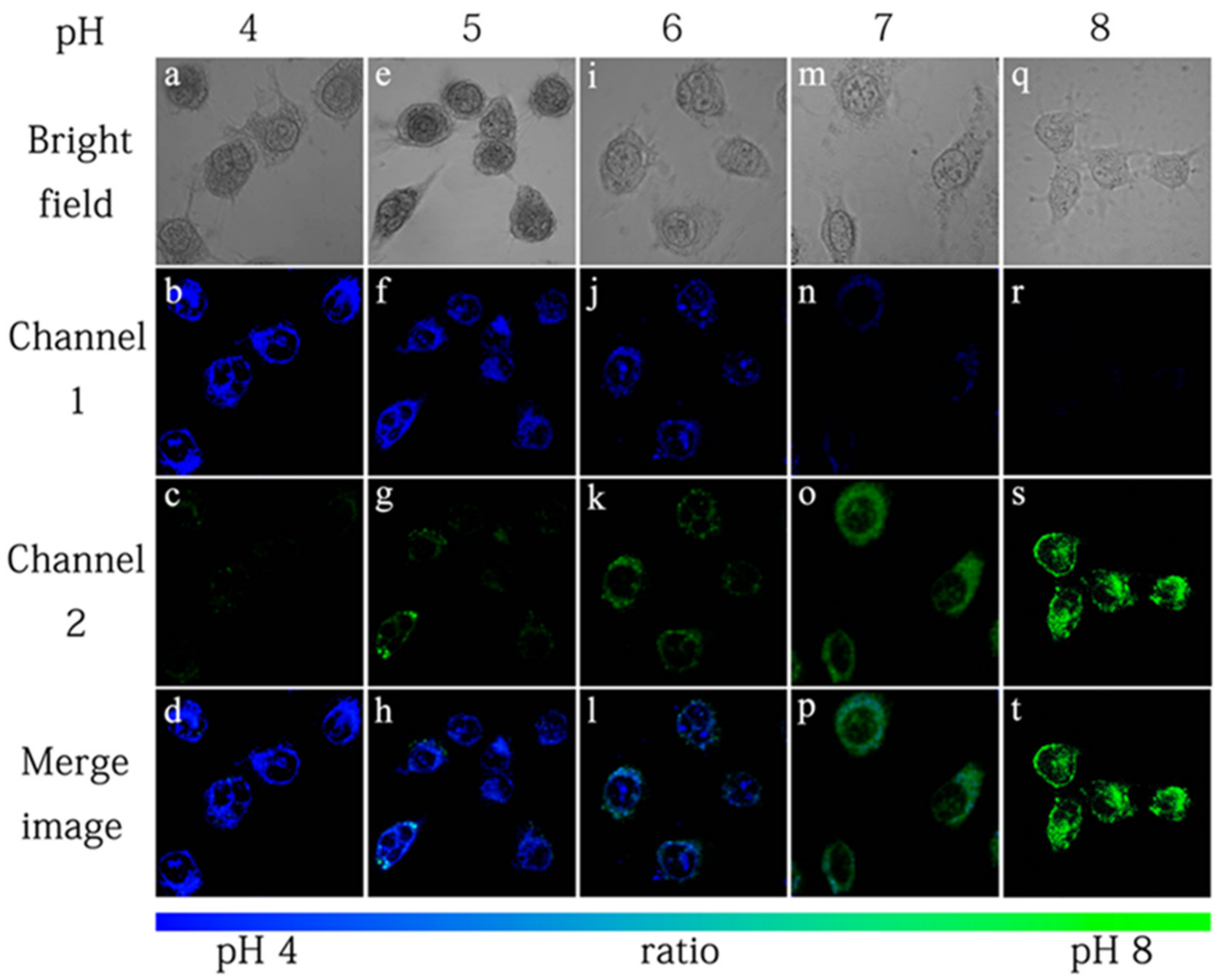
3.3. Benzothiazole-Based Probes
4. Conclusions and Outlook
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Winnik, F.M. Photophysics of preassociated pyrenes in aqueous polymer solutions and in other organized media. Chem. Rev. 1993, 93, 587–614. [Google Scholar] [CrossRef]
- Duarte, T.M.F.; Mullen, K. Pyrene-based materials for organic electronics. Chem. Rev. 2011, 111, 7260–7314. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, A.; Sengupta, A.; Chattopadhyay, A.; Das, D. Lysine triggered ratiometric conversion of dynamic to static excimer of a pyrene derivative: Aggregation-induced emission, nanomolar detection and human breast cancer cell (MCF7) imaging. Chem. Commun. 2015, 51, 11455–11458. [Google Scholar] [CrossRef]
- Hoche, J.; Schmitt, H.C.; Humeniuk, A.; Fischer, I.; Mitric, R.; Rohr, M.I.S. The mechanism of excimer formation: An experimental and theoretical study on the pyrene dimer. Phys. Chem. Chem. Phys. 2017, 19, 25002–25015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, M.; Okui, Y.; Hirade, T. Light-responsive microstructures capable of pyrene monomer fluorescence switching. J. Mater. Chem. C 2013, 1, 3448–3453. [Google Scholar]
- Li, W.; Wang, L.; Zhang, J.; Wang, H. Bis-pyrene-based supramolecular aggregates with reversibly mechanochromic and vapochromic responsiveness. J. Mater. Chem. C 2014, 2, 1887–1892. [Google Scholar] [CrossRef]
- Huang, J.; Wu, Y.; Chen, Y.; Zhu, Z.; Yang, X.; Yang, C.J.; Wang, K.; Tan, W. Pyrene-excimer probes based on the hybridization chain reaction for the detection of nucleic acids in complex biological fluids. Angew. Chem. Int. Ed. 2011, 50, 401–404. [Google Scholar]
- Conlon, P.; Yang, C.J.; Wu, Y.; Chen, Y.; Martinez, K.; Kim, Y.; Stevens, N.; Marti, A.A.; Jockusch, S.; Turro, N.J.; et al. Pyrene excimer signaling molecular beacons for probing nucleic acids. J. Am. Chem. Soc. 2008, 130, 336–342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mart, A.A.; Li, X.; Jockusch, S.; Li, Z.; Raveendra, B.; Kalachikov, S.; Russo, J.J.; Morozova, I.; Puthanveettil, S.V.; Ju, J.; et al. Pyrene binary probes for unambiguous detection of mRNA using time-resolved fluorescence spectroscopy. Nucleic Acids Res. 2006, 34, 3161–3168. [Google Scholar] [CrossRef] [Green Version]
- Østergaard, M.E.; Hrdlicka, P.J. Pyrene-functionalized oligonucleotides and locked nucleic acids (LNAs): Tools for fundamental research, diagnostics, and nanotechnologyw. Chem. Soc. Rev. 2011, 40, 5771–5788. [Google Scholar] [CrossRef]
- Bains, G.K.; Kim, S.H.; Sorin, E.J.; Narayanaswami, V. The extent of pyrene excimer fluorescence emission is a reflector of distance and flexibility: Analysis of the segment linking the LDL receptor-binding and tetramerization domains of apolipoprotein E3. Biochemistry 2012, 51, 6207–6219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, C.J.; Jockusch, S.; Vicens, M.; Turro, N.J.; Tan, W. Light-switching excimer probes for rapid protein monitoring in complex biological fluids. Proc. Natl. Acad. Sci. USA 2005, 102, 17278–17283. [Google Scholar] [PubMed]
- Bains, G.; Patel, A.B.; Narayanaswami, V. Pyrene: A probe to study protein conformation and conformational changes. Molecules 2011, 16, 7909–7935. [Google Scholar] [CrossRef] [PubMed]
- Yamana, K.; Ohtani, Y.; Nakano, H.; Saito, I. Bis-pyrene labeled DNA aptamer as an intelligent fluorescent biosensor. Bioorganic Med. Chem. Lett. 2003, 13, 3429–3431. [Google Scholar]
- Li, G.; Zhu, D.; Xue, L.; Jiang, H. Quinoline-based fluorescent probe for ratiometric detection of lysosomal pH. Org. Lett. 2013, 15, 5020–5023. [Google Scholar]
- Liu, X.D.; Xu, Y.; Sun, R.; Xu, Y.J.; Lu, J.M.; Ge, J.F. A coumarin–indole-based near-infrared ratiometric pH probe for intracellular fluorescence imaging. Analyst 2013, 138, 6542–6550. [Google Scholar]
- Zhou, X.; Su, F.; Lu, H.; Senechal-Willis, P.; Tian, Y.; Johnson, R.H.; Meldrum, D.R. An FRET-based ratiometric chemosensor for in vitro cellular fluorescence analyses of pH. Biomaterials 2012, 33, 171–180. [Google Scholar] [CrossRef] [Green Version]
- Yuan, L.; Lin, W.; Cao, Z.; Wang, J.; Chen, B. Development of FRET-based dual-ecitation ratiometric fluorescent pH probes and their photocaged derivatives. Chem. Eur. J. 2012, 18, 1247–1255. [Google Scholar]
- Grover, A.; Schmidt, B.F.; Salter, R.D.; Watkins, S.C.; Waggoner, A.S.; Bruchez, M.P. Genetically encoded pH sensor for tracking surface proteins through endocytosis. Angew. Chem. Int. Ed. 2012, 51, 4838–4842. [Google Scholar]
- Feng, X.; Qi, C.; Feng, H.T.; Zhao, Z.; Sung, H.H.Y.; Williams, I.D.; Kwok, R.T.K.; Lam, J.Y.; Qin, A.; Tang, B.Z. Dual fluorescence of tetraphenylethylene-substituted pyrenes with aggregation-induced emission characteristics for white-light emission. Chem. Sci. 2018, 9, 5679–5687. [Google Scholar] [CrossRef] [Green Version]
- Monarul Islam, M.; Hu, Z.; Wang, Q.; Redshaw, C.; Feng, X. Pyrene-based aggregation-induced emission luminogens and their applications. Mater. Chem. Front. 2019, 3, 762–781. [Google Scholar] [CrossRef]
- Casier, R.; Gauthier, M.; Duhamel, J. Using pyrene excimer fluorescence to probe polymer diffusion in latex films. Macromolecules 2017, 50, 1635–1644. [Google Scholar] [CrossRef] [Green Version]
- Bodenant, B.; Fages, F.; Delville, M.H. Metal-induced self-assembly of a pyrene-tethered hydroxamate ligand for the generation of multichromophoric supramolecular systems. The pyrene excimer as switch for iron(III)-driven intramolecular fluorescence quenching. J. Am. Chem. Soc. 1998, 120, 7511–7519. [Google Scholar]
- Chen, L.; Wu, D.; Yoon, J. Recent advances in the development of chromophore-based chemosensors for nerve agents and phosgene. ACS Sens. 2018, 3, 27–43. [Google Scholar] [PubMed] [Green Version]
- Verbitskiy, E.V.; Rusinov, G.L.; Chupakhin, O.N.; Charushin, V.N. Design of fluorescent sensors based on azaheterocyclic push-pull systems towards nitroaromatic explosives and related compounds: A review. Dyes Pigm. 2020, 180, 108414. [Google Scholar] [CrossRef]
- Verbitskiy, E.V.; Baranova, A.A.; Lugovik, K.I.; Khokhlov, K.O.; Chuvashov, R.D.; Dinastiya, E.M.; Rusinov, G.L.; Chupakhin, O.N.; Charushin, V.N. Linear and V-shaped push–pull systems on a base of pyrimidine scaffold with a pyrene-donative fragment for detection of nitroaromatic compounds. J. Iran. Chem. Soc. 2018, 4, 787–797. [Google Scholar] [CrossRef]
- Kadirvel, M.; Arsic, B.; Freeman, S.; Bichenkova, E.V. Exciplex and excimer molecular probes: Detection of conformational flip in a myo-inositol chair. Org. Biomol. Chem. 2008, 6, 1966–1972. [Google Scholar] [CrossRef]
- Fujimoto, K.; Shimizu, H.; Inouye, M. Unambiguous detection of target DNAs by excimer–monomer switching molecular beacons. J. Org. Chem. 2004, 69, 3271–3275. [Google Scholar]
- Chen, J.; Liao, D.; Wang, Y.; Zhou, H.; Li, W.; Yu, C. Real-time fluorometric assay for acetylcholinesterase activity and inhibitor screening through the pyrene probe monomerexcimer transition. Org. Lett. 2013, 15, 2132–2135. [Google Scholar]
- Dai, Q.; Liu, W.M.; Zhuang, X.Q.; Wu, J.S.; Zhang, H.Y.; Wang, P.F. Ratiometric fluorescence sensor based on a pyrene derivative and quantification detection of heparin in aqueous solution and serum. Anal. Chem. 2011, 83, 6559–6564. [Google Scholar] [CrossRef]
- Zhang, R.X.; Tang, D.; Lu, P.; Yang, X.Y.; Liao, D.L.; Zhang, Y.J.; Zhang, M.J.; Yu, C.; Yam, V.W.W. Nucleic acid-induced aggregation and pyrene excimer formation. Org. Lett. 2009, 11, 4302–4305. [Google Scholar] [PubMed]
- Ma, B.L.; Zeng, F.; Li, X.Z.; Wu, S.Z. A facile approach for sensitive, reversible and ratiometric detection of biothiols based on thymine-mediated excimer–monomer transformation. Chem. Commun. 2012, 48, 6007–6009. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fischbach, M.; Resch-Genger, U.; Seitz, O. Protease probes that enable excimer signaling upon scission. Angew. Chem. Int. Ed. 2014, 53, 11955–11959. [Google Scholar] [CrossRef] [PubMed]
- Klein, T.; Bischoff, R. Physiology and pathophysiology of matrix metalloproteases. Amino Acids 2011, 41, 271–290. [Google Scholar] [CrossRef] [Green Version]
- Kraskouskaya, D.; Bancerz, M.; Soor, H.S.; Gardiner, J.E.; Gunning, P.T. An excimer-based, turn-on fluorescent sensor for the selective detection of diphosphorylated proteins in aqueous solution and polyacrylamide gels. J. Am. Chem. Soc. 2014, 136, 1234–1237. [Google Scholar]
- Bai, Y.; Zhao, Q. Rapid fluorescence detection of immunoglobulin E using an aptamer switch based on a binding induced pyrene excimer. Anal. Methods 2017, 9, 3962–3967. [Google Scholar] [CrossRef]
- Pak, Y.L.; Park, S.J.; Xu, Q.; Kim, H.M.; Yoon, J. Ratiometric two-photon fluorescent probe for detecting and imaging hypochlorite. Anal. Chem. 2018, 90, 9510–9514. [Google Scholar] [CrossRef] [PubMed]
- Kettle, A.J.; Gedye, C.A.; Hampton, M.B.; Winterbourn, C.C. Inhibition of myeloperoxidase by benzoic acid hydrazides. Biochem. J. 1995, 308, 559–563. [Google Scholar] [CrossRef]
- Neve, J.; Parij, N.; Moguilevsky, N. Inhibition of the myeloperoxidase chlorinating activity by non-steroidal anti-inflammatory drugs investigated with a human recombinant enzyme. Eur. J. Pharmacol. 2001, 417, 37–43. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, J.; Zeng, F.; Huang, S.; Huang, J.; Xie, H.; Yu, C.; Wu, S. Pyrene derivative emitting red or near-infrared light with monomer/excimer conversion and its application to ratiometric detection of hypochlorite. ACS Appl. Mater. Interfaces 2016, 8, 1511–1519. [Google Scholar]
- Wang, B.S.; Li, P.; Yu, F.B.; Song, P.; Sun, X.F.; Yang, S.Q.; Lou, Z.G.; Han, Z.G. A reversible fluorescence probe based on Se–BODIPY for the redox cycle between HClO oxidative stress and H2S repair in living cells. Chem. Commun. 2013, 49, 1014–1016. [Google Scholar] [CrossRef] [PubMed]
- Jares-Erijman, E.A.; Jovin, T.M. FRET imaging. Nat. Biotechnol. 2003, 21, 1387–1395. [Google Scholar] [CrossRef] [PubMed]
- Sapsford, K.E.; Berti, L.; Medintz, I.L. Materials for fluorescence resonance energy transfer analysis: Beyond traditional donor–acceptor combinations. Angew. Chem. Int. Ed. 2006, 45, 4562–4589. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zhang, X.; Li, J.; Zhang, C.; Liang, H.; Mao, G.; Zhou, L.; Tan, W.; Yu, R. Bispyrene–fluorescein hybrid based FRET cassette: A convenient platform toward ratiometric time-resolved probe for bioanalytical applications. Anal. Chem. 2014, 86, 10389–10396. [Google Scholar] [PubMed]
- Tafani, M.; Cohn, J.A.; Karpinich, N.O.; Rothman, R.J.; Russo, M.A.; Farber, J.L. Regulation of intracellular pH mediates bax activation in HeLa cells treated with staurosporine or tumor necrosis factor-α. J. Biol. Chem. 2002, 277, 49569–49576. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.; Slattum, P.; Wang, C.Y.; Zang, L. Self-assembly of perylene imide molecules into 1D nanostructures: Methods, morphologies, and applications. Chem. Rev. 2015, 115, 11967–11998. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Trinh, M.T.; Chen, R.S.; Wang, W.; Khlyabich, P.P.; Kumar, B.; Xu, Q.Z.; Nam, C.Y.; Sfeir, M.Y.; Black, C.; et al. Efficient organic solar cells with helical perylene diimide electron acceptors. J. Am. Chem. Soc. 2014, 136, 15215–15221. [Google Scholar]
- Spenst, P.; Würthner, F. A perylene bisimide cyclophane as a “Turn-On” and “Turn-Off” fluorescence probe. Angew. Chem. Int. Ed. 2015, 54, 10165–10168. [Google Scholar]
- Würthner, F. Perylene bisimide dyes as versatile building blocks for functional supramolecular architectures. Chem. Commun. 2004, 14, 1564–1579. [Google Scholar] [CrossRef]
- Li, C.; Wonneberger, H. Perylene imides for organic photovoltaics: Yesterday, today, and tomorrow. Adv. Mater. 2012, 24, 613–636. [Google Scholar] [CrossRef]
- Gorman, J.; Pandya, R.; Allardice, J.R.; Price, M.B.; Schmidt, T.W.; Friend, R.H.; Rao, A.; Davis, L.K. Excimer formation in carboxylic acid-functionalized perylene diimides attached to silicon dioxide nanoparticles. J. Phys. Chem. C 2019, 123, 3433–3440. [Google Scholar]
- Reisch, A.; Trofymchuk, K.; Runser, A.; Fleith, G.; Rawiso, M.; Klymchenko, A.S. Tailoring fluorescence brightness and switching of nanoparticles through dye organization in the polymer matrix. ACS Appl. Mater. Interfaces 2017, 9, 43030–43042. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Zhou, H.; Zhang, Y.; Anjum Shahzad, S.; Yang, M.; Hu, Z.; Yu, C. Tuning of the perylene probe excimer emission with silver nanoparticles. Anal. Chim. Acta 2018, 1016, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Peneva, K.; Mihov, G.; Nolde, F.; Rocha, S.; Hotta, J.; Braeckmans, K.; Hofkens, J.; Uji-I, H.; Herrmann, A.; Müllen, K. Water-soluble monofunctional perylene and terrylene dyes: Powerful labels for single-enzyme tracking. Angew. Chem. Int. Ed. 2008, 47, 3372–3375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peneva, K.; Mihov, G.; Herrmann, A.; Zarrabi, N.; Borsch, M.; Duncan, T.M.; Müllen, K. Exploiting the nitrilotriacetic acid moiety for biolabeling with ultrastable perylene dyes. J. Am. Chem. Soc. 2008, 130, 5398–5399. [Google Scholar] [PubMed]
- Baumstark, D.; Wagenknecht, H.A. Perylene bisimide dimers as fluorescent “Glue” For DNA and for base-mismatch detection. Angew. Chem. Int. Ed. 2008, 47, 2612–2614. [Google Scholar] [CrossRef]
- Wang, B.; Yu, C. Fluorescence turn-on detection of a protein through the reduced aggregation of a perylene prob. Angew. Chem. Int. Ed. 2010, 49, 1485–1488. [Google Scholar] [CrossRef]
- Fan, Q.L.; Cheng, K.; Yang, Z.; Zhang, R.P.; Yang, M.; Hu, X.; Ma, X.W.; Bu, L.H.; Lu, X.M.; Xiong, X.X.; et al. Perylene-diimide-based nanoparticles as highly efficient photoacoustic agents for deep brain tumor imaging in living mice. Adv. Mater. 2015, 27, 843–847. [Google Scholar] [CrossRef]
- Niu, N.; Zhou, H.; Liu, N.; Ren, J.; Li, W.; Yu, C. A benzoperylene self-assembly complex with turn-on excimer emission for wash-free cell membrane fluorescence imaging. Chem. Commun. 2019, 55, 14446–14449. [Google Scholar]
- Jana, A.; Nguyen, K.T.; Li, X.; Zhu, P.; Tan, N.S.; Ågren, H.; Zhao, Y. Perylene-derived single-component organic nanoparticles with tunable emission: Efficient anticancer drug carriers with real time monitoring of drug release. ACS Nano 2014, 8, 5939–5952. [Google Scholar] [CrossRef]
- Shen, Y.; Zhang, Z.; Liu, H.; Yan, Y.; Zhang, S.; Yang, B.; Ma, Y. Highly efficient orange-red/red excimer fluorescence from dimeric π−π stacking of perylene and its nanoparticle applications. J. Phys. Chem. C 2019, 123, 13047–13056. [Google Scholar] [CrossRef]
- Görl, D.; Zhang, X.; Würthner, F. Molecular assemblies of perylene bisimide dyes in water. Angew. Chem. Int. Ed. 2012, 51, 6328–6348. [Google Scholar]
- Jana, A.; Bsai, L.; Li, X.; Ågren, H.; Zhao, Y. Morphology tuning of self-assembled perylene monoimide from nanoparticles to colloidosomes with enhanced excimeric NIR emission for bioimaging. ACS Appl. Mater. Interfaces 2016, 8, 2336–2347. [Google Scholar] [PubMed]
- Zhang, M.; Girault, H.H. SECM for imaging and detection of latent fingerprints. Analyst 2009, 134, 25–30. [Google Scholar] [PubMed]
- Leggett, R.; Lee-Smith, E.E.; Jickells, S.M.; Russell, D.A. “Intelligent” fingerprinting: Simultaneous identification of drug metabolites and individuals by using antibody-functionalized nanoparticles. Angew. Chem. Int. Ed. 2007, 46, 4100–4103. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Chen, J.; Zhou, H.; Li, W.; Zhang, Q.; Yu, C. Facile detection of latent fingerprints on various substrates based on perylene probe excimer emission. Anal. Methods 2014, 6, 654–657. [Google Scholar] [CrossRef]
- Heithoff, D.M.; Sinsheimer, R.L.; Low, D.A.; Mahan, M.J. An essential role for DNA adenine methylation in bacterial virulence. Science 1999, 284, 967–970. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, J.; Chen, Y.; Li, W.; Yu, C. Polymer-induced perylene probe excimer formation and selective sensing of DNA methyltransferase activity through the monomer–excimer transition. Anal. Chem. 2014, 86, 4371–4378. [Google Scholar] [CrossRef]
- Hanan, G.S.M.; Barbas, Y.W.; Hussain, E.K.; Shoham, Y. Hippocampal microRNA-132 mediates stress-inducible cognitive deficits through its acetylcholinesterase target. Brain Struct. Funct. 2013, 218, 59–72. [Google Scholar]
- Singh, M.; Kaur, M.; Kukreja, H.; Chugh, R.; Silakari, O.; Singh, D. Acetylcholinesterase inhibitors as Alzheimer therapy: From nerve toxins to neuroprotection. Eur. J. Med. Chem. 2013, 70, 165–188. [Google Scholar]
- Arribas, L.A.; Lomillon, M.A.; Renedo, O.D.; Martinez, M.J. Screen-printed biosensor based on the inhibition of the acetylcholinesterase activity for the determination of codeine. Talanta 2013, 111, 8–12. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Zhou, H.; Hussain, E.; Zhang, Y.; Niu, N.; Li, Y.; Ma, Y.; Yu, C. A ratiometric fluorescence assay for acetylcholinesterase activity and inhibitor screening based on supramolecular assembly induced monomer–excimer emission transition of a perylene probe. RSC Adv. 2018, 8, 12785–12790. [Google Scholar] [PubMed]
- Dey, N.; Samanta, S.K.; Bhattacharya, S. Heparin triggered dose dependent multicolor emissions witching in water: A convenient protocol for heparinaseI estimation in real-life biological fluids. Chem. Commun. 2017, 53, 1486–1489. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.Z.; Liao, H.; Feng, L.Y.; Wang, M.; Fu, W.S. Accelerating the peroxidase-like activity of gold nanoclusters at neutral pH for colorimetric detection of heparin and heparinase. Anal. Chem. 2018, 90, 6247–6252. [Google Scholar] [PubMed]
- Li, J.; Xu, J.; Guo, W.; Zhong, W.; Li, Q.; Tana, L.; Shang, L. Ratiometric fluorescence sensors for heparin and heparinase based on enhanced excimer emission of perylene probe induced by cationic silver nanoparticles. Sens. Actuators B 2020, 305, 127422. [Google Scholar] [CrossRef]
- Chughtai, A.H.; Ahmad, N.; Younus, H.A.; Laypkov, A.; Verpoort, F. Metal–organic frameworks: Versatile heterogeneous catalysts for efficient catalytic organic transformations. Chem. Soc. Rev. 2015, 44, 6804–6849. [Google Scholar] [CrossRef] [Green Version]
- Zhou, X.; Li, J.; Tan, L.L.; Lia, Q.; Shang, L. Novel perylene probe-encapsulated metal–organic framework nanocomposites for ratiometric fluorescence detection of ATP. J. Mater. Chem. B 2020, 8, 3661–3666. [Google Scholar]
- Han, G.; Kim, D.; Park, Y.; Bouffard, J.; Kim, Y. Excimers beyond pyrene: A far-red optical proximity reporter and its application to the label-free detection of DNA. Angew. Chem. Int. Ed. 2015, 54, 3912–3916. [Google Scholar] [CrossRef]
- Chapman, J.G.; Magee, W.P.; Stukenbrok, H.A.; Beckius, G.E.; Milici, A.J.; Tracey, W.R. A novel nonpeptidic caspase-3/7 inhibitor, (S)-(+)-5-[1-(2-methoxymethylpyrrolidinyl)sulfonyl]isatin reduces myocardial ischemic injury. Eur. J. Pharmacol. 2002, 456, 59–68. [Google Scholar] [CrossRef]
- Green, D.R.; Kroemer, G. Pharmacological manipulation of cell death: Clinical applications in sight? J. Clin. Investig. 2005, 115, 2610–2617. [Google Scholar] [CrossRef] [PubMed]
- Poreba, M.; Szalek, A.; Kasperkiewicz, P.; Rut, W.; Salvesen, G.S.; Drag, M. Small molecule active site directed tools for studying human caspases. Chem. Rev. 2015, 115, 12546–12629. [Google Scholar] [PubMed] [Green Version]
- Kim, T.-I.; Jin, H.; Bae, J.; Kim, Y. Excimer emission-based fluorescent probe targeting caspase-3. Anal. Chem. 2017, 89, 10565–10569. [Google Scholar] [PubMed]
- Ryou, S.-M.; Yeom, J.-H.; Kang, H.J.; Won, M.; Kim, J.-S.; Lee, B.; Seong, M.-J.; Ha, N.-C.; Bae, J.; Lee, K. Gold nanoparticle–DNA aptamer composites as a universal carrier for in vivo delivery of biologically functional proteins. J. Control. Release 2014, 196, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Holly, T.A.; Drincic, A.; Byun, Y.; Nakamura, S.; Harris, K.; Klocke, F.J.; Cryns, V.L. Caspase inhibition reduces myocyte cell death induced by myocardial ischemia and reperfusion in vivo. J. Mol. Cell. Cardiol. 1999, 31, 1709–1715. [Google Scholar]
- Xu, C.; Zhou, R.; Zhang, R.; Yang, L.; Wang, G. Label-free DNA sequence detection through FRET from a fluorescent polymer with pyrene excimer to SG. ACS Macro Lett. 2014, 3, 845–848. [Google Scholar]
- Zheng, F.; Guo, S.; Zeng, F.; Li, J.; Wu, S. Ratiometric fluorescent probe for alkaline phosphatase based on betaine-modified polyethylenimine via excimer/monomer conversion. Anal. Chem. 2014, 86, 9873–9879. [Google Scholar] [CrossRef]
- Chao, X.-J.; Wang, K.-N.; Sun, L.-L.; Cao, Q.; Ke, Z.-F.; Cao, D.-X.; Mao, Z.-W. Cationic organochalcogen with monomer/excimer emissions for dual-color live cell imaging and cell damage diagnosis. ACS Appl. Mater. Interfaces 2018, 10, 13264–13273. [Google Scholar] [CrossRef]
- Chatterjee, S.; Gohil, H.; Raval, I.; Chatterjee, S.; Ranjan Paital, A. An anthracene excimer fluorescence probe on mesoporous silica for dual functions of detection and adsorption of mercury (II) and copper (II) with biological in vivo applications. Small 2019, 15, 1804749. [Google Scholar] [CrossRef]
- Benni, I.; Cardoso Trabuco, M.; Di Stasio, E.; Arcovito, A.; Boffi, A.; Malatesta, F.; Bonamore, A.; De Panfilis, S.; de Turris, V.; Baiocco, P. Excimer based fluorescent pyrene–ferritin conjugate for protein oligomerization studies and imaging in living cells. RSC Adv. 2018, 8, 12815–12822. [Google Scholar] [CrossRef] [Green Version]
- Singh, A.; Singh, R.; Shellaiah, M.; Chandra Prakash, E.; Chang, H.-C.; Raghunath, P.; Lin, M.-C.; Lin, H.-C. A new pyrene-based aggregation induced ratiometric emission probe for selective detections of trivalent metal ions and its living cell application. Sens. Actuators B 2015, 207, 338–345. [Google Scholar] [CrossRef]
- Huang, J.; Zhu, Z.; Bamrungsap, S.; Zhu, G.; You, M.; He, X.; Wang, K.; Tan, W. Competition-mediated pyrene-switching aptasensor: Probing lysozyme in human serum with a monomer-excimer fluorescence switch. Anal. Chem. 2010, 82, 10158–10163. [Google Scholar] [PubMed]


































| Probe | a MA | b λem (nm) | c Δλ (nm) | Analyte | d LOD | f Ref. |
|---|---|---|---|---|---|---|
| 1 | A | 480 | 120 | protease MMP-7 | e ND | [33] |
| 2 | A | 480 | 130 | diphosphorylated protein | 0.6 µM | [35] |
| 3 | A | 485 | 120 | lgE | 1.6 nM | [36] |
| 4 | B | 482 | 122 | OCl− | 0.2 µM | [37] |
| 5 | B | 470 | 120 | OCl− | 0.35 µM | [40] |
| 6 | B | 459 | 114 | intracellular pH | - | [44] |
| 7 | A | 607 | 107 | cell imaging | - | [59] |
| 8 | A | 670 | 150 | cell imaging | - | [63] |
| 9 | A | 552 | 82 | fingerprint | - | [66] |
| 10 | B | 680 | 180 | DNA MTase | 0.2 U/mL | [68] |
| 10 | B | 680 | 180 | acetylcholinesterase | 5 mU/mL | [72] |
| 10 | B | 680 | 180 | heparin and heparinase | ND | [75] |
| 10 | B | 700 | 200 | ATP | 10 µM | [77] |
| 11 | B | 664 | 198 | dsDNA | ND | [78] |
| 12 | B | 660 | 189 | caspase-3 | 0.17 pM | [82] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y. Recent Advances in Excimer-Based Fluorescence Probes for Biological Applications. Molecules 2022, 27, 8628. https://doi.org/10.3390/molecules27238628
Chen Y. Recent Advances in Excimer-Based Fluorescence Probes for Biological Applications. Molecules. 2022; 27(23):8628. https://doi.org/10.3390/molecules27238628
Chicago/Turabian StyleChen, Yi. 2022. "Recent Advances in Excimer-Based Fluorescence Probes for Biological Applications" Molecules 27, no. 23: 8628. https://doi.org/10.3390/molecules27238628
APA StyleChen, Y. (2022). Recent Advances in Excimer-Based Fluorescence Probes for Biological Applications. Molecules, 27(23), 8628. https://doi.org/10.3390/molecules27238628







