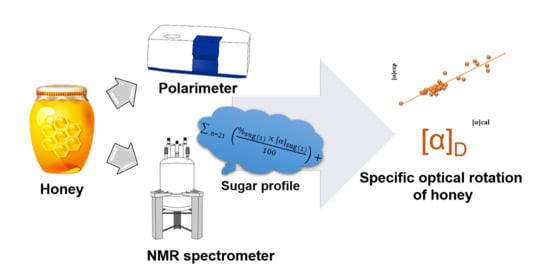
Optical Rotation—A Reliable Parameter for Authentication of Honey?
Abstract
Share and Cite
Gerginova, D.; Kurteva, V.; Simova, S. Optical Rotation—A Reliable Parameter for Authentication of Honey? Molecules 2022, 27, 8916. https://doi.org/10.3390/molecules27248916
Gerginova D, Kurteva V, Simova S. Optical Rotation—A Reliable Parameter for Authentication of Honey? Molecules. 2022; 27(24):8916. https://doi.org/10.3390/molecules27248916
Chicago/Turabian StyleGerginova, Dessislava, Vanya Kurteva, and Svetlana Simova. 2022. "Optical Rotation—A Reliable Parameter for Authentication of Honey?" Molecules 27, no. 24: 8916. https://doi.org/10.3390/molecules27248916
APA StyleGerginova, D., Kurteva, V., & Simova, S. (2022). Optical Rotation—A Reliable Parameter for Authentication of Honey? Molecules, 27(24), 8916. https://doi.org/10.3390/molecules27248916