Therapeutic Importance of Kaempferol in the Treatment of Cancer through the Modulation of Cell Signalling Pathways
Abstract
:1. Introduction
2. Major Mechanisms of Kaempferol (Kmp) in Management of Cancer
2.1. Inflammation
2.2. Reactive Oxygen Species (ROS)
2.3. Angiogenesis
2.4. Signal Transduction
2.5. Cell Cycle
2.6. Remodeling Tumor Metabolism
| Major Mechanism | Outcome of the Study | Refs |
|---|---|---|
| Inflammation | Kmp has been recognised as an effective inhibitor of pro-inflammatory molecules including vascular cell adhesion protein 1, prostaglandin-endoperoxide synthase (PTGS) and inducible nitric oxide synthase (NOSII) | [46,47] |
| Inflammation | Anti-inflammatory effects of Kmp are mostly facilitated by downregulation of numerous sequence-specific DNA-binding factors like STAT, nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) which have the capability to encourage the pro-inflammatory cytokines activation | [48] |
| Reactive Oxygen Species (ROS) | The ant-oxidant property of Kmp is linked with its up regulatory effects on antioxidant-response element- (ARE) mediative anti-oxidative enzymes like superoxide dismutase, catalase, and haem oxygenase in control of nuclear factor erythroid 2-related factor 2 signalling pathway | [59] |
| Reactive Oxygen Species (ROS) | Kmp reduced the thiobarbituric-acid reactive substances and red blood corpuscles lysates and upregulated the level of enzymatic antioxidants such as superoxide dismutase, glutathione perxidases (GSHPx) and catalase when 1,2-dimethylehydrazine (DMH)-induced-colon cancer male Wistar-rats treated with Kmp | [63] |
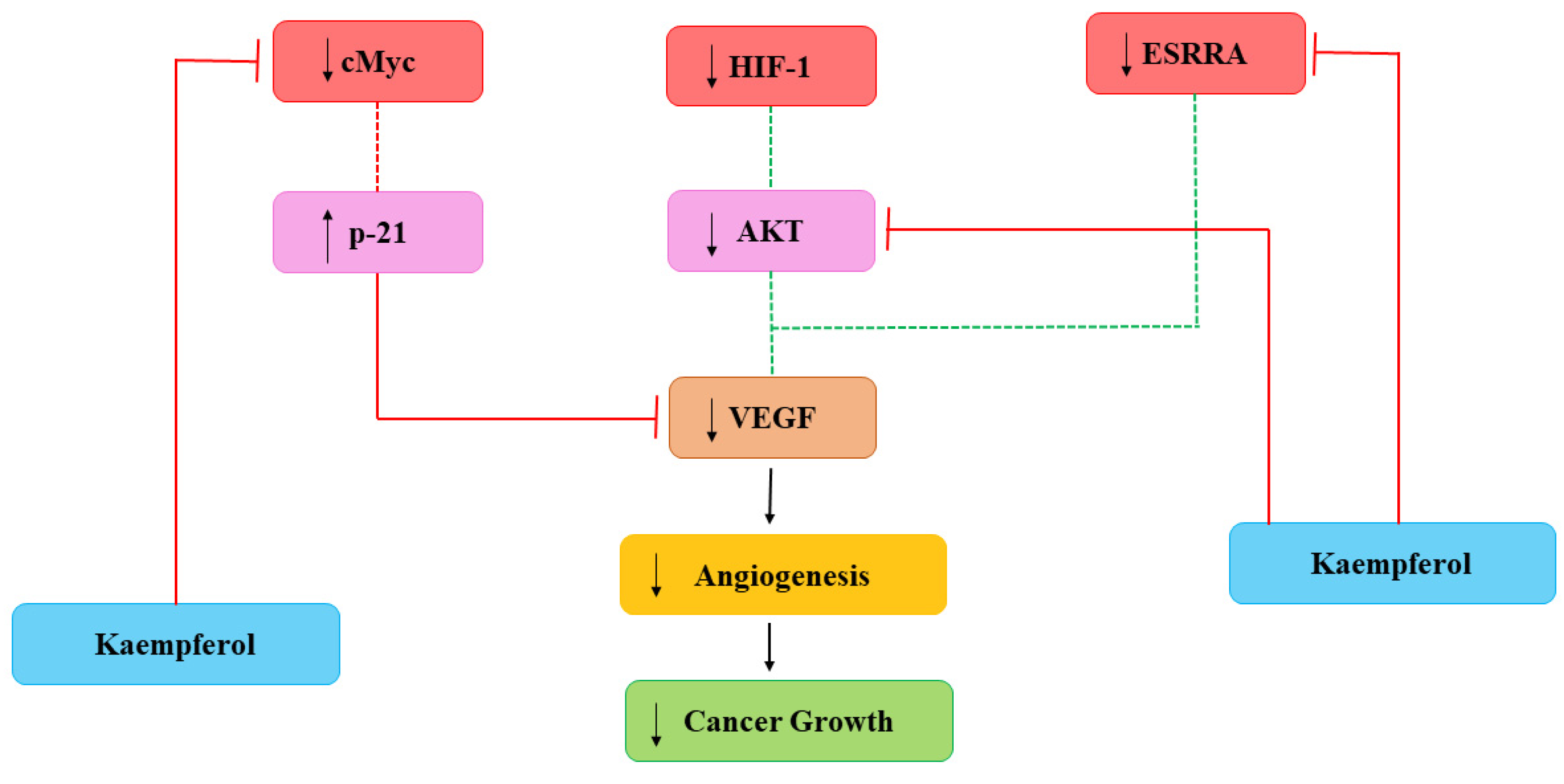
| Angiogenesis | Kmp prevented VEGF secretion in MDA-MB-231 cancerous cells and decreased the concentration of VEGF-mRNA among ovarian cancerous cell lines | [71] |
| Angiogenesis | Administered Kmp inhibited expression of NF-κB, c-Myc and phosphorylation of ERK and reduction of these encourages expression of p21 which antagonizes the release of VEGF | [70,71] |
| Signal transducer and activator of transcription 3 (STAT3) | At high concentration, Kmp prevented interleukin-6 induced-phosphorylation of STAT3 | [77] |
| Phosphatidylinositide-3-kinases (PI3K)-AKT pathways (PI3K-AKT) | Kmp repressed the growth of colorectal cancerous cells by preventing the activation of PI3K-AKT signalling pathways | [96] |
| Cell cycle | Kmp treatment induces G2-M phase cell cycle arrest through checkpoint kinase2 (CHK2) in ovarian cancerous cells or it has been shown that Kmp therapy can lead to G0-G1 cell cycle arrest in human esophageal squamous carcinoma Eca-109 cells | [105,106,107] |
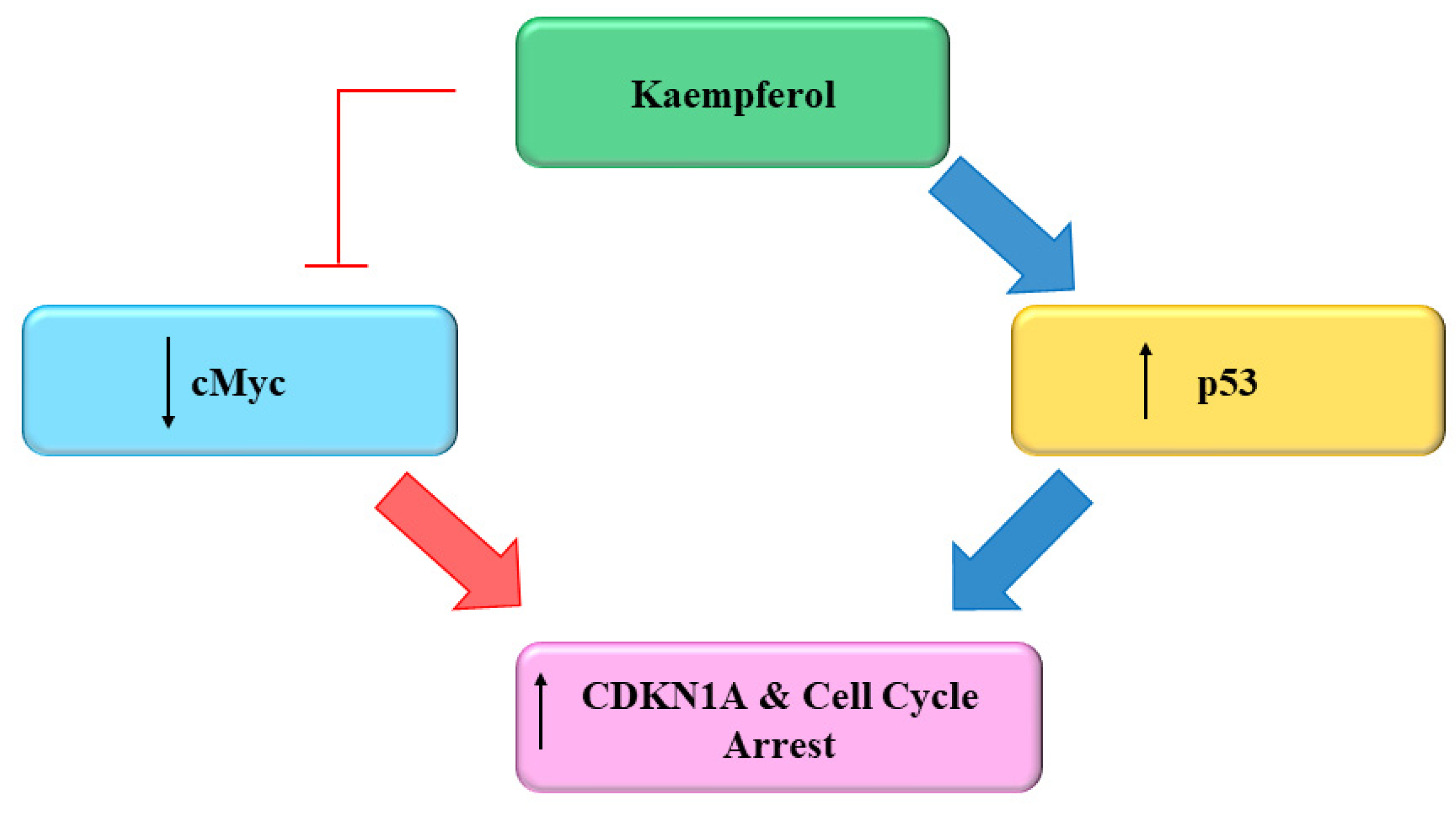
| Cell cycle | Administration of Kmp in combination with cisplatin reduces mRNA concentration of c-Myc and increases mRNA concentration of CDKN1A in ovarian cancerous cells | [111] |
3. Role of Kaempferol in Prevention and Inhibition of Various Types of Cancer
3.1. Hepatic Cancer
3.2. Lung Cancer
3.3. Prostate Cancer
3.4. Oral Cancer
3.5. Gastric Cancer
3.6. Breast Cancer
3.7. Leukaemia
3.8. Colon Cancer
3.9. Brain Tumor
3.10. Pancreatic Cancer
3.11. Bladder Cancer
3.12. Osteosarcoma
3.13. Cervical Cancer
3.14. Renal Cancer
3.15. Ovarian Cancer
| Types of Cancer | Mechanism/Outcome of the Study | Refs. |
|---|---|---|
| Hepatic cancer | Kmp in a dosage-dependent manner substantially prevent proliferation liver cancerous cells such as Huh-7, SKHEP-1 and Hep.G2 | [116,117] |
| Hepatic cancer | Additionally, 2-acetylaminofluorene and N-Nitrosodiethylamine-stimulated hepatocellular carcinoma from mice treated with combination of Kmp and luteolin prevented cancerous cells growth and caused apoptosis | [116,117] |
| Lung cancer | Kmp inhibited the NSCLC A549 cancerous cells, reduced formation of colonies and caused apoptosis | [125,126,127,128,129] |
| Lung cancer | Kmp reduced the number of metastasis and sub-cutaneous xenograft’s volume in comparison with control groups in lung-metastasis models | [129] |
| Prostate cancer | Kmp in dose-dependent manner prevents the proliferation of prostate cancer cells, through up-regulation of expression levels of PARP and caspase-3, -9, -8 proteins | [131,132] |
| Prostate cancer | Prostate cancerous cells transcriptome is significantly influenced by treatment of Kmp as it downregulated the expression of androgen-receptor genes | [133] |
| Oral cancer | In-vitro research demonstrated the anti-proliferative effect of Kmp on oral squamous cell cancer (SCC) cells such as SCC-4, -25, -QLL1, -1483, oesophageal squamous cell carcinoma such as Eca109 cells, oral cavity tumor cells such as PCI13 and pharyngeal squamous carcinoma cells such as FaDu and inhibited cell invasion and migration, formation of clones and caused apoptosis | [134,135,136] |
| Gastric cancer | Several studies observed the anti-proliferative activities of Kmp in SGC7901 and MKN29 stomach cancerous and promoted the G2-M stage cell cycle arrest, cell death and autophagy in these cancer cell lines | [137,138] |
| Breast cancer | At concentration in micro molars (μM), Kmp efficiently prevents the breast cancerous cells growth such as MCF7, MDA-MB231 | [140,141,142] |
| Leukaemia | Kmp in dosage-dependent manner (12.5 to 100 μM) reduced the viability of cells among leukaemia cells such as NB-4 and HL60 | [153,154] |
| Leukaemia | Kmp decreased the β-hexosaminidase release as a marker of de-granulation among leukemic cells such as RBL2H3 among mouse models, and enhanced the development of secretory granules in human leukaemia cells such as HMC1 (The biomarker of cancer cell lines is not always directly referred to the anti-cancer event) | [157,158] |
| Colon cancer | when Kmp combined to tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) resulted in apoptosis in colorectal cancerous cells via upregulation of death-receptor 5 (DR5) and receptors of TRAIL which increased the activity of TRAIL | [159] |
| Brain tumor | Kmp prevented migration and growth of GB cells and kmp-loaded mucoadhesive nano emulsion also prevented the growth of glioma tumor cells | [169,170,171] |
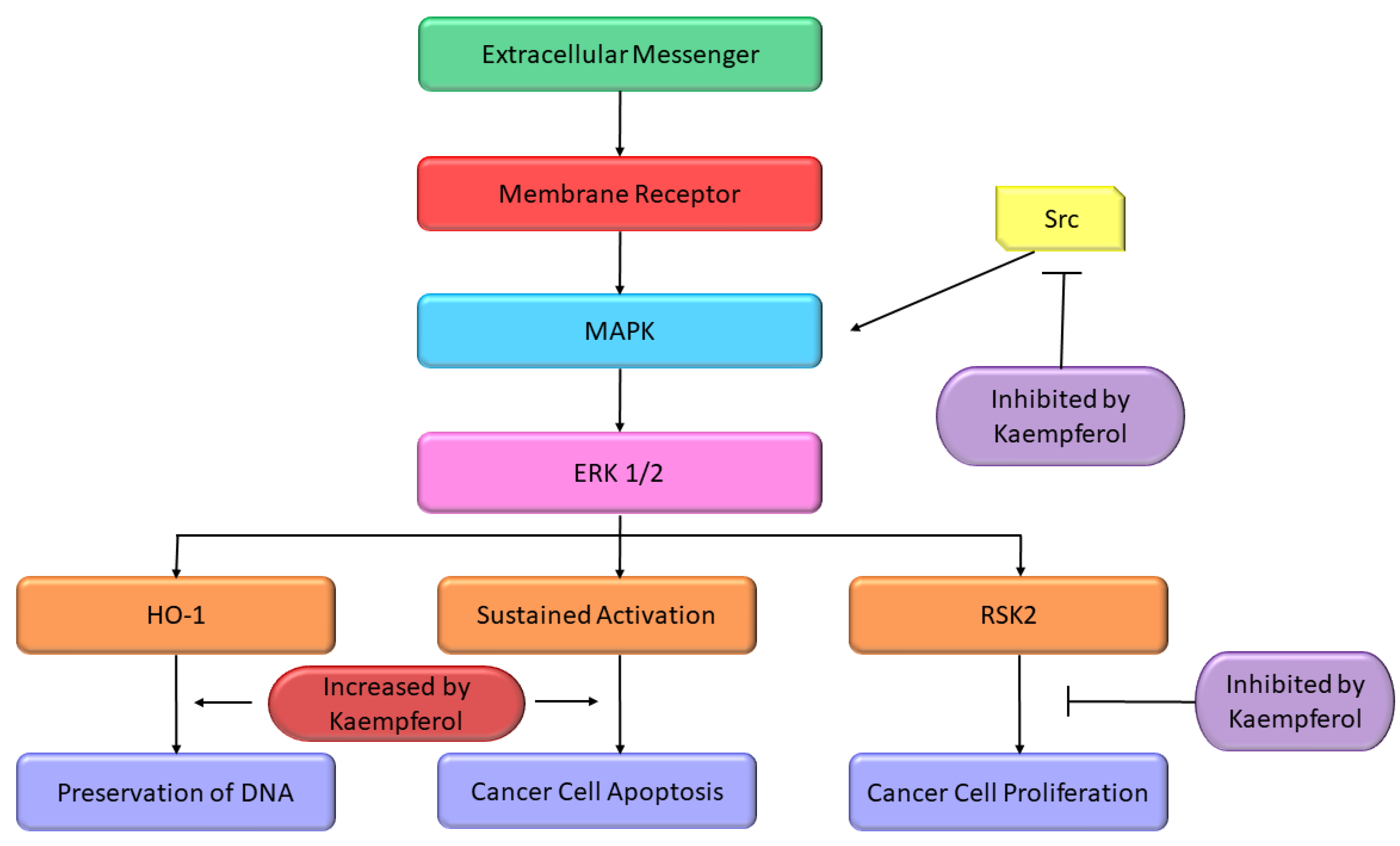
| Pancreatic cancer | Kmp in dosage-dependent manner prevents the pancreatic cancerous cells growth in PANC-1, MIA PaCa-2, and SNU213-pancreatic cancer cell line by causing apoptosis and efficiently preventing ERK-1/2, EGFR-related AKT and Src signalling pathways and migration of cells | [173,174] |
| Bladder cancer | Kmp inhibits growth of urinary bladder cancerous cells by encouraging apoptotic pathway and cell-cycle arrest | [177,178,179,180] |
| Osteosarcoma | Kmp in dosage-dependent manner prevents the growth of cancerous cells in bone cancerous cell lines such as HOB, 143B, U2OS and migration of U2OS cells with poorer toxicity in human fetal osteoblast cells | [182,183] |
| Cervical cancer | Kmp inhibited cancerous cells growth in SiHa, KB-V1 and HeLa cervical cancerous cell lines in comparison with HFF cell line and healthy cells | [186,187] |
| Renal cancer | Kmp substantially prevents the growth of cancerous cells and initiates apoptotic pathway in renal cancerous cell lines such as 769-P and 786-O | [189,190] |
| Renal cancer | Kmp uses its anti-cancer activities via inhibiting invasion and migration of cells and enhancing the focal adhesion kinase activity | [188] |
| Ovarian cancer | Kmp can prevent angiogenesis, proliferation, and growth of ovarian cancerous cells through reducing expression of VEGF | [69] |
4. Bioavailability of Kaempferol
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kashyap, D.; Garg, V.K.; Tuli, H.S.; Yerer, M.B.; Sak, K.; Sharma, A.K.; Kumar, M.; Aggarwal, V.; Sandhu, S.S. Fisetin and Quercetin: Promising Flavonoids with Chemopreventive Potential. Biomolecules 2019, 9, 174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- UICC. GLOBOCAN 2020: New Global Cancer Data. Available online: https://www.uicc.org/news/globocan-2020-new-global-cancer-data (accessed on 27 September 2022).
- Soto, A.M.; Sonnenschein, C. Environmental causes of cancer: Endocrine disruptors as carcinogens. Nat. Rev. Endocrinol. 2010, 6, 363–370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sawyers, C. Targeted cancer therapy. Nature 2004, 432, 294–297. [Google Scholar] [CrossRef] [PubMed]
- Housman, G.; Byler, S.; Heerboth, S.; Lapinska, K.; Longacre, M.; Snyder, N.; Sarkar, S. Drug resistance in cancer: An overview. Cancers 2014, 6, 1769–1792. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holohan, C.; Van Schaeybroeck, S.; Longley, D.B.; Johnston, P.G. Cancer drug resistance: An evolving paradigm. Nat. Rev. Cancer 2013, 13, 714–726. [Google Scholar] [CrossRef] [PubMed]
- Gottesman, M.M. Mechanisms of cancer drug resistance. Annu. Rev. Med. 2002, 53, 615–627. [Google Scholar] [CrossRef] [Green Version]
- Kashyap, D.; Sharma, A.; Tuli, H.S.; Sak, K.; Garg, V.K.; Buttar, H.S.; Setzer, W.N.; Sethi, G. Apigenin: A natural bioactive flavone-type molecule with promising therapeutic function. J. Funct. Foods 2018, 48, 457–471. [Google Scholar] [CrossRef]
- Kashyap, D.; Sharma, A.; Sak, K.; Tuli, H.S.; Buttar, H.S.; Bishayee, A. Fisetin: A bioactive phytochemical with potential for cancer prevention and pharmacotherapy. Life Sci. 2018, 194, 75–87. [Google Scholar] [CrossRef]
- Kashyap, D.; Sharma, A.; Tuli, H.S.; Sak, K.; Punia, S.; Mukherjee, T.K. Kaempferol—A dietary anticancer molecule with multiple mechanisms of action: Recent trends and advancements. J. Funct. 2017, 30, 203–219. [Google Scholar] [CrossRef]
- Kashyap, D.; Mondal, R.; Tuli, H.S.; Kumar, G.; Sharma, A.K. Molecular targets of gambogic acid in cancer: Recent trends and advancements. Tumor Biol. 2016, 37, 12915–12925. [Google Scholar] [CrossRef]
- Holland, T.M.; Agarwal, P.; Wang, Y.; Leurgans, S.E.; Bennett, D.A.; Booth, S.L.; Morris, M.C. Dietary flavonols and risk of Alzheimer dementia. Neurology 2020, 94, e1749–e1756. [Google Scholar] [CrossRef] [PubMed]
- Miean, K.H.; Mohamed, S. Flavonoid (myricetin, quercetin, kaempferol, luteolin, and apigenin) content of edible tropical plants. J. Agric. Food Chem. 2001, 49, 3106–3112. [Google Scholar] [CrossRef] [PubMed]
- de Vries, J.H.; Hollman, P.C.; Meyboom, S.; Buysman, M.N.; Zock, P.L.; van Staveren, W.A.; Katan, M.B. Plasma concentrations and urinary excretion of the antioxidant flavonols quercetin and kaempferol as biomarkers for dietary intake. Am. J. Clin. Nutr. 1998, 68, 60–65. [Google Scholar] [CrossRef] [Green Version]
- Marfak, A.; Trouillas, P.; Allais, D.P.; Champavier, Y.; Calliste, C.A.; Duroux, J.L. Radiolysis of kaempferol in water/methanol mixtures. Evaluation of antioxidant activity of kaempferol and products formed. J. Agric. Food Chem. 2003, 51, 1270–1277. [Google Scholar] [CrossRef] [PubMed]
- Dabeek, W.M.; Marra, M.V. Dietary Quercetin and Kaempferol: Bioavailability and Potential Cardiovascular-Related Bioactivity in Humans. Nutrients 2019, 11, 2288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leung, H.W.; Lin, C.J.; Hour, M.J.; Yang, W.H.; Wang, M.Y.; Lee, H.Z. Kaempferol induces apoptosis in human lung non-small carcinoma cells accompanied by an induction of antioxidant enzymes. Food Chem. Toxicol. 2007, 45, 2005–2013. [Google Scholar] [CrossRef]
- Cid-Ortega, S.; Monroy-Rivera, J.A. Extraction of Kaempferol and Its Glycosides Using Supercritical Fluids from Plant Sources: A Review. Food Technol. Biotechnol. 2018, 56, 480–493. [Google Scholar] [CrossRef]
- Bestwick, C.S.; Milne, L.; Duthie, S.J. Kaempferol induced inhibition of HL-60 cell growth results from a heterogeneous response, dominated by cell cycle alterations. Chem.-Biol. Interact. 2007, 170, 76–85. [Google Scholar] [CrossRef]
- Casagrande, F.; Darbon, J.M. Effects of structurally related flavonoids on cell cycle progression of human melanoma cells: Regulation of cyclin-dependent kinases CDK2 and CDK1. Biochem. Pharmacol. 2001, 61, 1205–1215. [Google Scholar] [CrossRef]
- Knowles, L.M.; Zigrossi, D.A.; Tauber, R.A.; Hightower, C.; Milner, J.A. Flavonoids suppress androgen-independent human prostate tumor proliferation. Nutr. Cancer 2000, 38, 116–122. [Google Scholar] [CrossRef]
- Shanmugam, M.K.; Lee, J.H.; Chai, E.Z.; Kanchi, M.M.; Kar, S.; Arfuso, F.; Dharmarajan, A.; Kumar, A.P.; Ramar, P.S.; Looi, C.Y.; et al. Cancer prevention and therapy through the modulation of transcription factors by bioactive natural compounds. Semin. Cancer Biol. 2016, 40–41, 35–47. [Google Scholar] [CrossRef]
- Shanmugam, M.K.; Kannaiyan, R.; Sethi, G. Targeting cell signaling and apoptotic pathways by dietary agents: Role in the prevention and treatment of cancer. Nutr. Cancer 2011, 63, 161–173. [Google Scholar] [CrossRef] [PubMed]
- van Ginkel, P.R.; Yan, M.B.; Bhattacharya, S.; Polans, A.S.; Kenealey, J.D. Natural products induce a G protein-mediated calcium pathway activating p53 in cancer cells. Toxicol. Appl. Pharmacol. 2015, 288, 453–462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rajendran, P.; Rengarajan, T.; Nandakumar, N.; Palaniswami, R.; Nishigaki, Y.; Nishigaki, I. Kaempferol, a potential cytostatic and cure for inflammatory disorders. Eur. J. Med. Chem. 2014, 86, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.Y.; Chen, Y.C. A review of the dietary flavonoid, kaempferol on human health and cancer chemoprevention. Food Chem. 2013, 138, 2099–2107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Imran, M.; Salehi, B.; Sharifi-Rad, J.; Aslam Gondal, T.; Saeed, F.; Imran, A.; Shahbaz, M.; Tsouh Fokou, P.V.; Umair Arshad, M.; Khan, H.; et al. Kaempferol: A Key Emphasis to Its Anticancer Potential. Molecules 2019, 24, 2277. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Ji, H.S.; Kang, J.H.; Shin, D.H.; Park, H.Y.; Choi, M.S.; Lee, C.H.; Lee, I.K.; Yun, B.S.; Jeong, T.S. Soy Leaf Extract Containing Kaempferol Glycosides and Pheophorbides Improves Glucose Homeostasis by Enhancing Pancreatic β-Cell Function and Suppressing Hepatic Lipid Accumulation in db/db Mice. J. Agric. Food Chem. 2015, 63, 7198–7210. [Google Scholar] [CrossRef]
- Sharifi-Rad, M.; Fokou, P.V.T.; Sharopov, F.; Martorell, M.; Ademiluyi, A.O.; Rajkovic, J.; Salehi, B.; Martins, N.; Iriti, M.; Sharifi-Rad, J. Antiulcer Agents: From Plant Extracts to Phytochemicals in Healing Promotion. Molecules 2018, 23, 1751. [Google Scholar] [CrossRef] [Green Version]
- Calderón-Montaño, J.M.; Burgos-Morón, E.; Pérez-Guerrero, C.; López-Lázaro, M. A review on the dietary flavonoid kaempferol. Mini Rev. Med. Chem. 2011, 11, 298–344. [Google Scholar] [CrossRef]
- Pei, J.; Chen, A.; Zhao, L.; Cao, F.; Ding, G.; Xiao, W. One-Pot Synthesis of Hyperoside by a Three-Enzyme Cascade Using a UDP-Galactose Regeneration System. J. Agric. Food Chem. 2017, 65, 6042–6048. [Google Scholar] [CrossRef]
- Neuhouser, M.L. Dietary flavonoids and cancer risk: Evidence from human population studies. Nutr. Cancer 2004, 50, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Weng, C.J.; Yen, G.C. Flavonoids, a ubiquitous dietary phenolic subclass, exert extensive in vitro anti-invasive and in vivo anti-metastatic activities. Cancer Metastasis Rev. 2012, 31, 323–351. [Google Scholar] [CrossRef] [PubMed]
- Elsharkawy, E.R. Isolation of phytoconstituents and evaluation of anticancer and Antioxidant potential of Launaea mucronata (Forssk.) Muschl. subsp. Pak. J. Pharm. Sci. 2017, 30, 399–405. [Google Scholar] [PubMed]
- Yi, X.; Zuo, J.; Tan, C.; Xian, S.; Luo, C.; Chen, S.; Yu, L.; Luo, Y. Kaempferol, a flavonoid compound from gynura medica induced apoptosis and growth inhibition in mcf-7 breast cancer cell. Afr. J. Tradit. Complement. Altern. Med. 2016, 13, 210–215. [Google Scholar] [CrossRef] [Green Version]
- Mishra, A.P.; Salehi, B.; Sharifi-Rad, M.; Pezzani, R.; Kobarfard, F.; Sharifi-Rad, J.; Nigam, M. Programmed Cell Death, from a Cancer Perspective: An Overview. Mol. Diagn. Ther. 2018, 22, 281–295. [Google Scholar] [CrossRef]
- Imran, M.; Rauf, A.; Shah, Z.A.; Saeed, F.; Imran, A.; Arshad, M.U.; Ahmad, B.; Bawazeer, S.; Atif, M.; Peters, D.G.; et al. Chemo-preventive and therapeutic effect of the dietary flavonoid kaempferol: A comprehensive review. Phytother. Res. 2019, 33, 263–275. [Google Scholar] [CrossRef]
- Marfe, G.; Tafani, M.; Indelicato, M.; Sinibaldi-Salimei, P.; Reali, V.; Pucci, B.; Fini, M.; Russo, M.A. Kaempferol induces apoptosis in two different cell lines via Akt inactivation, Bax and SIRT3 activation, and mitochondrial dysfunction. J. Cell. Biochem. 2009, 106, 643–650. [Google Scholar] [CrossRef]
- Kim, K.Y.; Jang, W.Y.; Lee, J.Y.; Jun, D.Y.; Ko, J.Y.; Yun, Y.H.; Kim, Y.H. Kaempferol Activates G2-Checkpoint of the Cell Cycle Resulting in G2-Arrest and Mitochondria-Dependent Apoptosis in Human Acute Leukemia Jurkat T Cells. J. Microbiol. Biotechnol. 2016, 26, 287–294. [Google Scholar] [CrossRef]
- Kim, B.; Jung, J.W.; Jung, J.; Han, Y.; Suh, D.H.; Kim, H.S.; Dhanasekaran, D.N.; Song, Y.S. PGC1α induced by reactive oxygen species contributes to chemoresistance of ovarian cancer cells. Oncotarget 2017, 8, 60299–60311. [Google Scholar] [CrossRef] [Green Version]
- Ferrero-Miliani, L.; Nielsen, O.H.; Andersen, P.S.; Girardin, S.E. Chronic inflammation: Importance of NOD2 and NALP3 in interleukin-1beta generation. Clin. Exp. Immunol. 2007, 147, 227–235. [Google Scholar] [CrossRef]
- Warren, C.A.; Paulhill, K.J.; Davidson, L.A.; Lupton, J.R.; Taddeo, S.S.; Hong, M.Y.; Carroll, R.J.; Chapkin, R.S.; Turner, N.D. Quercetin may suppress rat aberrant crypt foci formation by suppressing inflammatory mediators that influence proliferation and apoptosis. J. Nutr. 2009, 139, 101–105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tuli, H.S.; Chaudhary, P.; Beniwal, V.; Sharma, A.K. Microbial pigments as natural color sources: Current trends and future perspectives. J. Food Sci. Technol. 2015, 52, 4669–4678. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tuli, H.S.; Kashyap, D.; Sharma, A.K.; Sandhu, S.S. Molecular aspects of melatonin (MLT)-mediated therapeutic effects. Life Sci. 2015, 135, 147–157. [Google Scholar] [CrossRef] [PubMed]
- Tuli, H.S.; Kashyap, D.; Bedi, S.K.; Kumar, P.; Kumar, G.; Sandhu, S.S. Molecular aspects of metal oxide nanoparticle (MO-NPs) mediated pharmacological effects. Life Sci. 2015, 143, 71–79. [Google Scholar] [CrossRef]
- Kong, L.; Luo, C.; Li, X.; Zhou, Y.; He, H. The anti-inflammatory effect of kaempferol on early atherosclerosis in high cholesterol fed rabbits. Lipids Health Dis. 2013, 12, 115. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.K.; Xiao, H.B.; Fang, J. Anti-inflammatory properties of kaempferol via its inhibition of aldosterone signaling and aldosterone-induced gene expression. Can. J. Physiol. Pharmacol. 2014, 92, 117–123. [Google Scholar] [CrossRef]
- Hämäläinen, M.; Nieminen, R.; Vuorela, P.; Heinonen, M.; Moilanen, E. Anti-inflammatory effects of flavonoids: Genistein, kaempferol, quercetin, and daidzein inhibit STAT-1 and NF-kappaB activations, whereas flavone, isorhamnetin, naringenin, and pelargonidin inhibit only NF-kappaB activation along with their inhibitory effect on iNOS expression and NO production in activated macrophages. Mediat. Inflamm. 2007, 2007, 45673. [Google Scholar]
- García-Mediavilla, V.; Crespo, I.; Collado, P.S.; Esteller, A.; Sánchez-Campos, S.; Tuñón, M.J.; González-Gallego, J. The anti-inflammatory flavones quercetin and kaempferol cause inhibition of inducible nitric oxide synthase, cyclooxygenase-2 and reactive C-protein, and down-regulation of the nuclear factor kappaB pathway in Chang Liver cells. Eur. J. Pharmacol. 2007, 557, 221–229. [Google Scholar] [CrossRef]
- Kim, K.; Kim, S.; Moh, S.H.; Kang, H. Kaempferol inhibits vascular smooth muscle cell migration by modulating BMP-mediated miR-21 expression. Mol. Cell. Biochem. 2015, 407, 143–149. [Google Scholar] [CrossRef]
- Kim, S.H.; Park, J.G.; Lee, J.; Yang, W.S.; Park, G.W.; Kim, H.G.; Yi, Y.S.; Baek, K.S.; Sung, N.Y.; Hossen, M.J.; et al. The dietary flavonoid Kaempferol mediates anti-inflammatory responses via the Src, Syk, IRAK1, and IRAK4 molecular targets. Mediat. Inflamm. 2015, 2015, 904142. [Google Scholar] [CrossRef] [Green Version]
- Kadioglu, O.; Nass, J.; Saeed, M.E.; Schuler, B.; Efferth, T. Kaempferol Is an Anti-Inflammatory Compound with Activity towards NF-κB Pathway Proteins. Anticancer Res. 2015, 35, 2645–2650. [Google Scholar] [PubMed]
- Rakoff-Nahoum, S. Why cancer and inflammation? Yale J. Biol. Med. 2006, 79, 123–130. [Google Scholar] [PubMed]
- Bobe, G.; Albert, P.S.; Sansbury, L.B.; Lanza, E.; Schatzkin, A.; Colburn, N.H.; Cross, A.J. Interleukin-6 as a potential indicator for prevention of high-risk adenoma recurrence by dietary flavonols in the polyp prevention trial. Cancer Prev. Res. 2010, 3, 764–775. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adegoke, O.; Forbes, P.B. Challenges and advances in quantum dot fluorescent probes to detect reactive oxygen and nitrogen species: A review. Anal. Chim. Acta 2015, 862, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Hazra, B.; Das Sarma, M.; Sanyal, U. Separation methods of quinonoid constituents of plants used in Oriental traditional medicines. J. Chromatogr. B 2004, 812, 259–275. [Google Scholar] [CrossRef]
- Melo, P.S.; Massarioli, A.P.; Denny, C.; dos Santos, L.F.; Franchin, M.; Pereira, G.E.; Vieira, T.M.; Rosalen, P.L.; de Alencar, S.M. Winery by-products: Extraction optimization, phenolic composition and cytotoxic evaluation to act as a new source of scavenging of reactive oxygen species. Food Chem. 2015, 181, 160–169. [Google Scholar] [CrossRef] [Green Version]
- Yoshida, T.; Konishi, M.; Horinaka, M.; Yasuda, T.; Goda, A.E.; Taniguchi, H.; Yano, K.; Wakada, M.; Sakai, T. Kaempferol sensitizes colon cancer cells to TRAIL-induced apoptosis. Biochem. Biophys. Res. Commun. 2008, 375, 129–133. [Google Scholar] [CrossRef]
- Saw, C.L.; Guo, Y.; Yang, A.Y.; Paredes-Gonzalez, X.; Ramirez, C.; Pung, D.; Kong, A.N. The berry constituents quercetin, kaempferol, and pterostilbene synergistically attenuate reactive oxygen species: Involvement of the Nrf2-ARE signaling pathway. Food Chem. Toxicol. 2014, 72, 303–311. [Google Scholar] [CrossRef]
- Kowalski, J.; Samojedny, A.; Paul, M.; Pietsz, G.; Wilczok, T. Effect of kaempferol on the production and gene expression of monocyte chemoattractant protein-1 in J774.2 macrophages. Pharmacol. Rep. 2005, 57, 107–112. [Google Scholar]
- Wahab, A.; Begum, S. Luteolin and kaempferol from Cassia alata, antimicrobial and antioxidant activity of its methanolic extracts. FUUAST J. Biol. 2014, 4, 1–5. [Google Scholar]
- Choe, K.I.; Kwon, J.H.; Park, K.H.; Oh, M.H.; Kim, M.H.; Kim, H.H.; Cho, S.H.; Chung, E.K.; Ha, S.Y.; Lee, M.W. The antioxidant and anti-inflammatory effects of phenolic compounds isolated from the root of Rhodiola sachalinensis A. BOR. Molecules 2012, 17, 11484–11494. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nirmala, P.; Ramanathan, M. Effect of kaempferol on lipid peroxidation and antioxidant status in 1,2-dimethyl hydrazine induced colorectal carcinoma in rats. Eur. J. Pharmacol. 2011, 654, 75–79. [Google Scholar] [CrossRef]
- Wang, M.; Sun, J.; Jiang, Z.; Xie, W.; Zhang, X. Hepatoprotective effect of kaempferol against alcoholic liver injury in mice. Am. J. Chin. Med. 2015, 43, 241–254. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Tang, C.; Zhang, H. Hepatoprotective effects of kaempferol 3-O-rutinoside and kaempferol 3-O-glucoside from Carthamus tinctorius L. on CCl4-induced oxidative liver injury in mice. J. Food Drug Anal. 2015, 23, 310–317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bestwick, C.S.; Milne, L.; Pirie, L.; Duthie, S.J. The effect of short-term kaempferol exposure on reactive oxygen levels and integrity of human (HL-60) leukaemic cells. Biochim. Biophys. Acta 2005, 1740, 340–349. [Google Scholar] [CrossRef] [Green Version]
- Folkman, J. Angiogenesis: An organizing principle for drug discovery? Nat. Rev. Drug Discov. 2007, 6, 273–286. [Google Scholar] [CrossRef]
- Ferrara, N. Vascular endothelial growth factor as a target for anticancer therapy. Oncologist 2004, 9 (Suppl. S1), 2–10. [Google Scholar] [CrossRef]
- Luo, H.; Rankin, G.O.; Liu, L.; Daddysman, M.K.; Jiang, B.H.; Chen, Y.C. Kaempferol inhibits angiogenesis and VEGF expression through both HIF dependent and independent pathways in human ovarian cancer cells. Nutr. Cancer 2009, 61, 554–563. [Google Scholar] [CrossRef]
- Luo, H.; Rankin, G.O.; Juliano, N.; Jiang, B.H.; Chen, Y.C. Kaempferol inhibits VEGF expression and in vitro angiogenesis through a novel ERK-NFκB-cMyc-p21 pathway. Food Chem. 2012, 130, 321–328. [Google Scholar] [CrossRef] [Green Version]
- Schindler, R.; Mentlein, R. Flavonoids and vitamin E reduce the release of the angiogenic peptide vascular endothelial growth factor from human tumor cells. J. Nutr. 2006, 136, 1477–1482. [Google Scholar] [CrossRef] [Green Version]
- Hirano, T.; Nakajima, K.; Hibi, M. Signaling mechanisms through gp130: A model of the cytokine system. Cytokine Growth Factor Rev. 1997, 8, 241–252. [Google Scholar] [CrossRef]
- Liu, Q.; Li, G.; Li, R.; Shen, J.; He, Q.; Deng, L.; Zhang, C.; Zhang, J. IL-6 promotion of glioblastoma cell invasion and angiogenesis in U251 and T98G cell lines. J. Neuro-Oncol. 2010, 100, 165–176. [Google Scholar] [CrossRef] [PubMed]
- Xie, T.X.; Wei, D.; Liu, M.; Gao, A.C.; Ali-Osman, F.; Sawaya, R.; Huang, S. Stat3 activation regulates the expression of matrix metalloproteinase-2 and tumor invasion and metastasis. Oncogene 2004, 23, 3550–3560. [Google Scholar] [CrossRef] [PubMed]
- Michaud-Levesque, J.; Bousquet-Gagnon, N.; Béliveau, R. Quercetin abrogates IL-6/STAT3 signaling and inhibits glioblastoma cell line growth and migration. Exp. Cell Res. 2012, 318, 925–935. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, J.V.; Frank, D.A. Genome-wide analysis of STAT target genes: Elucidating the mechanism of STAT-mediated oncogenesis. Cancer Biol. Ther. 2004, 3, 1045–1050. [Google Scholar] [CrossRef] [Green Version]
- Basu, A.; Das, A.S.; Sharma, M.; Pathak, M.P.; Chattopadhyay, P.; Biswas, K.; Mukhopadhyay, R. STAT3 and NF-κB are common targets for kaempferol-mediated attenuation of COX-2 expression in IL-6-induced macrophages and carrageenan-induced mouse paw edema. Biochem. Biophys. Rep. 2017, 12, 54–61. [Google Scholar] [CrossRef]
- Yang, S.; Si, L.; Jia, Y.; Jian, W.; Yu, Q.; Wang, M.; Lin, R. Kaempferol exerts anti-proliferative effects on human ovarian cancer cells by inducing apoptosis, G0/G1 cell cycle arrest and modulation of MEK/ERK and STAT3 pathways. J. Buon 2019, 24, 975–981. [Google Scholar]
- Kok, K.; Geering, B.; Vanhaesebroeck, B. Regulation of phosphoinositide 3-kinase expression in health and disease. Trends Biochem. Sci. 2009, 34, 115–127. [Google Scholar] [CrossRef]
- Vogt, P.K.; Hart, J.R.; Gymnopoulos, M.; Jiang, H.; Kang, S.; Bader, A.G.; Zhao, L.; Denley, A. Phosphatidylinositol 3-kinase: The oncoprotein. Curr. Top. Microbiol. Immunol. 2010, 347, 79–104. [Google Scholar]
- Arcaro, A.; Guerreiro, A.S. The phosphoinositide 3-kinase pathway in human cancer: Genetic alterations and therapeutic implications. Curr. Genom. 2007, 8, 271–306. [Google Scholar] [CrossRef]
- Brown, K.K.; Toker, A. The phosphoinositide 3-kinase pathway and therapy resistance in cancer. F1000prime Rep. 2015, 7, 13. [Google Scholar] [CrossRef]
- Liu, P.; Cheng, H.; Roberts, T.M.; Zhao, J.J. Targeting the phosphoinositide 3-kinase pathway in cancer. Nat. Rev. Drug Discov. 2009, 8, 627–644. [Google Scholar] [CrossRef] [Green Version]
- Marone, R.; Cmiljanovic, V.; Giese, B.; Wymann, M.P. Targeting phosphoinositide 3-kinase: Moving towards therapy. Biochim. Biophys. Acta 2008, 1784, 159–185. [Google Scholar] [CrossRef]
- Maurya, A.K.; Vinayak, M. Abstract A07: Decline in the growth of murine T-cell lymphoma via modulation of PI3K signaling pathway: Key role of quercetin and PI-103. Mol. Cancer Ther. 2015, 14 (Suppl. S7), A07. [Google Scholar] [CrossRef]
- Maurya, A.K.; Vinayak, M. Quercetin regresses Dalton’s lymphoma growth via suppression of PI3K/AKT signaling leading to upregulation of p53 and decrease in energy metabolism. Nutr. Cancer 2015, 67, 354–363. [Google Scholar] [CrossRef]
- Maurya, A.K.; Vinayak, M. Anticarcinogenic action of quercetin by downregulation of phosphatidylinositol 3-kinase (PI3K) and protein kinase C (PKC) via induction of p53 in hepatocellular carcinoma (HepG2) cell line. Mol. Biol. Rep. 2015, 42, 1419–1429. [Google Scholar] [CrossRef]
- Vanhaesebroeck, B.; Guillermet-Guibert, J.; Graupera, M.; Bilanges, B. The emerging mechanisms of isoform-specific PI3K signalling. Nat. Reviews. Mol. Cell Biol. 2010, 11, 329–341. [Google Scholar] [CrossRef]
- Dibble, C.C.; Manning, B.D. A molecular link between AKT regulation and chemotherapeutic response. Cancer Cell 2009, 16, 178–180. [Google Scholar] [CrossRef] [Green Version]
- Chan, C.H.; Jo, U.; Kohrman, A.; Rezaeian, A.H.; Chou, P.C.; Logothetis, C.; Lin, H.K. Posttranslational regulation of Akt in human cancer. Cell Biosci. 2014, 4, 59. [Google Scholar] [CrossRef] [Green Version]
- Liao, Y.; Hung, M.C. Physiological regulation of Akt activity and stability. Am. J. Transl. Res. 2010, 2, 19–42. [Google Scholar]
- Fyffe, C.; Falasca, M. 3-Phosphoinositide-dependent protein kinase-1 as an emerging target in the management of breast cancer. Cancer Manag. Res. 2013, 5, 271–280. [Google Scholar]
- Osaki, M.; Oshimura, M.; Ito, H. PI3K-Akt pathway: Its functions and alterations in human cancer. Apoptosis 2004, 9, 667–676. [Google Scholar] [CrossRef]
- Testa, J.R.; Bellacosa, A. AKT plays a central role in tumorigenesis. Proc. Natl. Acad. Sci. USA 2001, 98, 10983–10985. [Google Scholar] [CrossRef] [Green Version]
- Chin, H.K.; Horng, C.T.; Liu, Y.S.; Lu, C.C.; Su, C.Y.; Chen, P.S.; Chiu, H.Y.; Tsai, F.J.; Shieh, P.C.; Yang, J.S. Kaempferol inhibits angiogenic ability by targeting VEGF receptor-2 and downregulating the PI3K/AKT, MEK and ERK pathways in VEGF-stimulated human umbilical vein endothelial cells. Oncol. Rep. 2018, 39, 2351–2357. [Google Scholar] [CrossRef]
- Li, Q.; Wei, L.; Lin, S.; Chen, Y.; Lin, J.; Peng, J. Synergistic effect of kaempferol and 5-fluorouracil on the growth of colorectal cancer cells by regulating the PI3K/Akt signaling pathway. Mol. Med. Rep. 2019, 20, 728–734. [Google Scholar] [CrossRef]
- Hong, J.T.; Yen, J.H.; Wang, L.; Lo, Y.H.; Chen, Z.T.; Wu, M.J. Regulation of heme oxygenase-1 expression and MAPK pathways in response to kaempferol and rhamnocitrin in PC12 cells. Toxicol. Appl. Pharmacol. 2009, 237, 59–68. [Google Scholar] [CrossRef]
- Luo, H.; Rankin, G.O.; Li, Z.; Depriest, L.; Chen, Y.C. Kaempferol induces apoptosis in ovarian cancer cells through activating p53 in the intrinsic pathway. Food Chem. 2011, 128, 513–519. [Google Scholar] [CrossRef]
- Cho, Y.Y.; Yao, K.; Pugliese, A.; Malakhova, M.L.; Bode, A.M.; Dong, Z. A regulatory mechanism for RSK2 NH2-terminal kinase activity. Cancer Res. 2009, 69, 4398–4406. [Google Scholar] [CrossRef] [Green Version]
- Lee, K.M.; Lee, K.W.; Jung, S.K.; Lee, E.J.; Heo, Y.S.; Bode, A.M.; Lubet, R.A.; Lee, H.J.; Dong, Z. Kaempferol inhibits UVB-induced COX-2 expression by suppressing Src kinase activity. Biochem. Pharmacol. 2010, 80, 2042–2049. [Google Scholar] [CrossRef] [Green Version]
- Athar, M.; An, K.P.; Morel, K.D.; Kim, A.L.; Aszterbaum, M.; Longley, J.; Epstein, E.H., Jr.; Bickers, D.R. Ultraviolet B(UVB)-induced cox-2 expression in murine skin: An immunohistochemical study. Biochem. Biophys. Res. Commun. 2001, 280, 1042–1047. [Google Scholar] [CrossRef]
- Meeran, S.M.; Katiyar, S.K. Cell cycle control as a basis for cancer chemoprevention through dietary agents. Front. Biosci. 2008, 13, 2191–2202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gescher, A.; Pastorino, U.; Plummer, S.M.; Manson, M.M. Suppression of tumour development by substances derived from the diet—Mechanisms and clinical implications. Br. J. Clin. Pharmacol. 1998, 45, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Milner, J.A.; McDonald, S.S.; Anderson, D.E.; Greenwald, P. Molecular targets for nutrients involved with cancer prevention. Nutr. Cancer 2001, 41, 1–16. [Google Scholar]
- Gao, Y.; Yin, J.; Rankin, G.O.; Chen, Y.C. Kaempferol Induces G2/M Cell Cycle Arrest via Checkpoint Kinase 2 and Promotes Apoptosis via Death Receptors in Human Ovarian Carcinoma A2780/CP70 Cells. Molecules 2018, 23, 1095. [Google Scholar] [CrossRef] [Green Version]
- Xu, W.; Liu, J.; Li, C.; Wu, H.Z.; Liu, Y.W. Kaempferol-7-O-beta-D-glucoside (KG) isolated from Smilax china L. rhizome induces G2/M phase arrest and apoptosis on HeLa cells in a p53-independent manner. Cancer Lett. 2008, 264, 229–240. [Google Scholar] [CrossRef]
- Li, R.; Mei, J.; Liu, G.J. Kaempferol-induced apoptosis of human esophageal squamous carcinoma Eca-109 cells and the mechanism. J. South. Med. Univ. 2011, 31, 1440–1442. [Google Scholar]
- Choi, E.J.; Ahn, W.S. Kaempferol induced the apoptosis via cell cycle arrest in human breast cancer MDA-MB-453 cells. Nutr. Res. Pract. 2008, 2, 322–325. [Google Scholar] [CrossRef] [Green Version]
- Jung, P.; Menssen, A.; Mayr, D.; Hermeking, H. AP4 encodes a c-MYC-inducible repressor of p21. Proc. Natl. Acad. Sci. USA 2008, 105, 15046–15051. [Google Scholar] [CrossRef] [Green Version]
- Luo, H.; Daddysman, M.K.; Rankin, G.O.; Jiang, B.H.; Chen, Y.C. Kaempferol enhances cisplatin’s effect on ovarian cancer cells through promoting apoptosis caused by down regulation of cMyc. Cancer Cell Int. 2010, 10, 16. [Google Scholar] [CrossRef]
- Ito, K.; Nakazato, T.; Yamato, K.; Miyakawa, Y.; Yamada, T.; Hozumi, N.; Segawa, K.; Ikeda, Y.; Kizaki, M. Induction of apoptosis in leukemic cells by homovanillic acid derivative, capsaicin, through oxidative stress: Implication of phosphorylation of p53 at Ser-15 residue by reactive oxygen species. Cancer Res. 2004, 64, 1071–1078. [Google Scholar] [CrossRef] [Green Version]
- Kashafi, E.; Moradzadeh, M.; Mohamadkhani, A.; Erfanian, S. Kaempferol increases apoptosis in human cervical cancer HeLa cells via PI3K/AKT and telomerase pathways. Biomed. Pharmacother. 2017, 89, 573–577. [Google Scholar] [CrossRef] [PubMed]
- Mu, J.J.; Zeng, Y.Y.; Huang, X.Y.; Zhao, X.H.; Song, B. Effects of Kaempferol on activation, proliferation and cell cycle of mouse T lymphocytes in vitro. Chin. J. Cell. Mol. Immunol. 2009, 25, 1106–1108. [Google Scholar]
- Chang, L.; Fang, S.; Gu, W. The Molecular Mechanism of Metabolic Remodeling in Lung Cancer. J. Cancer 2020, 11, 1403–1411. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seydi, E.; Salimi, A.; Rasekh, H.R.; Mohsenifar, Z.; Pourahmad, J. Selective Cytotoxicity of Luteolin and Kaempferol on Cancerous Hepatocytes Obtained from Rat Model of Hepatocellular Carcinoma: Involvement of ROS-Mediated Mitochondrial Targeting. Nutr. Cancer 2018, 70, 594–604. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.; Liu, X.; Li, H.; Yan, Y.; Hong, X.; Lin, Z. Kaempferol inhibits proliferation, migration, and invasion of liver cancer HepG2 cells by down-regulation of microRNA-21. Int. J. Immunopathol. Pharmacol. 2018, 32, 2058738418814341. [Google Scholar] [CrossRef] [Green Version]
- Mylonis, I.; Lakka, A.; Tsakalof, A.; Simos, G. The dietary flavonoid kaempferol effectively inhibits HIF-1 activity and hepatoma cancer cell viability under hypoxic conditions. Biochem. Biophys. Res. Commun. 2010, 398, 74–78. [Google Scholar] [CrossRef]
- Huang, W.W.; Tsai, S.C.; Peng, S.F.; Lin, M.W.; Chiang, J.H.; Chiu, Y.J.; Fushiya, S.; Tseng, M.T.; Yang, J.S. Kaempferol induces autophagy through AMPK and AKT signaling molecules and causes G2/M arrest via downregulation of CDK1/cyclin B in SK-HEP-1 human hepatic cancer cells. Int. J. Oncol. 2013, 42, 2069–2077. [Google Scholar] [CrossRef] [Green Version]
- Wonganan, O.; He, Y.J.; Shen, X.F.; Wongkrajang, K.; Suksamrarn, A.; Zhang, G.L.; Wang, F. 6-Hydroxy-3-O-methyl-kaempferol 6-O-glucopyranoside potentiates the anti-proliferative effect of interferon α/β by promoting activation of the JAK/STAT signaling by inhibiting SOCS3 in hepatocellular carcinoma cells. Toxicol. Appl. Pharmacol. 2017, 336, 31–39. [Google Scholar] [CrossRef]
- Allemani, C.; Weir, H.K.; Carreira, H.; Harewood, R.; Spika, D.; Wang, X.S.; Bannon, F.; Ahn, J.V.; Johnson, C.J.; Bonaventure, A.; et al. Global surveillance of cancer survival 1995–2009: Analysis of individual data for 25,676,887 patients from 279 population-based registries in 67 countries (CONCORD-2). Lancet 2015, 385, 977–1010. [Google Scholar] [CrossRef] [Green Version]
- Herbst, R.S.; Heymach, J.V.; Lippman, S.M. Lung cancer. N. Engl. J. Med. 2008, 359, 1367–1380. [Google Scholar] [CrossRef]
- Shivappa, N.; Wang, R.; Hébert, J.R.; Jin, A.; Koh, W.P.; Yuan, J.M. Association between inflammatory potential of diet and risk of lung cancer among smokers in a prospective study in Singapore. Eur. J. Nutr. 2019, 58, 2755–2766. [Google Scholar] [CrossRef] [PubMed]
- Zanoaga, O.; Braicu, C.; Jurj, A.; Rusu, A.; Buiga, R.; Berindan-Neagoe, I. Progress in Research on the Role of Flavonoids in Lung Cancer. Int. J. Mol. Sci. 2019, 20, 4291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Collins, I.; Workman, P. New approaches to molecular cancer therapeutics. Nat. Chem. Biol. 2006, 2, 689–700. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.T.; Tran, E.; Ong, C.K.; Lee, S.K.; Do, P.T.; Huynh, T.T.; Nguyen, T.H.; Lee, J.J.; Tan, Y.; Ong, C.S.; et al. Kaempferol-induced growth inhibition and apoptosis in A549 lung cancer cells is mediated by activation of MEK-MAPK. J. Cell. Physiol. 2003, 197, 110–121. [Google Scholar] [CrossRef] [PubMed]
- Sonoki, H.; Tanimae, A.; Endo, S.; Matsunaga, T.; Furuta, T.; Ichihara, K.; Ikari, A. Kaempherol and Luteolin Decrease Claudin-2 Expression Mediated by Inhibition of STAT3 in Lung Adenocarcinoma A549 Cells. Nutrients 2017, 9, 597. [Google Scholar] [CrossRef]
- Boadi, W.Y.; Lo, A. Effects of Quercetin, Kaempferol, and Exogenous Glutathione on Phospho- and Total-AKT in 3T3-L1 Preadipocytes. J. Diet. Suppl. 2018, 15, 814–826. [Google Scholar] [CrossRef]
- Han, X.; Liu, C.F.; Gao, N.; Zhao, J.; Xu, J. RETRACTED: Kaempferol suppresses proliferation but increases apoptosis and autophagy by up-regulating microRNA-340 in human lung cancer cells. Biomed. Pharmacother. 2018, 108, 809–816. [Google Scholar] [CrossRef]
- Qin, Y.; Cui, W.; Yang, X.; Tong, B. Kaempferol inhibits the growth and metastasis of cholangiocarcinoma in vitro and in vivo. Acta Biochim. Biophys. Sin. 2016, 48, 238–245. [Google Scholar] [CrossRef] [Green Version]
- Jo, E.; Park, S.J.; Choi, Y.S.; Jeon, W.K.; Kim, B.C. Kaempferol Suppresses Transforming Growth Factor-β1-Induced Epithelial-to-Mesenchymal Transition and Migration of A549 Lung Cancer Cells by Inhibiting Akt1-Mediated Phosphorylation of Smad3 at Threonine-179. Neoplasia 2015, 17, 525–537. [Google Scholar] [CrossRef] [Green Version]
- Halimah, E.; Diantini, A.; Destiani, D.P.; Pradipta, I.S.; Sastramihardja, H.S.; Lestari, K.; Subarnas, A.; Abdulah, R.; Koyama, H. Induction of caspase cascade pathway by kaempferol-3-O-rhamnoside in LNCaP prostate cancer cell lines. Biomed. Rep. 2015, 3, 115–117. [Google Scholar] [CrossRef]
- Bandyopadhyay, S.; Romero, J.R.; Chattopadhyay, N. Kaempferol and quercetin stimulate granulocyte-macrophage colony-stimulating factor secretion in human prostate cancer cells. Mol. Cell. Endocrinol. 2008, 287, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Mamouni, K.; Zhang, S.; Li, X.; Chen, Y.; Yang, Y.; Kim, J.; Bartlett, M.G.; Coleman, I.M.; Nelson, P.S.; Kucuk, O.; et al. A Novel Flavonoid Composition Targets Androgen Receptor Signaling and Inhibits Prostate Cancer Growth in Preclinical Models. Neoplasia 2018, 20, 789–799. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.W.; Chen, P.N.; Chen, M.K.; Yang, W.E.; Tang, C.H.; Yang, S.F.; Hsieh, Y.S. Kaempferol reduces matrix metalloproteinase-2 expression by down-regulating ERK1/2 and the activator protein-1 signaling pathways in oral cancer cells. PLoS ONE 2013, 8, e80883. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yao, S.; Wang, X.; Li, C.; Zhao, T.; Jin, H.; Fang, W. Kaempferol inhibits cell proliferation and glycolysis in esophagus squamous cell carcinoma via targeting EGFR signaling pathway. Tumour Biol. 2016, 37, 10247–10256. [Google Scholar] [CrossRef]
- Kang, J.W.; Kim, J.H.; Song, K.; Kim, S.H.; Yoon, J.H.; Kim, K.S. Kaempferol and quercetin, components of Ginkgo biloba extract (EGb 761), induce caspase-3-dependent apoptosis in oral cavity cancer cells. Phytother. Res. 2010, 24 (Suppl. S1), S77–S82. [Google Scholar] [CrossRef]
- Song, H.; Bao, J.; Wei, Y.; Chen, Y.; Mao, X.; Li, J.; Yang, Z.; Xue, Y. Kaempferol inhibits gastric cancer tumor growth: An in vitro and in vivo study. Oncol. Rep. 2015, 33, 868–874. [Google Scholar] [CrossRef] [Green Version]
- Kim, T.W.; Lee, S.Y.; Kim, M.; Cheon, C.; Ko, S.G. Kaempferol induces autophagic cell death via IRE1-JNK-CHOP pathway and inhibition of G9a in gastric cancer cells. Cell Death Dis. 2018, 9, 875. [Google Scholar] [CrossRef] [Green Version]
- Wang, R.; Yang, L.; Li, S.; Ye, D.; Yang, L.; Liu, Q.; Zhao, Z.; Cai, Q.; Tan, J.; Li, X. Quercetin Inhibits Breast Cancer Stem Cells via Downregulation of Aldehyde Dehydrogenase 1A1 (ALDH1A1), Chemokine Receptor Type 4 (CXCR4), Mucin 1 (MUC1), and Epithelial Cell Adhesion Molecule (EpCAM). Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2018, 24, 412–420. [Google Scholar] [CrossRef] [Green Version]
- Azevedo, C.; Correia-Branco, A.; Araújo, J.R.; Guimarães, J.T.; Keating, E.; Martel, F. The chemopreventive effect of the dietary compound kaempferol on the MCF-7 human breast cancer cell line is dependent on inhibition of glucose cellular uptake. Nutr. Cancer 2015, 67, 504–513. [Google Scholar] [CrossRef]
- Zhu, L.; Xue, L. Kaempferol Suppresses Proliferation and Induces Cell Cycle Arrest, Apoptosis, and DNA Damage in Breast Cancer Cells. Oncol. Res. 2019, 27, 629–634. [Google Scholar] [CrossRef]
- Li, S.; Yan, T.; Deng, R.; Jiang, X.; Xiong, H.; Wang, Y.; Yu, Q.; Wang, X.; Chen, C.; Zhu, Y. Low dose of kaempferol suppresses the migration and invasion of triple-negative breast cancer cells by downregulating the activities of RhoA and Rac1. OncoTargets Ther. 2017, 10, 4809–4819. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, G.A.; Choi, K.C.; Hwang, K.A. Treatment with Phytoestrogens Reversed Triclosan and Bisphenol A-Induced Anti-Apoptosis in Breast Cancer Cells. Biomol. Ther. 2018, 26, 503–511. [Google Scholar] [CrossRef] [PubMed]
- Diantini, A.; Subarnas, A.; Lestari, K.; Halimah, E.; Susilawati, Y.; Supriyatna, S.; Julaeha, E.; Achmad, T.H.; Suradji, E.W.; Yamazaki, C.; et al. Kaempferol-3-O-rhamnoside isolated from the leaves of Schima wallichii Korth. inhibits MCF-7 breast cancer cell proliferation through activation of the caspase cascade pathway. Oncol. Lett. 2012, 3, 1069–1072. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsiklauri, L.; An, G.; Ruszaj, D.M.; Alaniya, M.; Kemertelidze, E.; Morris, M.E. Simultaneous determination of the flavonoids robinin and kaempferol in human breast cancer cells by liquid chromatography-tandem mass spectrometry. J. Pharm. Biomed. Anal. 2011, 55, 109–113. [Google Scholar] [CrossRef]
- Kim, S.H.; Hwang, K.A.; Choi, K.C. Treatment with kaempferol suppresses breast cancer cell growth caused by estrogen and triclosan in cellular and xenograft breast cancer models. J. Nutr. Biochem. 2016, 28, 70–82. [Google Scholar] [CrossRef]
- Kang, G.Y.; Lee, E.R.; Kim, J.H.; Jung, J.W.; Lim, J.; Kim, S.K.; Cho, S.G.; Kim, K.P. Downregulation of PLK-1 expression in kaempferol-induced apoptosis of MCF-7 cells. Eur. J. Pharmacol. 2009, 611, 17–21. [Google Scholar] [CrossRef]
- Oh, S.M.; Kim, Y.P.; Chung, K.H. Biphasic effects of kaempferol on the estrogenicity in human breast cancer cells. Arch. Pharmacal Res. 2006, 29, 354–362. [Google Scholar] [CrossRef]
- Kim, B.W.; Lee, E.R.; Min, H.M.; Jeong, H.S.; Ahn, J.Y.; Kim, J.H.; Choi, H.Y.; Choi, H.; Kim, E.Y.; Park, S.P.; et al. Sustained ERK activation is involved in the kaempferol-induced apoptosis of breast cancer cells and is more evident under 3-D culture condition. Cancer Biol. Ther. 2008, 7, 1080–1089. [Google Scholar] [CrossRef] [Green Version]
- Lee, G.A.; Choi, K.C.; Hwang, K.A. Kaempferol, a phytoestrogen, suppressed triclosan-induced epithelial-mesenchymal transition and metastatic-related behaviors of MCF-7 breast cancer cells. Environ. Toxicol. Pharmacol. 2017, 49, 48–57. [Google Scholar] [CrossRef]
- Zheng, L.; Zhu, L.; Zhao, M.; Shi, J.; Li, Y.; Yu, J.; Jiang, H.; Wu, J.; Tong, Y.; Liu, Y.; et al. In Vivo Exposure of Kaempferol Is Driven by Phase II Metabolic Enzymes and Efflux Transporters. AAPS J. 2016, 18, 1289–1299. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhao, Y.; Yang, D.; Yu, Y.; Guo, H.; Zhao, Z.; Zhang, B.; Yin, X. Inhibitory effects of kaempferol on the invasion of human breast carcinoma cells by downregulating the expression and activity of matrix metalloproteinase-9. Biochem. Cell Biol. 2015, 93, 16–27. [Google Scholar] [CrossRef] [PubMed]
- Moradzadeh, M.; Tabarraei, A.; Sadeghnia, H.R.; Ghorbani, A.; Mohamadkhani, A.; Erfanian, S.; Sahebkar, A. Kaempferol increases apoptosis in human acute promyelocytic leukemia cells and inhibits multidrug resistance genes. J. Cell. Biochem. 2018, 119, 2288–2297. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.Y.; Lu, H.F.; Chou, Y.C.; Shih, Y.L.; Bau, D.T.; Chen, J.C.; Hsu, S.C.; Chung, J.G. Kaempferol induces DNA damage and inhibits DNA repair associated protein expressions in human promyelocytic leukemia HL-60 cells. Am. J. Chin. Med. 2015, 43, 365–382. [Google Scholar] [CrossRef] [PubMed]
- Benyahia, S.; Benayache, S.; Benayache, F.; Quintana, J.; López, M.; León, F.; Hernández, J.C.; Estévez, F.; Bermejo, J. Isolation from Eucalyptus occidentalis and identification of a new kaempferol derivative that induces apoptosis in human myeloid leukemia cells. J. Nat. Prod. 2004, 67, 527–531. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Daniel, K.G.; Chen, M.S.; Kuhn, D.J.; Landis-Piwowar, K.R.; Dou, Q.P. Dietary flavonoids as proteasome inhibitors and apoptosis inducers in human leukemia cells. Biochem. Pharmacol. 2005, 69, 1421–1432. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Matsuda, H.; Hata, H.; Sugawara, K.; Nakamura, S.; Yoshikawa, M. Structures of new flavonoids and benzofuran-type stilbene and degranulation inhibitors of rat basophilic leukemia cells from the Brazilian herbal medicine Cissus sicyoides. Chem. Pharm. Bull. 2009, 57, 1089–1095. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alexandrakis, M.; Letourneau, R.; Kempuraj, D.; Kandere-Grzybowska, K.; Huang, M.; Christodoulou, S.; Boucher, W.; Seretakis, D.; Theoharides, T.C. Flavones inhibit proliferation and increase mediator content in human leukemic mast cells (HMC-1). Eur. J. Haematol. 2003, 71, 448–454. [Google Scholar] [CrossRef] [Green Version]
- Boateng, J.; Verghese, M.; Shackelford, L.; Walker, L.T.; Khatiwada, J.; Ogutu, S.; Williams, D.S.; Jones, J.; Guyton, M.; Asiamah, D.; et al. Selected fruits reduce azoxymethane (AOM)-induced aberrant crypt foci (ACF) in Fisher 344 male rats. Food Chem. Toxicol. 2007, 45, 725–732. [Google Scholar] [CrossRef]
- Riahi-Chebbi, I.; Souid, S.; Othman, H.; Haoues, M.; Karoui, H.; Morel, A.; Srairi-Abid, N.; Essafi, M.; Essafi-Benkhadir, K. The Phenolic compound Kaempferol overcomes 5-fluorouracil resistance in human resistant LS174 colon cancer cells. Sci. Rep. 2019, 9, 195. [Google Scholar] [CrossRef] [Green Version]
- Choi, J.B.; Kim, J.H.; Lee, H.; Pak, J.N.; Shim, B.S.; Kim, S.H. Reactive Oxygen Species and p53 Mediated Activation of p38 and Caspases is Critically Involved in Kaempferol Induced Apoptosis in Colorectal Cancer Cells. J. Agric. Food Chem. 2018, 66, 9960–9967. [Google Scholar] [CrossRef]
- Lee, H.S.; Cho, H.J.; Yu, R.; Lee, K.W.; Chun, H.S.; Park, J.H. Mechanisms underlying apoptosis-inducing effects of Kaempferol in HT-29 human colon cancer cells. Int. J. Mol. Sci. 2014, 15, 2722–2737. [Google Scholar] [CrossRef] [PubMed]
- Deepa, M.; Sureshkumar, T.; Satheeshkumar, P.K.; Priya, S. Antioxidant rich Morus alba leaf extract induces apoptosis in human colon and breast cancer cells by the downregulation of nitric oxide produced by inducible nitric oxide synthase. Nutr. Cancer 2013, 65, 305–310. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Du, B.; Wang, T.; Wang, S.; Zhang, J. Kaempferol induces apoptosis in human HCT116 colon cancer cells via the Ataxia-Telangiectasia Mutated-p53 pathway with the involvement of p53 Upregulated Modulator of Apoptosis. Chem.-Biol. Interact. 2009, 177, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, Y.; Chang, C.C.; Mori, T.; Sato, K.; Ohtsuki, K.; Upham, B.L.; Trosko, J.E. Augmentation of differentiation and gap junction function by kaempferol in partially differentiated colon cancer cells. Carcinogenesis 2005, 26, 665–671. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Cui, M.; Li, C.; Li, H.; Dai, Y.; Cui, K.; Li, Z. Kaempferol reverses aerobic glycolysis via miR-339-5p-mediated PKM alternative splicing in colon cancer cells. J. Agric. Food Chem. 2021, 69, 3060–3068. [Google Scholar] [CrossRef]
- Ostrom, Q.T.; Gittleman, H.; Fulop, J.; Liu, M.; Blanda, R.; Kromer, C.; Wolinsky, Y.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS Statistical Report: Primary Brain and Central Nervous System Tumors Diagnosed in the United States in 2008–2012. Neuro-Oncology 2015, 17 (Suppl. S4), iv1–iv62. [Google Scholar] [CrossRef]
- Afshari, A.R.; Jalili-Nik, M.; Soukhtanloo, M.; Ghorbani, A.; Sadeghnia, H.R.; Mollazadeh, H.; Karimi Roshan, M.; Rahmani, F.; Sabri, H.; Vahedi, M.M.; et al. Auraptene-induced cytotoxicity mechanisms in human malignant glioblastoma (U87) cells: Role of reactive oxygen species (ROS). EXCLI J. 2019, 18, 576–590. [Google Scholar]
- Sharma, V.; Joseph, C.; Ghosh, S.; Agarwal, A.; Mishra, M.K.; Sen, E. Kaempferol induces apoptosis in glioblastoma cells through oxidative stress. Mol. Cancer Ther. 2007, 6, 2544–2553. [Google Scholar] [CrossRef] [Green Version]
- Jeong, J.C.; Kim, M.S.; Kim, T.H.; Kim, Y.K. Kaempferol induces cell death through ERK and Akt-dependent down-regulation of XIAP and survivin in human glioma cells. Neurochem. Res. 2009, 34, 991–1001. [Google Scholar] [CrossRef]
- Colombo, M.; Figueiró, F.; de Fraga Dias, A.; Teixeira, H.F.; Battastini, A.M.O.; Koester, L.S. Kaempferol-loaded mucoadhesive nanoemulsion for intranasal administration reduces glioma growth in vitro. Int. J. Pharm. 2018, 543, 214–223. [Google Scholar] [CrossRef]
- Siegelin, M.D.; Reuss, D.E.; Habel, A.; Herold-Mende, C.; von Deimling, A. The flavonoid kaempferol sensitizes human glioma cells to TRAIL-mediated apoptosis by proteasomal degradation of survivin. Mol. Cancer Ther. 2008, 7, 3566–3574. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, A.Y.; Li, M.; Chen, C.; Yao, Q. Ginkgo biloba extract kaempferol inhibits cell proliferation and induces apoptosis in pancreatic cancer cells. J. Surg. Res. 2008, 148, 17–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.; Kim, J.H. Kaempferol Inhibits Pancreatic Cancer Cell Growth and Migration through the Blockade of EGFR-Related Pathway In Vitro. PLoS ONE 2016, 11, e0155264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, F.; Luo, X.; Tsun, A.; Li, Z.; Li, D.; Li, B. Kaempferol enhances the suppressive function of Treg cells by inhibiting FOXP3 phosphorylation. Int. Immunopharmacol. 2015, 28, 859–865. [Google Scholar] [CrossRef] [PubMed]
- Nöthlings, U.; Murphy, S.P.; Wilkens, L.R.; Boeing, H.; Schulze, M.B.; Bueno-de-Mesquita, H.B.; Michaud, D.S.; Roddam, A.; Rohrmann, S.; Tjønneland, A.; et al. A food pattern that is predictive of flavonol intake and risk of pancreatic cancer. Am. J. Clin. Nutr. 2008, 88, 1653–1662. [Google Scholar] [CrossRef] [Green Version]
- Wu, P.; Meng, X.; Zheng, H.; Zeng, Q.; Chen, T.; Wang, W.; Zhang, X.; Su, J. Kaempferol Attenuates ROS-Induced Hemolysis and the Molecular Mechanism of Its Induction of Apoptosis on Bladder Cancer. Molecules 2018, 23, 2592. [Google Scholar] [CrossRef] [Green Version]
- Dang, Q.; Song, W.; Xu, D.; Ma, Y.; Li, F.; Zeng, J.; Zhu, G.; Wang, X.; Chang, L.S.; He, D.; et al. Kaempferol suppresses bladder cancer tumor growth by inhibiting cell proliferation and inducing apoptosis. Mol. Carcinog. 2015, 54, 831–840. [Google Scholar] [CrossRef]
- Garcia, R.; Gonzalez, C.A.; Agudo, A.; Riboli, E. High intake of specific carotenoids and flavonoids does not reduce the risk of bladder cancer. Nutr. Cancer 1999, 35, 212–214. [Google Scholar] [CrossRef]
- Xie, F.; Su, M.; Qiu, W.; Zhang, M.; Guo, Z.; Su, B.; Liu, J.; Li, X.; Zhou, L. Kaempferol promotes apoptosis in human bladder cancer cells by inducing the tumor suppressor, PTEN. Int. J. Mol. Sci. 2013, 14, 21215–21226. [Google Scholar] [CrossRef] [Green Version]
- Meyers, P.A.; Gorlick, R. Osteosarcoma. Pediatr. Clin. North Am. 1997, 44, 973–989. [Google Scholar] [CrossRef]
- Huang, W.W.; Chiu, Y.J.; Fan, M.J.; Lu, H.F.; Yeh, H.F.; Li, K.H.; Chen, P.Y.; Chung, J.G.; Yang, J.S. Kaempferol induced apoptosis via endoplasmic reticulum stress and mitochondria-dependent pathway in human osteosarcoma U-2 OS cells. Mol. Nutr. Food Res. 2010, 54, 1585–1595. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.J.; Lin, C.M.; Lee, C.Y.; Shih, N.C.; Peng, S.F.; Tsuzuki, M.; Amagaya, S.; Huang, W.W.; Yang, J.S. Kaempferol suppresses cell metastasis via inhibition of the ERK-p38-JNK and AP-1 signaling pathways in U-2 OS human osteosarcoma cells. Oncol. Rep. 2013, 30, 925–932. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Afroze, N.; Pramodh, S.; Almutary, A.G.; Rizvi, T.A.; Rais, N.; Raina, R.; Faiyazuddin, M.; Alnuqaydan, A.M.; Hussain, A. Kaempferol Regresses Carcinogenesis through a Molecular Cross Talk Involved in Proliferation, Apoptosis and Inflammation on Human Cervical Cancer Cells, HeLa. Appl. Sci. 2022, 12, 3155. [Google Scholar] [CrossRef]
- Liao, W.; Chen, L.; Ma, X.; Jiao, R.; Li, X.; Wang, Y. Protective effects of kaempferol against reactive oxygen species-induced hemolysis and its antiproliferative activity on human cancer cells. Eur. J. Med. Chem. 2016, 114, 24–32. [Google Scholar] [CrossRef]
- Limtrakul, P.; Khantamat, O.; Pintha, K. Inhibition of P-glycoprotein function and expression by kaempferol and quercetin. J. Chemother. 2005, 17, 86–95. [Google Scholar] [CrossRef]
- Tu, L.Y.; Bai, H.H.; Cai, J.Y.; Deng, S.P. The mechanism of kaempferol induced apoptosis and inhibited proliferation in human cervical cancer SiHa cell: From macro to nano. Scanning 2016, 38, 644–653. [Google Scholar] [CrossRef] [Green Version]
- Hung, T.W.; Chen, P.N.; Wu, H.C.; Wu, S.W.; Tsai, P.Y.; Hsieh, Y.S.; Chang, H.R. Kaempferol Inhibits the Invasion and Migration of Renal Cancer Cells through the Downregulation of AKT and FAK Pathways. Int. J. Med. Sci. 2017, 14, 984–993. [Google Scholar] [CrossRef] [Green Version]
- An, G.; Gallegos, J.; Morris, M.E. The bioflavonoid kaempferol is an Abcg2 substrate and inhibits Abcg2-mediated quercetin efflux. Drug Metab. Dispos. 2011, 39, 426–432. [Google Scholar] [CrossRef] [Green Version]
- Song, W.; Dang, Q.; Xu, D.; Chen, Y.; Zhu, G.; Wu, K.; Zeng, J.; Long, Q.; Wang, X.; He, D.; et al. Kaempferol induces cell cycle arrest and apoptosis in renal cell carcinoma through EGFR/p38 signaling. Oncol. Rep. 2014, 31, 1350–1356. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Tian, B.; Wang, Y.; Ding, H. Kaempferol Sensitizes Human Ovarian Cancer Cells-OVCAR-3 and SKOV-3 to Tumor Necrosis Factor-Related Apoptosis-Inducing Ligand (TRAIL)-Induced Apoptosis via JNK/ERK-CHOP Pathway and Up-Regulation of Death Receptors 4 and 5. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2017, 23, 5096–5105. [Google Scholar] [CrossRef] [Green Version]
- Xu, G.; Li, B.; Wang, T.; Wan, J.; Zhang, Y.; Huang, J.; Shen, Y. Enhancing the anti-ovarian cancer activity of quercetin using a self-assembling micelle and thermosensitive hydrogel drug delivery system. RSC Adv. 2018, 8, 21229–21242. [Google Scholar] [CrossRef] [PubMed]
- Amidon, G.L.; Lennernäs, H.; Shah, V.P.; Crison, J.R. A theoretical basis for a biopharmaceutic drug classification: The correlation of in vitro drug product dissolution and in vivo bioavailability. Pharm. Res. 1995, 12, 413–420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cutler, D. Assessment of rate and extent of drug absorption. Pharmacol. Ther. 1981, 14, 123–160. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, S.; Shukla, D.; Mishra, B.; Singh, S. Lipid—An emerging platform for oral delivery of drugs with poor bioavailability. Eur. J. Pharm. Biopharm. 2009, 73, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Skolnik, S. Mitigating permeability-mediated risks in drug discovery. Expert Opin. Drug Metab. Toxicol. 2010, 6, 171–187. [Google Scholar] [CrossRef]
- Bohets, H.; Annaert, P.; Mannens, G.; Van Beijsterveldt, L.; Anciaux, K.; Verboven, P.; Meuldermans, W.; Lavrijsen, K. Strategies for absorption screening in drug discovery and development. Curr. Top. Med. Chem. 2001, 1, 367–383. [Google Scholar] [CrossRef] [Green Version]
- Barve, A.; Chen, C.; Hebbar, V.; Desiderio, J.; Saw, C.L.; Kong, A.N. Metabolism, oral bioavailability and pharmacokinetics of chemopreventive kaempferol in rats. Biopharm. Drug Dispos. 2009, 30, 356–365. [Google Scholar] [CrossRef] [Green Version]
- Li, C.; Li, X.; Choi, J.S. Enhanced bioavailability of etoposide after oral or intravenous administration of etoposide with kaempferol in rats. Arch. Pharmacal Res. 2009, 32, 133–138. [Google Scholar] [CrossRef]
- Bobe, G.; Weinstein, S.J.; Albanes, D.; Hirvonen, T.; Ashby, J.; Taylor, P.R.; Virtamo, J.; Stolzenberg-Solomon, R.Z. Flavonoid intake and risk of pancreatic cancer in male smokers (Finland). Cancer Epidemiol. Biomark. Prev. 2008, 17, 553–562. [Google Scholar] [CrossRef] [Green Version]
- Denison, M.S.; Nagy, S.R. Activation of the aryl hydrocarbon receptor by structurally diverse exogenous and endogenous chemicals. Annu. Rev. Pharmacol. Toxicol. 2003, 43, 309–334. [Google Scholar] [CrossRef]
- Puppala, D.; Gairola, C.G.; Swanson, H.I. Identification of kaempferol as an inhibitor of cigarette smoke-induced activation of the aryl hydrocarbon receptor and cell transformation. Carcinogenesis 2007, 28, 639–647. [Google Scholar] [CrossRef] [PubMed]
- Gates, M.A.; Tworoger, S.S.; Hecht, J.L.; De Vivo, I.; Rosner, B.; Hankinson, S.E. A prospective study of dietary flavonoid intake and incidence of epithelial ovarian cancer. Int. J. Cancer 2007, 121, 2225–2232. [Google Scholar] [CrossRef] [PubMed]
- Gates, M.A.; Vitonis, A.F.; Tworoger, S.S.; Rosner, B.; Titus-Ernstoff, L.; Hankinson, S.E.; Cramer, D.W. Flavonoid intake and ovarian cancer risk in a population-based case-control study. Int. J. Cancer 2009, 124, 1918–1925. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.; Lee, I.M.; Zhang, S.M.; Blumberg, J.B.; Buring, J.E.; Sesso, H.D. Dietary intake of selected flavonols, flavones, and flavonoid-rich foods and risk of cancer in middle-aged and older women. Am. J. Clin. Nutr. 2009, 89, 905–912. [Google Scholar] [CrossRef] [Green Version]
- Schinkel, A.H.; Jonker, J.W. Mammalian drug efflux transporters of the ATP binding cassette (ABC) family: An overview. Adv. Drug Deliv. Rev. 2003, 55, 3–29. [Google Scholar] [CrossRef]
- Zhou, S.F.; Wang, L.L.; Di, Y.M.; Xue, C.C.; Duan, W.; Li, C.G.; Li, Y. Substrates and inhibitors of human multidrug resistance associated proteins and the implications in drug development. Curr. Med. Chem. 2008, 15, 1981–2039. [Google Scholar] [CrossRef]
- Kazmi, I.; Al-Abbasi, F.A.; Afzal, M.; Altayb, H.N.; Nadeem, M.S.; Gupta, G. Formulation and Evaluation of Kaempferol Loaded Nanoparticles against Experimentally Induced Hepatocellular Carcinoma: In Vitro and In Vivo Studies. Pharmaceutics 2021, 13, 2086. [Google Scholar] [CrossRef]
- Alyami, N.M.; Alyami, H.M.; Almeer, R. Using green biosynthesized kaempferol-coated sliver nanoparticles to inhibit cancer cells growth: An in vitro study using hepatocellular carcinoma (HepG2). Cancer Nanotechnol. 2022, 13, 26. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qattan, M.Y.; Khan, M.I.; Alharbi, S.H.; Verma, A.K.; Al-Saeed, F.A.; Abduallah, A.M.; Al Areefy, A.A. Therapeutic Importance of Kaempferol in the Treatment of Cancer through the Modulation of Cell Signalling Pathways. Molecules 2022, 27, 8864. https://doi.org/10.3390/molecules27248864
Qattan MY, Khan MI, Alharbi SH, Verma AK, Al-Saeed FA, Abduallah AM, Al Areefy AA. Therapeutic Importance of Kaempferol in the Treatment of Cancer through the Modulation of Cell Signalling Pathways. Molecules. 2022; 27(24):8864. https://doi.org/10.3390/molecules27248864
Chicago/Turabian StyleQattan, Malak Yahia, Mohammad Idreesh Khan, Shudayyed Hasham Alharbi, Amit Kumar Verma, Fatimah A. Al-Saeed, Alduwish Manal Abduallah, and Azza A. Al Areefy. 2022. "Therapeutic Importance of Kaempferol in the Treatment of Cancer through the Modulation of Cell Signalling Pathways" Molecules 27, no. 24: 8864. https://doi.org/10.3390/molecules27248864
APA StyleQattan, M. Y., Khan, M. I., Alharbi, S. H., Verma, A. K., Al-Saeed, F. A., Abduallah, A. M., & Al Areefy, A. A. (2022). Therapeutic Importance of Kaempferol in the Treatment of Cancer through the Modulation of Cell Signalling Pathways. Molecules, 27(24), 8864. https://doi.org/10.3390/molecules27248864






