Synthesis and Preclinical Evaluation of Small-Molecule Prostate-Specific Membrane Antigen-Targeted Abiraterone Conjugate
Abstract
1. Introduction
2. Results and Discussion
2.1. Synthesis
2.2. In Vitro Evaluation
2.2.1. Evaluation of CC50 on Prostate Cancer Cell Lines 22Rv1, PC-3 and Human Adult Dermal Fibroblasts
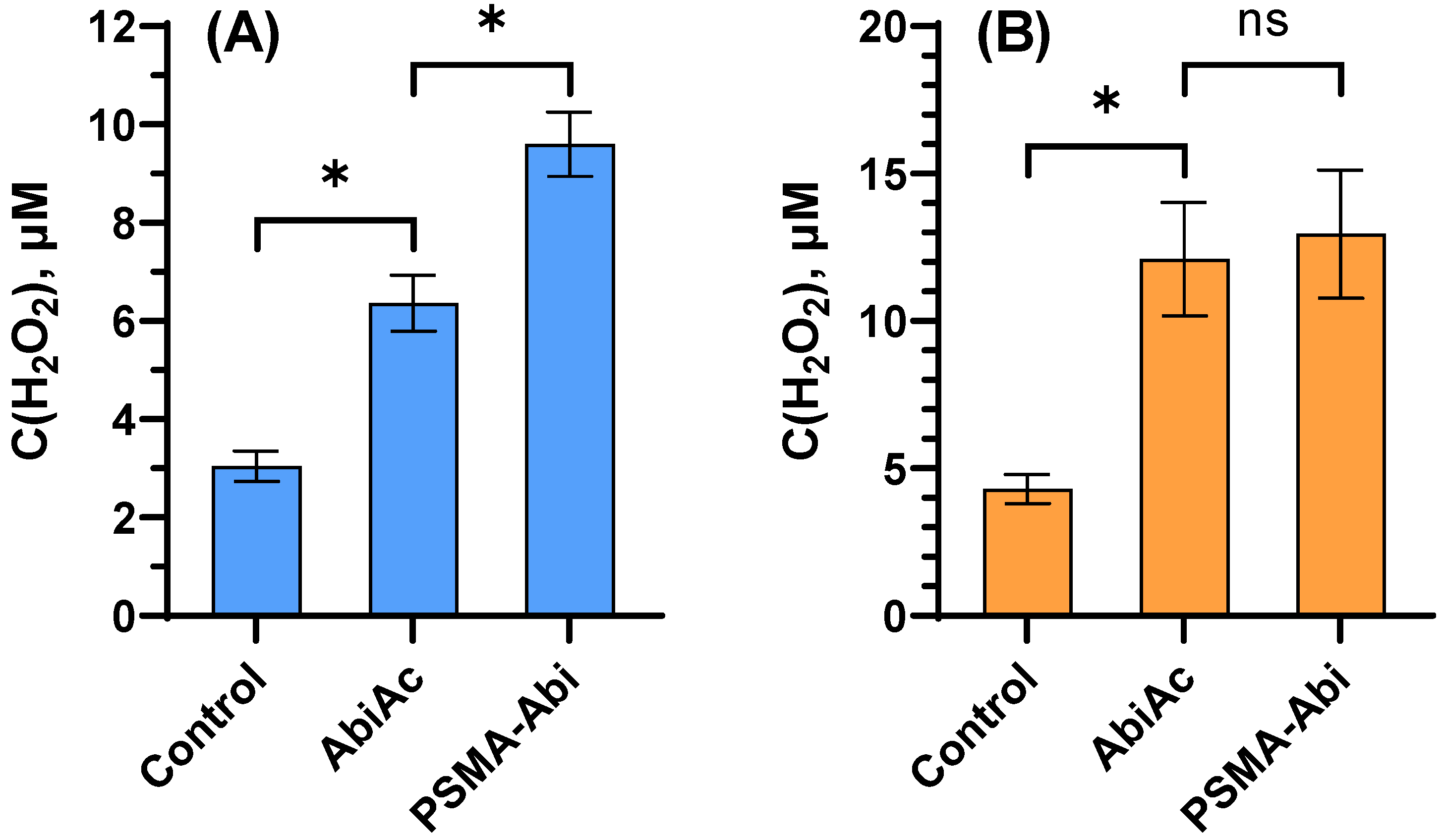
2.2.2. Measurement of Reactive Oxygen Species Level
2.2.3. Cell Cycle Analysis
2.3. In Vivo Evaluation
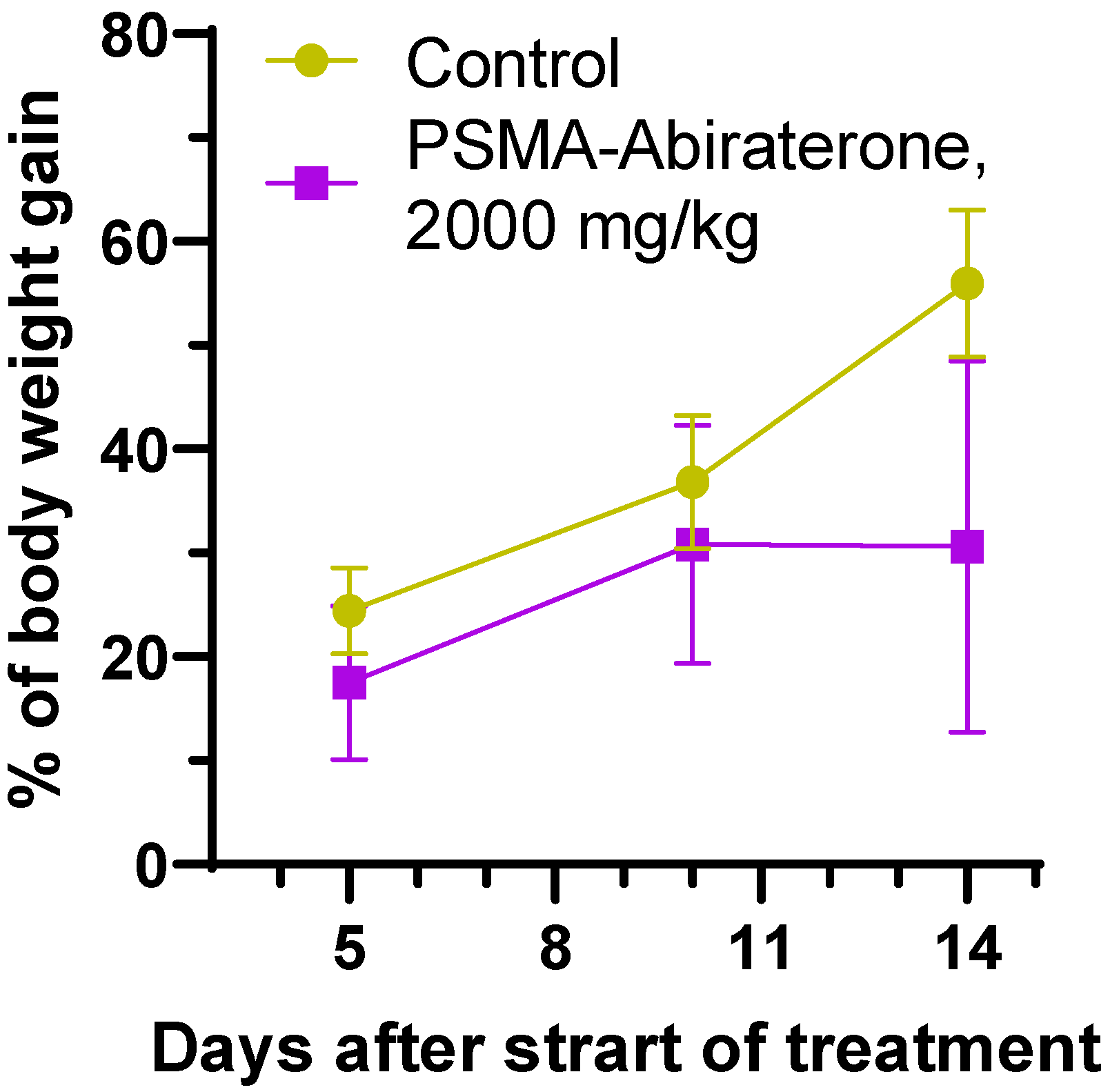
2.3.1. Acute Toxicity
2.3.2. Evaluation of Anticancer Effect
2.3.3. Pharmacokinetics
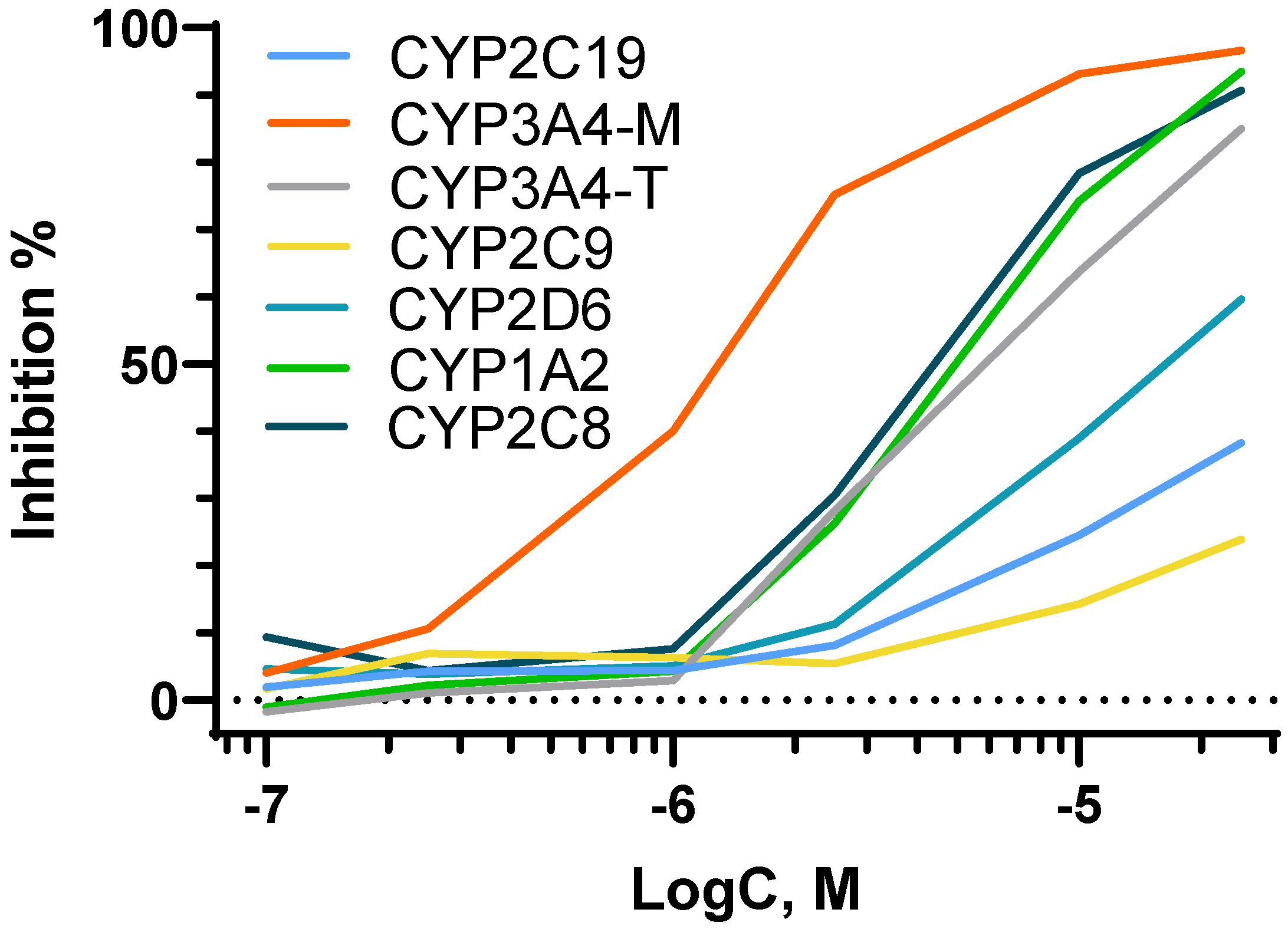
2.3.4. Cytochrome Study
3. Conclusions
4. Experimental Section
4.1. Synthesis
4.2. Synthesis of Abiraterone
4.3. Synthesis of Abiraterone Hexyn-5-oate
4.4. Synthesis of Conjugate PSMA-Abi
4.5. Cell Lines
4.6. Single Cell ROS Measurement by Using Pt-Nanoelectrodes
4.7. Cell Cycle Analysis
4.8. Human Tumor Xenograft Mouse Model
4.9. Acute Toxicity
4.10. Pharmacokinetics
4.11. Study of PSMA-Abi’s Ability to Inhibit the Activity of Human Liver Cytochromes (1A2, 2C8, 2C9, 2C19, 2D6, and 3A4)
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
Abbreviations
References
- Hellerstedt, B.A.; Pienta, K.J. The Current State of Hormonal Therapy for Prostate Cancer. CA Cancer J. Clin. 2002, 52, 154–174. [Google Scholar] [CrossRef] [PubMed]
- Harris, W.P.; Mostaghel, E.A.; Nelson, P.S.; Montgomery, B. Androgen deprivation therapy: Progress in understanding mechanisms of resistance and optimizing androgen depletion. Nat. Clin. Pract. Urol. 2009, 6, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, P.L.; Alibhai, S.M.H.; Basaria, S.; D’Amico, A.V.; Kantoff, P.W.; Keating, N.L.; Penson, D.F.; Rosario, D.J.; Tombal, B.; Smith, M.R. Adverse effects of androgen deprivation therapy and strategies to mitigate them. Eur. Urol. 2015, 67, 825–836. [Google Scholar] [CrossRef] [PubMed]
- Rehman, Y.; Rosenberg, J.E. Abiraterone acetate: Oral androgen biosynthesis inhibitor for treatment of castration-resistant prostate cancer. Drug Des. Dev. Ther. 2012, 6, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Sommer, U.; Siciliano, T.; Ebersbach, C.; Beier, A.-M.K.; Stope, M.B.; Jöhrens, K.; Baretton, G.B.; Borkowetz, A.; Thomas, C.; Erb, H.H.H. Impact of Androgen Receptor Activity on Prostate-Specific Membrane Antigen Expression in Prostate Cancer Cells. Int. J. Mol. Sci. 2022, 23, 1046. [Google Scholar] [CrossRef]
- Taplin, M.E.; Balk, S.P. Androgen receptor: A key molecule in the progression of prostate cancer to hormone independence. J. Cell. Biochem. 2004, 91, 483–490. [Google Scholar] [CrossRef] [PubMed]
- Crawford, E.D.; Heidenreich, A.; Lawrentschuk, N.; Tombal, B.; Pompeo, A.C.L.; Mendoza-Valdes, A.; Miller, K.; Debruyne, F.M.J.; Klotz, L. Androgen-targeted therapy in men with prostate cancer: Evolving practice and future considerations. Prostate Cancer Prostatic Dis. 2019, 22, 24–38. [Google Scholar] [CrossRef]
- Rice, M.A.; Malhotra, S.V.; Stoyanova, T. Second-Generation Antiandrogens: From Discovery to Standard of Care in Castration Resistant Prostate Cancer. Front. Oncol. 2019, 9, 801. [Google Scholar] [CrossRef]
- Miller, K.; Carles, J.; Gschwend, J.E.; van Poppel, H.; Diels, J.; Brookman-May, S.D. The Phase 3 COU-AA-302 Study of Abiraterone Acetate Plus Prednisone in Men with Chemotherapy-naïve Metastatic Castration-resistant Prostate Cancer: Stratified Analysis Based on Pain, Prostate-specific Antigen, and Gleason Score. Eur. Urol. 2018, 74, 17–23. [Google Scholar] [CrossRef]
- Fizazi, K.; Scher, H.I.; Molina, A.; Logothetis, C.J.; Chi, K.N.; Jones, R.J.; Staffurth, J.N.; North, S.; Vogelzang, N.J.; Saad, F.; et al. Abiraterone acetate for treatment of metastatic castration-resistant prostate cancer: Final overall survival analysis of the COU-AA-301 randomised, double-blind, placebo-controlled phase 3 study. Lancet Oncol. 2012, 13, 983–992. [Google Scholar] [CrossRef] [PubMed]
- Katagiri, M.; Kagawa, N.; Waterman, M.R. The Role of Cytochrome b5 in the Biosynthesis of Androgens by Human P450c17. Arch. Biochem. Biophys. 1995, 317, 343–347. [Google Scholar] [CrossRef] [PubMed]
- Attard, G.; Reid, A.H.M.; Olmos, D.; de Bono, J.S. Antitumor activity with CYP17 blockade indicates that castration-resistant prostate cancer frequently remains hormone driven. Cancer Res. 2009, 69, 4937–4940. [Google Scholar] [CrossRef] [PubMed]
- Gala, U.; Miller, D.; Williams, R.O. Improved Dissolution and Pharmacokinetics of Abiraterone through KinetiSol® Enabled Amorphous Solid Dispersions. Pharmaceutics 2020, 12, 357. [Google Scholar] [CrossRef] [PubMed]
- Beg, S.; Malik, A.K.; Ansari, M.J.; Malik, A.A.; Ali, A.M.A.; Theyab, A.; Algahtani, M.; Almalki, W.H.; Alharbi, K.S.; Alenezi, S.K.; et al. Systematic Development of Solid Lipid Nanoparticles of Abiraterone Acetate with Improved Oral Bioavailability and Anticancer Activity for Prostate Carcinoma Treatment. ACS Omega 2022, 7, 16968–16979. [Google Scholar] [CrossRef] [PubMed]
- Katekar, R.; Sen, S.; Riyazuddin, M.; Husain, A.; Garg, R.; Verma, S.; Mitra, K.; Gayen, J.R. Augmented experimental design for bioavailability enhancement: A robust formulation of abiraterone acetate. J. Liposome Res. 2022, 32, 1–12. [Google Scholar] [CrossRef]
- Baker, A.; Khalid, M.; Uddin, I.; Khan, M.S. Targeted non AR mediated smart delivery of abiraterone to the prostate cancer. PLoS ONE 2022, 17, e0272396. [Google Scholar] [CrossRef]
- Basak, D.; Arrighi, S.; Darwiche, Y.; Deb, S. Comparison of Anticancer Drug Toxicities: Paradigm Shift in Adverse Effect Profile. Life 2022, 12, 48. [Google Scholar] [CrossRef]
- Machulkin, A.; Uspenskaya, A.; Zyk, N.; Nimenko, E.; Ber, A.; Petrov, S.; Shafikov, R.; Skvortsov, D.; Smirnova, G.; Borisova, Y.; et al. PSMA-targeted small-molecule docetaxel conjugate: Synthesis and preclinical evaluation. Eur. J. Med. Chem. 2021, 227, 113936. [Google Scholar] [CrossRef]
- Machulkin, A.E.; Uspenskaya, A.A.; Zyk, N.Y.; Nimenko, E.A.; Ber, A.P.; Petrov, S.A.; Polshakov, V.I.; Shafikov, R.R.; Skvortsov, D.A.; Plotnikova, E.A.; et al. Synthesis, characterization and preclinical evaluation of small-molecule prostate-specific membrane antigen targeted monomethyl auristatin E conjugate. J. Med. Chem. 2021, 64, 17123–17145. [Google Scholar] [CrossRef]
- Hupe, M.C.; Philippi, C.; Roth, D.; Kümpers, C.; Ribbat-Idel, J.; Becker, F.; Joerg, V.; Duensing, S.; Lubczyk, V.H.; Kirfel, J.; et al. Expression of prostate-specific membrane antigen (PSMA) on biopsies is an independent risk stratifier of prostate cancer patients at time of initial diagnosis. Front. Oncol. 2018, 8, 623. [Google Scholar] [CrossRef]
- Queisser, A.; Hagedorn, S.A.; Braun, M.; Vogel, W.; Duensing, S.; Perner, S. Comparison of different prostatic markers in lymph node and distant metastases of prostate cancer. Mod. Pathol. 2015, 28, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Heynickx, N.; Herrmann, K.; Vermeulen, K.; Baatout, S.; Aerts, A. The salivary glands as a dose limiting organ of PSMA- targeted radionuclide therapy: A review of the lessons learnt so far. Nucl. Med. Biol. 2021, 98–99, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, Y.; Kuratsukuri, K.; Landas, S.; Imaida, K.; Rovito, P.M.; Wang, C.Y.; Haas, G.P. Expression of prostate-specific membrane antigen in normal and malignant human tissues. World J. Surg. 2006, 30, 628–636. [Google Scholar] [CrossRef] [PubMed]
- Klein Nulent, T.J.W.; Valstar, M.H.; de Keizer, B.; Willems, S.M.; Smit, L.A.; Al-Mamgani, A.; Smeele, L.E.; van Es, R.J.J.; de Bree, R.; Vogel, W.V. Physiologic distribution of PSMA-ligand in salivary glands and seromucous glands of the head and neck on PET/CT. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2018, 125, 478–486. [Google Scholar] [CrossRef]
- Bouchelouche, K.; Choyke, P.L.; Capala, J. Prostate specific membrane antigen- a target for imaging and therapy with radionuclides. Discov. Med. 2010, 9, 55–61. [Google Scholar]
- Pomper, M.G.; Chen, Y.; Slusher, B.S.; Pullambhatla, M.; Byun, Y.; Rojas, C.; Banerjee, S.R.; Mease, R.C.; Stathis, M. Synthesis and Biological Evaluation of Low Molecular Weight Fluorescent Imaging Agents for the Prostate-Specific Membrane Antigen. Bioconjug. Chem. 2012, 23, 2377–2385. [Google Scholar]
- Schottelius, M.; Wurzer, A.; Wissmiller, K.; Beck, R.; Koch, M.; Gorpas, D.; Notni, J.; Buckle, T.; Van Oosterom, M.N.; Steiger, K.; et al. Synthesis and preclinical characterization of the PSMA-targeted hybrid tracer PSMA-I&F for nuclear and fluorescence imaging of prostate cancer. J. Nucl. Med. 2019, 60, 71–78. [Google Scholar]
- Kularatne, S.A.; Thomas, M.; Myers, C.H.; Gagare, P.; Kanduluru, A.K.; Crian, C.J.; Cichocki, B.N. Evaluation of novel prostate-specific membrane antigen-targeted near-infrared imaging agent for fluorescence-guided surgery of prostate cancer. Clin. Cancer Res. 2019, 25, 177–187. [Google Scholar] [CrossRef]
- Machulkin, A.E.; Shafikov, R.R.; Uspenskaya, A.A.; Petrov, S.A.; Ber, A.P.; Skvortsov, D.A.; Nimenko, E.A.; Zyk, N.Y.; Smirnova, G.B.; Pokrovsky, V.S.; et al. Synthesis and biological evaluation of PSMA ligands with aromatic residues and fluorescent conjugates based on them. J. Med. Chem. 2021, 64, 4532–4552. [Google Scholar] [CrossRef]
- Kularatne, S.A.; Venkatesh, C.; Santhapuram, H.K.R.; Wang, K.; Vaitilingam, B.; Henne, W.A.; Low, P.S. Synthesis and biological analysis of prostate-specific membrane antigen-targeted anticancer prodrugs. J. Med. Chem. 2010, 53, 7767–7777. [Google Scholar] [CrossRef]
- Leamon, C.P.; Reddy, J.A.; Bloomfield, A.; Dorton, R.; Nelson, M.; Vetzel, M.; Kleindl, P.; Hahn, S.; Wang, K.; Vlahov, I.R. Prostate-Specific Membrane Antigen-Specific Antitumor Activity of a Self-Immolative Tubulysin Conjugate. Bioconjug. Chem. 2019, 30, 1805–1813. [Google Scholar] [CrossRef] [PubMed]
- Morris, M.J.; Vogelzang, N.J.; Sartor, O.; Armour, A.; Groaning, M.; Robarts, A.; Petrylak, D.P.; Tolcher, A.W.; Gordon, M.S.; Babiker, H.M. Phase 1 study of the PSMA-targeted small-molecule drug conjugate EC1169 in patients with metastatic castrate-resistant prostate cancer (mCRPC). J. Clin. Oncol. 2017, 35, 5038. [Google Scholar] [CrossRef]
- Rogers, O.C.; Rosen, D.M.; Antony, L.; Harper, H.M.; Das, D.; Yang, X.; Minn, I.; Mease, R.C.; Pomper, M.G.; Denmeade, S.R. Targeted delivery of cytotoxic proteins to prostate cancer via conjugation to small molecule urea-based PSMA inhibitors. Sci. Rep. 2021, 11, 14925. [Google Scholar] [CrossRef]
- Suvorov, N.V.; MacHulkin, A.E.; Ivanova, A.V.; Popkov, A.M.; Bondareva, E.A.; Plotnikova, E.A.; Yakubovskaya, R.I.; Majouga, A.G.; Mironov, A.F.; Grin, M.A. Synthesis of PSMA-targeted 131- and 152-substituted chlorin e6 derivatives and their biological properties. J. Porphyr. Phthalocyanines 2018, 22, 1030–1038. [Google Scholar] [CrossRef]
- Chen, Y.; Chatterjee, S.; Lisok, A.; Minn, I.; Pullambhatla, M.; Wharram, B.; Wang, Y.; Jin, J.; Bhujwalla, Z.M.; Nimmagadda, S.; et al. A PSMA-targeted theranostic agent for photodynamic therapy. J. Photochem. Photobiol. B Biol. 2017, 167, 111–116. [Google Scholar] [CrossRef]
- Ngen, E.J.; Chen, Y.; Azad, B.B.; Boinapally, S.; Jacob, D.; Lisok, A.; Shen, C.; Hossain, M.S.; Jin, J.; Bhujwalla, Z.M.; et al. Prostate-specific membrane antigen (PSMA)-targeted photodynamic therapy enhances the delivery of PSMA-targeted magnetic nanoparticles to PSMA-expressing prostate tumors. Nanotheranostics 2021, 5, 182–196. [Google Scholar] [CrossRef]
- FDA Approves Second PSMA-Targeted PET Imaging Drug for Men with Prostate Cancer. Available online: https://www.fda.gov/news-events/press-announcements/fda-approves-first-psma-targeted-pet-imaging-drug-men-prostate-cancer (accessed on 1 December 2020).
- Cheong, E.J.Y.; Nair, P.C.; Neo, R.W.Y.; Tu, H.T.; Lin, F.; Chiong, E.; Esuvaranathan, K.; Fan, H.; Szmulewitz, R.Z.; Peer, C.J.; et al. Slow-, Tight-Binding Inhibition of CYP17A1 by Abiraterone Redefines Its Kinetic Selectivity and Dosing Regimen. J. Pharmacol. Exp. Ther. 2020, 374, 438–451. [Google Scholar] [CrossRef]
- Kaighn, M.E.; Narayan, K.S.; Ohnuki, Y.; Lechner, J.F.; Jones, L.W. Establishment and characterization of a human prostatic carcinoma cell line (PC-3). Investig. Urol. 1979, 17, 16–23. [Google Scholar]
- Sramkoski, R.M.; Pretlow, T.G.; Giaconia, J.M.; Pretlow, T.P.; Schwartz, S.; Sy, M.-S.; Marengo, S.R.; Rhim, J.S.; Zhang, D.; Jacobberger, J.W. A new human prostate carcinoma cell line, 22Rv1. Vitr. Cell. Dev. Biol.-Anim. 1999, 35, 403–409. [Google Scholar] [CrossRef]
- Shi, Y.K.; Wang, B.; Shi, X.L.; Zhao, Y.D.; Yu, B.; Liu, H.M. Synthesis and biological evaluation of new steroidal pyridines as potential anti-prostate cancer agents. Eur. J. Med. Chem. 2018, 145, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.Y.; Ko, C.Y.; Kao, T.J.; Yang, W.B.; Tsai, Y.T.; Chuang, J.Y.; Hu, S.L.; Yang, P.Y.; Lo, W.L.; Hsu, T.I.; et al. CYP17A1 Maintains the Survival of Glioblastomas by Regulating SAR1-Mediated Endoplasmic Reticulum Health and Redox Homeostasis. Cancers 2019, 11, 1378. [Google Scholar] [CrossRef]
- Vaneev, A.N.; Gorelkin, P.V.; Garanina, A.S.; Lopatukhina, H.V.; Vodopyanov, S.S.; Alova, A.V.; Ryabaya, O.O.; Akasov, R.A.; Zhang, Y.; Novak, P.; et al. In Vitro and In Vivo Electrochemical Measurement of Reactive Oxygen Species After Treatment with Anticancer Drugs. Anal. Chem. 2020, 92, 8010–8014. [Google Scholar] [CrossRef] [PubMed]
- Erofeev, A.; Gorelkin, P.; Garanina, A.; Alova, A.; Efremova, M.; Vorobyeva, N. Novel method for rapid toxicity screening of magnetic nanoparticles. Sci. Rep. 2018, 8, 7462. [Google Scholar] [CrossRef] [PubMed]
- Simon, I.; Perales, S.; Casado-Medina, L.; Rodríguez-Martínez, A.; Garrido-Navas, M.D.C.; Puche-Sanz, I.; Diaz-Mochon, J.J.; Alaminos, C.; Lupiañez, P.; Lorente, J.A.; et al. Cross-Resistance to Abiraterone and Enzalutamide in Castration Resistance Prostate Cancer Cellular Models Is Mediated by AR Transcriptional Reactivation. Cancers 2021, 13, 1483. [Google Scholar] [CrossRef] [PubMed]
- Janssen Pharmaceutica. Safety Data Sheet, Zytiga (Abiraterone Acetate) Abiraterone Acetate 250MG/Tablet; Janssen Pharmaceutica: Beerse, Belgium, 2013. [Google Scholar]
- Silverman, R.B. Chemical Modifications Influencing the Pharmacokinetic Properties. In The Practice of Medicinal Chemistry, 3rd ed.; Wermuth, C.G., Ed.; Elsevier Science Publishing Co. Inc.: San Diego, CA, United States, 2008; Section VI; pp. 699–803. [Google Scholar]
- Belpaire, F.M.; Bogaert, M.G. Cytochrome P450: Genetic polymorphism and drug interactions. Acta Clin. Belg. 1996, 51, 254–260. [Google Scholar] [CrossRef]



| Compound | CC50, μM | ||
|---|---|---|---|
| PC-3 | 22Rv1 | HDFa | |
| PSMA-Abi | 8.1 ± 2.8 | 8.2 ± 3.1 | >100 |
| AbiAc | 5.9 ± 0.8 [41] | 22.7 ± 3.3 | >100 |
| Mean ± SD | |
|---|---|
| Cmax, ng/mL | 318 ± 190 |
| Tmax, h | 3.8 |
| AUC0–t, h× ng/mL | 3791 ± 875 |
| AUC0–∞, h× ng/mL | 4623 ± 1260 |
| AUCt–∞/AUC0–∞, % | 21 ± 5 |
| Kel, 1/h | 0.023 ± 0.006 |
| T1/2, h | 31.3 ± 8.7 |
| MRT0–t, h | 27.2 ± 1.7 |
| Cytochrome | Substrate | Standard Inhibitor | IC50, nM | PSMA-Abi, µM |
|---|---|---|---|---|
| 1A2 | Phenacetin | α-Naphthoflavone | 57 ± 6 | 4.5 ± 1.1 |
| 3A4-M | Midazolam | Ketoconazole | 90 ± 8 | 1.1 ± 0.2 |
| 3A4-T | Testosterone | Ketoconazole | 184 ± 41 | 5.7 ± 0.8 |
| 2C8 | Paclitaxel | Montelukast | 1270 ± 262 | 4.4 ± 1.1 |
| 2C9 | Tolbutamide | Sulfaphenazole | 641 ± 77 | >25 |
| 2C19 | Mephenytoin | Tranylcypromine | 11,700 ± 940 | >25 |
| 2D6 | Dextromethorphan | Quinidine | 64 ± 5 | 17 ± 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Machulkin, A.E.; Nimenko, E.A.; Zyk, N.U.; Uspenskaia, A.A.; Smirnova, G.B.; Khan, I.I.; Pokrovsky, V.S.; Vaneev, A.N.; Timoshenko, R.V.; Mamed-Nabizade, V.V.; et al. Synthesis and Preclinical Evaluation of Small-Molecule Prostate-Specific Membrane Antigen-Targeted Abiraterone Conjugate. Molecules 2022, 27, 8795. https://doi.org/10.3390/molecules27248795
Machulkin AE, Nimenko EA, Zyk NU, Uspenskaia AA, Smirnova GB, Khan II, Pokrovsky VS, Vaneev AN, Timoshenko RV, Mamed-Nabizade VV, et al. Synthesis and Preclinical Evaluation of Small-Molecule Prostate-Specific Membrane Antigen-Targeted Abiraterone Conjugate. Molecules. 2022; 27(24):8795. https://doi.org/10.3390/molecules27248795
Chicago/Turabian StyleMachulkin, Aleksei E., Ekaterina A. Nimenko, Nikolay U. Zyk, Anastasiia A. Uspenskaia, Galina B. Smirnova, Irina I. Khan, Vadim S. Pokrovsky, Alexander N. Vaneev, Roman V. Timoshenko, Vugara V. Mamed-Nabizade, and et al. 2022. "Synthesis and Preclinical Evaluation of Small-Molecule Prostate-Specific Membrane Antigen-Targeted Abiraterone Conjugate" Molecules 27, no. 24: 8795. https://doi.org/10.3390/molecules27248795
APA StyleMachulkin, A. E., Nimenko, E. A., Zyk, N. U., Uspenskaia, A. A., Smirnova, G. B., Khan, I. I., Pokrovsky, V. S., Vaneev, A. N., Timoshenko, R. V., Mamed-Nabizade, V. V., Zavertkina, M. V., Erofeev, A., Gorelkin, P., Majouga, A. G., Zyk, N. V., Khazanova, E. S., & Beloglazkina, E. K. (2022). Synthesis and Preclinical Evaluation of Small-Molecule Prostate-Specific Membrane Antigen-Targeted Abiraterone Conjugate. Molecules, 27(24), 8795. https://doi.org/10.3390/molecules27248795






