Purification Process and In Vitro and In Vivo Bioactivity Evaluation of Pectolinarin and Linarin from Cirsium japonicum
Abstract
:1. Introduction
2. Results and Discussion
2.1. Macroporous Resin Enrichment Process
2.1.1. Selection of Macroporous Resins
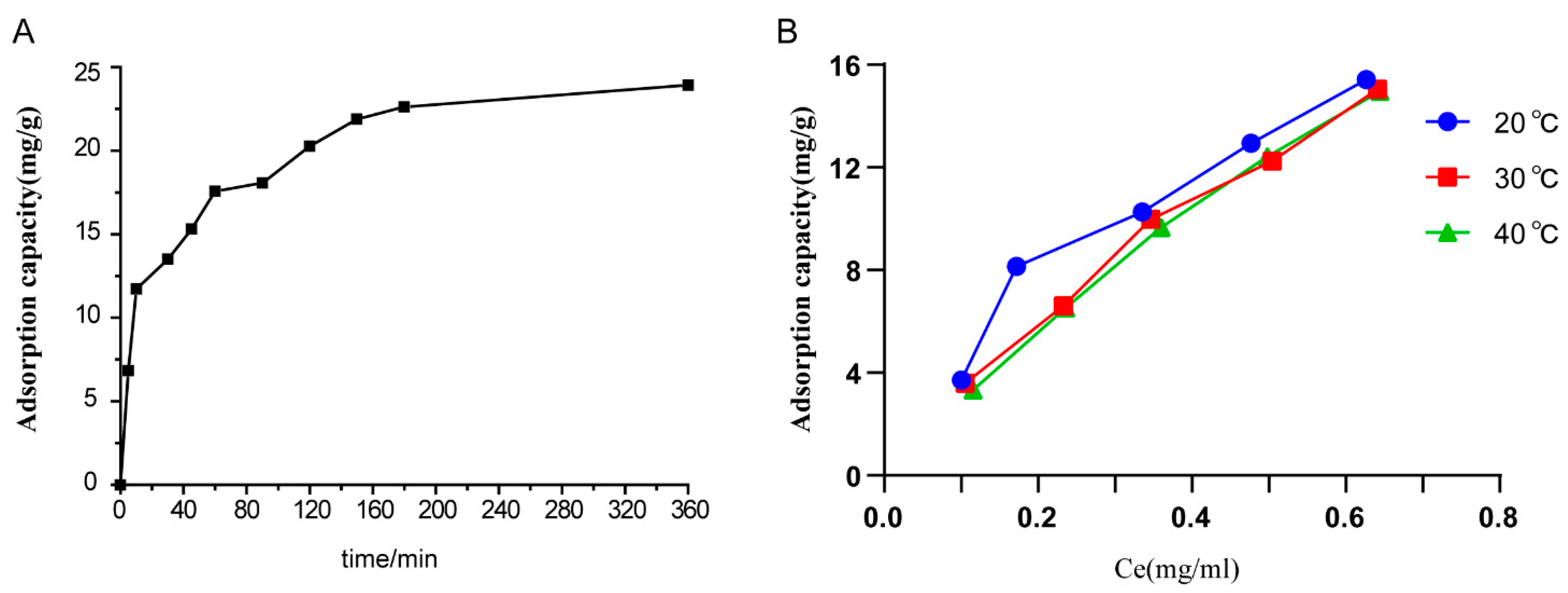
2.1.2. Adsorption Kinetics of Resins
2.1.3. Adsorption Isotherms on Resins
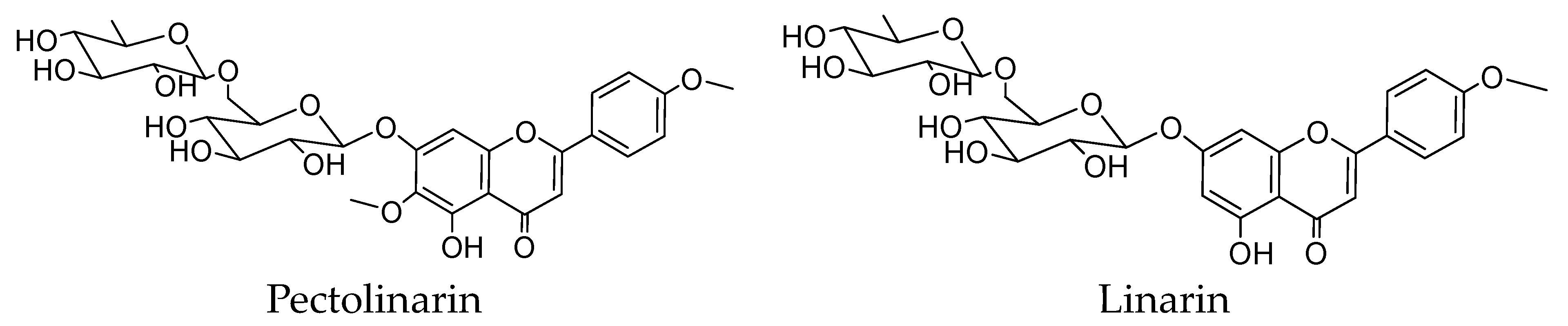
2.2. Purification and Identification of Pectolinarin and Linarin
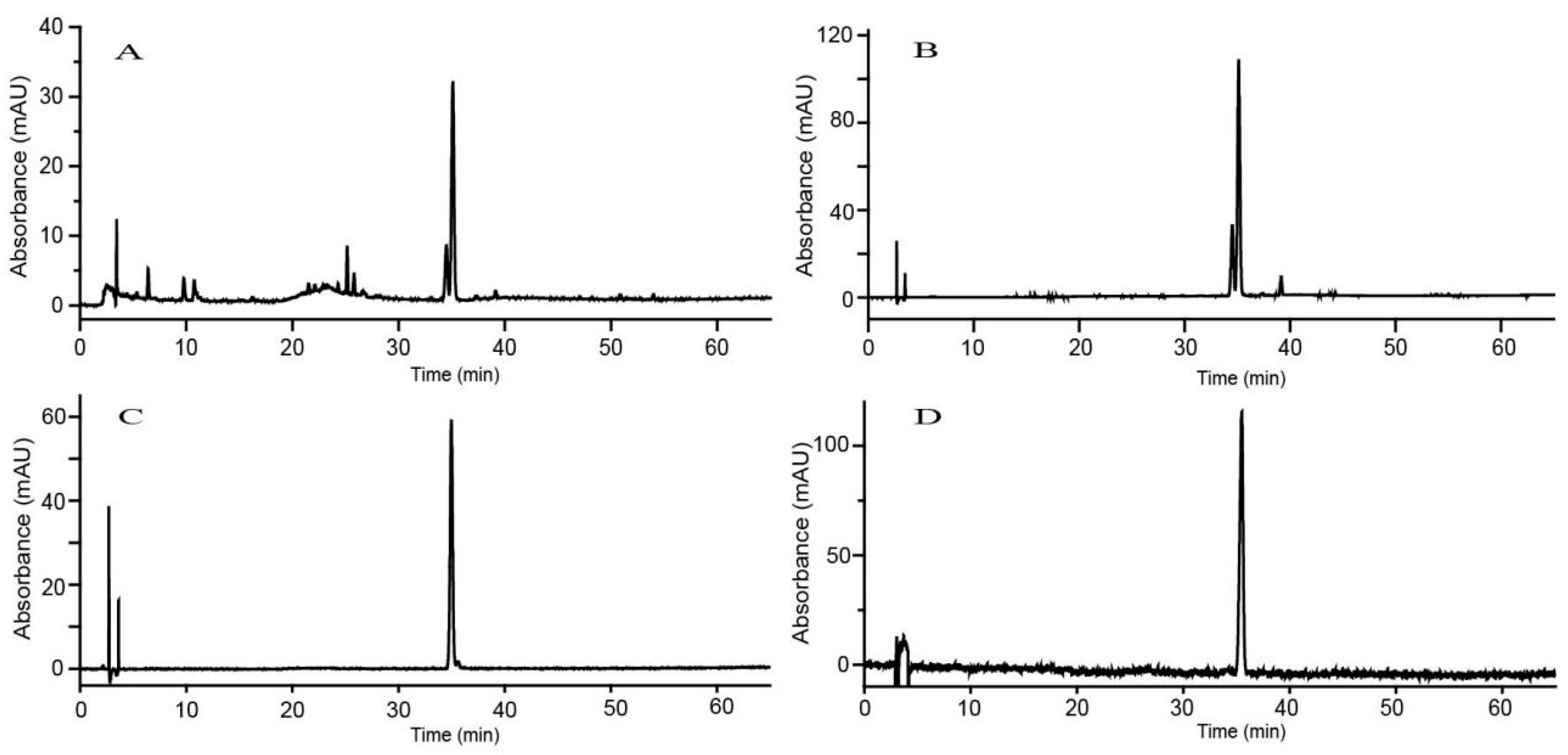
2.2.1. HPLC Analysis
2.2.2. Purification and Confirmation of Linarin and Pectolinarin
2.3. Bioactivity Assay In Vitro
2.3.1. ORAC Assay
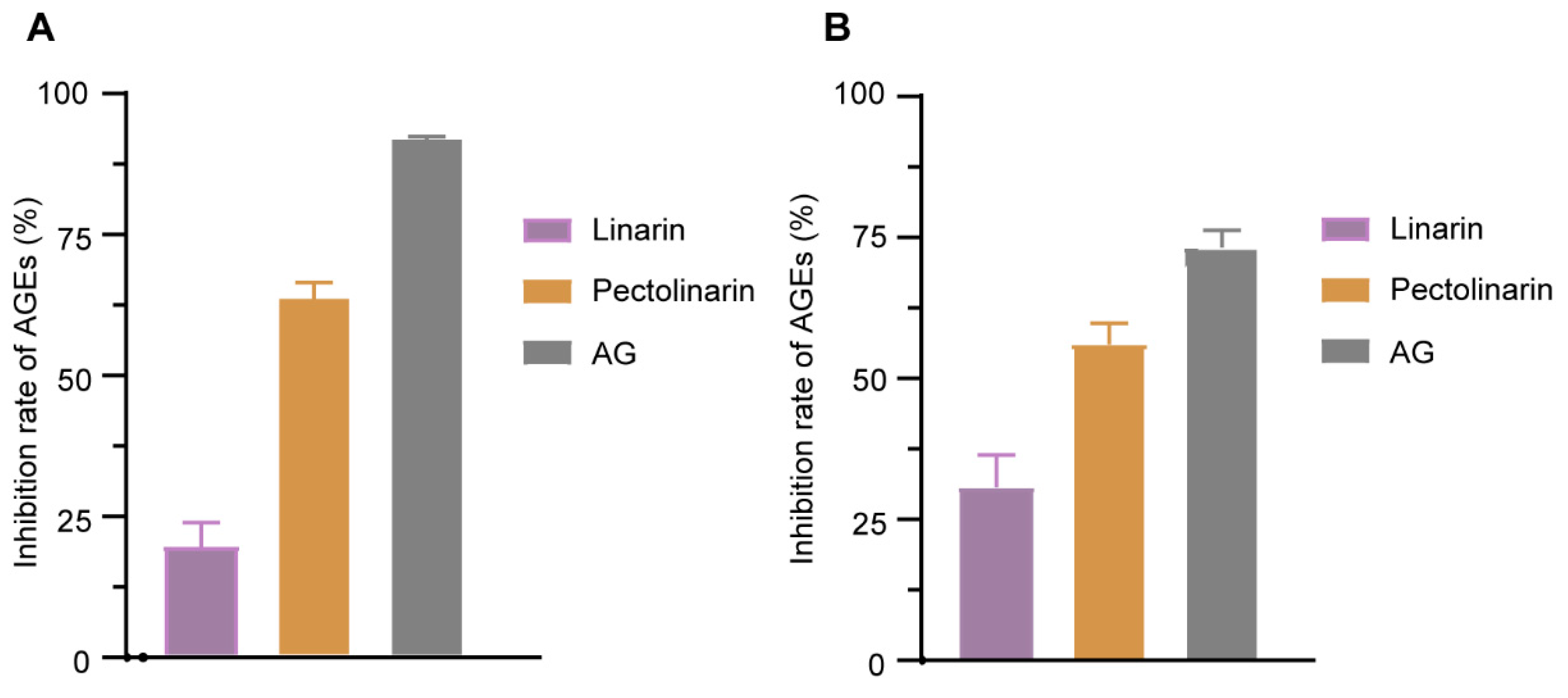
2.3.2. Anti-AGEs Assay
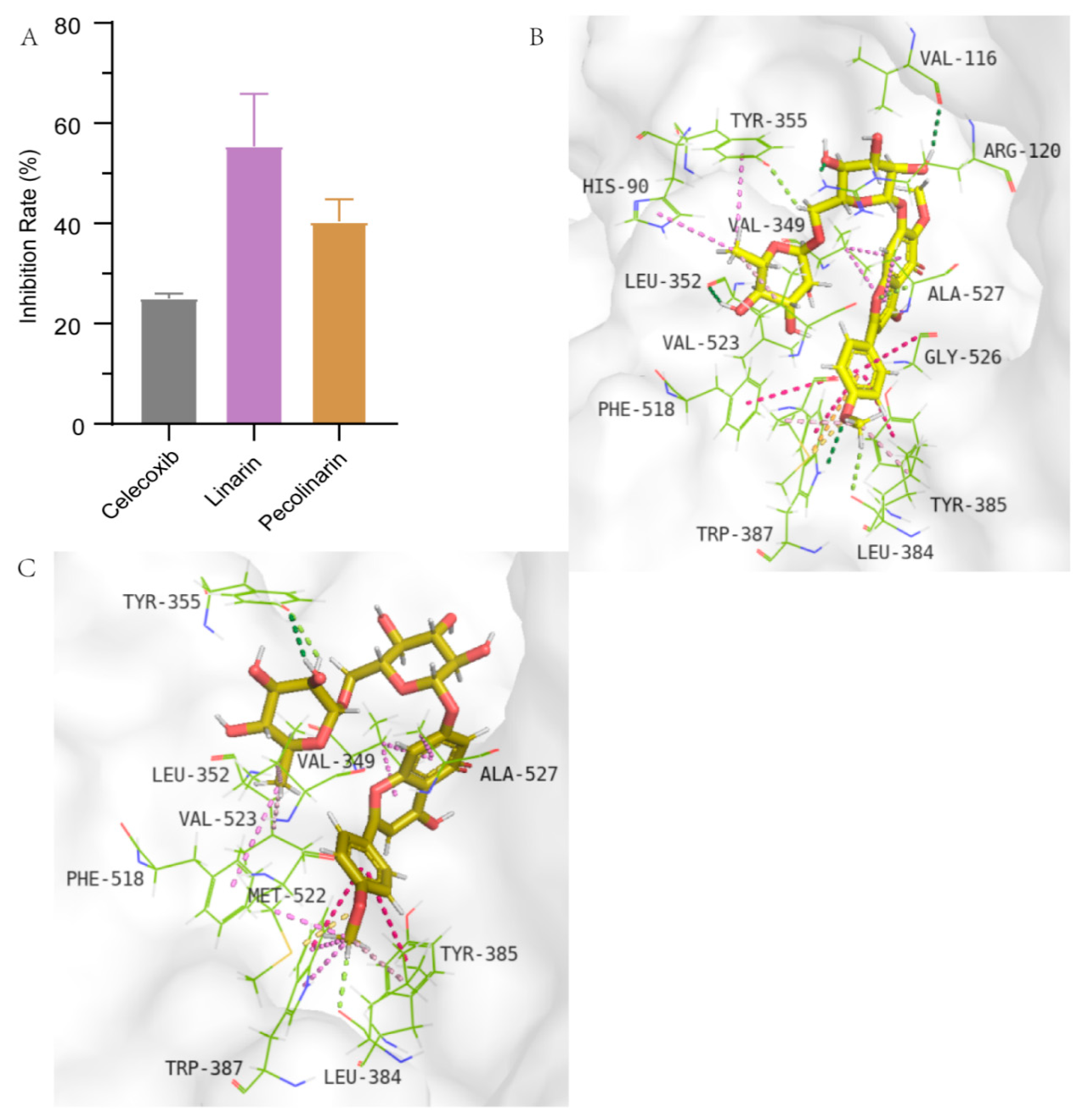
2.3.3. COX-2 Inhibition Assay
2.4. Bioassay In Vivo
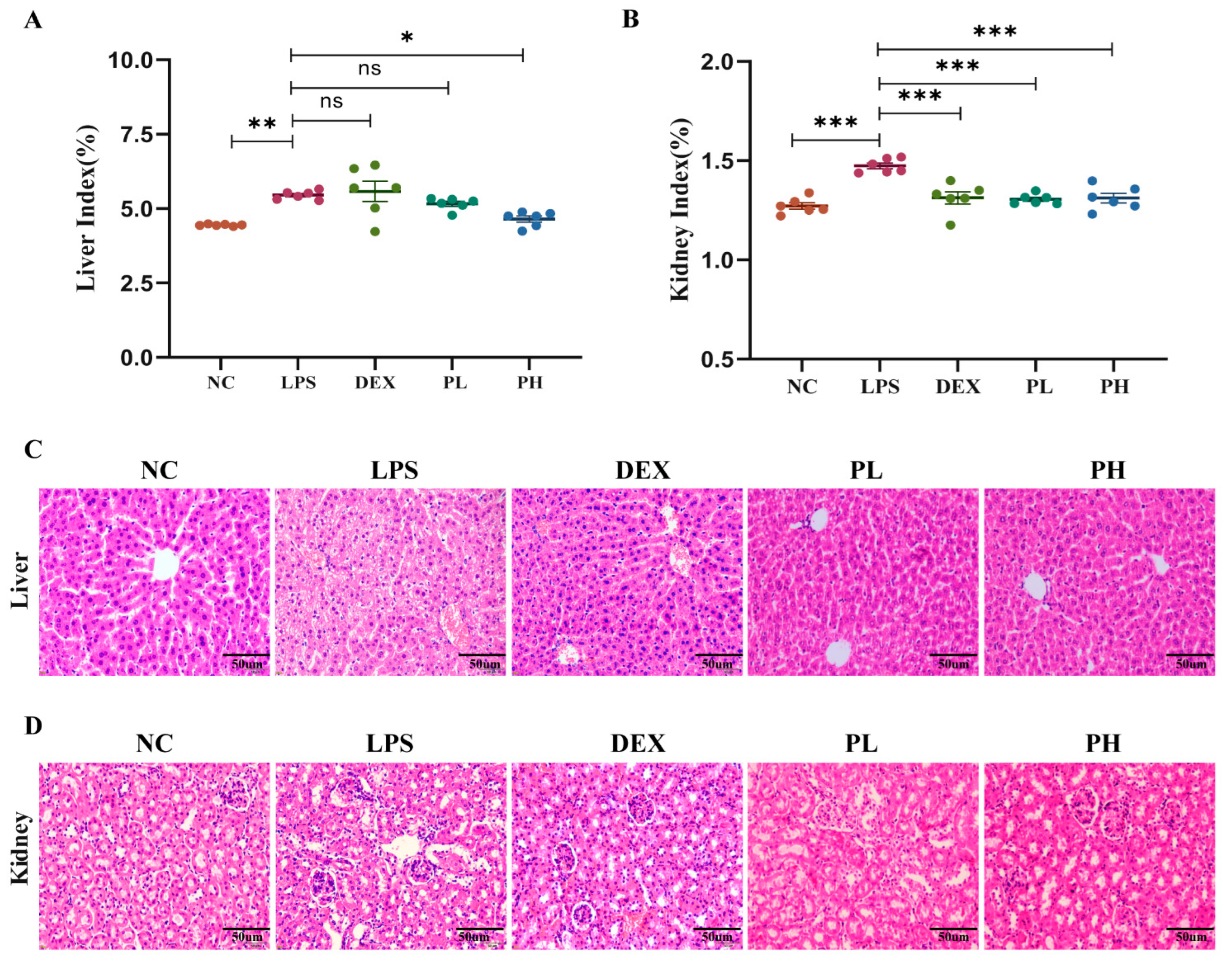
2.4.1. Pectolinarin Alleviates LPS-Induced Liver and Kidney Histopathological Injuries
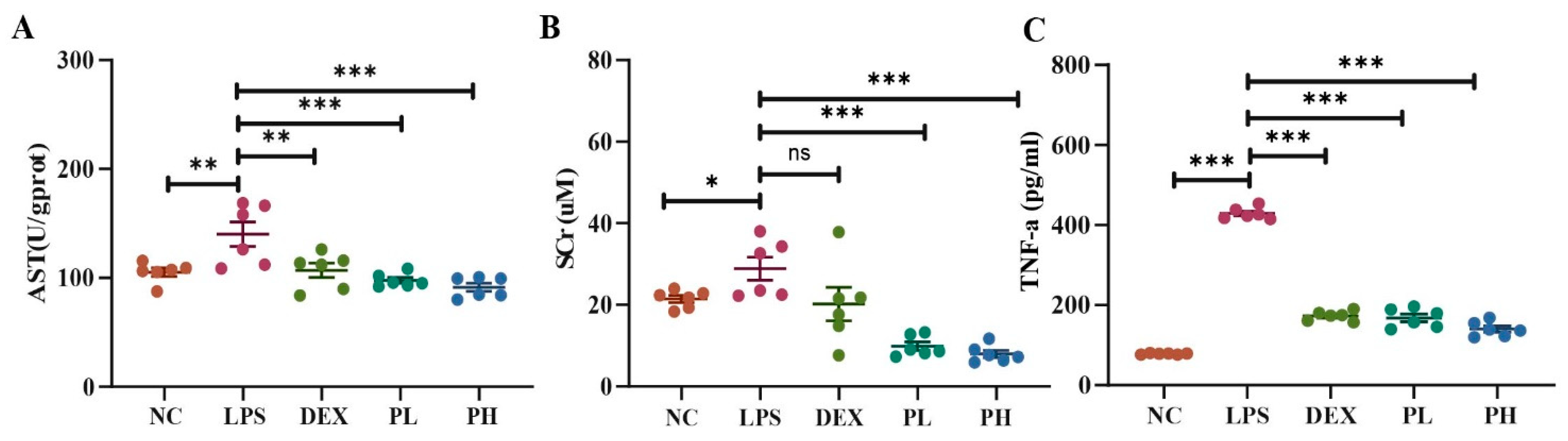
2.4.2. Biochemical Assessment
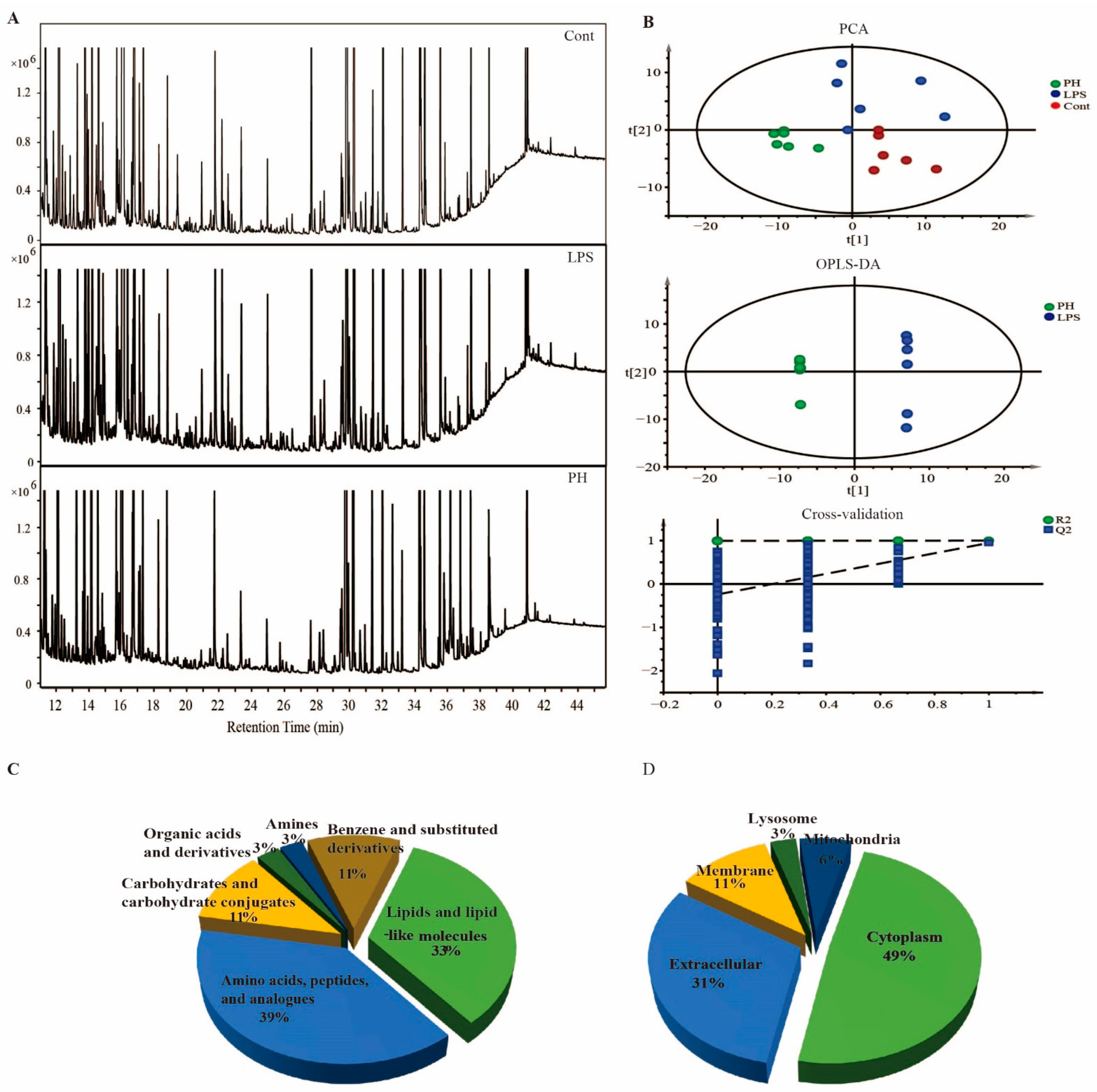
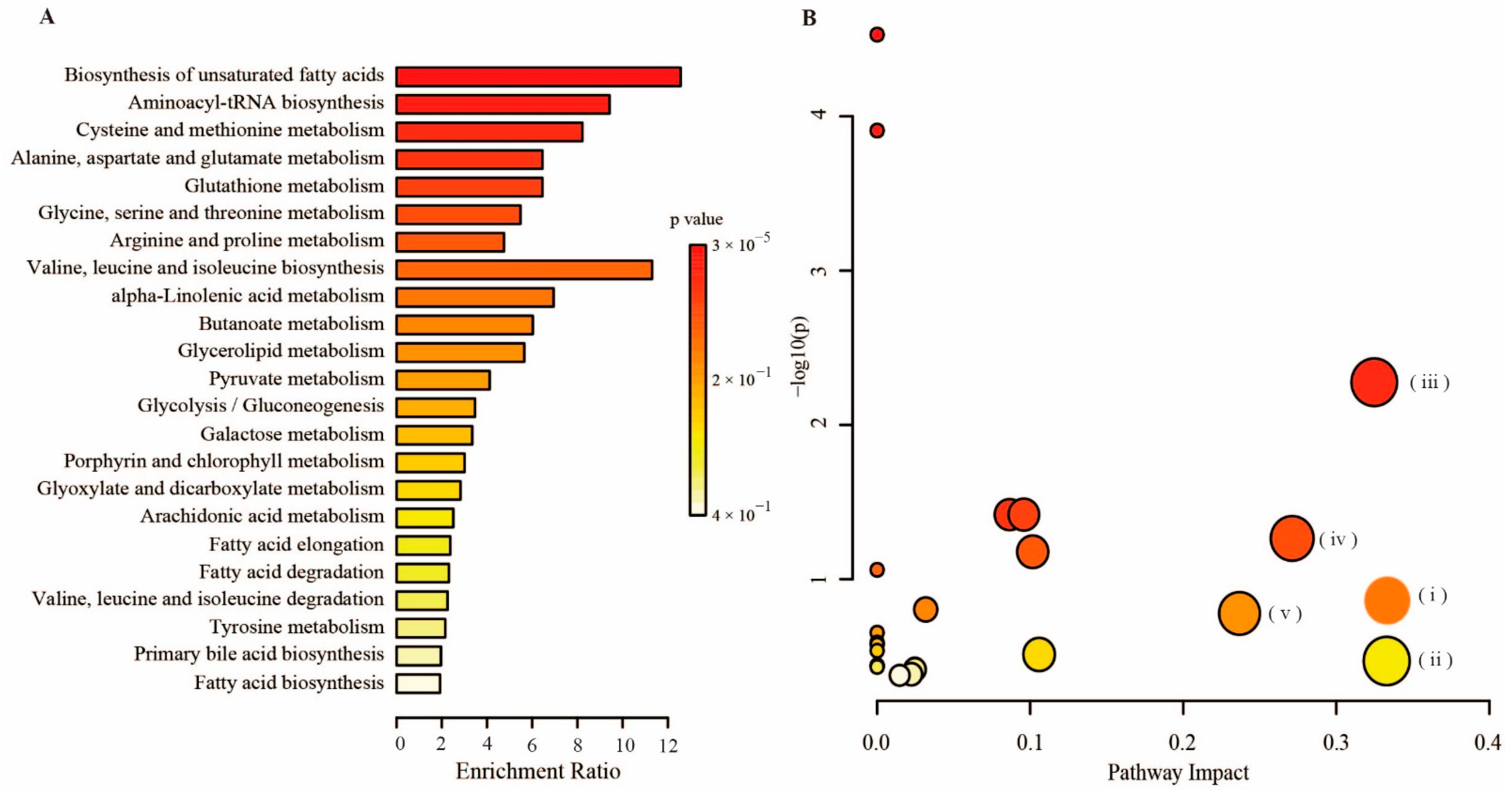
2.5. Metabolomics Analysis
3. Materials and Methods
3.1. Materials and Reagents
3.2. Macroporous Resin Enrichment Process
3.2.1. Preparation of the Crude Extract of C. japonicum
3.2.2. Determination of Total Flavonoid Content
3.2.3. Screening of Macroporous Resin
3.2.4. Adsorption Kinetics
3.2.5. Adsorption Isotherms
3.3. Purification and Identification of Pectolinarin and Linarin
3.3.1. HPLC Analysis
3.3.2. Purification of two Compounds by Prep-HPLC
3.3.3. Identification of Pectolinarin and Linarin
3.4. Bioassay In Vitro
3.4.1. Antioxidant Activity
3.4.2. Anti-AGEs Activity
3.4.3. Anti-Inflammation and Molecular Docking
3.5. Bioassay In Vivo
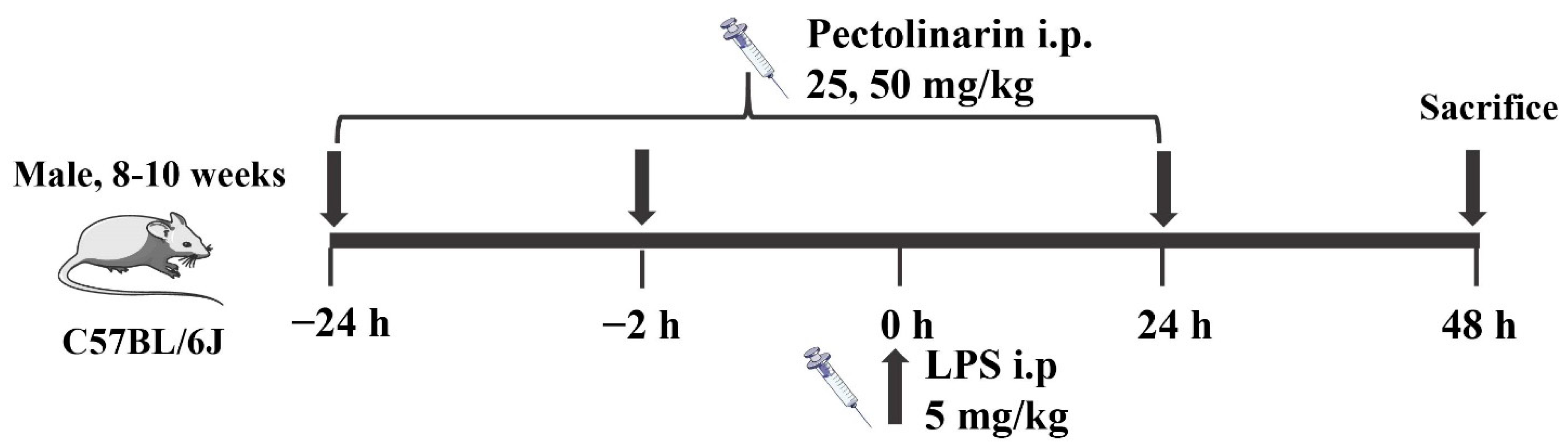
3.5.1. Animal Experiments
3.5.2. Histopathological Evaluation and Serum Biochemical Analysis
3.6. Metabolomics Analysis
3.7. Statistics
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Ge, H.; Turhong, M.; Abudkrem, M.; Tang, Y. Fingerprint analysis of Cirsium japonicum DC. using high performance liquid chromatography. J. Pharm. Anal. 2013, 3, 278–284. [Google Scholar] [CrossRef] [Green Version]
- Ma, Q.; Jiang, J.-G.; Zhang, X.-M.; Zhu, W. Identification of luteolin 7-O-β-D-glucuronide from Cirsium japonicum and its anti-inflammatory mechanism. J. Funct. Foods 2018, 46, 521–528. [Google Scholar] [CrossRef]
- Cheriet, T.; Ben-Bachir, B.; Thamri, O.; Seghiri, R.; Mancini, I. Isolation and Biological Properties of the Natural Flavonoids Pectolinarin and Pectolinarigenin—A Review. Antibiotics 2020, 9, 417. [Google Scholar] [CrossRef] [PubMed]
- Liao, Z.; Chen, X.; Wu, M. Antidiabetic effect of flavones from Cirsium japonicum DC in diabetic rats. Arch. Pharmacal Res. 2010, 33, 353–362. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Liao, M.; Cheng, X.; Liang, C.; Diao, X.; Zhang, L. Ultrahigh-performance liquid chromatography coupled with triple quadrupole and time-of-flight mass spectrometry for the screening and identification of the main flavonoids and their metabolites in rats after oral administration of Cirsium japonicum DC. ex. Rapid Commun. Mass Spectrom. 2018, 32, 1451–1461. [Google Scholar] [CrossRef]
- Shen, D.; Labreche, F.; Wu, C.; Fan, G.; Li, T.; Dou, J.; Zhu, J. Ultrasound-assisted adsorption/desorption of jujube peel flavonoids using macroporous resins. Food Chem. 2022, 368, 130800. [Google Scholar] [CrossRef]
- Wang, D.; Du, N.; Wen, L.; Zhu, H.; Liu, F.; Wang, X.; Du, J.; Li, S. An Efficient Method for the Preparative Isolation and Purification of Flavonoid Glycosides and Caffeoylquinic Acid Derivatives from Leaves of Lonicera japonica Thunb. Using High Speed Counter-Current Chromatography (HSCCC) and Prep-HPLC Guided by DPPH-HPLC Experiments. Molecules 2017, 22, 229. [Google Scholar] [CrossRef] [Green Version]
- Xie, L.; Deng, Z.; Zhang, J.; Dong, H.; Wang, W.; Xing, B.; Liu, X. Comparison of Flavonoid O-Glycoside, C-Glycoside and Their Aglycones on Antioxidant Capacity and Metabolism during In Vitro Digestion and In Vivo. Foods 2022, 11, 882. [Google Scholar] [CrossRef]
- Van Hung, P. Phenolic Compounds of Cereals and Their Antioxidant Capacity. Crit. Rev. Food Sci. Nutr. 2016, 56, 25–35. [Google Scholar] [CrossRef]
- Peng, Y.; Gan, R.; Li, H.; Yang, M.; McClements, D.J.; Gao, R.; Sun, Q. Absorption, metabolism, and bioactivity of vitexin: Recent advances in understanding the efficacy of an important nutraceutical. Crit. Rev. Food Sci. Nutr. 2021, 61, 1049–1064. [Google Scholar] [CrossRef]
- Macovei, D.-G.; Irimes, M.-B.; Hosu, O.; Cristea, C.; Tertis, M. Point-of-care electrochemical testing of biomarkers involved in inflammatory and inflammatory-associated medical conditions. Anal. Bioanal. Chem. 2022, 414, 1–31. [Google Scholar] [CrossRef] [PubMed]
- Kotas, M.E.; Medzhitov, R. Homeostasis, Inflammation, and Disease Susceptibility. Cell 2015, 160, 816–827. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, W.; Wang, S.; Liu, Q.; Wang, X.; Shan, T.; Wang, Y. Cathelicidin-WA attenuates LPS-induced inflammation and redox imbalance through activation of AMPK signaling. Free Radic. Biol. Med. 2018, 129, 338–353. [Google Scholar] [CrossRef] [PubMed]
- Cooke, J. Dietary Reduction of Advanced Glycation End Products: An Opportunity for Improved Nutrition Care. J. Ren. Nutr. 2017, 27, e23–e26. [Google Scholar] [CrossRef] [Green Version]
- Nowotny, K.; Jung, T.; Höhn, A.; Weber, D.; Grune, T.; Nowotny, K.; Jung, T.; Höhn, A.; Weber, D.; Grune, T. Advanced Glycation End Products and Oxidative Stress in Type 2 Diabetes Mellitus. Biomolecules 2015, 5, 194–222. [Google Scholar] [CrossRef] [Green Version]
- Jia, L.; Zhang, L.; Ye, Y.-H.; Li, J.-L.; Cong, M.; Yuan, T. Effect and Mechanism of Elaeagnus angustifolia Flower and Its Major Flavonoid Tiliroside on Inhibiting Non-enzymatic Glycosylation. J. Agric. Food Chem. 2019, 67, 13960–13968. [Google Scholar] [CrossRef]
- Tian, C.; Zhang, P.; Yang, C.; Gao, X.; Wang, H.; Guo, Y.; Liu, M. Extraction Process, Component Analysis, and In Vitro Antioxidant, Antibacterial, and Anti-Inflammatory Activities of Total Flavonoid Extracts from Abutilon theophrasti Medic. Leaves. Mediat. Inflamm. 2018, 2018, 3508506. [Google Scholar] [CrossRef] [Green Version]
- Gutierrez, R.M.P.; Velazquez, E.G. Glucopyranoside flavonoids isolated from leaves of Spinacia oleracea (spinach) inhibit the formation of advanced glycation end products (AGEs) and aldose reductase activity (RLAR). Biomed. Pharmacother. 2020, 128, 110299. [Google Scholar] [CrossRef]
- Hou, M.; Hu, W.; Xiu, Z.; Shi, Y.; Hao, K.; Cao, D.; Guan, Y.; Yin, H. Efficient enrichment of total flavonoids from Pteris ensiformis Burm. extracts by macroporous adsorption resins and in vitro evaluation of antioxidant and antiproliferative activities. J. Chromatogr. B 2020, 1138, 121960. [Google Scholar] [CrossRef]
- Chu, M.-J.; Liu, X.-M.; Yan, N.; Wang, F.-Z.; Du, Y.-M.; Zhang, Z.-F. Partial Purification, Identification, and Quantitation of Antioxidants from Wild Rice (Zizania latifolia). Molecules 2018, 23, 2782. [Google Scholar] [CrossRef]
- Wan, P.; Sheng, Z.; Han, Q.; Zhao, Y.; Cheng, G.; Li, Y. Enrichment and purification of total flavonoids from Flos Populi extracts with macroporous resins and evaluation of antioxidant activities in vitro. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2014, 945–946, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Li, K.P.; Yuan, M.; Wu, Y.L.; Pineda, M.; Zhang, C.M.; Chen, Y.F.; Chen, Z.Q.; Rong, X.L.; Turnbull, J.E.; Guo, J. A high-fat high-fructose diet dysregulates the homeostatic crosstalk between gut microbiome, metabolome, and immunity in an experimental model of obesity. Mol. Nutr. Food Res. 2022, 66, e2100950. [Google Scholar] [CrossRef] [PubMed]
- Zain, M.S.C.; Lee, S.Y.; Teo, C.Y.; Shaari, K. Adsorption and Desorption Properties of Total Flavonoids from Oil Palm (Elaeis guineensis Jacq.) Mature Leaf on Macroporous Adsorption Resins. Molecules 2020, 25, 778. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dong, Y.; Zhao, M.; Sun-Waterhouse, D.; Zhuang, M.; Chen, H.; Feng, M.; Lin, L. Absorption and desorption behaviour of the flavonoids from Glycyrrhiza glabra L. leaf on macroporous adsorption resins. Food Chem. 2015, 168, 538–545. [Google Scholar] [CrossRef]
- Chang, X.-L.; Wang, D.; Chen, B.-Y.; Feng, Y.-M.; Wen, S.-H.; Zhan, P.-Y. Adsorption and Desorption Properties of Macroporous Resins for Anthocyanins from the Calyx Extract of Roselle (Hibiscus sabdariffa L.). J. Agric. Food Chem. 2012, 60, 2368–2376. [Google Scholar] [CrossRef]
- Hong, L.T.T.; Huyen, T.T.; Tri, L.M.; Hien, T.D.; Loi, H. New iridoid from Valeriana hardwickii Wall. Vietnam J. Chem. 2021, 59, 12–16. [Google Scholar] [CrossRef]
- Valgimigli, L.; Amorati, R.; Petrucci, S.; Pedulli, G.F.; Hu, D.; Hanthorn, J.J.; Pratt, D.A. Unexpected Acid Catalysis in Reactions of Peroxyl Radicals with Phenols. Angew. Chem. Int. Ed. 2009, 48, 8348–8351. [Google Scholar] [CrossRef]
- Boisard, S.; Le Ray, A.-M.; Gatto, J.; Aumond, M.-C.; Blanchard, P.; Derbré, S.; Flurin, C.; Richomme, P. Chemical Composition, Antioxidant and Anti-AGEs Activities of a French Poplar Type Propolis. J. Agric. Food Chem. 2014, 62, 1344–1351. [Google Scholar] [CrossRef] [Green Version]
- Chaudhuri, J.; Bains, Y.; Guha, S.; Kahn, A.; Hall, D.; Bose, N.; Gugliucci, A.; Kapahi, P. The Role of Advanced Glycation End Products in Aging and Metabolic Diseases: Bridging Association and Causality. Cell Metab. 2018, 28, 337–352. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.-J.; Cho, H.-I.; Kim, S.-J.; Park, J.-H.; Kim, J.-S.; Kim, Y.H.; Lee, S.K.; Kwak, J.-H.; Lee, S.-M. Protective effect of linarin against d-galactosamine and lipopolysaccharide-induced fulminant hepatic failure. Eur. J. Pharmacol. 2014, 738, 66–73. [Google Scholar] [CrossRef]
- Fan, J.-H.; Feng, G.-G.; Huang, L.; Tang, G.-D.; Jiang, H.-X.; Xu, J. Naofen promotes TNF-α-mediated apoptosis of hepatocytes by activating caspase-3 in lipopolysaccharide-treated rats. World J. Gastroenterol. 2014, 20, 4963–4971. [Google Scholar] [CrossRef] [PubMed]
- Beale, D.J.; Pinu, F.R.; Kouremenos, K.A.; Poojary, M.M.; Narayana, V.K.; Boughton, B.A.; Kanojia, K.; Dayalan, S.; Jones, O.A.H.; Dias, D.A. Review of recent developments in GC–MS approaches to metabolomics-based research. Metabolomics 2018, 14, 152. [Google Scholar] [CrossRef] [PubMed]
- Zeki, O.C.; Eylem, C.C.; Reçber, T.; Kir, S.; Nemutlu, E. Integration of GC–MS and LC–MS for untargeted metabolomics profiling. J. Pharm. Biomed. Anal. 2020, 190, 113509. [Google Scholar] [CrossRef] [PubMed]
- Li, K.-P.; He, Z.; Wang, X.; Pineda, M.; Chen, R.; Liu, H.; Ma, K.; Shen, H.; Wu, C.; Huang, N.; et al. Apigenin C-glycosides of Microcos paniculata protects lipopolysaccharide induced apoptosis and inflammation in acute lung injury through TLR4 signaling pathway. Free Radic. Biol. Med. 2018, 124, 163–175. [Google Scholar] [CrossRef] [Green Version]
- Del Bufalo, A.; Bernad, J.; Dardenne, C.; Verda, D.; Meunier, J.R.; Rousset, F.; Martinozzi-Teissier, S.; Pipy, B. Contact sensitizers modulate the arachidonic acid metabolism of PMA-differentiated U-937 monocytic cells activated by LPS. Toxicol. Appl. Pharmacol. 2011, 256, 35–43. [Google Scholar] [CrossRef]
- Taha, A.Y.; Blanchard, H.C.; Cheon, Y.; Ramadan, E.; Chen, M.; Chang, L.; Rapoport, S.I. Dietary Linoleic Acid Lowering Reduces Lipopolysaccharide-Induced Increase in Brain Arachidonic Acid Metabolism. Mol. Neurobiol. 2016, 54, 4303–4315. [Google Scholar] [CrossRef]
- Shi, C.; Han, X.; Mao, X.; Fan, C.; Jin, M. Metabolic profiling of liver tissues in mice after instillation of fine particulate matter. Sci. Total. Environ. 2019, 696, 133974. [Google Scholar] [CrossRef]
- Tamanna, N.; Mayengbam, S.; House, J.D.; Treberg, J.R. Methionine restriction leads to hyperhomocysteinemia and alters hepatic H2S production capacity in Fischer-344 rats. Mech. Ageing Dev. 2018, 176, 9–18. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, L.; Wang, L.; Zhao, Y. Total phenols, flavonoids, and procyanidins levels and total antioxidant activity of different Korean pine (Pinus koraiensis) varieties. J. For. Res. 2018, 30, 1743–1754. [Google Scholar] [CrossRef]
- Wang, X.; Wang, S.; Huang, S.; Zhang, L.; Ge, Z.; Sun, L.; Zong, W. Purification of Polyphenols from Distiller’s Grains by Macroporous Resin and Analysis of the Polyphenolic Components. Molecules 2019, 24, 1284. [Google Scholar] [CrossRef]
- Sun, J.; Su, X.; Zhang, Z.; Hu, D.; Hou, G.; Zhao, F.; Sun, J.; Cong, W.; Wang, C.; Li, H. Separation of three chromones from Saposhnikovia divaricata using macroporous resins followed by preparative high-performance liquid chromatography. J. Sep. Sci. 2021, 44, 3287–3294. [Google Scholar] [CrossRef] [PubMed]
- Spagnuolo, L.; Della Posta, S.; Fanali, C.; Dugo, L.; De Gara, L. Antioxidant and Antiglycation Effects of Polyphenol Compounds Extracted from Hazelnut Skin on Advanced Glycation End-Products (AGEs) Formation. Antioxidants 2021, 10, 424. [Google Scholar] [CrossRef] [PubMed]
- Jiao, J.; Yang, Y.; Wu, Z.; Li, B.; Zheng, Q.; Wei, S.; Wang, Y.; Yang, M. Screening cyclooxygenase-2 inhibitors from Andrographis paniculata to treat inflammation based on bio-affinity ultrafiltration coupled with UPLC-Q-TOF-MS. Fitoterapia 2019, 137, 104259. [Google Scholar] [CrossRef] [PubMed]
- Uddin, J.; Xu, S.; Crews, B.C.; Aleem, A.M.; Ghebreselasie, K.; Banerjee, S.; Marnett, L.J. Harmaline Analogs as Substrate-Selective Cyclooxygenase-2 Inhibitors. ACS Med. Chem. Lett. 2020, 11, 1881–1885. [Google Scholar] [CrossRef]
- Anantharaju, P.G.; Reddy, B.D.; Padukudru, M.A.; Chitturi, C.M.K.; Vimalambike, M.G.; Madhunapantula, S.V. Naturally occurring benzoic acid derivatives retard cancer cell growth by inhibiting histone deacetylases (HDAC). Cancer Biol. Ther. 2017, 18, 492–504. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoo, Y.M.; Nam, J.H.; Kim, M.Y.; Choi, J.W.; Park, H.-J. Pectolinarin and pectolinarigenin of Cirsium setidens prevent the hepatic injury in rats caused by d-galactosamine via an antioxidant mechanism. Biol. Pharm. Bull. 2008, 31, 760–764. [Google Scholar] [CrossRef]

| Resin | Polarity | Adsorption Capacity (mg·g−1) | Desorption Capacity (mg·g−1) |
|---|---|---|---|
| HPD500 | Polar | 2.24 ± 0.08 | 1.68 ± 0.06 |
| HPD600 | Polar | 1.83 ± 0.02 | 1.70 ± 0.06 |
| NKA-9 | Polar | 1.92 ± 0.04 | 1.72 ± 0.40 |
| DM130 | Weak polar | 1.78 ± 0.11 | 1.77 ± 0.13 |
| AB-8 | Weak polar | 2.03 ± 0.07 | 1.82 ± 0.06 |
| D-101 | Non-polar | 1.92 ± 0.03 | 2.04 ± 0.18 |
| HPD100 | Non-polar | 1.05 ± 0.31 | 1.84 ± 0.11 |
| HP-20 | Non-polar | 1.65 ± 0.14 | 1.68 ± 0.09 |
| Kinetics Equations | Dynamic Parameters | ||
|---|---|---|---|
| Qe (mg·g−1) | K1 | R2 | |
| Pseudo-first-order model | 21.0497 | 0.0407 | 0.8768 |
| Pseudo-second-order model | 23.2981 | 0.0027 | 0.9493 |
| T/°C | Langmuir Model | Freundlich Model | ||||
|---|---|---|---|---|---|---|
| Qm (mg·g−1) | KL (mL·mg−1) | R2 | KF ((mg·g−1)(mL·mg−1)1/n) | n | R2 | |
| 20 | 34.2411 | 0.0234 | 0.9938 | 21.2050 | 1.4208 | 0.9877 |
| 30 | 43.1711 | 0.0282 | 0.9921 | 21.2261 | 1.2949 | 0.9911 |
| 40 | 56.7479 | 0.0315 | 0.9992 | 21.9174 | 1.2003 | 0.9959 |
| No. | Metabolite | RT (min) | p-Value (t-Test) | VIP Value | Fold Change * |
|---|---|---|---|---|---|
| 1 | Tyramine | 12.04 | 3.50 × 10−2 | 1.38 | −0.79 |
| 2 | L-Lactic acid | 12.21 | 4.60 × 10−2 | 1.16 | −0.58 |
| 3 | 2-Phenylbutyric acid | 12.40 | 2.40 × 10−2 | 1.47 | −1.8 |
| 4 | n-Butylamine | 13.08 | 1.00 × 10−2 | 1.09 | −2.6 |
| 5 | 4-Aminobutanoic acid | 13.39 | 1.20 × 10−2 | 1.08 | −0.87 |
| 6 | L-alpha-Aminobutyric acid | 13.98 | 3.30 × 10−2 | 1.27 | −1.28 |
| 7 | L-Isoleucine | 14.87 | 4.20 × 10−2 | 1.31 | −1.03 |
| 8 | Acetylglycine | 15.53 | 5.00 × 10−3 | 1.35 | 2.19 |
| 9 | L-Asparagine | 16.14 | 2.60 × 10−2 | 1.06 | −0.54 |
| 10 | Glycine | 17.35 | 6.00 × 10−3 | 1.09 | 1 |
| 11 | Cystathionine | 17.55 | 1.50 × 10−2 | 1.01 | −1.81 |
| 12 | L-Norleucine | 17.71 | 1.00 × 10−3 | 1.08 | −1.64 |
| 13 | L-Asparagine | 18.40 | 5.00 × 10−3 | 1.38 | −1.37 |
| 14 | L-Methionine | 19.24 | 3.30 × 10−2 | 1.55 | −0.63 |
| 15 | N-α-Acetyl-L-Lysine | 20.32 | 4.00 × 10−3 | 1.09 | −1.8 |
| 16 | Glutamic acid | 21.15 | 1.10 × 10−2 | 1.06 | −0.43 |
| 17 | Methyl alpha-D-galactopyranoside | 21.45 | 1.20 × 10−2 | 1.25 | −1.7 |
| 18 | Benzoic acid | 22.17 | 4.00 × 10−3 | 1.41 | −3.66 |
| 19 | D-Phenylalanine | 22.23 | 1.80 × 10−2 | 1.09 | −0.36 |
| 20 | L-Proline | 22.96 | 2.10 × 10−2 | 1.07 | −0.87 |
| 21 | 4-Hydroxybenzoic acid | 23.77 | 1.90 × 10−2 | 1.31 | 0.02 |
| 22 | 5,8,11-Eicosatrienoic acid | 24.27 | 1.10 × 10−2 | 1.04 | −1.19 |
| 23 | Glycerol | 30.50 | 3.00 × 10−2 | 1.35 | −1.58 |
| 24 | Palmitoleic acid | 31.74 | 1.50 × 10−2 | 1.26 | −1.96 |
| 25 | Palmitic Acid | 32.05 | 1.40 × 10−2 | 1.41 | −1.22 |
| 26 | Linolenic acid | 34.3 | 2.00 × 10−3 | 1.33 | −1.1 |
| 27 | Oleic Acid | 34.34 | 5.00 × 10−3 | 1.32 | −1.08 |
| 28 | Stearic acid | 34.61 | 2.40 × 10−2 | 1.34 | −0.78 |
| 29 | Arachidonic acid | 35.85 | 2.00 × 10−3 | 1.51 | −0.79 |
| 30 | 9-Octadecenamide | 35.95 | 0.00 × 10 | 1.18 | 2.68 |
| 31 | Oleamide | 36.21 | 6.00 × 10−3 | 1.22 | 3.26 |
| 32 | Methyl galactoside | 36.36 | 2.50 × 10−2 | 1.35 | 2.67 |
| 33 | 2-Palmitoylglycerol | 37.22 | 3.70 × 10−2 | 1.54 | −0.85 |
| 34 | 1-Monopalmitin | 37.44 | 4.50 × 10−2 | 1.66 | −1.68 |
| 35 | Glycerol monostearate | 38.55 | 2.50 × 10−2 | 1.69 | −2.43 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ye, Y.; Chen, Z.; Wu, Y.; Gao, M.; Zhu, A.; Kuai, X.; Luo, D.; Chen, Y.; Li, K. Purification Process and In Vitro and In Vivo Bioactivity Evaluation of Pectolinarin and Linarin from Cirsium japonicum. Molecules 2022, 27, 8695. https://doi.org/10.3390/molecules27248695
Ye Y, Chen Z, Wu Y, Gao M, Zhu A, Kuai X, Luo D, Chen Y, Li K. Purification Process and In Vitro and In Vivo Bioactivity Evaluation of Pectolinarin and Linarin from Cirsium japonicum. Molecules. 2022; 27(24):8695. https://doi.org/10.3390/molecules27248695
Chicago/Turabian StyleYe, Yana, Zhenlin Chen, Yonglin Wu, Mengmeng Gao, Anqi Zhu, Xinyuan Kuai, Duosheng Luo, Yanfen Chen, and Kunping Li. 2022. "Purification Process and In Vitro and In Vivo Bioactivity Evaluation of Pectolinarin and Linarin from Cirsium japonicum" Molecules 27, no. 24: 8695. https://doi.org/10.3390/molecules27248695
APA StyleYe, Y., Chen, Z., Wu, Y., Gao, M., Zhu, A., Kuai, X., Luo, D., Chen, Y., & Li, K. (2022). Purification Process and In Vitro and In Vivo Bioactivity Evaluation of Pectolinarin and Linarin from Cirsium japonicum. Molecules, 27(24), 8695. https://doi.org/10.3390/molecules27248695







