Metabolite Profiling, Antioxidant and Key Enzymes Linked to Hyperglycemia Inhibitory Activities of Satureja hispidula: An Underexplored Species from Algeria
Abstract
:1. Introduction
2. Results
2.1. Total Bioactive Content
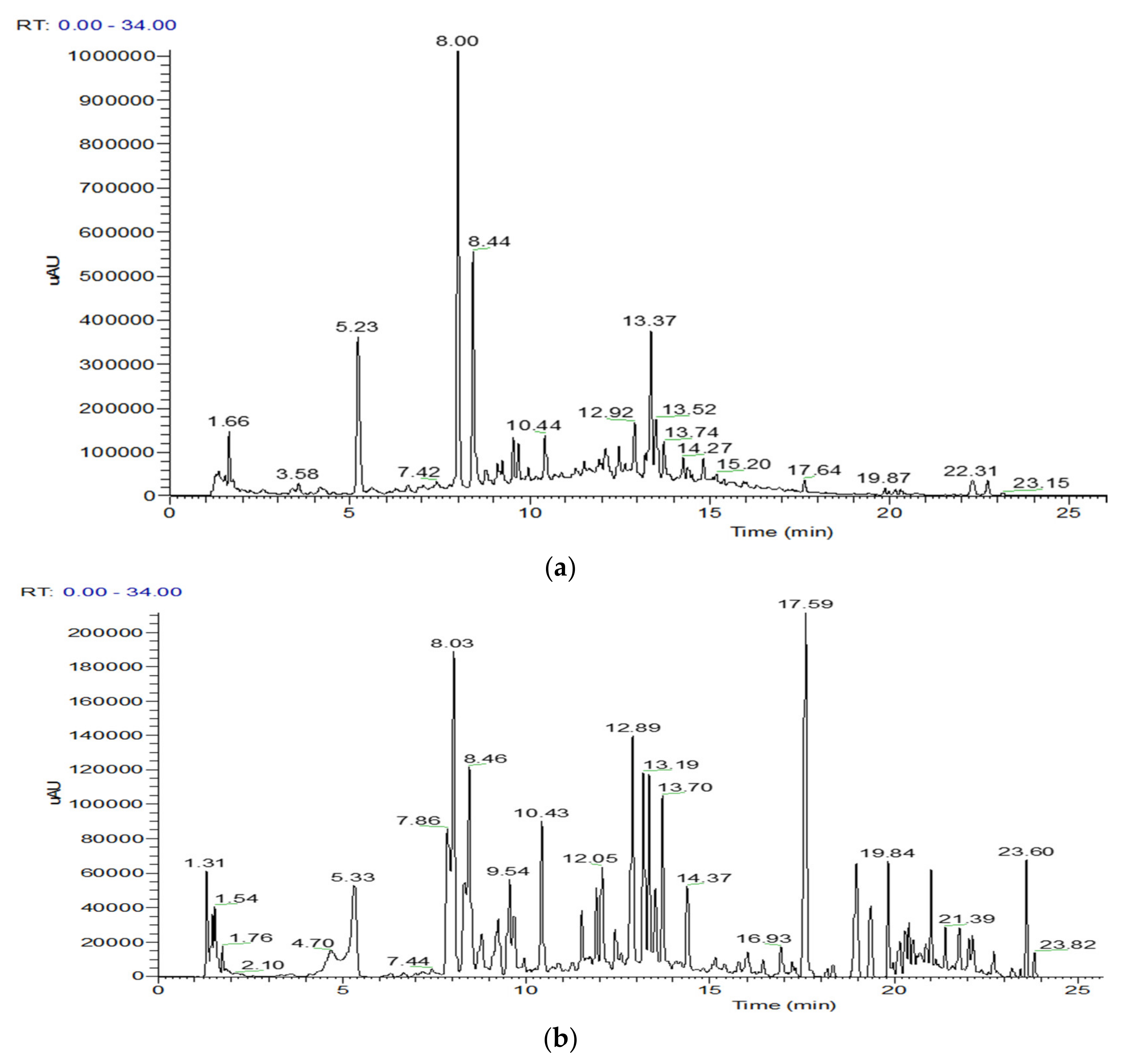
2.2. UHPLC-DAD-ESI/MS Characterization of S. hispidula Extracts
2.3. Antioxidant Activity
2.4. Inhibition of Enzymatic Activities
3. Discussion
3.1. Compound Identification
3.2. Biological Activities
4. Materials and Methods
4.1. Chemicals
4.2. Extract Preparation
4.3. Total Bioactive Content
4.4. UHPLC-DAD-ESI/MS Characterization of S. hispidula Extracts
4.5. Antioxidant Activities
4.5.1. Determination of 1,1-Diphenyl-2-Picrylhydrazyl Radical Scavenging Activity
4.5.2. Determination of 2,2′-Azinobis(3-Ethylbenzothiazoline-6-Sulfonic Acid) Scavenging Activity
4.5.3. Ferric Reducing Antioxidant Power Assay
4.6. Inhibition of Enzymatic Activities
4.6.1. Inhibition of α-Glucosidase Activity
4.6.2. Inhibition of α-Amylase Activity
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Asmat, U.; Abad, K.; Ismail, K. Diabetes mellitus and oxidative stress-A concise review. Saudi. Pharm. J. 2016, 24, 547–553. [Google Scholar] [CrossRef] [Green Version]
- Hamidpour, R.; Hamidpour, S.; Hamidpour, M.; Shalari, M.; Sohraby, M. Summer savory: From the selection of traditional applications to the novel effect in relief, prevention, and treatment of a number of serious illnesses such as diabetes, cardiovascular disease, Alzheimer’s disease, and cancer. J. Tradit. Complement. Med. 2014, 4, 140–144. [Google Scholar] [CrossRef] [Green Version]
- Benarba, B. Medicinal plants used by traditional healers from South-West Algeria: An ethnobotanical study. J. Intercult. Ethnopharmacol. 2016, 5, 320–330. [Google Scholar] [CrossRef]
- Hamza, N.; Berke, B.; Umar, A.; Cheze, C.; Gin, H.; Moore, N. A review of Algerian medicinal plants used in the treatment of diabetes. J. Ethnopharmacol. 2019, 238, 111841. [Google Scholar] [CrossRef]
- Momtaz, S.; Abdollahi, M. A systematic review of the biological activities of Satureja L. species. Pharmacologyonline 2008, 2, 34–58. [Google Scholar]
- Fierascu, I.; Dinu-Pirvu, C.E.; Fierascu, R.C.; Velescu, B.S.; Anuta, V.; Ortan, A.; Jinga, V. Phytochemical profile and biological activities of Satureja hortensis L.: A review of the last decade. Molecules 2018, 23, 2458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yousefzadi, M.; Riahi-Madvar, A.; Hadian, J.; Rezaee, F.; Rafiee, R.; Biniaz, M. Toxicity of essential oil of Satureja khuzistanica: In vitro cytotoxicity and antimicrobial activity. J. Immunotoxicol. 2014, 11, 50–55. [Google Scholar] [CrossRef] [Green Version]
- Clifford, M.N.; Johnston, K.L.; Knight, S.; Kuhnert, N. Hierarchical scheme for LC-MSn identification of chlorogenic acids. J. Agric. Food Chem. 2003, 51, 2900–2911. [Google Scholar] [CrossRef] [PubMed]
- Magaña, A.A.; Kamimura, N.; Soumyanath, A.; Stevens, J.F.; Maier, C.S. Caffeoylquinic acids: Chemistry, biosynthesis, occurrence, analytical challenges, and bioactivity. Plant. J. 2021, 107, 1299–1319. [Google Scholar] [CrossRef]
- Rechek, H.; Haouat, A.; Hamaidia, K.; Allal, H.; Boudiar, T.; Pinto, D.C.G.A.; Cardoso, S.M.; Bensouici, C.; Soltani, N.; Silva, A.M.S. Chemical composition and antioxidant, anti-inflammatory, and enzyme inhibitory activities of an endemic species from Southern Algeria: Warionia saharae. Molecules 2021, 26, 5257. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Dong, Z.; Wu, H.; Ding, W.; Zhao, M.; Shi, Q.; Wang, Q. Comparative studies on ophiopogonis and liriopes based on the determination of 11 bioactive components using LC–MS/MS and hierarchical clustering analysis. Food Res. Int. 2014, 57, 15–25. [Google Scholar] [CrossRef]
- Li, X.; Yin, D.; Sun, Y. Identification of the metabolite of ophiopogonanone A by liquid chromatography/quadrupole time-of-flight mass spectrometry. Rapid Commun. Mass Spectrom. 2022, 36, e9311. [Google Scholar] [CrossRef]
- Ye, M.; Guo, D. Analysis of homoisoflavonoids in Ophiopogon japonicus by HPLC-DAD-ESI-MSn. Am. Soc. Mass Spectrom. Elsevier Inc. 2005, 16, 234–243. [Google Scholar] [CrossRef] [Green Version]
- March, R.E.; Miao, X.-S. A fragmentation study of kaempferol using electrospray quadrupole time-of-flight mass spectrometry at high mass resolution. Int. J. Mass Spectrom. 2004, 231, 157–167. [Google Scholar] [CrossRef]
- Kerebba, N.; Oyedeji, A.O.; Byamukama, R.; Kuria, K.S.; Oyedeji, O.O. UHPLC-ESI-QTOF-MS/MS Characterisation of phenolic compounds from Tithonia diversifolia (Hemsl) A. Gray and antioxidant activity. Chemistrselect 2022, 7, e202104406. [Google Scholar] [CrossRef]
- Cao, J.; Yin, C.; Qin, Y.; Cheng, Z.; Chen, D. Approach to the study of flavone di-C-glycosides by HPLC tandem ion trap MS and its application to characterization of flavonoid composition in Viola yedoensis. J. Mass Spectrom. 2014, 49, 1010–1024. [Google Scholar] [CrossRef] [PubMed]
- Oliva, F.; Fanti, F.; Palmieri, S.; Viteritti, E.; Eugelio, F.; Pepe, A.; Compagnone, D.; Serg, M. Predictive multi experiment approach for the determination of conjugated phenolic compounds in vegetal matrices by means of LC-MS/MS. Molecules 2022, 27, 3089. [Google Scholar] [CrossRef] [PubMed]
- Grati, W.; Samet, S.; Bouzyani, B.; Ayachi, A.; Treilhou, M.; Téné, N.; Mezghani-Jarraya, R. HESI-MS/MS Analysis of phenolic compounds from Calendula aegyptiaca fruits extracts and evaluation of their antioxidant activities. Molecules 2022, 27, 2314. [Google Scholar] [CrossRef] [PubMed]
- Bekkai, D.; Ouled el Majdoub, Y.; Bekkai, H.; Cacciola, F.; Miceli, N.; Taviano, M.F.; Cavo, E.; Errabii, T.; Vinci, R.L.; Mondello, L.; et al. Determination of the phenolic profileby liquid chromatography, evaluation of antioxidant activity and toxicity of Moroccan Erica multifora, Erica scoparia, and Calluna vulgaris (Ericaceae). Molecules 2022, 27, 3979. [Google Scholar] [CrossRef]
- Barros, L.; Dueñas, M.; Dias, M.I.; Sousa, M.J.; Santos-Buelga, C.; Ferreira, I.C. Phenolic profiles of cultivated, in vitro cultured and commercial samples of Melissa officinalis L. infusions. Food Chem. 2013, 136, 1–8. [Google Scholar] [CrossRef]
- Li, F.; Zhang, Y.B.; Wei, X.; Song, C.H.; Qlao, M.Q.; Zhang, H.Y. Metabolic profiling of Shu-Yu capsule in rat serum based on metabolic fingerprinting analysis using HPLC-ESI-MSn. Mol. Rep. 2016, 13, 4191–4204. [Google Scholar] [CrossRef]
- Yao, H.; Chen, B.; Zhang, Y.; Ou, H.; Li, Y.; Li, S.; Shi, P.; Lin, X. Analysis of the total biflavonoids extract from Selaginella doederleinii by HPLC-QTOF-MS and its in vitro anticancer effects. Molecules 2017, 22, 325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdelaziz, S.; Al yousef, H.M.; Al-Qahtani, A.S.; Hassa, W.H.B.; Fantouk, O.I.; El-Sayed, M.A. Phytochemical profile, antioxidant and cytoxic potential of Parkinsonia aculeata L. growing in Saudi Arabia. Saudi Pharm. J. 2020, 28, 1129–1137. [Google Scholar] [CrossRef]
- Gouveia, S.C.; Castilho, C.P. Characterisation of phenolic compounds in Helichrysum melaleucum by HPLC with on-line UV and MS detection. Rapid Commun. Mass Spectrom. 2010, 24, 1851–1868. [Google Scholar] [CrossRef] [PubMed]
- Zeghad, N.; Ahmed, E.; Belkhiri, A.; Heyden, Y.V.; Demeyer, K. Antioxidant activity of Vitis vinifera, Punica granatum, Citrus aurantium and Opuntia ficus indica fruits cultivated in Algeria. Heliyon 2019, 5, e01575. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Li, K.; Xie, H.; Xie, Y.; Li, Y.; Zhao, X.; Jiang, X.; Chen, D. Antioxidant and cytoprotective effects of the di-O-caffeoylquinic acid family: The mechanism, structure-activity relationship, and conformational effect. Molecules 2018, 23, 222. [Google Scholar] [CrossRef] [Green Version]
- Hamed, Y.S.; Abdin, M.; Chen, G.; Akhtar, H.M.S.; Zeng, X. Effects of impregnate temperature on extraction of caffeoylquinic acid derivatives from Moringa oleifera leaves and evaluation of inhibitory activity digestive enzyme, antioxidant, antiproliferative and antibacterial activities of the extract. Int. J. Food Sci. 2020, 55, 3082–3090. [Google Scholar] [CrossRef]
- Trendafilova, A.; Genova, V.; Rangelov, M.; Todorova, M.; Ozek, G.; Yur, S.; Ozek, T.; Aneva, I.; Veleva, R.; Moskova-Doumanova, V.; et al. Caffeoylquinic acids, cytotoxic, antioxidant, acetylcholinesterase and tyrosinase enzyme inhibitory activities of six Inula species from Bulgaria. Chem. Biodivers. 2020, 17, e2000051. [Google Scholar] [CrossRef] [PubMed]
- World Health Organisation (WHO). Diabetes. 2020. Available online: https://www.who.int/health-topics/diabetes#tab=tab_1 (accessed on 2 November 2022).
- Ogurtsova, K.; Fernandes, J.D.R.; Huang, Y.; Linnenkamp, Y.; Guariguata, L.; Cho, N.H.; Cavan, D.; Shaw, J.E.; Makarov, L.E. IDF Diabetes atlas: Global estimates for the prevalence of diabetes for 2015 and 2040. Elsevier 2017, 128, 40–50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van de Laar, F.A. Alpha-glucosidase inhibitors in the early treatment of type 2 diabetes. Vasc. Health Risk Manag. 2008, 4, 1189–1195. [Google Scholar] [CrossRef] [Green Version]
- Gupta, P.D.; De, A. Diabetes mellitus and its herbal treatment. IJRPBS 2012, 3, 706–721. [Google Scholar]
- Chen, W.-C.; Wang, S.-W.; Li, C.-W.; Lin, H.-R.; Yang, C.-S.; Chu, Y.-C.; Lee, T.-H.; Chen, J.-J. Comparison of various solvent extracts and major bioactive components from Portulaca oleracea for antioxidant, anti-tyrosinase, and anti-α-glucosidase activities. Antioxidants 2022, 11, 398. [Google Scholar] [CrossRef] [PubMed]
- Kwon, Y.-I.; Apostolidis, E.; Shetty, K. Inhibitory potential of wine and tea against α-amylase and α-glucosidase for management of hyperglyemia linked to type 2 diabetes. J. Food Biochem. 2006, 32, 15–31. [Google Scholar] [CrossRef]
- Kim, K.-T.; Rioux, L.-E.; Turgeon, S.L. Alpha-amylase and alpha-glucosidase inhibition is differentially modulated by fucoidan obtained from Fucus vesiculosus and Ascophyllum nodosum. Phytochemistry 2014, 98, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Rahimi-Madiseh, M.; Malekpour-Tehrani, A.; Bahmani, M.; Rafieian-Kopaei, M. The research and development on the antioxidants in prevention of diabetic complications. Asian Pacific J. Tropical Med. 2016, 9, 825–831. [Google Scholar] [CrossRef] [Green Version]
- Pieme, C.A.; Tatangmo, J.A.; Simo, G.; Nya, P.C.B.; Moor, V.J.A.M.; Moukette, B.M.; Nzufo, F.T.; Nono, B.L.N.; Sobngwi, E. Relationship between hyperglycemia, antioxidant capacity and some enzymatic and non-enzymatic antioxidants in African patients with type 2 diabetes. BMC Res. Notes 2017, 10, 141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iacobini, C.; Vitale, M.; Pesce, C.; Pugliese, G.; Menini, S. Diabetic complications and oxidative stress: A 20-year voyage back in time and back to the future. Antioxidants 2021, 10, 727. [Google Scholar] [CrossRef]
- Ramakrishna, V.; Jailkhani, R. Evaluation of oxidative stress in insulin dependent diabetes mellitus (IDDM) patients. Diagn. Pathol. 2007, 2, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventos, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin Ciocalteu reagent. Elsevier 1999, 299, 152–178. [Google Scholar] [CrossRef]
- Türkoglu, A.; Duru, M.E.; Mercan, N.; Kivrak, I.; Gezer, K. Antioxidant and antimicrobial activities of Laetiporus sulphureus (Bull.) Murrill. Food Chem. 2007, 101, 267–273. [Google Scholar] [CrossRef]
- Neto, R.; Marçal, C.; Queirós, A.; Abreu, H.; Silva, A.; Cardoso, S. Screening of Ulva rigida, Gracilaria sp., Fucus vesiculosus and Saccharina latissima as functional ingredients. Int. J. Mol. Sci. 2018, 19, 2987. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wasli, H.; Jelali, N.; Silva, A.M.S.; Ksouri, R.; Cardoso, S.M. Variation of polyphenolic composition, antioxidants and physiological characteristics of dill (Anethum graveolens L.) as affected by bicarbonate-induced iron deficiency conditions. Ind. Crops Prod. 2018, 126, 466–476. [Google Scholar] [CrossRef]
- Catarino, M.D.; Silva, A.M.S.; Cruz, M.T.; Cardoso, S.M. Antioxidant and anti-inflammatory activities of Geranium robertianum L. decoctions. Food Funct. 2018, 8, 3355–3365. [Google Scholar] [CrossRef] [PubMed]
- Pereira, O.R.; Catarino, M.D.; Afonso, F.; Silva, A.M.S.; Cardoso, S.M. Salvia elegans, Salvia greggii and Salvia officinalis decoctions: Antioxidant activities and inhibition of carbohydrate and lipid metabolic enzymes. Molecules 2018, 23, 3169. [Google Scholar] [CrossRef] [PubMed]
| Sample | TPC (mg GAE/g of Extract) | TFC (mg QE/g of Extract) |
|---|---|---|
| S. hispidula EE | 85.7 ± 0.46 | 22.9 ± 0.21 |
| S. hispidula AE | 131.24 ± 0.81 | 22.5 ± 1.55 |
| N° | Rt (min) | [M-H]− | MS2 Fragments | Assigned Identification | Quantification (µg/mg of Extract) | |
|---|---|---|---|---|---|---|
| EE | AE | |||||
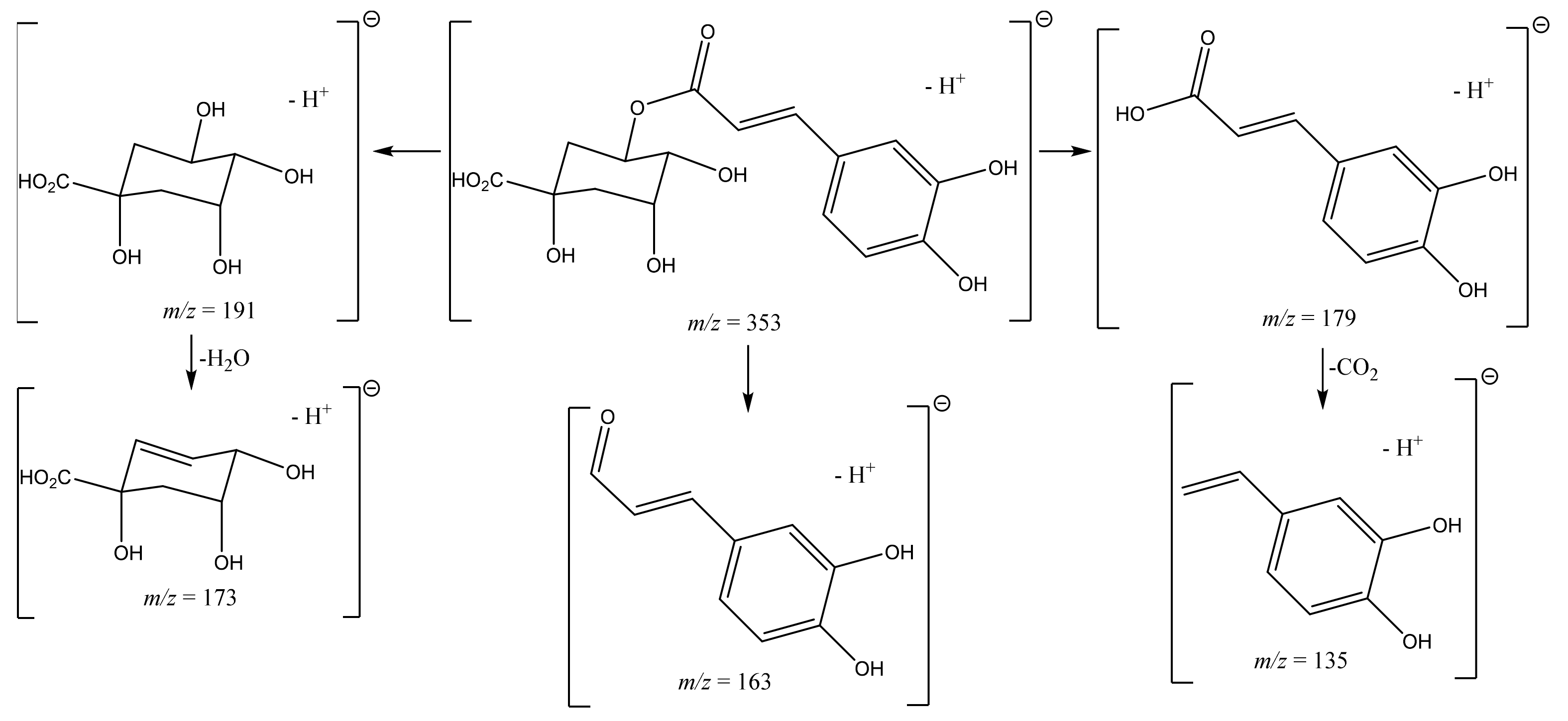
| 1 | 5.23 5.33 | 353 | 191 [M-H-caffeoyl]− 179 [M-H-quinic]− 173 [M-H-caffeoyl-H2O]− 135 [M-H-quinic-CO2]− | 3-O-CQA | 4.54 ± 0.49 | 13.39 ± 2.39 |
| 2 | 7.86 | 353 | 191 [M-H-caffeoyl]− 179 [M-H-quinic]− 163 [M-H-O-quinic]− 135 [M-H-quinic-CO2]− | 5-O-CQA * | 3.02 ± 0.00 | / |
| 3 | 8.00 8.03 | 353 | 191 [M-H-caffeoyl]− 179 [M-H-quinic]− 173 [M-H-caffeoyl-H2O]− 163 [M-H-O-quinic]− 135 [M-H-quinic-CO2]− | 5-O-CQA | 9.85 ± 2.04 | 26.05 ± 4.89 |
| 4 | 8.33 | 353 | 191 [M-H-caffeoyl]− 179 [M-H-quinic]− 163 [M-H-O-quinic]− | 4-O-CQA * | 1.46 ± 0.60 | / |
| 5 | 8.44 8.46 | 353 | 191 [M-H-caffeoyl]− 179 [M-H-quinic]− 173 [M-H-caffeoyl-H2O]− 163 [M-H-O-quinic]− | 4-O-CQA | 6.32 ± 1.14 | 13.97 ± 2.52 |
| 6 | 8.85 | 179 | 135 [M-H-CO2]− | Caffeic acid | 0.55 ± 0.04 | NQ |
| 7 | 9.12 | 225 | 97 | Unknown | NQ | NQ |
| 8 | 9.34 | 609 | 447 [M-H-glucosyl]− 285 [M-H-diglucosyl]− | Kaempferol-O-diglucoside | 0.69 ± 0.23 | 1.91 ± 0.65 |
| 9 | 9.58 | 353 | 191 [M-H-caffeoyl]− 179 [M-H-quinic]− 173 [M-H-caffeoyl-H2O]− 135 [M-H-quinic-CO2]− | 1-O-CQA | NQ | NQ |
| 10 | 9.64 | 667 | 505 [M-H-glucosyl]− 463 [M-H-6″-malonylglucoside]− 301 [quercetin moiety]− | Quercetin 3-O-(6″-malonylglucoside)-7-O-glucoside | 2.84 ± 0.28 | / |
| 11 | 9.77 | 593 | 575 [M-H-H2O]− 503 [M-H-C3H6O3]− 473 [M-H-C4H8O4]− 431 [M-H-glucosyl]− 268 [apigenin moiety]− | Apigenin 6,8 di-C-glucoside | 1.59 ± 0.17 | 4.44 ± 0.78 |
| 12 | 10.43 10.44 | 651 | 531 [M-H-C4H8O4]− 489 [M-H-glucosyl]− 447 [M-H-glucosyl-CH3CO]− 285 [kaempferol moiety]− | Kaempferol 3-O-(6”-acetylglucoside)-7-O-glucoside | 4.05 ± 0.40 | 5.11 ± 1.57 |
| 13 | 11.63 | 609 | 462 [M-H-rhamnosyl]− 342 [M-H-rhamnosyl-C4H8O4]− 301 [quercetin moiety]− | Rutin | 0.52 ± 0.13 | 2.21 ± 0.80 |
| 14 | 12.03 | 463 | 301 [quercetin moiety]− | Quercetin-glucoside | 1.07 ± 0.20 | 3.38 ± 0.93 |
| 15 | 12.15 | 461 | 285 [luteolin moiety]− | Luteolin-7-O-glucuronide | / | 6.27 ± 0.75 |
| 16 | 12.19 | 447 | 327 [M-H-C3H6O3]− 285 [luteolin moiety]− 178 0,4B− 151 1,3A− | Luteolin-7-O-glucoside | 2.12 ± 0.30 | / |
| 17 | 12.89 12.92 | 515 | 353 [M-H-caffeoyl]− 335 [M-H-caffeoyl-H2O]− 309 [M-H-caffeoyl-CO2]− 191 [quinic moiety]− 179 [caffeoyl moiety]− | 4,5-O-diCQA | 7.43 ± 0.39 | 4.98 ± 0.05 |
| 18 | 13.19 | 447 | 285 [kaempferol moiety]− | Kaempferol-O-glucoside | 9.06 ± 0.79 | / |
| 19 | 13.34 13.37 | 505 | 462 [M-H-CH3CO]− 301 [quercetin moiety]− | Quercetin-O-acetyl glucoside | 5.72 ± 0.53 | 3.44 ± 1.01 |
| 20 | 13.49 13.52 | 445 | 269 [apigenin moiety]− | Apigenin 7-O-glucuronide | 1.20 ± 0.37 | 9.47 ± 4.09 |
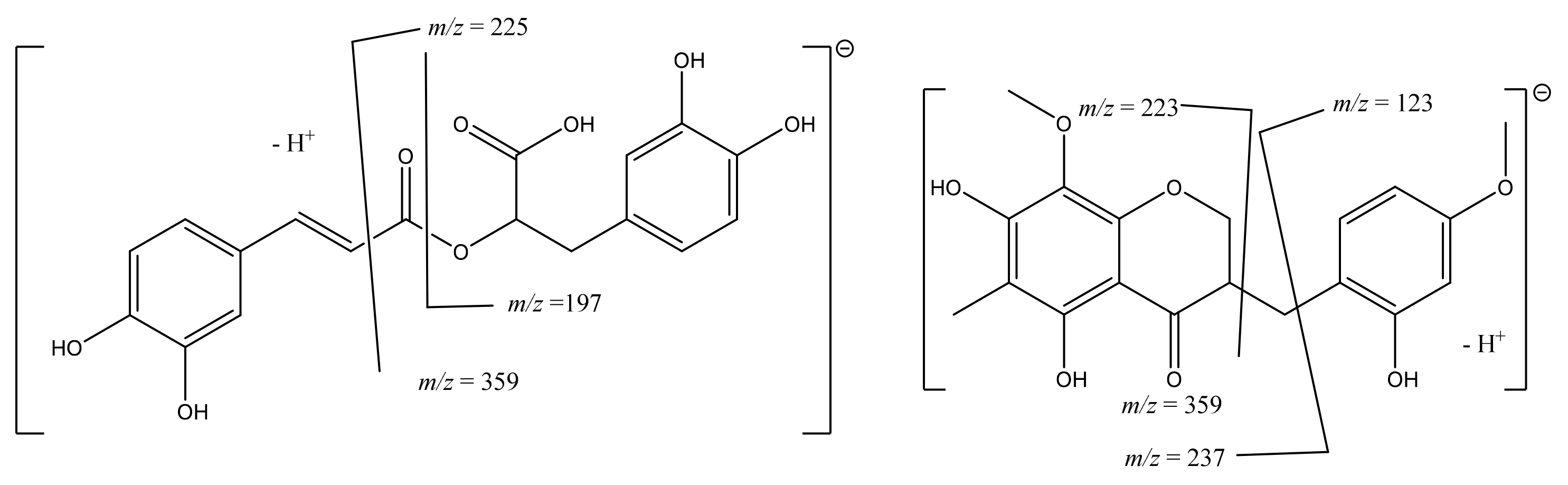
| 21 | 13.70 13.74 | 359 | 315 [M-H-CO2]− 225 [M-H-C8H8O2]− 197 [M-H-caffeoyl]− 181 [M-H-O-caffeoyl]− 179 [caffeoyl moiety]− | Rosmarinic acid | 8.73 ± 0.29 | 2.18 ± 0.49 |
| 22 | 14.37 | 489 | 447 [M-H-CH3CO]− 285 [luteolin moiety]− 151 1,3A− | Luteolin-O-acetylglucoside | 1.43 ± 0.19 | / |
| 23 | 14.89 | 537 | 519 [M-H-H2O]− 269 [apigenin moiety]− | 2,8”-Biapigenin | / | 0.73 ± 0.09 |
| 24 | 17.59 17.64 | 359 | 344 [M-H-CH3]− 237 [M-H-C7H6O2]− 223 [M-H-C8H8O2]− 221 [M-H-C8H10O2]− 123 [3-methoxyphenyl moiety]− | Ophiopogonanone E | NQ | NQ |
| 25 | 19.05 | 329 | 314 [M-H-CH3]− 301 [quercetin moiety]− 299 [M-H-2CH3]− | Dimethylquercetin | 2.72 ± 0.27 | / |
| 26 | 19.53 | 359 | 344 [M-H-CH3]− 237 [M-H-C7H6O2]− 223 [M-H-C8H8O2]− | Ophiopogonanone E isomer | NQ | / |
| 27 | 19.84 19.87 | 309 | 291 [M-H-H2O]− 265 [M-H-CO2]− | Oxodihydroxy octadecenoic acid | NQ | NQ |
| 28 | 21.09 | 329 | 311 [M-H-H2O]− 285 [M-H-CO2]− | Trihydroxy octadecenoic acid | NQ | / |
| 29 | 21.39 | 343 | 328 [M-H-CH3]− 196 1,3A− | 5,2’-Dihydroxy-7,8,6’-trimethoxyflavone | 0.71 ± 0.11 | / |
| 30 | 21.81 | 311 | 293 [M-H-H2O]− 267 [M-H-CO2]− | Dihydroxy octadecadienoic acid | NQ | / |
| 31 | 23.60 | 339 | 339, 275, 183 | Unknown | NQ | / |
| Sample | DPPH● | ABTS●+ | FRAP |
|---|---|---|---|
| IC50 (μg/mL) | A0.5 | ||
| S. hispidula EE | 5.67 ± 0.07 | 5.95 ± 0.82 | 10.77 ± 0.12 |
| S. hispidula AE | 2.43 ± 0.11 | 2.16 ± 0.1 | 5.82 ± 0.17 |
| Ascorbic acid | 3.86 ± 0.23 | 4.01 ± 0.09 | 10.03 ± 0.22 |
| Sample | Enzyme Inhibitory Activity | |
|---|---|---|
| α-Glucosidase IC50 (μg/mL) | α-Amylase (% inhibition) | |
| S. hispidula EE | 106.94 ± 1.55 a | 30.34 ± 4.58 |
| S. hispidula AE | 23.52 ± 6.33 b | 4.86 ± 0.04 |
| Acarbose | 405.77 ± 34.83 c | nd * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haouat, A.; Rechek, H.; Pinto, D.C.G.A.; Cardoso, S.M.; Válega, M.S.G.A.; Boudjerda, A.; Silva, A.M.S.; Mekkiou, R. Metabolite Profiling, Antioxidant and Key Enzymes Linked to Hyperglycemia Inhibitory Activities of Satureja hispidula: An Underexplored Species from Algeria. Molecules 2022, 27, 8657. https://doi.org/10.3390/molecules27248657
Haouat A, Rechek H, Pinto DCGA, Cardoso SM, Válega MSGA, Boudjerda A, Silva AMS, Mekkiou R. Metabolite Profiling, Antioxidant and Key Enzymes Linked to Hyperglycemia Inhibitory Activities of Satureja hispidula: An Underexplored Species from Algeria. Molecules. 2022; 27(24):8657. https://doi.org/10.3390/molecules27248657
Chicago/Turabian StyleHaouat, Ammar, Habiba Rechek, Diana C. G. A. Pinto, Susana M. Cardoso, Mónica S. G. A. Válega, Abdelhamid Boudjerda, Artur M. S. Silva, and Ratiba Mekkiou. 2022. "Metabolite Profiling, Antioxidant and Key Enzymes Linked to Hyperglycemia Inhibitory Activities of Satureja hispidula: An Underexplored Species from Algeria" Molecules 27, no. 24: 8657. https://doi.org/10.3390/molecules27248657





