Abstract
The escalating demand for crop production, environmental protection, and food safety warrants the development of new fungicides with greater efficiency, environmental friendliness, and innocuous metabolites to fight against destructive phytopathogens. Herein, we report on the synthesis and antifungal activity of dipeptide-based stilbene derivatives bearing a thiophene-substituted 1,3,4-oxadiazole fragment for the first time. In vitro bioassay indicated that the target compounds had remarkable antifungal potency superior to previously reported counterparts without a dipeptidyl group, of which compound 3c exhibited the highest activity against Botrytis cinerea with EC50 values of 106.1 μg/mL. Moreover, the in vivo protective effect of compound 3c (59.1%) against tomato gray mold was more potent than that of carboxin (42.0%). Preliminary investigations on the mode of action showed that compound 3c induced marked hyphal malformations and increased the membrane permeability of B. cinerea as well as inhibiting mycelial respiration. These promising results suggest that this novel type of molecular framework has great potential to be further developed as alternative fungicides.
1. Introduction
Plant fungal infections have remarkably brought about yield reduction and product deterioration as primary plant diseases that have resulted in economic losses in crops [1]. Especially, Botrytis cinerea is among the top-ten phytopathogenic fungi given scientific or economic importance, as its causes damage during plant cultivation as well as after harvest [2,3,4]. B. cinerea, known as gray mold, is intractable because of its broad host range of more than 220 eudicot plants and manifold attack strategies that include cell wall-degrading enzymes, phytotoxins, and detoxification proteins [5,6]. Several characteristics of chemical fungicides, consisting of low cost, high efficiency, rapid action, and long efficacy duration, remain the mainstay of fungal disease control. Nevertheless, it is the appearance of drug-resistant pathogens [7], and environmental residuals toxic to other organisms [8] resulting from inappropriate or long-term use of available chemicals, that warrant the development of antifungal surrogates with an innovative framework, outstanding bioactivity, and good biocompatibility.
In this context, extensive research efforts have been focused on the exploitation of natural products as an inestimable source of prospective candidates with new modes of action and reasonable degradability. Stilbenes possessing a 1,2-diphenylethylene backbone, a group of plant secondary metabolites, such as well-known resveratrol, are involved in plant defense against multiple biotic and abiotic stresses [9]. Their biological activities [10] and clinical potential [11] have been elaborately documented in the literature. However, although they could inhibit phytopathogenic fungal growth in vitro [12,13], the paucity [10,14], rapid oxidation, and microbial metabolism [15,16] of naturally occurring stilbenes make their external applications on crops hard to achieve, and therefore necessitate synthesis and modification of chemical mimics. Heterocyclic compounds are widely used as components of many biologically active molecules for the optimization of lead compounds in drug development. Thereinto, 1,3,4-oxadiazole substructure prevalent in pesticides and drugs is commonly integrated into the target structures to innovate their biopharmaceutical activities, including antimicrobial [17], anti-inflammatory [18], antitumor [19], and anti-diabetic [20] activities. As another privileged building block, thiophene moiety has drawn the considerable interest of researchers since its derivatives show appreciable diversity in biological effects, such as insecticidal [21], fungicidal [22], and herbicidal [23] effects. Thus, exploration of stilbene backbone modified by these molecular motifs should be expected.
Due to the important role of peptides in living organisms, peptides have been reported as resourceful pharmacological vehicles, and to date, more than 60 peptide therapies have been approved in the United States and other major markets [24,25,26]. Moreover, dipeptide agents, the shortest peptides, are not only easy to prepare and devoid of toxic metabolites, but they are capable of penetrating biological barriers and are more stable when compared to oligopeptides [27]. Dipeptide derivatives, natural and synthetic, have been investigated for applications in diverse aspects [28,29,30,31]. For example, the cyclic Leu-leu dipeptide was isolated and purified from the ethyl acetate extract of a broth of the genus Gordonia sp. (WA4-31). Antibacterial experiments showed that it had good inhibitory effects on Candida albicans, Aspergillus niger, and so on [32]. Khalaf et al. reported Gly-Gly dipeptide derivatives as effective inhibitors against Bacillus subtilits and Candida albicans [33].
In a previous study, we synthesized a series of 5-(2-thienyl)-1,3,4-oxadiazole-containing stilbene derivatives that displayed promising antifungal activities and could be considered as a potential bioactive scaffold [34]. As a part of our ongoing efforts to develop novel stilbenes of potent fungicidal competence, three different dipeptide fragments (Gly-Gly, Gly-Met, Gly-Leu) were integrated into this stilbene scaffold based on the molecular hybridization principle. The newly synthesized substances were first screened for their in vitro antifungal activities against B. cinerea. In addition, their in vivo protective efficacies against tomato gray mold were tested to explore their practical potential in agriculture. The antifungal mechanism of these designed molecules was preliminarily studied in terms of hyphal morphology, membrane permeability, and mycelial respiration.
2. Results and Discussion
2.1. Chemistry
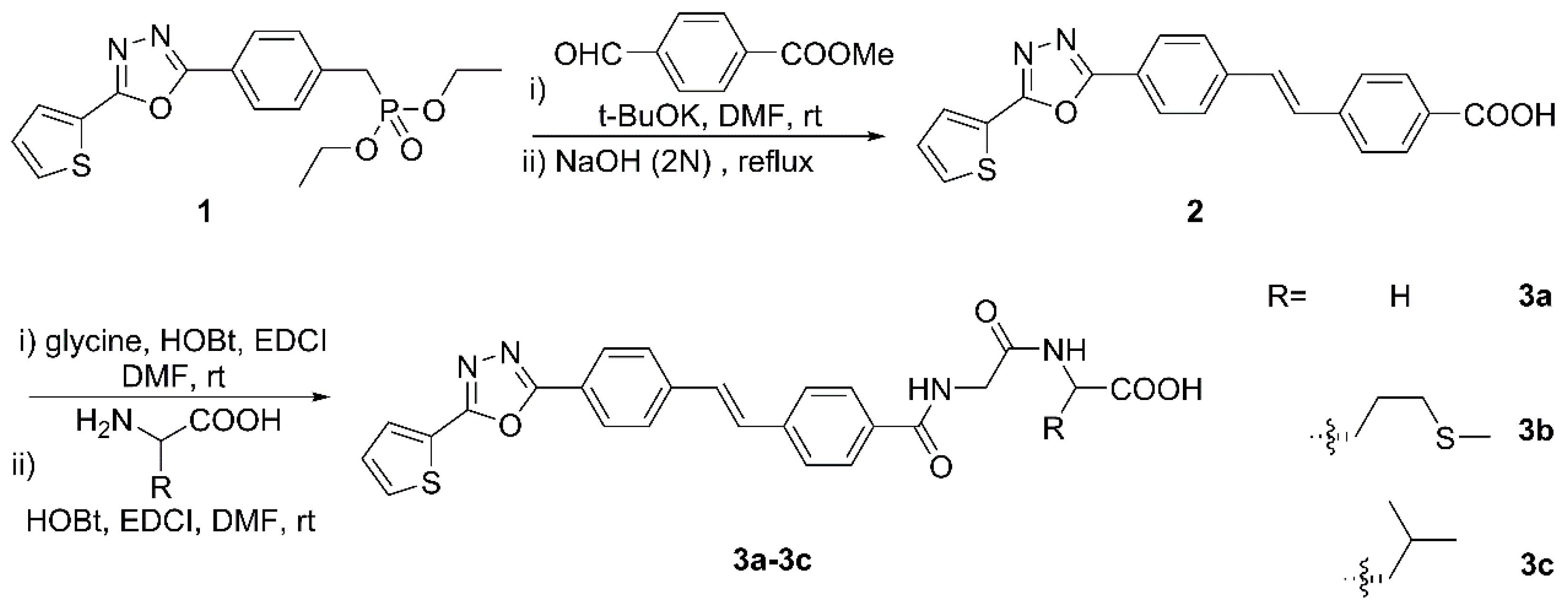
Preparation of target compounds 3a–3c was achieved as depicted in Figure 1 starting from the previously described heterocyclic-substituted benzylphosphonate. The stilbene was obtained by the Wittig–Horner reaction of compound 1 with p-(Methoxycarbonyl)benzaldehyde, followed by basic hydrolysis to restore the carboxylic acid functionality. The condensation reaction with glycine in the presence of HOBt and EDCI to provide amide was next, which was finally coupled with a certain L-amino acid under the same conditions as one of the former steps for forming the peptide linkage. It is noteworthy that the whole experiment proceeded smoothly without laborious column chromatographic purification, and the target compounds were obtained in good yields. The structures of newly synthesized compounds were confirmed by NMR and HRMS spectral analysis.
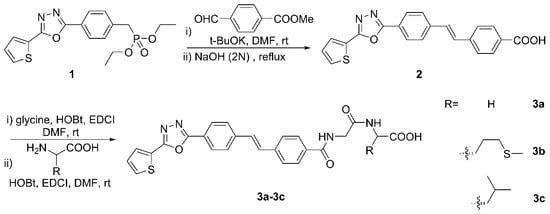
Figure 1.
Synthetic route for the target molecules 3a–3c.
2.2. In Vitro Antifungal Activities
For the required compounds, the bactericidal activity against two important fungal pathogens was evaluated in vitro. These compounds were preliminarily evaluated at 200 µg/mL, and successfully, the evaluation results (Table 1) revealed that the synthesized molecules showed moderate to good inhibition effects toward the tested fungi. The EC50 values for dipeptidyl stilbene derivatives, along with the commercial amide fungicide carboxin, were measured for further exploration of their antifungal potential and are listed in Table 1. It can be clearly seen that compounds 3a–3c were all more potent against B. cinerea, with EC50 values ranging from 106.1 to 119.6 µg/mL compared to carboxin (EC50 = 138.7 µg/mL), out of which 3c presented the highest level of activity (EC50 = 106.1 µg/mL) and was over twice more effective than resveratrol (EC50 = 263.1 µg/mL). Despite having less potency against C. lagenarium, compounds 3b and 3c still exerted fungicidal performance comparable to or better than that of the positive control carboxin. For instance, the EC50 value of 3c was 186.7 µg/mL, lower than that of carboxin (EC50 = 201.7 µg/mL). There was an interesting phenomenon in that the final integrated structures displayed growing inhibitory activities with the increasing hydrophobicity of dipeptide substituents due to changes of the external exposed amino acid (Gly < Met < Leu [35]). Furthermore, in contrast with our previous work where the best compound inhibited B. cinerea in vitro with EC50 = 155.4 μg/mL [36], this test outcome indicated that the introduction of simple dipeptidyl moiety was beneficial to antifungal action.

Table 1.
In vitro antifungal activities of the target compounds against B. cinerea.
2.3. Effect on Gray Mold of Tomatoes
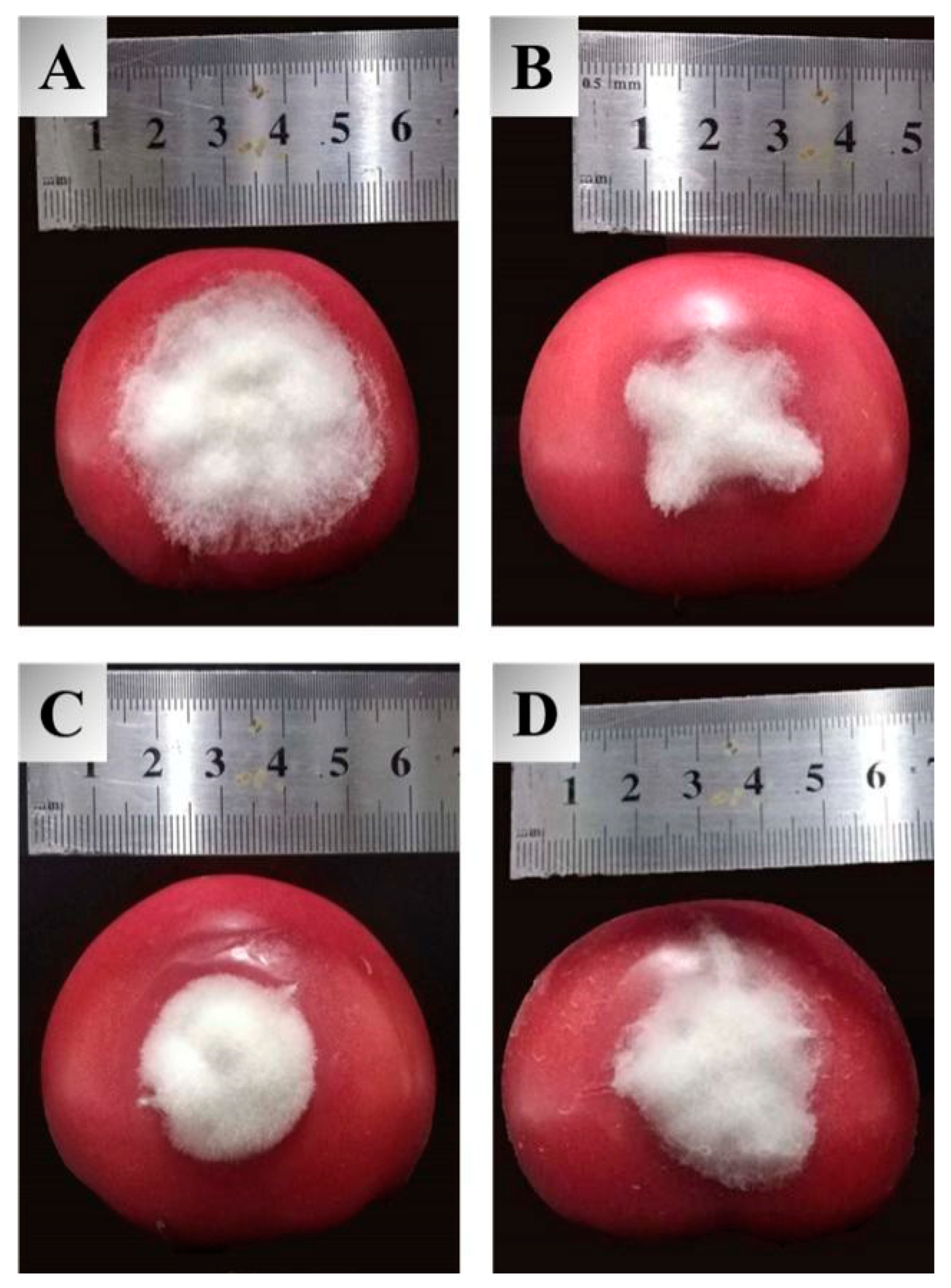
Inspired by the ameliorated antifungal activities of the target compounds in the in vitro assay, an in vivo experiment for these molecules against tomato gray mold was carried out to examine their practical potential, and the obtained results are summarized in Table 2. The inhibitory effects of synthetic compounds against B. cinerea in vivo were identical to those observed against mycelial growth in the Petri dishes. Compounds 3a–3c exhibited excellent protective impacts with the control efficacy of 55.2%, 56.1%, and 59.1%, respectively, at a concentration of 400 µg/mL. Meanwhile, they were all more effective than carboxin (42.0%), among which 3c can availably prevent the extension of lesions on tomatoes, as is illustrated in Figure 2. Considering the unique chemical structure and efficient bioactivities of dipeptidyl stilbene derivatives containing heterocycles, it is suggested that this type of molecular scaffold could be regarded as prospective agrochemicals for the management of B. cinerea.

Table 2.
Protective activities of compounds 3a–3c against tomato gray mold at 400 µg/mL under greenhouse conditions.
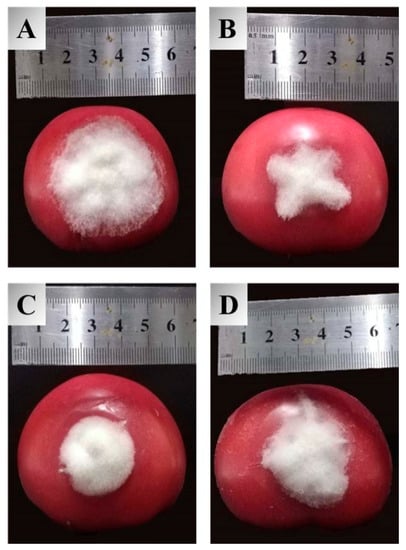
Figure 2.
Protective efficacy of compound 3c, resveratrol, and carboxin against tomato gray mold at 400 µg/mL, (A) blank control, (B) 3c, (C) resveratrol, (D) carboxin.
2.4. Optical Microscopy Analysis
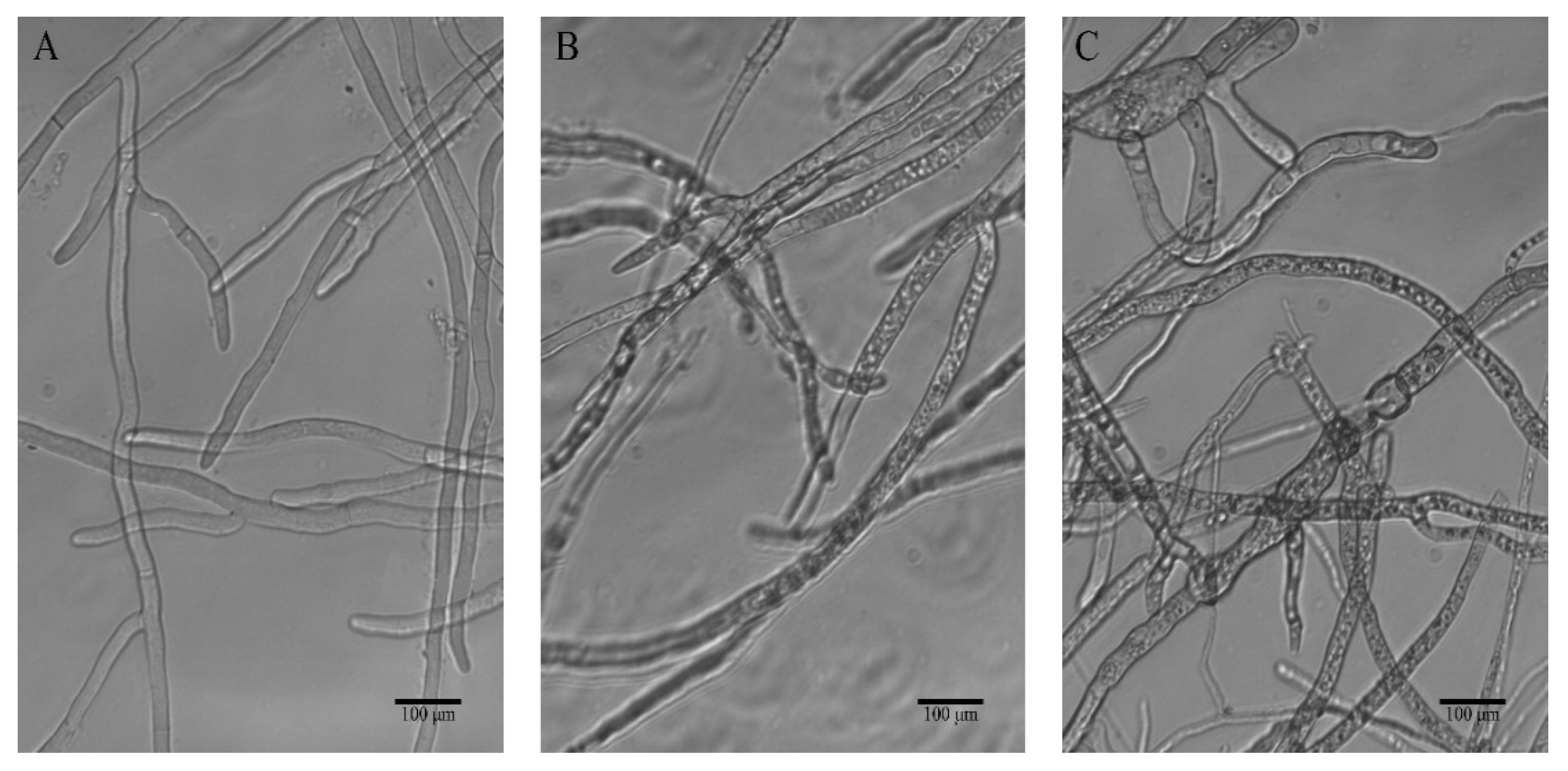
The hyphal morphological alterations of B. cinerea in response to treatment with 3c were monitored by light microscopy. In the control sample, the hyphae appeared linear and intact tubular, and homogenous with distinguishable septa, while as shown in Figure 3B, the fungus subjected to the action of compound 3c at 100 µg/mL presented evident changes, of which hyphal vesiculation was particularly visible. The imprints on the mycelia were more significant after exposure to 200 µg/mL 3c, and microscopic examination showed irregularly tortuous hyphae without a relatively uniform diameter, swollen or elongated. These observed mycelial malformations implied that destroying the structure of the cell wall and membrane system might be one of the antifungal mechanisms of compound 3c against B. cinerea.
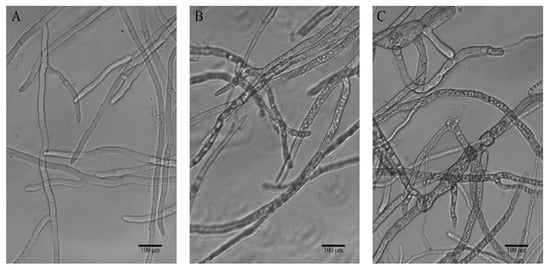
Figure 3.
Effect of different concentrations of compound 3c on the hyphal morphology of B. cinerea strains observed by a light microscope (×200), (A) blank control, (B) 100 µg/mL, (C) 200 µg/mL.
2.5. Effect on Cell Membrane Permeability
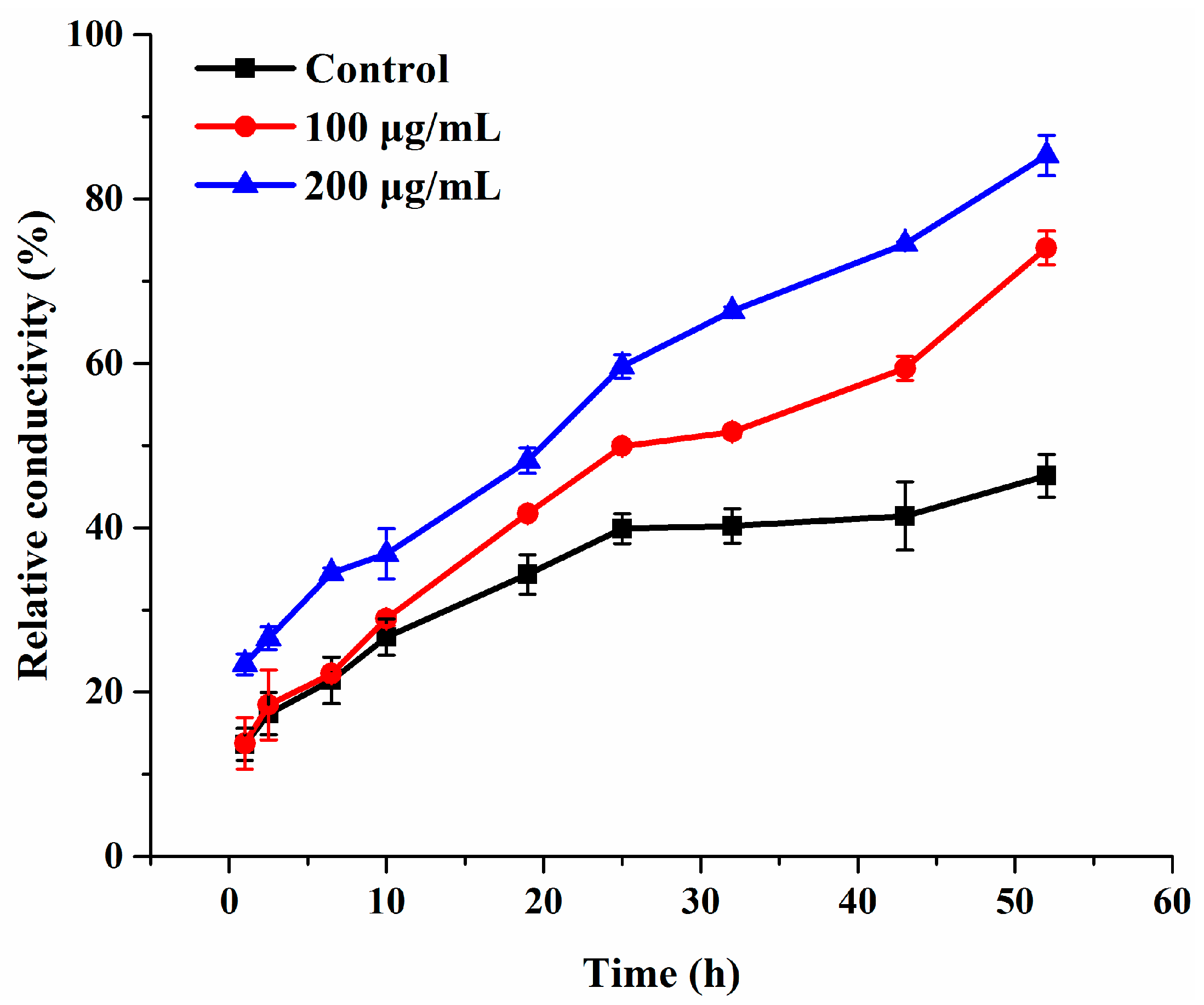
Cell membrane plays a pivotal role in maintaining cellular basic metabolism and defending the cell against exogenous disturbance [36]. Electrolyte leakage is generally taken as an indicator of cell membrane permeability. Hence, in order to confirm whether compound 3c affects the membrane permeability of B. cinerea, the electrical conductivity of suspensions of intact mycelia was measured. Compared to the control group, the relative permeability in the 3c-treated groups was higher and continually elevated during the entire time of treatment, even 25 h later (Figure 4). Besides, the extent of the damage induced by 3c to the mycelial cell membrane system increased in a concentration-dependent manner, which was distinctly revealed by significant effects at 200 µg/mL within 10 h. These findings proved that the exposure to compound 3c permeabilized the membrane, led to electrolyte leakage and thereby augmented the conductivity of the solution.
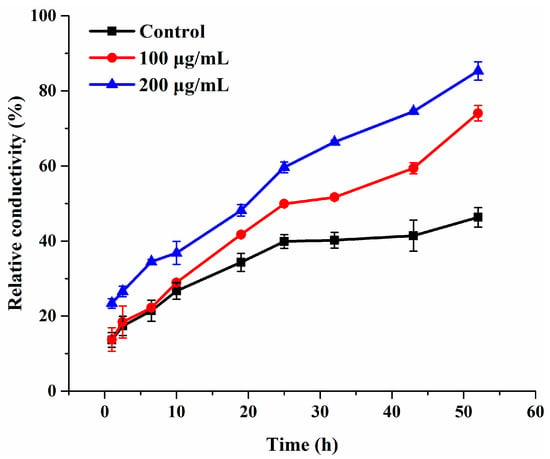
Figure 4.
Electrolyte leakage from B. cinerea suspensions treated with compound 3c. Each treatment was carried out in triplicate.
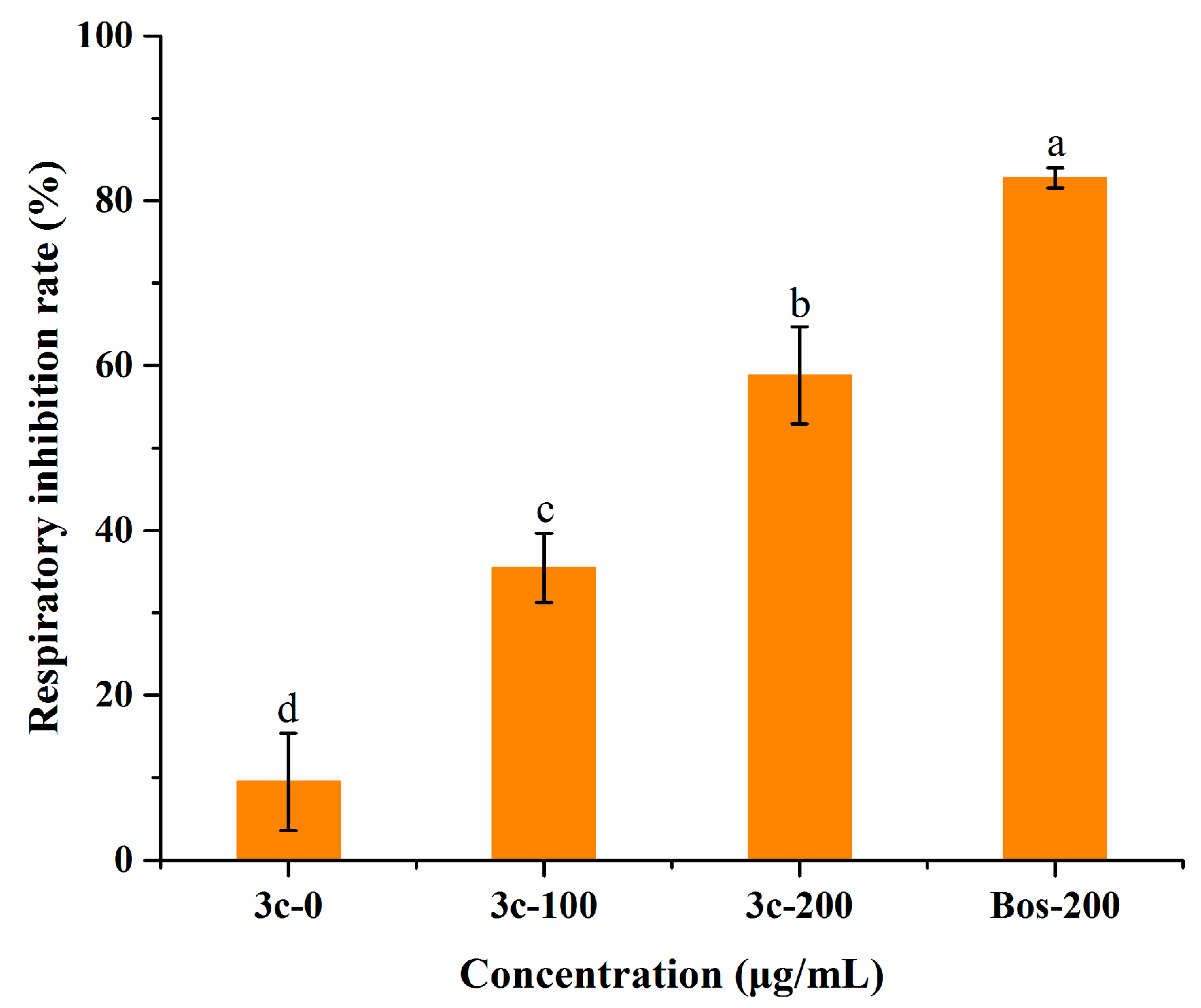
2.6. Effect on Mycelial Respiration
According to related reports, pterostilbene can interact with the mitochondrial membrane of cells and inhibit cell respiration [37]. To further explore the antifungal mechanism, we tested the mycelial oxygen consumption rate of B. cinerea when treated with compound 3c, utilizing a respiration inhibitor, boscalid, as a reference. Notwithstanding that the respiration inhibitory activity (58.8 ± 5.9%) was poorer than that of boscalid (82.8 ± 1.2%) at an equal dose (Figure 5), treatment with 3c had a statistically significant impact on mycelial respiration, which suggested that this compound might also function in the same way as boscalid and partly suppress the mycelium growth of B. cinerea by inhibiting the mitochondrial respiratory chain. Likewise, the low level of compound 3c resulted in less prominent inhibition of oxygen consumption. Combined with the results of the membrane permeability assay, it was inferred that 3c exerted antifungal activity against B. cinerea through multiple pathways, favorable to retarding the development of fungal resistance.
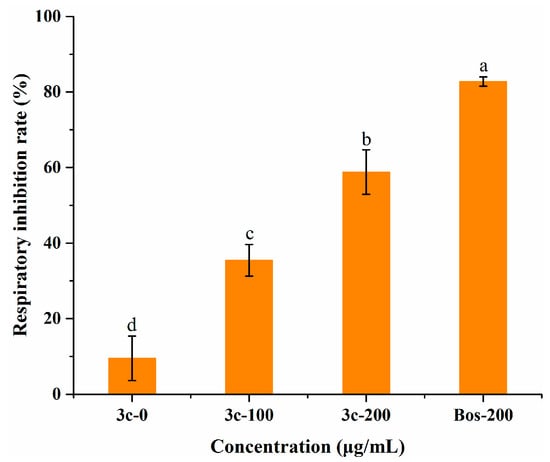
Figure 5.
Evaluation of the respiratory inhibition rate on B. cinerea mycelia exposed to compound 3c and the commercial respiration inhibitor boscalid (BOS). Different letters among treatments mean statistically significant differences using Tukey’s test (p < 0.05).
To sum up, we designed and synthesized structurally tunable dipeptide-based stilbene derivatives bearing thiophene and 1,3,4-oxidazole moieties by innovatively introducing easy-to-prepare and diverse dipeptides. The in vitro bioassay indicated that all the compounds possessed better fungicidal activities against tested fungal strains than that of resveratrol. Furthermore, in vivo experiments demonstrated their effectiveness for the control of tomato gray mold caused by B. cinerea. It was also observed that abnormal hyphal morphology, increased membrane permeability, and the repressed mycelial respiration of B. cinerea took place after interaction with compound 3c. Given their high efficacies and versatile effects on gray mold, dipeptidyl stilbenes are of promising development value for seeking novel agricultural fungicides.
3. Materials and Methods
3.1. Chemicals and Instrumentation
All chemicals, reagents, and solvents were obtained commercially and used without further purification. Melting points were determined employing a BUCHI Melting Point M-565. 1H, and 13C NMR spectra were recorded in deuterated dimethyl sulfoxide (DMSO-d6) on a Bruker AVANCE 400 (400 MHz for 1H and 100 MHz for 13C) spectrometer (Switzerland) using TMS as an internal standard. Chemical shifts (δ) were expressed in parts per million (ppm) and coupling constants (J) were given in hertz (Hz). The following abbreviations were used to explain the multiplicities: s = singlet; d = doublet; t = triplet; q = quartet; m = multiplet. Mass spectra were registered on a high-resolution electrospray ionization mass spectrometer (maXis impact, Bruker, Germany).
3.2. In Vitro Antifungal Test
The in vitro fungicidal activity of the target compounds was demonstrated by examining the inhibition of the mycelial radial growth of representative phytopathogenic fungus, B. cinerea. These compounds were initially diluted to the testing concentration of 200 µg/mL with PDA medium at 50–60 °C and transferred into sterilized Petri dishes. After solidification, 5 mm-diameter mycelial disks taken from the peripheral part of a colony of each active fungus were placed at the center of the dishes aseptically. The plates were then incubated at 25 °C for 3 days. DMSO served as the negative control whereas carboxin served as the positive control. Each experiment was carried out in triplicate. The inhibitory effect of the test compounds on both fungi was calculated by the formula , where I represented the inhibition rate, and a and b were the mean colony diameters in the untreated and treated Petri dishes, respectively. Additionally, the corresponding inhibition rates of all test compounds at a series of concentrations (400, 200, 100, 50, 25, 12.5, 6.25 µg/mL) were measured under the same conditions as described above to compute medium effective concentration (EC50) values with SPSS 17.0 software.
3.3. In Vivo Assay against Tomato Gray Mold
In vivo assay was conducted on tomatoes (Lycopersicum esculentum) artificially inoculated with B. cinerea. Fruits were selected as experimental material based on uniformity and absence of physical injuries or infections, then surfaced-disinfected with 75% ethanol, rinsed with tap water, and air-dried before treatment. Test solutions (400 µg/mL) of synthesized compounds, prepared by dissolution in DMSO and dilution with distilled water comprising 0.1% Tween 80, were sprayed evenly on fruits and allowed to dry at room temperature. Commercial carboxin and an equivalent amount of DMSO were used as controls. The fruits were wounded with a sterile inoculation needle at the equatorial region and inoculated with the pathogen afterward. Each treatment consisted of three replicates. Treated fruits were stored at 25 °C and high relative humidity (90–95%) for one week. The efficacy of test compounds was expressed by the percentage of reduction in lesion diameter that was determined using the cross method.
3.4. Optical Microscopy of Hyphal Morphology of B. cinerea
Three-day-old B. cinerea mycelia were cultured in 50 mL potato dextrose broth (PDB) containing different concentrations of 3c (100 µg/mL and 200 µg/mL). The blank control had equal content (0.5%) of DMSO. After incubation at 25 °C for 22.5 h, the mycelia were collected, washed with 0.2 M phosphate-buffered saline (PBS) at pH 7.2, resuspended in PBS (0.2 M, pH 7.2), and observed using a light microscope at ×200 magnification.
3.5. Assessment of Cell Membrane Permeability
The change in the membrane permeability of B. cinerea was examined by measuring relative conductivity with a DDS-307 conductivity meter (Shanghai INESA Scientific Instrument Co. Ltd., Shanghai, China). The mycelia of 3-day-old B. cinerea were collected from PDB medium and washed with sterile distilled water, then treated with 100 or 200 µg/mL compound 3c. 0.01% DMF at the same dosage as the solutions mentioned above, and served as the blank control. Thereafter, the electrical conductivity of the mycelia suspension was determined at 0 (L0), 1, 2.5, 6.5, 10, 19, 25, 32, 43, and 52 h (L1) with the final conductivity (L2) of mycelia suspension being after it was boiled and cooled. The equation for the relative permeability was .
3.6. Determination of Oxygen Consumption
The influence of compound 3c on the mycelial respiration of B. cinerea was evaluated as stated by Yan et al [38]. Mycelial blocks from a one-week-old culture of B. cinerea were placed in 50 mL PDB and incubated at 25 °C for 3 days with 200 rpm shaking. After being harvested and rinsed, mycelia were suspended in 0.1 M PBS (pH 7.2, 50 mg fresh weight of mycelia mL−1) amended with 2% glucose, comprising 3c (100 or 200 µg/mL) or boscalid (200 µg/mL, positive control) and 0.01% DMF (blank control). Each treatment was repeated three times. A JPB-607A dissolved oxygen meter (Shanghai INESA Scientific Instrument Co. Ltd., Shanghai, China) was employed to determine mycelial oxygen consumption. The inhibition rate of respiration (IR) was calculated by the following formula , where R1 and R0 were the ratios of mycelial oxygen uptake with or without treatment, respectively. Statistical analysis of the results was performed using SPSS 17.0.
3.7. General Method for the Synthesis of Carboxylic Acid 2
Phosphonate 1 (1.89 g, 5 mmol) prepared according to the procedure reported (see Supplementary Materials) was dissolved in DMF (30 mL) and added to a 100 mL flask containing p-(Methoxycarbonyl)benzaldehyde (0.82 g, 5 mmol); then, an ethanol solution of potassium tert-butoxide (15 mL, 6 mmol) was introduced and the mixture was stirred for 6 h. The formed precipitate was filtered, washed with water, and dried to obtain intermediate ester.
The hydrolyzation of ester was carried out with 2 N NaOH aqueous solution under reflux (6 h). After cooling to room temperature, the solution was acidified to pH 3–4 with 1 M HCl, followed by filtration to obtain a crude product that was purified by recrystallization from DMSO/EtOH to give 1.31 g of 2.
3.8. General Method for the Synthesis of the Target Compounds
A two-step synthetic procedure was applied to obtain the target compounds. Acid 2 (1.20 g, 3.20 mmol) was stirred for 2 h in DMF (30 mL) at ambient temperature with 1-hydroxybenzotriazole (HOBt, 0.43 g, 3.20 mmol) and 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (EDCI, 0.61 g, 3.20 mmol). Glycine (0.24 g, 3.20 mmol) was then added and the reaction system was left to stir for 7 h. Then, 50 mL of water was added and the resultant yellow solid was removed by filtration and washed successively with 0.5 M HCl and methanol to remove impurities.
The coupling of the above resultant with glycine, L-methionine and L-leucine, respectively, was performed in a similar manner to its own synthetic protocols to afford target compounds 3a–3c that were recrystallized from DMSO/EtOH.
3.8.1. (E)-4-(4-(5-(Thiophen-2-yl)-1,3,4-oxadiazol-2-yl)styryl)benzoic acid (2)
1H NMR (400 MHz, DMSO-d6) δ 12.91 (s, COOH, 1H), 8.07 (d, J = 8.0 Hz, Th-H, 2H), 7.96 (d, J = 7.5 Hz, Ph-H, 4H), 7.85 (d, J = 8.1 Hz, Ph-H, 2H), 7.75 (d, J = 8.0 Hz, Ph-H, 2H), 7.48 (s, CH = CH, 2H), 7.32 (t, J = 4.3 Hz, Th-H, 1H); 13C NMR (101 MHz, DMSO-d6) δ 167.46, 163.76, 160.78, 141.38,140.65, 132.12, 131.00, 130.45, 130.29,130.24, 130.18, 129.20, 128.05, 127.49, 127.27, 124.76, 122.67; HRMS (ESI), m/z calcd for C21H15N2O3S [M + H] + 375.0798; found, 375.0793.
3.8.2. (E)-(4-(4-(5-(Thiophen-2-yl)-1,3,4-oxadiazol-2-yl)styryl)benzoyl)glycylglycine (3a)
A yellow solid, yield 54%, mp 217–218 °C; 1H NMR (400 MHz, DMSO-d6) δ 12.56 (s, 1H), 8.86 (dt, J = 17.9, 5.7 Hz, 1H), 8.24 (t, J = 5.9 Hz, 1H), 8.12 (d, J = 8.0 Hz, 2H), 7.99 (t, J = 4.0 Hz, 2H), 7.94 (d, J = 8.1 Hz, 2H), 7.89 (d, J = 8.1 Hz, 2H), 7.78 (d, J = 8.1 Hz, 2H), 7.52 (s, 2H), 7.35 (t, J = 4.4 Hz, 1H), 3.94 (d, J = 5.8 Hz, 2H), 3.79 (d, J = 5.8 Hz, 2H); 13C NMR (101 MHz, DMSO-d6) δ 171.64, 169.83, 166.47, 163.80, 160.80, 140.84, 139.97, 133.71, 132.19, 131.06, 130.47, 129.54, 129.26, 128.39, 127.99, 127.54, 127.06, 124.77, 122.55, 42.93, 41.18; HRMS (ESI) [M + Na]+ calcd for C25H20N4O5S: 511.1047, found: 511.1040.
3.8.3. (E)-(4-(4-(5-(Thiophen-2-yl)-1,3,4-oxadiazol-2-yl)styryl)benzoyl)glycyl-L-methionine (3b)
A yellow solid, yield 50%, mp 196–197 °C; 1H NMR (400 MHz, DMSO-d6) δ 12.61 (s, 1H), 8.76 (t, J = 6.0 Hz, 1H), 8.24 (d, J = 7.9 Hz, 1H), 8.11 (d, J = 8.0 Hz, 2H), 7.98 (t, J = 4.0 Hz, 2H), 7.93 (dd, J = 8.3, 3.4 Hz, 2H), 7.88 (d, J = 8.1 Hz, 2H), 7.77 (d, J = 8.1 Hz, 2H), 7.51 (s, 2H), 7.34 (t, J = 4.4 Hz, 1H), 4.38 (td, J = 8.5, 4.5 Hz, 1H), 4.03–3.89 (m, 2H), 2.55 (s, 4H), 2.05 (s, 3H); 13C NMR (101 MHz, DMSO-d6) δ 173.67, 169.55, 166.48, 163.80, 160.80, 140.84, 139.95, 133.75, 132.18, 131.06, 130.47, 129.54, 129.26, 128.33, 127.99, 127.54, 127.08, 124.76, 122.55, 51.41, 40.90, 31.30, 30.11, 15.05; HRMS (ESI) [M + H]+ calcd for C28H27N4O5S2: 563.1378, found: 563.1418.
3.8.4. (E)-(4-(4-(5-(Thiophen-2-yl)-1,3,4-oxadiazol-2-yl)styryl)benzoyl)glycyl-L-leucine (3c)
A yellow solid, yield 56%, mp 215–216 °C; 1H NMR (400 MHz, DMSO-d6) δ 12.68 (s, 1H), 8.85–8.71 (m, 1H), 8.18 (d, J = 8.1 Hz, 1H), 8.12 (s, 2H), 7.99 (d, J = 5.0 Hz, 2H), 7.93 (d, J = 7.1 Hz, 2H), 7.88 (d, J = 8.0 Hz, 2H), 7.77 (d, J = 5.8 Hz, 2H), 7.51 (s, 2H), 7.34 (d, J = 4.4 Hz, 1H), 4.29 (td, J = 8.6, 5.9 Hz, 1H), 3.96 (t, J = 4.5 Hz, 2H), 1.67 (q, J = 6.8 Hz, 1H), 1.54 (dq, J = 12.6, 7.9, 6.4 Hz, 2H), 0.89 (dd, J = 16.2, 6.5 Hz, 6H); 13C NMR (101 MHz, DMSO-d6) δ 174.49, 171.82, 169.38, 166.46, 163.80, 160.79, 140.84, 139.93, 133.79, 133.54, 132.18, 131.06, 130.44, 129.25, 128.32, 128.24, 127.99, 127.54, 127.15, 127.08, 124.77, 122.55, 24.76, 23.33, 21.91; HRMS (ESI) [M + H]+ calcd for C29H29N4O5S: 545.1814, found: 545.1856.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules27248755/s1, Synthetic procedure and characterization data for intermediate 1, citation of references [34,39]
Author Contributions
Conceptualization, Y.Z. and D.H.; methodology, X.L.; software, X.L; validation, L.W., Y.Z. and D.H.; formal analysis, X.L.; investigation, Y.Z.; resources, X.L.; data curation, L.W.; writing—original draft preparation, Y.Z.; writing—review and editing, D.H.; visualization, X.L.; supervision, D.H.; project administration, Y.Z.; funding acquisition, D.H. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by the Natural Science Foundation of Guangdong Province (2018A030313224).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article.
Conflicts of Interest
The authors declare no competing financial interests.
Sample Availability
Samples of the compounds are not available from the authors.
References
- Horbach, R.; Navarro-Quesada, A.R.; Knogge, W.; Deising, H.B. When and how to kill a plant cell: Infection strategies of plant pathogenic fungi. J. Plant Physiol. 2011, 168, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Dean, R.; Van Kan, J.A.L.; Pretorius, Z.A.; Hammond-Kosack, K.E.; Di Pietro, A.; Spanu, P.D.; Rudd, J.J.; Dickman, M.; Kahmann, R.; Ellis, J.; et al. The Top 10 fungal pathogens in molecular plant pathology. Mol. Plant Pathol. 2012, 13, 414–430. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zheng, X.J.; Khaskheli, M.I.; Sun, X.F.; Chang, X.L.; Gong, G.S. Identification of Colletotrichum species associated with blueberry anthracnose in Sichuan, China. Pathogens. 2020, 9, 718. [Google Scholar] [CrossRef] [PubMed]
- Noor, N.M.; Zakaria, L. Identification and characterization of Colletotrichum spp. associated with chili anthracnose in peninsular Malaysia. Eur. J. Plant Pathol. 2018, 151, 961–973. [Google Scholar] [CrossRef]
- Fournier, E.; Gladieux, P.; Giraud, T. The ‘Dr Jekyll and Mr Hyde fungus’: Noble rot versus gray mold symptoms of Botrytis cinerea on grapes. Evol. Appl. 2013, 6, 960–969. [Google Scholar] [CrossRef]
- Han, S.H.; Song, M.H.; Keum, Y.S. Effects of azole fungicides on secreted metabolomes of Botrytis cinerea. J. Agric. Food Chem. 2020, 68, 5309–5317. [Google Scholar] [CrossRef]
- Veloukas, T.; Markoglou, A.N.; Karaoglanidis, G.S. Differential effect of SdhB gene mutations on the sensitivity to SDHI fungicides in Botrytis cinerea. Plant Dis. 2013, 97, 118–122. [Google Scholar] [CrossRef]
- Chambers, J.E.; Greim, H.; Kendall, R.J.; Segner, H.; Sharpe, R.M.; Van Der Kraak, G. Human and ecological risk assessment of a crop protection chemical: A case study with the azole fungicide epoxiconazole. Crit. Rev. Toxicol. 2014, 44, 176–210. [Google Scholar] [CrossRef]
- Shen, T.; Wang, X.N.; Lou, H.X. Natural stilbenes: An overview. Nat. Prod. Rep. 2009, 26, 916–935. [Google Scholar] [CrossRef]
- Singh, D.; Mendonsa, R.; Koli, M.; Subramanian, M.; Nayak, S.K. Antibacterial activity of resveratrol structural analogues: A mechanistic evaluation of the structure-activity relationship. Toxicol. Appl. Pharmacol. 2019, 367, 23–32. [Google Scholar] [CrossRef]
- Roupe, K.A.; Remsberg, C.M.; Yanez, J.A.; Davies, N.M. Pharmacometrics of stilbenes: Seguing towards the clinic. Curr. Clin. Pharmacol. 2006, 1, 81–101. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.D.; Deng, Y.Z.; Han, T.Y.; Jiang, L.Q.; Xi, P.G.; Wang, Q.; Jiang, Z.D.; Gao, L.W. In vitro and in vivo effectiveness of phenolic compounds for the control of postharvest gray mold of table grapes. Postharvest Biol. Technol. 2018, 139, 106–114. [Google Scholar] [CrossRef]
- Gabaston, J.; Richard, T.; Biais, B.; Waffo-Teguo, P.; Pedrot, E.; Jourdes, M.; Corio-Costet, M.F.; Mérillon, J.M. Stilbenes from common spruce (Picea abies) bark as natural antifungal agent against downy mildew (Plasmopara viticola). Ind. Crop. Prod. 2017, 103, 267–273. [Google Scholar] [CrossRef]
- Wang, Y.; Catana, F.; Yang, Y.N.; Roderick, R.; van Breemen, R.B. An LC-MS method for analyzing total resveratrol in grape juice, cranberry juice, and in wine. J. Agric. Food Chem. 2002, 50, 431–435. [Google Scholar] [CrossRef]
- De Filippis, B.; Ammazzalorso, A.; Amoroso, R.; Giampietro, L. Stilbene derivatives as new perspective in antifungal medicinal chemistry. Drug Dev. Res. 2019, 80, 285–293. [Google Scholar] [CrossRef]
- Sbaghi, M.; Jeandet, P.; Bessis, R.; Leroux, P. Degradation of stilbene-type phytoalexins in relation to the pathogenicity of Botrytis cinerea to grapevines. Plant Pathol. 1996, 45, 139–144. [Google Scholar] [CrossRef]
- Tao, Q.Q.; Liu, L.W.; Wang, P.Y.; Long, Q.S.; Zhao, Y.L.; Jin, L.H.; Xu, W.M.; Chen, Y.; Li, Z.; Yang, S. Synthesis and in vitro and in vivo biological activity evaluation and quantitative proteome profiling of oxadiazoles bearing flexible heterocyclic patterns. J. Agric. Food Chem. 2019, 67, 7626–7639. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.J.; Li, C.S.; Cui, M.Y.; Song, Z.W.; Bai, X.Q.; Liang, C.W.; Wan-g, H.Y.; Zhang, T.Y. Synthesis, biological evaluation of benzothiazole derivatives bearing a 1,3,4-oxadiazole moiety as potential anti-oxidant and anti-inflammatory agents. Bioorg. Med. Chem. Lett. 2020, 30, 127237. [Google Scholar] [CrossRef]
- Sharma, V.; Kumar, R.; Angeli, A.; Supuran, C.T.; Sharma, P.K. Tail approach synthesis of novel benzenesulfonamides incorporating 1,3,4-oxadiazole hybrids as potent inhibitor of carbonic anhydrase I, II, IX, and XII isoenzymes. Eur. J. Med. Chem. 2020, 193, 112219. [Google Scholar] [CrossRef]
- Bhutani, R.; Pathak, D.P.; Kapoor, G.; Husain, A.; Iqbal, M.A. Novel hybrids of benzothiazole-1,3,4-oxadiazole-4-thiazolidinone: Synthesis, in silico ADME study, molecular docking and in vivo anti-diabetic assessment. Bioorg. Chem. 2019, 83, 6–19. [Google Scholar] [CrossRef]
- Silverio, M.R.S.; Espindola, L.S.; Lopes, N.P.; Vieira, P.C. Plant natural products for the control of Aedes aegypti: The main vector of important arboviruses. Molecules 2020, 25, 3484. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.T.; Wu, H.B.; Jiang, H.Y. Thiophenes from Echinops grijsii as a preliminary approach to control disease complex of root-knot nematodes and soil-borne fungi: Isolation, activities, and structure-nonphototoxic activity relationship analysis. J. Agric. Food Chem. 2019, 67, 6160–6168. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.L.; Shi, Y.X.; Zhan, Y.Z.; Zhang, L.Y.; Zhang, Y.; Wang, L.Z.; Zhang, X.; Li, Y.H.; Li, Z.M.; Li, B.J. Synthesis and biological activity of novel furan/thiophene and piperazine-containing (bis)1,2,4-triazole Mannich bases. Chin. J. Chem. 2015, 33, 1124–1134. [Google Scholar] [CrossRef]
- Ezugwu, J.A.; Okoro, U.C.; Ezeokonkwo, M.A.; Bhimapaka, C.R.; Okafor, S.N.; Ugwu, D.I.; Ekoh, O.C.; Attah, S.I. Novel Leu-Val based dipeptide as antimicrobial and antimalarial agents: Synthesis and molecular docking. Front. Chem. 2020, 8, 583926. [Google Scholar] [CrossRef] [PubMed]
- Day, T.; Greenfield, S.A. Bioactivity of a peptide derived from acetylcholinesterase in hippocampal organotypic cultures. Exp. Brain Res. 2004, 155, 500–508. [Google Scholar] [CrossRef] [PubMed]
- Lau, J.L.; Dunn, M.K. Therapeutic peptides: Historical perspectives, current development trends, and future directions. Bioorg. Med. Chem. 2018, 26, 2700–2707. [Google Scholar] [CrossRef]
- Gudasheva, T.A. Theoretical grounds and technologies for dipeptide drug development. Russ. Chem. Bull. 2015, 64, 2012–2021. [Google Scholar] [CrossRef]
- Miao, J.Y.; Guo, H.X.; Chen, F.L.; Zhao, L.C.; He, L.P.; Ou, Y.W.; Huang, M.M.; Zhang, Y.; Guo, B.Y.; Cao, Y.; et al. Antibacterial effects of a cell-pe-netrating peptide isolated from Kefir. J. Agric. Food Chem. 2016, 64, 3234–3242. [Google Scholar] [CrossRef]
- Tareq, F.S.; Lee, M.A.; Lee, H.-S.; Lee, Y.-J.; Lee, J.S.; Hasan, C.M.; Islam, M.T.; Shin, H.J. Gageotetrins A-C, noncytotoxic antimicrobial linear lipopeptides from a marine bacterium Bacillus subtilis. Org. Lett. 2014, 16, 928–931. [Google Scholar] [CrossRef]
- Khan, F.A.; Nasim, N.; Wang, Y.; Alhazmi, A.; Sanam, M.; Ul-Haq, Z.; Yalamati, D.; Ulanova, M.; Jiang, Z.H. Amphiphilic desmuramyl peptides for the rational design of new vaccine adjuvants: Synthesis, in vitro modulation of inflammatory response and molecular docking studies. Eur. J. Med. Chem. 2021, 209, 112863. [Google Scholar] [CrossRef]
- Lu, X.; Jia, C.; Gao, J.H.; Wang, R.D.; Zhang, L.X.; Sun, Q.; Huang, J.N. Structure-activity relationship and molecular docking analysis of cysteine-containing dipeptides as antioxidant and ACE inhibitory. Int. J. Food Sci. Technol. 2020, 56, 2789–2803. [Google Scholar] [CrossRef]
- Ma, Y.; Xu, M.H.; Liu, H.C.; Yu, T.T.; Guo, P.; Liu, W.B.; Jin, X.B. Antimicrobial compounds were isolated from the secondary metabolites of gordonia, a resident of intestinal tract of Periplaneta americana. AMB Express 2021, 11, 111. [Google Scholar] [CrossRef] [PubMed]
- Khalaf, H.S.; Naglah, A.M.; Al-Omar, M.A.; Moustafa, G.O.; Awad, H.M.; Bakheit, A.H. Synthesis, docking, computational studies, and antimicrobial evaluations of new dipeptide derivatives based on nicotinoylglycylglycine hydrazide. Molecules 2020, 25, 3589. [Google Scholar] [CrossRef]
- Wen, L.; Jian, W.L.; Shang, J.B.; He, D.H. Synthesis and antifungal activities of novel thiophene-based stilbene derivatives bearing an 1,3,4-oxadiazole unit. Pest Manag. Sci. 2019, 75, 1123–1130. [Google Scholar] [CrossRef]
- Monera, O.D.; Sereda, T.J.; Zhou, N.E.; Kay, C.M.; Hodges, R.S. Relationship of sidechain hydrophobicity and α-helical propensity on the stability of the single-stranded amphipathic α-helix. J. Pept. Sci. 1995, 1, 319–329. [Google Scholar] [CrossRef]
- Liu, G.S.; Zhang, S.; Yang, K.; Zhu, L.Z.; Lin, D.H. Toxicity of perfluorooctane sulfonate and perfluorooctanoic acid to Escherichia coli: Membrane disruption, oxidative stress, and DNA damage induced cell inactivation and/or death. Environ. Pollut. 2016, 214, 806–815. [Google Scholar] [CrossRef] [PubMed]
- Serazetdinova, L.; Oldach, K.H.; Lörz, H. Expression of transgenic stilbene synthases in wheat causes the accumulation of unknown stilbene derivatives with antifungal activity. J. Plant Physiol. 2005, 162, 985–1002. [Google Scholar] [CrossRef]
- Yan, X.J.; Liang, X.M.; Jin, S.H.; Lv, J.P.; Yu, C.X.; Qi, W.Y.; Li, B.J.; Yuan, H.Z.; Qi, S.H.; Shi, Y.X.; et al. Primary study on mode of action for macrocyclic fungicide candidates (7B3, D1) against Rhizoctonia solani Kühn. J. Agric. Food Chem. 2010, 58, 2726–2729. [Google Scholar] [CrossRef]
- Jian, W.L.; He, D.H.; Xi, P.G.; Li, X.W. Synthesis and biological evaluation of novel fluorine-containing stilbene derivatives as fungicidal agents against phytopathogenic fungi. J. Agric. Food. Chem. 2015, 63, 9963–9969. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).