Biochemical Reactions and Their Biological Contributions in Honey
Abstract
:1. Introduction
2. Enzymatic Reactions in Honey
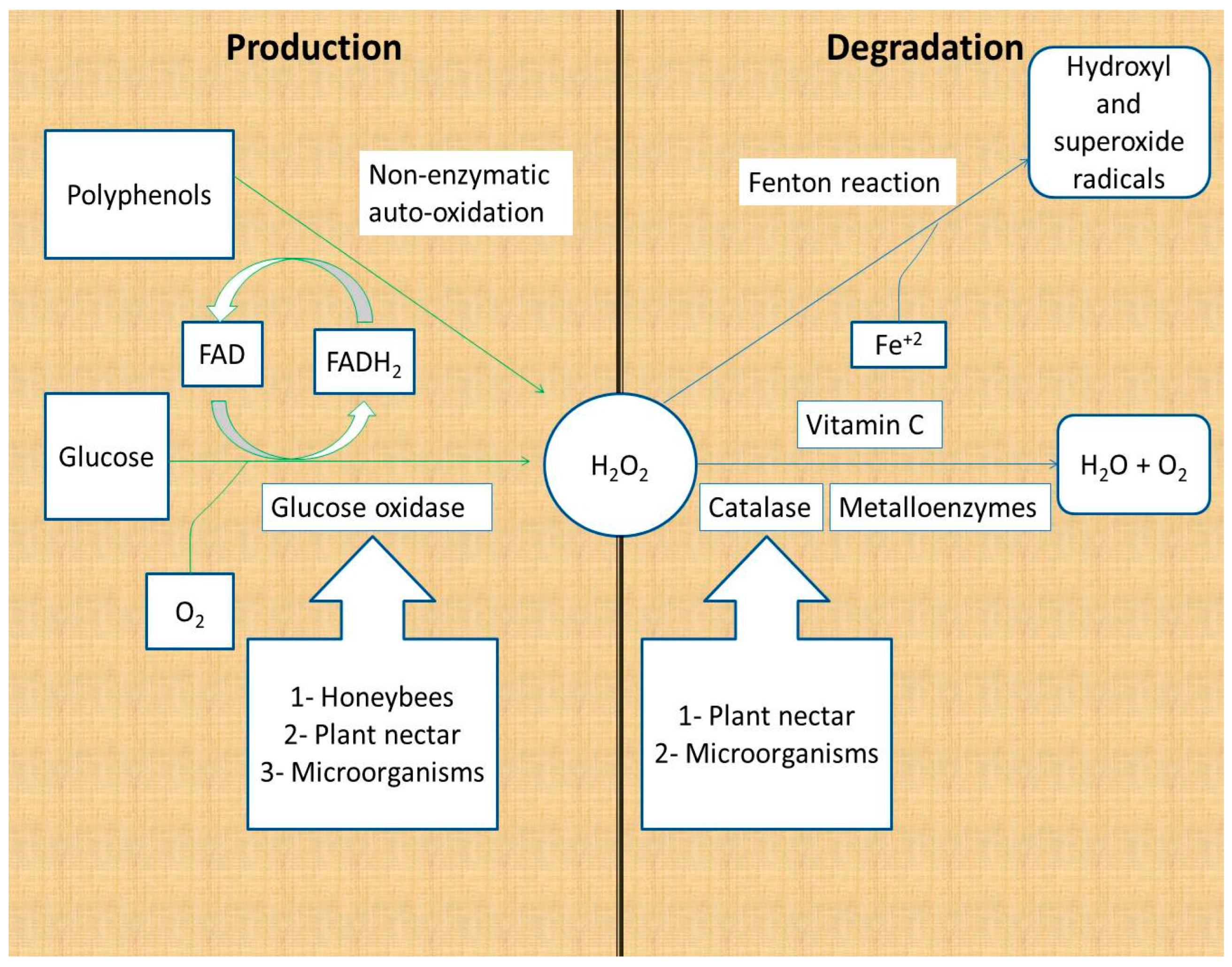
2.1. Production and Degradation of Hydrogen Peroxide
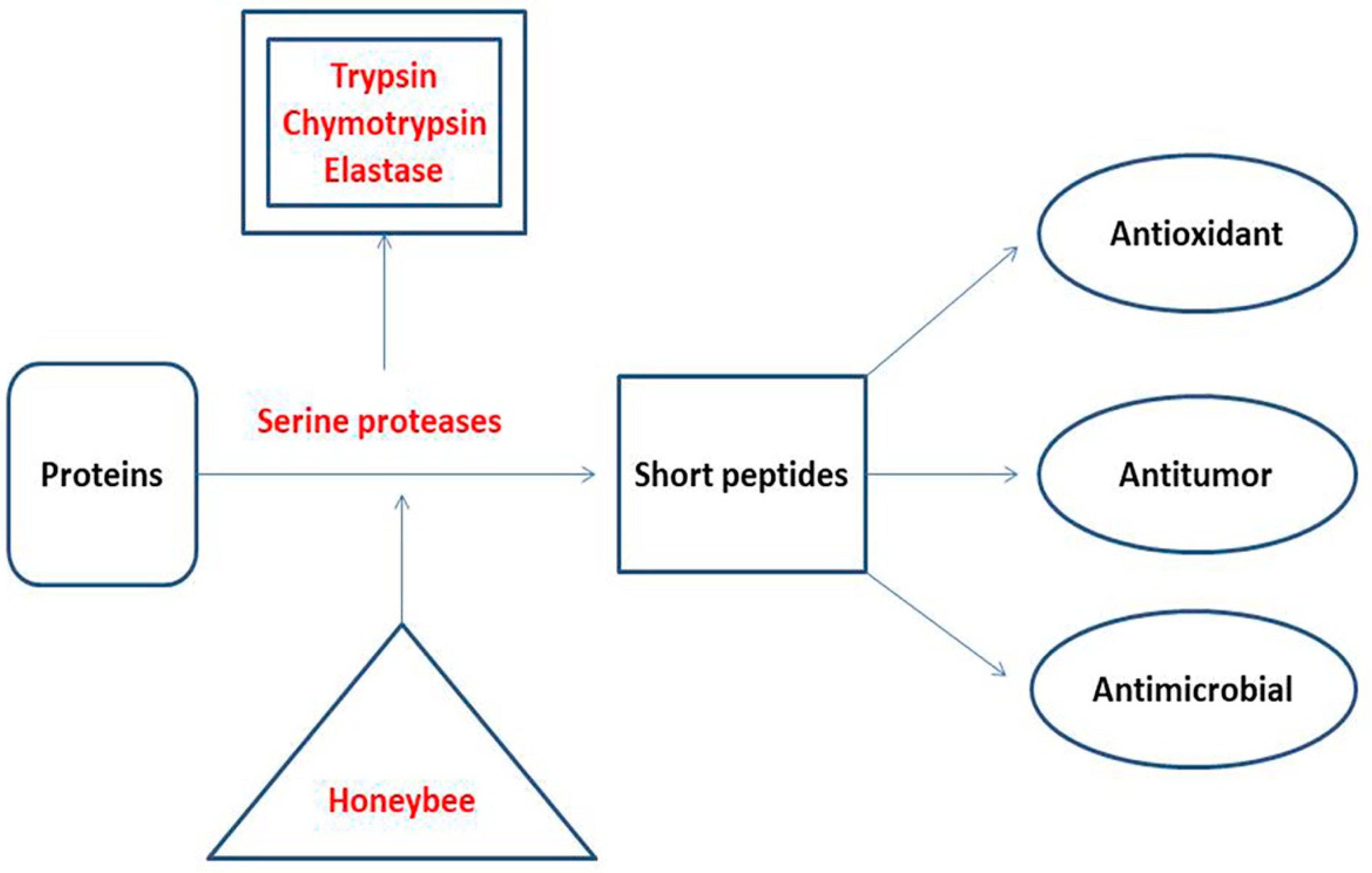
2.2. Proteases
3. Enzymes in Honey as Quality Parameters and Indicators for Floral Origin, Honeybee Disease and the Presence of Yeast
3.1. Diastase and Invertase
3.2. β-Glucosidase
3.3. Glucosylceramidase (Glucocerebrosidase or Ceramide Glucosidase)
3.4. Acid Phosphatase
4. Non-Enzymatic Reactions in Honey
4.1. Non-Enzymatic Trans-Glycosylation
4.2. Production and Degradation of Dicarbonyl Compounds
4.3. Production and Degradation of HMF
4.4. Maillard Reaction in Honey
4.5. Caramelization in Honey
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Codex Alimentarius. International Food Standards. CXS 12-1981. Standard for Honey 1981. Revised 2019. Available online: https://www.fao.org/fao-who-codexalimentarius/sh-proxy/en/?lnk=1&url=https%253A%252F%252Fworkspace.fao.org%252Fsites%252Fcodex%252FStandards%252FCXS%2B12-1981%252Fcxs_012e.pdf (accessed on 17 July 2022).
- Viuda-Martos, M.; Ruiz-Navajas, Y.; Fernández-López, J.; Pérez-Álvarez, J.A. Functional properties of honey, propolis, and royal jelly. J. Food Sci. 2008, 73, R117–R124. [Google Scholar] [CrossRef] [PubMed]
- da Silva, P.M.; Gauche, C.; Gonzaga, L.V.; Costa, A.C.O.; Fett, R. Honey: Chemical composition, stability and authenticity. Food Chem. 2016, 196, 309–323. [Google Scholar] [CrossRef] [PubMed]
- Erkmen, O.; Bozoglu, T.F. Enzymatic and Nonenzymatic Food Spoilage. In Food Microbiology: Principles into Practice; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2016; pp. 401–406. [Google Scholar] [CrossRef]
- Wang, J.; Li, Q.X. Chemical Composition, Characterization, and Differentiation of Honey Botanical and Geographical Origins. Adv. Food Nutr. Res. 2011, 62, 89–137. [Google Scholar] [CrossRef] [PubMed]
- Rossano, R.; LaRocca, M.; Polito, T.; Perna, A.M.; Padula, M.C.; Martelli, G.; Riccio, P. What Are the Proteolytic Enzymes of Honey and What They Do Tell Us? A Fingerprint Analysis by 2-D Zymography of Unifloral Honeys. PLoS ONE 2012, 7, e49164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Utzeri, V.J.; Schiavo, G.; Ribani, A.; Tinarelli, S.; Bertolini, F.; Bovo, S.; Fontanesi, L. Entomological signatures in honey: An environmental DNA metabarcoding approach can disclose information on plant-sucking insects in agricultural and forest landscapes. Sci. Rep. 2018, 8, 996. [Google Scholar] [CrossRef] [Green Version]
- Borutinskaite, V.; Treigyte, G.; Čeksteryte, V.; Kurtinaitiene, B.; Navakauskiene, R. Proteomic identification and enzymatic activity of buckwheat (Fagopyrum esculentum) honey based on different assays. J. Food Nutr. Res. 2018, 57, 57–69. [Google Scholar]
- Nolan, V.C.; Harrison, J.; Cox, J.A. Dissecting the antimicrobial composition of honey. Antibiotics 2019, 8, 251. [Google Scholar] [CrossRef] [Green Version]
- Bizerra, F.C.; Da Silva, P.I.; Hayashi, M.A.F. Exploring the antibacterial properties of honey and its potential. Front. Microbiol. 2012, 3, 398. [Google Scholar] [CrossRef] [Green Version]
- Barboni, E.; Kemeny, D.; Campos, S.; Vernon, C. The purification of acid phosphatase from honey bee venom (Apis mellifica). Toxicon 1987, 25, 1097–1103. [Google Scholar] [CrossRef]
- Boonchiangma, S.; Chanthai, S.; Srijaranai, S.; Srijaranai, S. Chemical Compositions and Non-Enzymatic Browning Compounds of Thai Honey: A Kinetic Study. J. Food Process Eng. 2009, 34, 1584–1596. [Google Scholar] [CrossRef]
- Silva, S.P.; Moreira, A.S.P.; Domingues, M.D.R.M.; Evtuguin, D.V.; Coelho, E.; Coimbra, M.A. Contribution of non-enzymatic transglycosylation reactions to the honey oligosaccharides origin and diversity. Pure Appl. Chem. 2019, 91, 1231–1242. [Google Scholar] [CrossRef]
- Nagai, T.; Kai, N.; Tanoue, Y.; Suzuki, N. Chemical properties of commercially available honey species and the functional properties of caramelization and Maillard reaction products derived from these honey species. J. Food Sci. Technol. 2017, 55, 586–597. [Google Scholar] [CrossRef] [PubMed]
- Hellwig, M.; Rückriemen, J.; Sandner, D.; Henle, T. Unique Pattern of Protein-Bound Maillard Reaction Products in Manuka (Leptospermum scoparium) Honey. J. Agric. Food Chem. 2017, 65, 3532–3540. [Google Scholar] [CrossRef] [PubMed]
- Brudzynski, K. A current perspective on hydrogen peroxide production in honey. A review. Food Chem. 2020, 332, 127229. [Google Scholar] [CrossRef] [PubMed]
- Grzesik, M.; Bartosz, G.; Stefaniuk, I.; Pichla, M.; Namieśnik, J.; Sadowska-Bartosz, I. Dietary antioxidants as a source of hydrogen peroxide. Food Chem. 2018, 278, 692–699. [Google Scholar] [CrossRef]
- Carter, C.; Thornburg, R.W. Tobacco Nectarin I. J. Biol. Chem. 2000, 275, 36726–36733. [Google Scholar] [CrossRef] [Green Version]
- Carter, C.J.; Thornburg, R.W. Tobacco Nectarin V Is a Flavin-Containing Berberine Bridge Enzyme-Like Protein with Glucose Oxidase Activity. Plant Physiol. 2004, 134, 460–469. [Google Scholar] [CrossRef] [Green Version]
- Silva, M.S.; Rabadzhiev, Y.; Eller, M.R.; Iliev, I.; Ivanova, I.; Santana, W.C. Microorganisms in Honey; Intech Open: Vienna, Austria, 2017. [Google Scholar]
- Endo, A.; Salminen, S. Honeybees and beehives are rich sources for fructophilic lactic acid bacteria. Syst. Appl. Microbiol. 2013, 36, 444–448. [Google Scholar] [CrossRef]
- Brudzynski, K.; Abubaker, K.; Laurent, M.; Castle, A. Re-Examining the Role of Hydrogen Peroxide in Bacteriostatic and Bactericidal Activities of Honey. Front. Microbiol. 2011, 2, 213. [Google Scholar] [CrossRef] [Green Version]
- Gillette, C.C. Honey catalase. J. Econ. Entomol. 1931, 24, 605–606. [Google Scholar] [CrossRef]
- Zámocký, M.; Gasselhuber, B.; Furtmüller, P.G.; Obinger, C. Molecular evolution of hydrogen peroxide degrading enzymes. Arch. Biochem. Biophys. 2012, 525, 131–144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Majtan, J.; Sojka, M.; Palenikova, H.; Bucekova, M.; Majtan, V. Vitamin C Enhances the Antibacterial Activity of Honey against Planktonic and Biofilm-Embedded Bacteria. Molecules 2020, 25, 992. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brudzynski, K.; Lannigan, R. Mechanism of Honey Bacteriostatic Action against MRSA and VRE Involves Hydroxyl Radicals Generated from Honey’s Hydrogen Peroxide. Front. Microbiol. 2012, 3, 36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cos, P.; Calomme, M.; Pieters, L.; Vlietinck, A.; Berghe, D.V. Structure-Activity Relationship of Flavonoids as Antioxidant and Pro-Oxidant Compounds. Stud. Nat. Prod. Chem. 2000, 22, 307–341. [Google Scholar] [CrossRef]
- Brudzynski, K.; Miotto, D.; Kim, L.; Sjaarda, C.; Maldonado-Alvarez, L.; Fukś, H. Active macromolecules of honey form colloidal particles essential for honey antibacterial activity and hydrogen peroxide production. Sci. Rep. 2017, 7, 7637. [Google Scholar] [CrossRef] [Green Version]
- Hossain, L.; Lim, L.; Hammer, K.; Hettiarachchi, D.; Locher, C. Honey-Based Medicinal Formulations: A Critical Review. Appl. Sci. 2021, 11, 5159. [Google Scholar] [CrossRef]
- Alaerjani, W.M.A.; Abu-Melha, S.A.; Khan, K.A.; Ghramh, H.A.; Alalmie, A.Y.A.; Alshareef, R.M.H.; Al-Shehri, B.M.; Mohammed, M.E.A. Presence of short and cyclic peptides in Acacia and Ziziphus honeys may potentiate their medicinal values. Open Chem. 2021, 19, 1162–1173. [Google Scholar] [CrossRef]
- Zheng, L.; Zhao, Y.; Dong, H.; Su, G.; Zhao, M. Structure–activity relationship of antioxidant dipeptides: Dominant role of Tyr, Trp, Cys and Met residues. J. Funct. Foods 2016, 21, 485–496. [Google Scholar] [CrossRef]
- Guo, H.; Kouzuma, Y.; Yonekura, M. Structures and properties of antioxidative peptides derived from royal jelly protein. Food Chem. 2009, 113, 238–245. [Google Scholar] [CrossRef]
- Karami, Z.; Akbari-Adergani, B. Bioactive food derived peptides: A review on correlation between structure of bioactive peptides and their functional properties. J. Food Sci. Technol. 2019, 56, 535–547. [Google Scholar] [CrossRef]
- Kuroda, K.; Caputo, G.A. Antimicrobial polymers as synthetic mimics of host-defense peptides. Rev. Nanomed. Nanobiotechnol. 2012, 5, 49–66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joo, S.-H. Cyclic Peptides as Therapeutic Agents and Biochemical Tools. Biomol. Ther. 2012, 20, 19–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bogdanov, S.; Lüllmann, C.; Martin, P.; von der Ohe, W.; Russmann, H.; Vorwohl, G.; Oddo, L.P.; Sabatini, A.G.; Marcazzan, G.L.; Piro, R.; et al. Honey Quality and International Regulatory Standards. European Honey Standards 1999. Available online: https://www.apiservices.biz/en/articles/sort-by-date-up-online/768-honey-quality-and-international-regulatory-standards-1999 (accessed on 17 July 2022).
- Aušević, B.; Haurdic, B.; Jašić, M.; Bašić, M. Enzymatic activities in honey. In Proceedings of the Second Conference of Bee Keeping and Bee Products- with International Participation- Bee Keeping and Bee Products, Gradačac, Bosnia and Herzegovina, 20 August 2017; pp. 90–97. [Google Scholar]
- Alonso-Torre, S.; Cavia, M.M.; Fernández-Muiño, M.; Moreno, G.; Huidobro, J.; Sancho, M. Evolution of acid phosphatase activity of honeys from different climates. Food Chem. 2006, 97, 750–755. [Google Scholar] [CrossRef]
- Miłek, M.; Bocian, A.; Kleczyńska, E.; Sowa, P.; Dżugan, M. The Comparison of Physicochemical Parameters, Antioxidant Activity and Proteins for the Raw Local Polish Honeys and Imported Honey Blends. Molecules 2021, 26, 2423. [Google Scholar] [CrossRef]
- Mussatto, S.I.; Aguilar, C.N.; Rodrigues, L.R.; Teixeira, J. Fructooligosaccharides and β-fructofuranosidase production by Aspergillus japonicus immobilized on lignocellulosic materials. J. Mol. Catal. B Enzym. 2009, 59, 76–81. [Google Scholar] [CrossRef] [Green Version]
- Slevin, M.M.; Allsopp, P.J.; Magee, P.J.; Bonham, M.P.; Naughton, V.R.; Strain, J.J.; Duffy, M.E.; Wallace, J.M.; Mc Sorley, E.M. Supplementation with Calcium and Short-Chain Fructo-Oligosaccharides Affects Markers of Bone Turnover but Not Bone Mineral Density in Postmenopausal Women. J. Nutr. 2013, 144, 297–304. [Google Scholar] [CrossRef] [Green Version]
- Flamm, G.; Glinsmann, W.; Kritchevsky, D.; Prosky, L.; Roberfroid, M. Inulin and Oligofructose as Dietary Fiber: A Review of the Evidence. Crit. Rev. Food Sci. Nutr. 2001, 41, 353–362. [Google Scholar] [CrossRef]
- Miguel, M.G.; Antunes, M.D.; Faleiro, M.L. Honey as a Complementary Medicine. Integr. Med. Insights 2017, 12, 1178633717702869. [Google Scholar] [CrossRef] [Green Version]
- Hellwig, M.; Gensberger-Reigl, S.; Henle, T.; Pischetsrieder, M. Food-derived 1,2-dicarbonyl compounds and their role in diseases. Semin. Cancer Biol. 2018, 49, 1–8. [Google Scholar] [CrossRef]
- Rizzi, G.P. The Strecker Degradation of Amino Acids: Newer Avenues for Flavor Formation. Food Rev. Int. 2008, 24, 416–435. [Google Scholar] [CrossRef]
- Dunkel, A.; Steinhaus, M.; Kotthoff, M.; Nowak, B.; Krautwurst, D.; Schieberle, P.; Hofmann, T. Nature’s Chemical Signatures in Human Olfaction: A Foodborne Perspective for Future Biotechnology. Angew. Chem. Int. Ed. 2014, 53, 7124–7143. [Google Scholar] [CrossRef] [PubMed]
- Frischmann, M.; Bidmon, C.; Angerer, A.J.; Pischetsrieder, M. Identification of DNA Adducts of Methylglyoxal. Chem. Res. Toxicol. 2005, 18, 1586–1592. [Google Scholar] [CrossRef] [PubMed]
- Kocadağlı, T.; Gökmen, V. Effects of Sodium Chloride, Potassium Chloride, and Calcium Chloride on the Formation of α-Dicarbonyl Compounds and Furfurals and the Development of Browning in Cookies during Baking. J. Agric. Food Chem. 2016, 64, 7838–7848. [Google Scholar] [CrossRef]
- Cämmerer, B.; Jalyschko, W.; Kroh, L.W. Intact Carbohydrate Structures as Part of the Melanoidin Skeleton. J. Agric. Food Chem. 2002, 50, 2083–2087. [Google Scholar] [CrossRef] [PubMed]
- Almasaudi, S. The antibacterial activities of honey. Saudi J. Biol. Sci. 2020, 28, 2188–2196. [Google Scholar] [CrossRef] [PubMed]
- Johnston, M.; McBride, M.; Dahiya, D.; Owusu-Apenten, R.; Nigam, P.S. Antibacterial activity of Manuka honey and its components: An overview. AIMS Microbiol. 2018, 4, 655–664. [Google Scholar] [CrossRef]
- Svendsen, C.; Høie, A.H.; Alexander, J.; Murkovic, M.; Husøy, T. The food processing contaminant glyoxal promotes tumour growth in the multiple intestinal neoplasia (Min) mouse model. Food Chem. Toxicol. 2016, 94, 197–202. [Google Scholar] [CrossRef]
- Krainer, S.; Brodschneider, R.; Vollmann, J.; Crailsheim, K.; Riessberger-Gallé, U. Effect of hydroxymethylfurfural (HMF) on mortality of artificially reared honey bee larvae (Apis mellifera carnica). Ecotoxicology 2015, 25, 320–328. [Google Scholar] [CrossRef]
- Bailey, L. The Effect of Acid-Hydrolysed Sucrose on Honeybees. J. Apic. Res. 1966, 5, 127–136. [Google Scholar] [CrossRef]
- Surh, Y.-J.; Liem, A.; Miller, J.A.; Tannenbaum, S.R. 5-Sulfooxymethylfurfural as a possible ultimate mutagenic and carcinogenic metabolite of the Maillard reaction product, 5-hydroxymethylfurfural. Carcinogenesis 1994, 15, 2375–2377. [Google Scholar] [CrossRef]
- Bakhiya, N.; Monien, B.; Frank, H.; Seidel, A.; Glatt, H. Renal organic anion transporters OAT1 and OAT3 mediate the cellular accumulation of 5-sulfooxymethylfurfural, a reactive, nephrotoxic metabolite of the Maillard product 5-hydroxymethylfurfural. Biochem. Pharmacol. 2009, 78, 414–419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morales, F.J. Hydroxymethylfurfural (HMF) and related compounds. In Process-Induced Food Toxicants: Occurrence, Formation, Mitigation, and Health Risks; John Wiley & Sons: Hoboken, NJ, USA, 2008; ISBN 978-0-470-07475-6. [Google Scholar]
- Zhao, L.; Chen, J.; Su, J.; Li, L.; Hu, S.; Li, B.; Zhang, X.; Xu, Z.; Chen, T. In Vitro Antioxidant and Antiproliferative Activities of 5-Hydroxymethylfurfural. J. Agric. Food Chem. 2013, 61, 10604–10611. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh, M.; Khodaei, H.; Abbasi, M.M.; Saleh-Ghadimi, S. Assessing the effect of 5-hydroxymethylfurfural on selected components of immune responses in mice immunised with ovalbumin. J. Sci. Food Agric. 2017, 97, 3979–3984. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.-M.; Wu, J.-Y.; Su, C.; Ferng, S.; Lo, C.-Y.; Chiou, R.Y.-Y. Identification and Mode of Action of 5-Hydroxymethyl-2-furfural (5-HMF) and 1-Methyl-1,2,3,4-tetrahydro-β-carboline-3-carboxylic Acid (MTCA) as Potent Xanthine Oxidase Inhibitors in Vinegars. J. Agric. Food Chem. 2012, 60, 9856–9862. [Google Scholar] [CrossRef]
- Wu, S.; Hu, J.; Wei, L.; Du, Y.; Shi, X.; Zhang, L. Antioxidant and antimicrobial activity of Maillard reaction products from xylan with chitosan/chitooligomer/glucosamine hydrochloride/taurine model systems. Food Chem. 2014, 148, 196–203. [Google Scholar] [CrossRef]
- Starowicz, M.; Ostaszyk, A.; Zieliński, H. The Relationship between the Browning Index, Total Phenolics, Color, and Antioxidant Activity of Polish-Originated Honey Samples. Foods 2021, 10, 967. [Google Scholar] [CrossRef]
- Brudzynski, K. Honey melanoidins: Emerging novel understanding on the mechanism of antioxidant and antibacterial action of honey. In Honey: Current Research and Clinical Applications; Majtan, J., Ed.; Nova Science Publisher, Inc.: Hauppauge, NY, USA, 2012; pp. 17–38. [Google Scholar]
- Antony, S.M.; Han, I.Y.; Rieck, J.R.; Dawson, P.L. Antioxidative Effect of Maillard Reaction Products Formed from Honey at Different Reaction Times. J. Agric. Food Chem. 2000, 48, 3985–3989. [Google Scholar] [CrossRef]
- Sengar, G.; Sharma, H.K. Food caramels: A review. J. Food Sci. Technol. 2012, 51, 1686–1696. [Google Scholar] [CrossRef] [Green Version]
- Kroh, L.W. Caramelisation in food and beverages. Food Chem. 1994, 51, 373–379. [Google Scholar] [CrossRef]
- Brenna, O.V.; Ceppi, E.L.; Giovanelli, G. Antioxidant capacity of some caramel-containing soft drinks. Food Chem. 2009, 115, 119–123. [Google Scholar] [CrossRef]
- Tsai, P.-J.; Yu, T.-Y.; Chen, S.-H.; Liu, C.-C.; Sun, Y.-F. Interactive role of color and antioxidant capacity in caramels. Food Res. Int. 2009, 42, 380–386. [Google Scholar] [CrossRef]
- Rawlings, N.; Barrett, A. Evolutionary families of peptidases. Biochem. J. 1993, 290, 205–218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rawlings, N.D. Twenty-five years of nomenclature and classification of proteolytic enzymes. Biochim. Biophys. Acta (BBA)-Proteins Proteom. 2019, 1868, 140345. [Google Scholar] [CrossRef]
- Simpson, R.J. Fragmentation of Protein Using Trypsin. Cold Spring Harb. Protoc. 2006, 2006, prot4550. [Google Scholar] [CrossRef]
- Gráf, L.; Szilágyi, L.; Venekei, I. Chymotrypsin. In Handbook of Proteolytic Enzymes, 3rd ed.; Rawlings, N.D., Salvesen, G., Eds.; Academic Press: Cambridge, MA, USA, 2013; pp. 2626–2633. [Google Scholar] [CrossRef]
- Cotten, S.W. Evaluation of exocrine pancreatic function. In Contemporary Practice in Clinical Chemistry, 4th ed.; Clarke, W., Marzinke, M.A., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 573–585. [Google Scholar] [CrossRef]
- Gillette, M.A.; Carr, S.A. Quantitative analysis of peptides and proteins in biomedicine by targeted mass spectrometry. Nat. Methods 2013, 10, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Lichtenberg-Kraag, B. Evidence for correlation between invertase activity and sucrose content during the ripening process of honey. J. Apic. Res. 2014, 53, 364–373. [Google Scholar] [CrossRef]
- Huang, Z.; Liu, L.; Li, G.; Li, H.; Ye, D.; Li, X. Nondestructive Determination of Diastase Activity of Honey Based on Visible and Near-Infrared Spectroscopy. Molecules 2019, 24, 1244. [Google Scholar] [CrossRef] [Green Version]
- Huidobro, J.F.; Santana, F.J.; Sanchez, M.P.; Sancho, M.T.; Muniategui, S.; Simal-Lozano, J. Diastase, invertase and β-glucosidase activities in fresh honey from north-west Spain. J. Apic. Res. 1995, 34, 39–44. [Google Scholar] [CrossRef]
- Serrano, S.; Espejo, R.; Villarejo, M.; Jodral, M.L. Diastase and invertase activities in Andalusian honeys. Int. J. Food Sci. Technol. 2007, 42, 76–79. [Google Scholar] [CrossRef]
- Pontoh, J.; Low, N. Purification and characterization of β-glucosidase from honey bees (Apis mellifera). Insect Biochem. Mol. Biol. 2002, 32, 679–690. [Google Scholar] [CrossRef]
- Gloster, T.M.; Madsen, R.; Davies, G.J. Structural basis for cyclophellitol inhibition of a β-glucosidase. Org. Biomol. Chem. 2006, 5, 444–446. [Google Scholar] [CrossRef] [PubMed]
- Tagad, C.K.; Sabharwal, S.G. Purification and characterization of acid phosphatase from Macrotyloma uiflorum seeds. J. Food Sci. Technol. 2017, 55, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Carman, G.M.; Han, G.-S. Fat-regulating phosphatidic acid phosphatase: A review of its roles and regulation in lipid homeostasis. J. Lipid Res. 2019, 60, 2–6. [Google Scholar] [CrossRef] [PubMed]
- Widlanski, T.S.; Taylor, W. Chemistry and enzymology of phosphatases. In Comprehensive Natural Products Chemistry; Barton, S.D., Nakanishi, K., Meth-Cohn, O., Eds.; Elsevier: Amsterdam, The Netherlands, 1999; pp. 139–162. [Google Scholar] [CrossRef]
- Weston, R.J.; Brocklebank, L.K. The oligosaccharide composition of some New Zealand honeys. Food Chem. 1999, 64, 33–37. [Google Scholar] [CrossRef]
- Belorkar, S.A.; Gupta, A.K. Oligosaccharides: A boon from nature’s desk. AMB Express 2016, 6, 82. [Google Scholar] [CrossRef] [Green Version]
- Zheng, J.; Ou, J.; Ou, S. Alpha-Dicarbonyl Compounds; Springer: Singapore, 2019; pp. 19–46. [Google Scholar] [CrossRef]
- Arena, E.; Ballistreri, G.; Tomaselli, F.; Fallico, B. Survey of 1,2-Dicarbonyl Compounds in Commercial Honey of Different Floral Origin. J. Food Sci. 2011, 76, C1203–C1210. [Google Scholar] [CrossRef]
- Marceau, E.; Yaylayan, V.A. Profiling of α-Dicarbonyl Content of Commercial Honeys from Different Botanical Origins: Identification of 3,4-Dideoxyglucoson-3-ene (3,4-DGE) and Related Compounds. J. Agric. Food Chem. 2009, 57, 10837–10844. [Google Scholar] [CrossRef]
- Adams, C.J.; Manley-Harris, M.; Molan, P.C. The origin of methylglyoxal in New Zealand manuka (Leptospermum scoparium) honey. Carbohydr. Res. 2009, 344, 1050–1053. [Google Scholar] [CrossRef]
- Jojima, T.; Igari, T.; Gunji, W.; Suda, M.; Inui, M.; Yukawa, H. Identification of a HAD superfamily phosphatase, HdpA, involved in 1,3-dihydroxyacetone production during sugar catabolism in Corynebacterium glutamicum. FEBS Lett. 2012, 586, 4228–4232. [Google Scholar] [CrossRef] [Green Version]
- Shapla, U.M.; Solayman, M.; Alam, N.; Khalil, M.I.; Gan, S.H. 5-Hydroxymethylfurfural (HMF) levels in honey and other food products: Effects on bees and human health. Chem. Cent. J. 2018, 12, 35. [Google Scholar] [CrossRef]
- Yang, W.; Zhang, C.; Li, C.; Huang, Z.Y.; Miao, X. Pathway of 5-hydroxymethyl-2-furaldehyde formation in honey. J. Food Sci. Technol. 2019, 56, 2417–2425. [Google Scholar] [CrossRef] [PubMed]
- Fallico, B.; Zappalà, M.; Arena, E.; Verzera, A. Effects of conditioning on HMF content in unifloral honeys. Food Chem. 2004, 85, 305–313. [Google Scholar] [CrossRef]
- Khalil, M.; Sulaiman, S.; Gan, S. High 5-hydroxymethylfurfural concentrations are found in Malaysian honey samples stored for more than one year. Food Chem. Toxicol. 2010, 48, 2388–2392. [Google Scholar] [CrossRef]
- Islam, A.; Khalil, I.; Islam, N.; Moniruzzaman, M.; Mottalib, A.; Sulaiman, S.A.; Gan, S.H. Physicochemical and antioxidant properties of Bangladeshi honeys stored for more than one year. BMC Complement. Altern. Med. 2012, 12, 177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, I.; Singh, S. Honey moisture reduction and its quality. J. Food Sci. Technol. 2018, 55, 3861–3871. [Google Scholar] [CrossRef]
- Moniruzzaman, M.; Khalil, M.I.; Sulaiman, S.A.; Gan, S.H. Physicochemical and antioxidant properties of Malaysian honeys produced by Apis cerana, Apis dorsata and Apis mellifera. BMC Complement. Altern. Med. 2013, 13, 43. [Google Scholar] [CrossRef] [Green Version]
- Chou, W.-M.; Liao, H.-C.; Yang, Y.-C.; Peng, C.-C. Evaluation of Honey Quality with Stored Time and Temperatures. J. Food Nutr. Res. 2020, 8, 591–599. [Google Scholar] [CrossRef]
- ACS Network Chemistry Community Online. Bio-Based Platform Chemicals and Alternative Feedstocks. 2014. Available online: https://communities.acs.org/t5/GCI-Nexus-Blog/Bio-based-Platform-Chemicals-and-Alternative-Feedstocks/ba-p/15814 (accessed on 17 July 2022).
- Fallico, B.; Arena, E.; Zappala, M. Degradation of 5-Hydroxymethylfurfural in Honey. J. Food Sci. 2008, 73, C625–C631. [Google Scholar] [CrossRef]
- Khan, K.A.; Ghramh, H.A.; Babiker, M.; Ahmad, Z.; El-Niweiri, M.A.A.; Ibrahim, E.H.; Brima, E.I.; Mohammed, M.E.A. Tolerance of Ziziphus and Acacia honeys to one year storage conditions and altitude. J. King Saud Univ.-Sci. 2021, 33, 101577. [Google Scholar] [CrossRef]
- Zeng, X.; Bai, W.; Zhu, X.; Dong, H. Browning Intensity and Taste Change Analysis of Chicken Protein-Sugar Maillard Reaction System with Antioxidants and Different Drying Processes. J. Food Process. Preserv. 2016, 41, e13117. [Google Scholar] [CrossRef]
- Yan, S.; Wang, X.; Wu, Y.; Wang, K.; Shan, J.; Xue, X. A metabolomics approach revealed an Amadori compound distinguishes artificially heated and naturally matured acacia honey. Food Chem. 2022, 385, 132631. [Google Scholar] [CrossRef] [PubMed]
- Amaya-Farfan, J.; Rodriguez-Amaya, D.B. The Maillard reactions. In Chemical Changes during Processing and Storage of Foods; Rodriguez-Amaya, D.B., Amaya-Farfan, J., Eds.; Academic Press: Cambridge, MA, USA, 2021; pp. 215–263. [Google Scholar] [CrossRef]
- Kumar, N.; Raghavendra, M.; Tokas, J.; Singal, H.R. Flavor addition in dairy products. In Nutrients in Dairy and their Implications on Health and Disease; Watson, R.R., Collier, R.J., Preedy, V.R., Eds.; Academic Press: Cambridge, MA, USA, 2017; pp. 123–135. [Google Scholar] [CrossRef]
- Brudzynski, K.; Sjaarda, C.; Maldonado-Alvarez, L. A New Look on Protein-Polyphenol Complexation during Honey Storage: Is This a Random or Organized Event with the Help of Dirigent-Like Proteins? PLoS ONE 2013, 8, e72897. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rahardjo, M.; Sihombing, M.; Anggraeni, M.K. Color development and antioxidant activity in honey caramel. IOP Conf. Ser. Earth Environ. Sci. 2020, 443. [Google Scholar] [CrossRef]
- Paravisini, L.; Gourrat-Pernin, K.; Gouttefangeas, C.; Moretton, C.; Nigay, H.; Dacremont, C.; Guichard, E. Identification of compounds responsible for the odorant properties of aromatic caramel. Flavour Fragr. J. 2012, 27, 424–432. [Google Scholar] [CrossRef]
- Chappel, C.; Howell, J. Caramel colours—A historical introduction. Food Chem. Toxicol. 1992, 30, 351–357. [Google Scholar] [CrossRef]

| Reaction | Enzyme | Products | Biological Activity | Ref. | |
|---|---|---|---|---|---|
| 1 | Production of hydrogen peroxide | Glucose oxidase | Hydrogen peroxide and gluconic acid | Antibacterial and wound and burn dressings | [16,28,29] |
| 2 | Production of short peptides | Proteases | Short peptides | Antimicrobial, antioxidant, antitumor and weight loss inducers | [30,31,32,33,34,35] |
| 3 | Degradation of amylose | Diastase | Glucose and maltose | Honey quality parameter that indicates storage conditions | [1] |
| 4 | Degradation of sucrose | Invertase | Glucose and fructose | Indicator for honey storage conditions | [36] |
| 5 | Degradation of organic phosphates | Acid phosphatase | Inorganic phosphate | Marker of honey floral origin and indicator of honey fermentation | [37,38,39] |
| 6 | Trans-glycosylation | Non-enzymatic | Disaccharides Oligosacharides | Artificial sweeteners, increase bone mineral density in postmenopausal women (fructooligosaccharides), and classified as prebiotic molecules | [40,41,42,43] |
| 7 | Production and degradation of dicarbonyls | Enzymatic (dihydroxyacetone phosphatase) and non-enzymatic | Dicarbonyls (glyoxal, methylglyoxal and 3-deoxyglucosone), methional and methylbutanal, AGEs and nucleoside AGEs and melanoidins | 1-Antibacterial, antitumor, antioxidant and contribution to the honey color, flavor and odorant. 2-High level of dicarbonyl molecules are reported to be with some toxicity to the humans such as tumerigenic, negative impact on blood vessels and induction of diabtes and uremia | [44,45,46,47,48,49,50,51,52] |
| 8 | Production and degradation of HMF | Non-enzymatic | HMF, formic and levulinic acids, soluble HMF polymers and insoluble humins | Threatening honeybee life, human hepatorenal toxicity, induction of neoplastic changes, and irritation of mucous membranes HMF has positive impacts on human health, such as antioxidant, anti-carcinogen, anti-allergenic, and anti-hyperuricemic activities | [53,54,55,56,57,58,59,60] |
| 9 | Maillard reaction | Non-enzymatic | Complexes of sugars and amino acids, amino aldoses and ketoses, dicarbonyls, enediols, 2-amino-2-deoxy-ald-oses and melanoidins | Antibacterial and antioxidant | [14,61,62,63,64] |
| 10 | Caramelization | Non-enzymatic | Deoxyosones, furan and pyran derivatives, HMF, hydroxydimethylfuranone (HDF) and hydroxyacetilfuran (HAF) | Contribution to the color, aroma, and flavor of honey and antioxidants | [65,66,67,68] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alaerjani, W.M.A.; Abu-Melha, S.; Alshareef, R.M.H.; Al-Farhan, B.S.; Ghramh, H.A.; Al-Shehri, B.M.A.; Bajaber, M.A.; Khan, K.A.; Alrooqi, M.M.; Modawe, G.A.; et al. Biochemical Reactions and Their Biological Contributions in Honey. Molecules 2022, 27, 4719. https://doi.org/10.3390/molecules27154719
Alaerjani WMA, Abu-Melha S, Alshareef RMH, Al-Farhan BS, Ghramh HA, Al-Shehri BMA, Bajaber MA, Khan KA, Alrooqi MM, Modawe GA, et al. Biochemical Reactions and Their Biological Contributions in Honey. Molecules. 2022; 27(15):4719. https://doi.org/10.3390/molecules27154719
Chicago/Turabian StyleAlaerjani, Wed Mohammed Ali, Sraa Abu-Melha, Rahaf Mohammed Hussein Alshareef, Badriah Saad Al-Farhan, Hamed A. Ghramh, Badria Mohammed Abdallah Al-Shehri, Majed A. Bajaber, Khalid Ali Khan, Munira M. Alrooqi, Gad Allah Modawe, and et al. 2022. "Biochemical Reactions and Their Biological Contributions in Honey" Molecules 27, no. 15: 4719. https://doi.org/10.3390/molecules27154719
APA StyleAlaerjani, W. M. A., Abu-Melha, S., Alshareef, R. M. H., Al-Farhan, B. S., Ghramh, H. A., Al-Shehri, B. M. A., Bajaber, M. A., Khan, K. A., Alrooqi, M. M., Modawe, G. A., & Mohammed, M. E. A. (2022). Biochemical Reactions and Their Biological Contributions in Honey. Molecules, 27(15), 4719. https://doi.org/10.3390/molecules27154719











