Pharmacological Effects and Clinical Prospects of Cepharanthine
Abstract
:1. Introduction
2. Important Physicochemical Properties of Cepharanthine
3. Pharmacological Effects of Cepharanthine
3.1. Antiviral Effects
3.2. Prevention of Leukopenia
3.3. Antitumor Effects
3.4. Anti-Inflammatory Effects
3.5. Immunomodulation
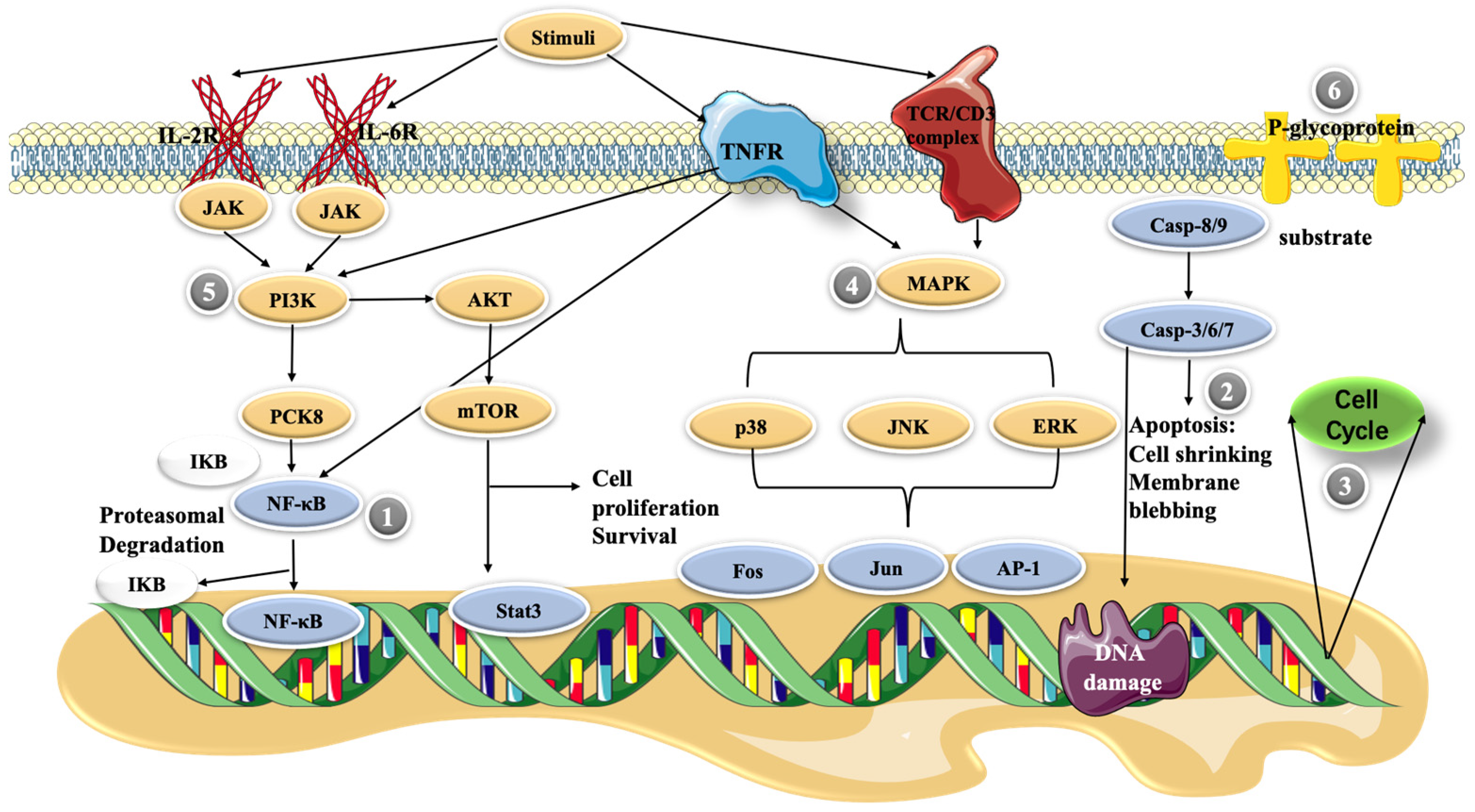
4. Molecular Mechanisms of Cepharanthine
4.1. NF-κB Pathway
4.2. Apoptosis
4.3. Cell Cycle Control
4.4. MAPK Pathway
4.5. PI3K/Akt/mTOR Signaling Pathway
4.6. P-glycoprotein Expression
5. New Dosage Forms of Cepharanthine
5.1. Oral Formulation
5.1.1. Oral Disintegrating Tablets
5.1.2. Dropping Pills
5.2. Injections
5.3. Pulmonary Drug Delivery Systems—DPIs
5.4. Nanoformulations
5.4.1. Liposomes
5.4.2. Nanoparticles
5.5. Summary
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rdel, C.O.; Kellogg, E.A.; Werff, H.V. Molecular phylogeny of the moonseed family (Menispermaceae): Implications for morphological diversification. Am. J. Bot. 2007, 94, 1425–1438. [Google Scholar]
- Thavamani, B.S.; Mathew, M.; Dhanabal, S.P. In vitro cytotoxic activity of menispermaceae plants against HeLa cell line. Anc. Sci. Life 2013, 33, 81–84. [Google Scholar] [PubMed]
- Kao, M.-C.; Yang, C.-H.; Chou, W.-C.; Sheu, J.-R.; Huang, C.-J. Cepharanthine mitigates lung injury in lower limb ischemia–reperfusion. J. Surg. Res. 2015, 199, 647–656. [Google Scholar] [CrossRef]
- Hifumi, T.; Yamamoto, A.; Morokuma, K.; Okada, I.; Kiriu, N.; Ogasawara, T.; Hasegawa, E.; Kato, H.; Inoue, J.; Koido, Y.; et al. Clinical Efficacy of Antivenom and Cepharanthine for the Treatment of Mamushi (Gloydius blomhoffii) Bites in Tertiary Care Centers in Japan. Jpn. J. Infect. Dis. 2013, 66, 26–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rogosnitzky, M.; Okediji, P.; Koman, I. Cepharanthine: A review of the antiviral potential of a Japanese-approved alopecia drug in COVID-19. Pharmacol. Rep. 2020, 72, 1509–1516. [Google Scholar] [CrossRef] [PubMed]
- Desgrouas, C.; Dormoi, J.; Chapus, C.; Ollivier, E.; Parzy, D.; Taudon, N. In vitro and in vivo combination of cepharanthine with anti-malarial drugs. Malar. J. 2014, 13, 90. [Google Scholar] [CrossRef] [Green Version]
- Bailly, C. Cepharanthine: An update of its mode of action, pharmacological properties and medical applications. Phytomedicine 2019, 62, 152956. [Google Scholar] [CrossRef]
- Fan, H.; He, S.T.; Han, P.; Hong, B.; Liu, K.; Li, M.; Wang, S.; Tong, Y. Cepharanthine: A Promising Old Drug against SARS-CoV-2. Adv. Biol. (Weinh.) 2022, e2200148. [Google Scholar] [CrossRef]
- Hong, L.; Guo, Z.; Huang, K.; Wei, S.; Liu, B.; Meng, S.; Long, C. Ethnobotanical study on medicinal plants used by Maonan people in China. J. Ethnobiol. Ethnomed. 2015, 11, 32. [Google Scholar] [CrossRef] [Green Version]
- Rogosnitzky, M.; Danks, R. Therapeutic potential of the biscoclaurine alkaloid, cepharanthine, for a range of clinical conditions. Pharmacol. Rep. 2011, 63, 337–347. [Google Scholar] [CrossRef]
- Kikukawa, Y.; Okuno, Y.; Tatetsu, H.; Nakamura, M.; Harada, N.; Ueno, S.; Kamizaki, Y.; Mitsuya, H.; Hata, H. Induction of cell cycle arrest and apoptosis in myeloma cells by cepharanthine, a biscoclaurine alkaloid. Int. J. Oncol. 2008, 33, 807–814. [Google Scholar]
- Yasuda, K.; Moro, M.; Akasu, M.; Ohnishi, A. Pharmacokinetic disposition of Cepharanthin following single and multiple intravenous doses in healthy subjects. Rinsho Yakuri/Jpn. J. Clin. Pharmacol. Ther. 1989, 20, 741–749. [Google Scholar] [CrossRef]
- He, C.L.; Huang, L.Y.; Wang, K.; Gu, C.J.; Hu, J.; Zhang, G.J.; Xu, W.; Xie, Y.H.; Tang, N.; Huang, A.L. Identification of bis-benzylisoquinoline alkaloids as SARS-CoV-2 entry inhibitors from a library of natural products. Signal Transduct. Target 2021, 6, 131. [Google Scholar] [CrossRef]
- Okamoto, M.; Ono, M.; Baba, M. Potent Inhibition of HIV Type 1 Replication by an Antiinflammatory Alkaloid, Cepharanthine, in Chronically Infected Monocytic Cells. AIDS Res. Hum. Retrovir. 1998, 14, 1239–1245. [Google Scholar] [CrossRef]
- Matsuda, K.; Hattori, S.; Komizu, Y.; Kariya, R.; Ueoka, R.; Okada, S. Cepharanthine inhibited HIV-1 cell–cell transmission and cell-free infection via modification of cell membrane fluidity. Bioorg. Med. Chem. Lett. 2014, 24, 2115–2117. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.-B.; Wang, Y.-F.; Zhang, Y.; Zheng, L.-Y.; Yang, X.-A.; Wang, N.; Jiang, J.-H.; Ma, F.; Yin, D.-T.; Sun, C.-Y.; et al. In vitro activity of cepharanthine hydrochloride against clinical wild-type and lamivudine-resistant hepatitis B virus isolates. Eur. J. Pharmacol. 2012, 683, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Tang, Q.; Rao, Z.; Fang, Y.; Jiang, X.; Liu, W.; Luan, F.; Zeng, N. Inhibition of herpes simplex virus 1 by cepharanthine via promoting cellular autophagy through up-regulation of STING/TBK1/P62 pathway. Antivir. Res. 2021, 193, 105143. [Google Scholar] [CrossRef] [PubMed]
- Toyama, M.; Hamasaki, T.; Uto, T.; Aoyama, H.; Okamoto, M.; Hashmoto, Y.; Baba, M. Synergistic inhibition of HTLV-1-infected cell proliferation by combination of cepharanthine and a tetramethylnaphthalene derivative. Anticancer Res. 2012, 32, 2639–2645. [Google Scholar]
- Guo, G.; Ye, L.; Pan, K.; Chen, Y.; Xing, D.; Yan, K.; Chen, Z.; Ding, N.; Li, W.; Huang, H.; et al. New Insights of Emerging SARS-CoV-2: Epidemiology, Etiology, Clinical Features, Clinical Treatment, and Prevention. Front. Cell Dev. Biol. 2020, 8, 410. [Google Scholar] [CrossRef]
- Beigel, J.H.; Tomashek, K.M.; Dodd, L.E.; Mehta, A.K.; Zingman, B.S.; Kalil, A.C.; Hohmann, E.; Chu, H.Y.; Luetkemeyer, A.; Kline, S.; et al. Remdesivir for the Treatment of COVID-19—Preliminary report. N. Engl. J. Med. 2020, 383, 1813–1826. [Google Scholar] [CrossRef]
- Cao, B.; Wang, Y.; Wen, D.; Liu, W.; Wang, J.; Fan, G.; Ruan, L.; Song, B.; Cai, Y.; Wei, M.; et al. A Trial of Lopinavir–Ritonavir in Adults Hospitalized with Severe COVID-19. N. Engl. J. Med. 2020, 382, 1787–1799. [Google Scholar] [CrossRef] [PubMed]
- Boulware, D.R.; Pullen, M.F.; Bangdiwala, A.S.; Pastick, K.A.; Lofgren, S.M.; Okafor, E.C.; Skipper, C.P.; Nascene, A.A.; Nicol, M.R.; Abassi, M.; et al. A Randomized Trial of Hydroxychloroquine as Postexposure Prophylaxis for Covid-19. N. Engl. J. Med. 2020, 383, 517–525. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.; Hu, S.; Gao, J. Discovering drugs to treat coronavirus disease 2019 (COVID-19). Drug Discov. Ther. 2020, 14, 58–60. [Google Scholar] [CrossRef]
- Fan, H.; Liu, K.; Hong, B.; He, S.; Han, P.; Li, M.; Wang, S.; Tong, Y. Progress in the study of antiviral activity of cepharanthine against SARS-CoV-2. Nan Fang Yi Ke Da Xue Xue Bao 2022, 42, 955–956. [Google Scholar] [PubMed]
- Ohashi, H.; Watashi, K.; Saso, W.; Shionoya, K.; Iwanami, S.; Hirokawa, T.; Shirai, T.; Kanaya, S.; Ito, Y.; Kim, K.S.; et al. Multidrug treatment with nelfinavir and cepharanthine against COVID-19. bioRxiv 2020, 039925. [Google Scholar] [CrossRef] [Green Version]
- White, M.A.; Lin, W.; Cheng, X. Discovery of COVID-19 Inhibitors Targeting the SARS-CoV-2 Nsp13 Helicase. bioRxiv 2020, 243246. [Google Scholar] [CrossRef] [PubMed]
- Ohashi, H.; Watashi, K.; Saso, W.; Shionoya, K.; Iwanami, S.; Hirokawa, T.; Shirai, T.; Kanaya, S.; Ito, Y.; Kim, K.S.; et al. Potential anti-COVID-19 agents, cepharanthine and nelfinavir, and their usage for combination treatment. Iscience 2021, 24, 102367. [Google Scholar] [CrossRef]
- Li, S.; Liu, W.; Chen, Y.; Wang, L.; An, W.; An, X.; Song, L.; Tong, Y.; Fan, H.; Lu, C. Transcriptome analysis of cepharanthine against a SARS-CoV-2-related coronavirus. Brief Bioinf. 2021, 22, 1378–1386. [Google Scholar] [CrossRef]
- Drayman, N.; De Marco, J.K.; Jones, K.A.; Azizi, S.A.; Froggatt, H.M.; Tan, K.; Maltseva, N.I.; Chen, S.; Nicolaescu, V.; Dvorkin, S.; et al. Masitinib is a broad coronavirus 3CL inhibitor that blocks replication of SARS-CoV-2. Science 2021, 373, 931–936. [Google Scholar] [CrossRef]
- Oyaizu, H.; Adachi, Y.; Yasumizu, R.; Ono, M.; Ikebukuro, K.; Fukuhara, S.; Ikehara, S. Protection of T cells from radiation-induced apoptosis by Cepharanthin®. Int. Immunopharmacol. 2001, 1, 2091–2099. [Google Scholar] [CrossRef]
- Terasaki, M.; Abe, T.; Miyagi, N.; Ogo, E.; Shigemori, M. Feasibility and response to 1-(4-amino-2-methyl-5-pyrimidynyl) methyl-3-(2-chloroethyl)-3-nitrosourea hydrochloride chemotherapy with pre-treated procarbazine for elderly patients with newly diagnosed glioblastoma. J. Neuro-Oncol. 2007, 81, 265–269. [Google Scholar] [CrossRef] [PubMed]
- Ito, H.; Ito, H.; Amano, H.; Noda, H. Inhibitory effect of a biscoclaurine alkaloid, cepharanthin, on lung metastasis of Lewis lung carcinoma. Jpn. J. Pharmacol. 1991, 56, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Suzuki, H.; Zhou, Y.-W.; Liu, W.; Yoshihara, M.; Kato, M.; Akhand, A.A.; Hayakawa, A.; Takeuchi, K.; Hossain, K.; et al. Cepharanthine activates caspases and induces apoptosis in Jurkat and K562 human leukemia cell lines. J. Cell. Biochem. 2001, 82, 200–214. [Google Scholar] [CrossRef] [PubMed]
- Tamatani, A.M.; Motegi, K.; Takamaru, N.; Kawashima, Y.; Bando, T. Cepharanthin-enhanced radiosensitivity through the inhibition of radiation-induced nuclear factor-kappaB activity in human oral squamous cell carcinoma cells. Int. J. Oncol. 2007, 31, 761–768. [Google Scholar]
- Paradowska-Gorycka, A.; Grzybowska-Kowalczyk, A.; Wojtecka-Lukasik, E.; Maslinski, S. IL-23 in the Pathogenesis of Rheumatoid Arthritis. Scand. J. Immunol. 2010, 71, 134–145. [Google Scholar] [CrossRef]
- Zhou, Y.; Hopper-Borge, E.; Shen, T.; Huang, X.-C.; Shi, Z.; Kuang, Y.-H.; Furukawa, T.; Akiyama, S.-I.; Peng, X.-X.; Ashby, C.R.; et al. Cepharanthine is a potent reversal agent for MRP7(ABCC10)-mediated multidrug resistance. Biochem. Pharmacol. 2009, 77, 993–1001. [Google Scholar] [CrossRef]
- Kathawala, R.J.; Wang, Y.-J.; Ashby, C.R., Jr.; Chen, Z.-S. Recent advances regarding the role of ABC subfamily C member 10 (ABCC10) in the efflux of antitumor drugs. Chin. J. Cancer 2014, 33, 223–230. [Google Scholar] [CrossRef] [Green Version]
- Huang, C.Z.; Wang, Y.F.; Zhang, Y.; Peng, Y.M.; Liu, Y.X.; Ma, F.; Jiang, J.H.; Wang, Q.D. Cepharanthine hydrochloride reverses P-glycoprotein-mediated multidrug resistance in human ovarian carcinoma A2780/Taxol cells by inhibiting the PI3K/Akt signaling pathway. Oncol. Rep. 2017, 38, 2558–2564. [Google Scholar] [CrossRef] [Green Version]
- Kadioglu, O.; Law, B.Y.K.; Mok, S.W.F.; Xu, S.-W.; Efferth, T.; Wong, V.K.W. Mode of Action Analyses of Neferine, a Bisbenzylisoquinoline Alkaloid of Lotus (Nelumbo nucifera) against Multidrug-Resistant Tumor Cells. Front. Pharmacol. 2017, 8, 238. [Google Scholar] [CrossRef] [Green Version]
- Okada, K.; Sakusabe, N.; Kobayashi, A.; Hoshi, N.; Sato, K. Prevention of Lung Metastasis by Intra-tumoral Injection of Cepharanthin and Staphylococcal Enterotoxin B in Transplantable Rat Osteosarcoma. Jpn. J. Cancer Res. 1999, 90, 928–934. [Google Scholar] [CrossRef]
- Ershun, Z.; Yunhe, F.; Zhengkai, W.; Yongguo, C.; Naisheng, Z.; Zhengtao, Y. Cepharanthine attenuates lipopolysaccharide-induced mice mastitis by suppressing the NF-kappaB signaling pathway. Inflammation 2014, 37, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Morikawa, K.; Oseko, F.; Morikawa, S. Inhibition of proliferation and differentiation of human B-lymphocytes by a biscoclaurine alkaloid. Int. J. Immunopharmacol. 1992, 14, 941–949. [Google Scholar] [CrossRef] [PubMed]
- Tabata, R.; Tabata, C.; Tazoh, A.; Nagai, T. Low dose cepharanthine ameliorates immune thrombocytopenic purpura associated with multiple myeloma. Int. Immunopharmacol. 2012, 13, 242–244. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.-L.; Hirano, T.; Tanaka, S.; Onda, K.; Oka, K. Persistent reversal of P-glycoprotein-mediated daunorubicin resistance by tetrandrine in multidrug-resistant human T lymphoblastoid leukemia MOLT-4 cells. J. Pharm. Pharmacol. 2003, 55, 1531–1537. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Wang, X.; Tu, Y.; Masaki, H.; Tanaka, S.; Onda, K.; Sugiyama, K.; Yamada, H.; Hirano, T. Tetrandrine and cepharanthine induce apoptosis through caspase cascade regulation, cell cycle arrest, MAPK activation and PI3K/Akt/mTOR signal modification in glucocorticoid resistant human leukemia Jurkat T cells. Chem. Interact. 2019, 310, 108726. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Chen, S.; Wang, X.; Tanaka, S.; Onda, K.; Sugiyama, K.; Yamada, H.; Hirano, T. Molecular mechanisms and therapeutic implications of tetrandrine and cepharanthine in T cell acute lymphoblastic leukemia and autoimmune diseases. Pharmacol. Ther. 2020, 217, 107659. [Google Scholar] [CrossRef]
- Huang, H.; Hu, G.; Wang, C.; Xu, H.; Chen, X.; Qian, A. Cepharanthine, an Alkaloid from Stephania cepharantha Hayata, Inhibits the Inflammatory Response in the RAW264.7 Cell and Mouse Models. Inflammation 2014, 37, 235–246. [Google Scholar] [CrossRef]
- Samra, Y.A.; Said, H.S.; Elsherbiny, N.M.; Liou, G.I.; El-Shishtawy, M.M.; Eissa, L.A. Cepharanthine and Piperine ameliorate diabetic nephropathy in rats: Role of NF-kappaB and NLRP3 inflammasome. Life Sci. 2016, 157, 187–199. [Google Scholar] [CrossRef]
- Golan-Goldhirsh, A.; Gopas, J. Plant derived inhibitors of NF-κB. Phytochem. Rev. 2013, 13, 107–121. [Google Scholar] [CrossRef]
- Gerschenson, L.E.; Rotello, R.J. Apoptosis: A different type of cell death. FASEB J. 1992, 6, 2450–2455. [Google Scholar] [CrossRef]
- Degterev, A.; Yuan, J. Expansion and evolution of cell death programmes. Nat. Rev. Mol. Cell Biol. 2008, 9, 378–390. [Google Scholar] [CrossRef] [PubMed]
- Kurokawa, M.; Kornbluth, S. Caspases and Kinases in a Death Grip. Cell 2009, 138, 838–854. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uto, T.; Toyama, M.; Yoshinaga, K.; Baba, M. Cepharanthine induces apoptosis through the mitochondria/caspase pathway in murine dendritic cells. Immunopharmacol. Immunotoxicol. 2016, 38, 238–243. [Google Scholar] [CrossRef] [PubMed]
- Vermeulen, K.; Van Bockstaele, D.R.; Berneman, Z.N. The cell cycle: A review of regulation, deregulation and therapeutic targets in cancer. Cell Prolif. 2003, 36, 131–149. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Zhang, R.; Wang, Y.; Xu, D.; Zhang, L.; Qin, J.; Su, G.; Feng, Y.; Chen, H.; You, S.; et al. Correction: Cepharanthine hydrochloride reverses the mdr1 (P-glycoprotein)-mediated esophageal squamous cell carcinoma cell cisplatin resistance through JNK and p53 signals. Oncotarget 2021, 12, 61–62. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Flies, D.B. Molecular mechanisms of T cell co-stimulation and co-inhibition. Nat. Rev. Immunol. 2013, 13, 227–242. [Google Scholar] [CrossRef]
- Xu, W.; Meng, K.; Tu, Y.; Tanaka, S.; Onda, K.; Sugiyama, K.; Hirano, T.; Yamada, H. Tetrandrine potentiates the glucocorticoid pharmacodynamics via inhibiting P-glycoprotein and mitogen-activated protein kinase in mitogen-activated human peripheral blood mononuclear cells. Eur. J. Pharmacol. 2017, 807, 102–108. [Google Scholar] [CrossRef]
- Durinck, K.; Goossens, S.; Peirs, S.; Wallaert, A.; Van Loocke, W.; Matthijssens, F.; Pieters, T.; Milani, G.; Lammens, T.; Rondou, P.; et al. Novel biological insights in T-cell acute lymphoblastic leukemia. Exp. Hematol. 2015, 43, 625–639. [Google Scholar] [CrossRef]
- Tewari, D.; Patni, P.; Bishayee, A.; Sah, A.N.; Bishayee, A. Natural products targeting the PI3K-Akt-mTOR signaling pathway in cancer: A novel therapeutic strategy. Semin. Cancer Biol. 2022, 80, 1–17. [Google Scholar] [CrossRef]
- Polivka, J., Jr.; Janku, F. Molecular targets for cancer therapy in the PI3K/AKT/mTOR pathway. Pharmacol. Ther. 2014, 142, 164–175. [Google Scholar] [CrossRef]
- Takahashi-Yanaga, F.; Sasaguri, T. GSK-3beta regulates cyclin D1 expression: A new target for chemotherapy. Cell Signal 2008, 20, 581–589. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Li, X.; Ding, X.; Qi, W.; Yang, Q. Cepharanthine Induces Autophagy, Apoptosis and Cell Cycle Arrest in Breast Cancer Cells. Cell. Physiol. Biochem. 2017, 41, 1633–1648. [Google Scholar] [CrossRef] [PubMed]
- Faes, S.; Dormond, O. PI3K and AKT: Unfaithful Partners in Cancer. Int. J. Mol. Sci. 2015, 16, 21138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahajan, K.; Mahajan, N.P. PI3K-independent AKT activation in cancers: A treasure trove for novel therapeutics. J. Cell. Physiol. 2012, 227, 3178–3184. [Google Scholar] [CrossRef] [PubMed]
- Levatić, J.; Ćurak, J.; Kralj, M.; Šmuc, T.; Osmak, M.; Supek, F. Accurate Models for P-gp Drug Recognition Induced from a Cancer Cell Line Cytotoxicity Screen. J. Med. Chem. 2013, 56, 5691–5708. [Google Scholar] [CrossRef]
- Genovese, I.; Ilari, A.; Assaraf, Y.G.; Fazi, F.; Colotti, G. Not only P-glycoprotein: Amplification of the ABCB1-containing chromosome region 7q21 confers multidrug resistance upon cancer cells by coordinated overexpression of an assortment of resistance-related proteins. Drug Resist. Updat. 2017, 32, 23–46. [Google Scholar] [CrossRef]
- Li, H.; Yan, Z.; Ning, W.; Xiao-Juan, G.; Cai-Hong, Z.; Jin-Hua, J.; Fang, M.; Qing-Duan, W. Using Rhodamine 123 Accumulation in CD8+ Cells as a Surrogate Indicator to Study the P-Glycoprotein Modulating Effect of Cepharanthine Hydrochloride In Vivo. J. Biomed. Biotechnol. 2011, 2011, 281651. [Google Scholar] [CrossRef] [Green Version]
- Xu, W.; Wang, X.; Chen, S.; Wu, H.; Tanaka, S.; Onda, K.; Sugiyama, K.; Yamada, H.; Hirano, T. Tetrandrine enhances glucocorticoid receptor translocation possibly via inhibition of P-glycoprotein in daunorubicin-resistant human T lymphoblastoid leukemia cells. Eur. J. Pharmacol. 2020, 881, 173232. [Google Scholar] [CrossRef]
- Vanbillemont, B.; De Beer, T. Application of polyvinyl acetate in an innovative formulation strategy for lyophilized orally disintegrating tablets. Int. J. Pharm. 2020, 588, 119717. [Google Scholar] [CrossRef]
- Cantor, S.L.; Khan, M.A.; Gupta, A. Development and optimization of taste-masked orally disintegrating tablets (ODTs) of clindamycin hydrochloride. Drug Dev. Ind. Pharm. 2015, 41, 1156–1164. [Google Scholar] [CrossRef]
- Yıldız, S.; Aytekin, E.; Yavuz, B.; Pehlivan, S.B.; Vural, I.; Ünlü, N. Development and evaluation of orally disintegrating tablets comprising taste-masked mirtazapine granules. Pharm. Dev. Technol. 2018, 23, 488–495. [Google Scholar] [CrossRef]
- Comoglu, T.; Ozyilmaz, E.D. Orally disintegrating tablets and orally disintegrating mini tablets-novel dosage forms for pediatric use. Pharm. Dev. Technol. 2019, 24, 902–914. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, A.A.; Salah, S. Fast relief from migraine attacks using fast-disintegrating sublingual zolmitriptan tablets. Drug Dev. Ind. Pharm. 2012, 38, 762–769. [Google Scholar] [CrossRef]
- Song, H.; Moon, C.; Lee, B.-J.; Oh, E. Mesoporous Pravastatin Solid Dispersion Granules Incorporable into Orally Disintegrating Tablets. J. Pharm. Sci. 2018, 107, 1886–1895. [Google Scholar] [CrossRef] [PubMed]
- Shanmugam, S. Granulation techniques and technologies: Recent progresses. BioImpacts 2015, 5, 55–63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gryczke, A.; Schminke, S.; Maniruzzaman, M.; Beck, J.; Douroumis, D. Development and evaluation of orally disintegrating tablets (ODTs) containing Ibuprofen granules prepared by hot melt extrusion. Colloids Surfaces B Biointerfaces 2011, 86, 275–284. [Google Scholar] [CrossRef] [PubMed]
- Badgujar, B.; Mundada, A. The technologies used for developing orally disintegrating tablets: A review. Acta Pharm. 2011, 61, 117–139. [Google Scholar] [CrossRef] [PubMed]
- Kande, K.V.; Kotak, D.J.; Degani, M.S.; Kirsanov, D.; Legin, A.; Devarajan, P.V. Microwave-Assisted Development of Orally Disintegrating Tablets by Direct Compression. AAPS PharmSciTech 2017, 18, 2055–2066. [Google Scholar] [CrossRef]
- Hannan, P.; Khan, J.; Safiullah, S. Oral dispersible system: A new approach in drug delivery system. Indian J. Pharm. Sci. 2016, 78, 2–7. [Google Scholar] [CrossRef] [Green Version]
- Khaled, S.A.; Alexander, M.R.; Wildman, R.D.; Wallace, M.J.; Sharpe, S.; Yoo, J.; Roberts, C.J. 3D extrusion printing of high drug loading immediate release paracetamol tablets. Int. J. Pharm. 2018, 538, 223–230. [Google Scholar] [CrossRef]
- Li, G.F. Research progress on dosage form and clinical application of drop pills. West China Pharm. J. 2020, 35, 579–583. [Google Scholar]
- Hu, L.; Shi, Y.; Li, J.H.; Gao, N.; Ji, J.; Niu, F.; Chen, Q.; Yang, X.; Wang, S. Enhancement of Oral Bioavailability of Curcumin by a Novel Solid Dispersion System. AAPS PharmSciTech 2015, 16, 1327–1334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vo, C.L.-N.; Park, C.; Lee, B.-J. Current trends and future perspectives of solid dispersions containing poorly water-soluble drugs. Eur. J. Pharm. Biopharm. 2013, 85, 799–813. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhao, J. Comparison of the dissolution rate of cepharanthine tablets and Qianjintiansu dropping pills. J. Jinggangshan Univ. 2009, 30, 83–84, 88. [Google Scholar]
- Goldberg, M.; Gomez-Orellana, I. Challenges for the oral delivery of macromolecules. Nat. Rev. Drug Discov. 2003, 2, 289–295. [Google Scholar] [CrossRef]
- Chaudhary, K.; Patel, M.M.; Mehta, P.J. Long-Acting Injectables: Current Perspectives and Future Promise. Crit. Rev. Ther. Drug Carr. Syst. 2019, 36, 137–181. [Google Scholar] [CrossRef]
- Ma, Q.; Xie, Y.; Wang, Z.; Lei, B.; Chen, R.; Liu, B.; Jiang, H.; Wang, Y.; Liu, Q.; Yang, Z. Efficacy and safety of ReDuNing injection as a treatment for COVID-19 and its inhibitory effect against SARS-CoV-2. J. Ethnopharmacol. 2021, 279, 114367. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Lv, L.; Zhou, Y.L.; Xie, L.D.; Xu, Q.; Zou, X.F.; Ding, Y.; Tian, J.; Fan, J.L.; Fan, H.W.; et al. Efficacy and safety of Xiyanping injection in the treatment of COVID-19: A multicenter, prospective, open-label and randomized controlled trial. Phytother. Res. 2021, 35, 4401–4410. [Google Scholar] [CrossRef]
- Xing, Y.; Hua, Y.-R.; Shang, J.; Ge, W.-H.; Liao, J. Traditional Chinese medicine network pharmacology study on exploring the mechanism of Xuebijing Injection in the treatment of coronavirus disease 2019. Chin. J. Nat. Med. 2020, 18, 941–951. [Google Scholar] [CrossRef]
- Kim, Y.-K.; Xing, L.; Chen, B.-A.; Xu, F.; Jiang, H.-L.; Zhang, C. Aerosol delivery of programmed cell death protein 4 using polysorbitol-based gene delivery system for lung cancer therapy. J. Drug Target. 2014, 22, 829–838. [Google Scholar] [CrossRef]
- Kaur, S.S. Pulmonary drug delivery system: Newer patents. Pharm. Pat. Anal. 2017, 6, 225–244. [Google Scholar] [CrossRef] [PubMed]
- Muralidharan, P.; Malapit, M.; Mallory, E.; Hayes, D.; Mansour, H.M. Inhalable nanoparticulate powders for respiratory delivery. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 1189–1199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bharti, N.; Kumar, S.L.H.; Budhiraja, A. Pulmonary drug delivery as a vital route for delivering nanoparticles e a review. World J. Pharm. Pharm. Sci. 2012, 2, 4037–4060. [Google Scholar] [CrossRef]
- Buttini, F.; Colombo, P.; Rossi, A.; Sonvico, F.; Colombo, G. Particles and powders: Tools of innovation for non-invasive drug administration. J. Control. Release 2012, 161, 693–702. [Google Scholar] [CrossRef]
- Nisini, R.; Poerio, N.; Mariotti, S.; De Santis, F.; Fraziano, M. The Multirole of Liposomes in Therapy and Prevention of Infectious Diseases. Front. Immunol. 2018, 9, 155. [Google Scholar] [CrossRef] [Green Version]
- Guimarães, D.; Cavaco-Paulo, A.; Nogueira, E. Design of liposomes as drug delivery system for therapeutic applications. Int. J. Pharm. 2021, 601, 120571. [Google Scholar] [CrossRef]
- D’Mello, S.R.; Cruz, C.N.; Chen, M.-L.; Kapoor, M.; Lee, S.L.; Tyner, K.M. The evolving landscape of drug products containing nanomaterials in the United States. Nat. Nanotechnol. 2017, 12, 523–529. [Google Scholar] [CrossRef]
- Hoshyar, N.; Gray, S.; Han, H.; Bao, G. The effect of nanoparticle size on in vivo pharmacokinetics and cellular interaction. Nanomedicine 2016, 11, 673–692. [Google Scholar] [CrossRef] [Green Version]
- Pandey, R.; Khuller, G.K. Antitubercular inhaled therapy: Opportunities, progress and challenges. J. Antimicrob. Chemother. 2005, 55, 430–435. [Google Scholar] [CrossRef]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 2021, 20, 101–124. [Google Scholar] [CrossRef]
- Al-Hallak, M.K.; Sarfraz, M.K.; Azarmi, S.; Roa, W.H.; Finlay, W.H.; Löbenberg, R. Pulmonary delivery of inhalable nanoparticles: Dry powder inhalers. Ther. Deliv. 2011, 2, 1313–1324. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Hardie, J.; Zhang, X.; Rotello, V.M. Effects of engineered nanoparticles on the innate immune system. Semin. Immunol. 2017, 34, 25–32. [Google Scholar] [CrossRef] [PubMed]
- de Braganca, L.; Ferguson, G.J.; Santos, J.L.; Derrick, J.P. Adverse immunological responses against non-viral nanoparticle (NP) delivery systems in the lung. J. Immunotoxicol. 2021, 18, 61–73. [Google Scholar] [CrossRef] [PubMed]
- Thorp, E.B.; Boada, C.; Jarbath, C.; Luo, X. Nanoparticle Platforms for Antigen-Specific Immune Tolerance. Front. Immunol. 2020, 11, 945. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, W.; Li, Y.; Chang, J.; Tian, F.; Zhao, F.; Ma, Y.; Sun, J. Microfluidic Sonication to Assemble Exosome Membrane-Coated Nanoparticles for Immune Evasion-Mediated Targeting. Nano Lett. 2019, 19, 7836–7844. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Zheng, J.; Ding, Y.; Meng, Y.; Tan, F.; Gong, W.; Chu, X.; Kong, X.; Gao, C. Cepharanthine loaded nanoparticles coated with macrophage membranes for lung inflammation therapy. Drug Deliv. 2021, 28, 2582–2593. [Google Scholar] [CrossRef] [PubMed]
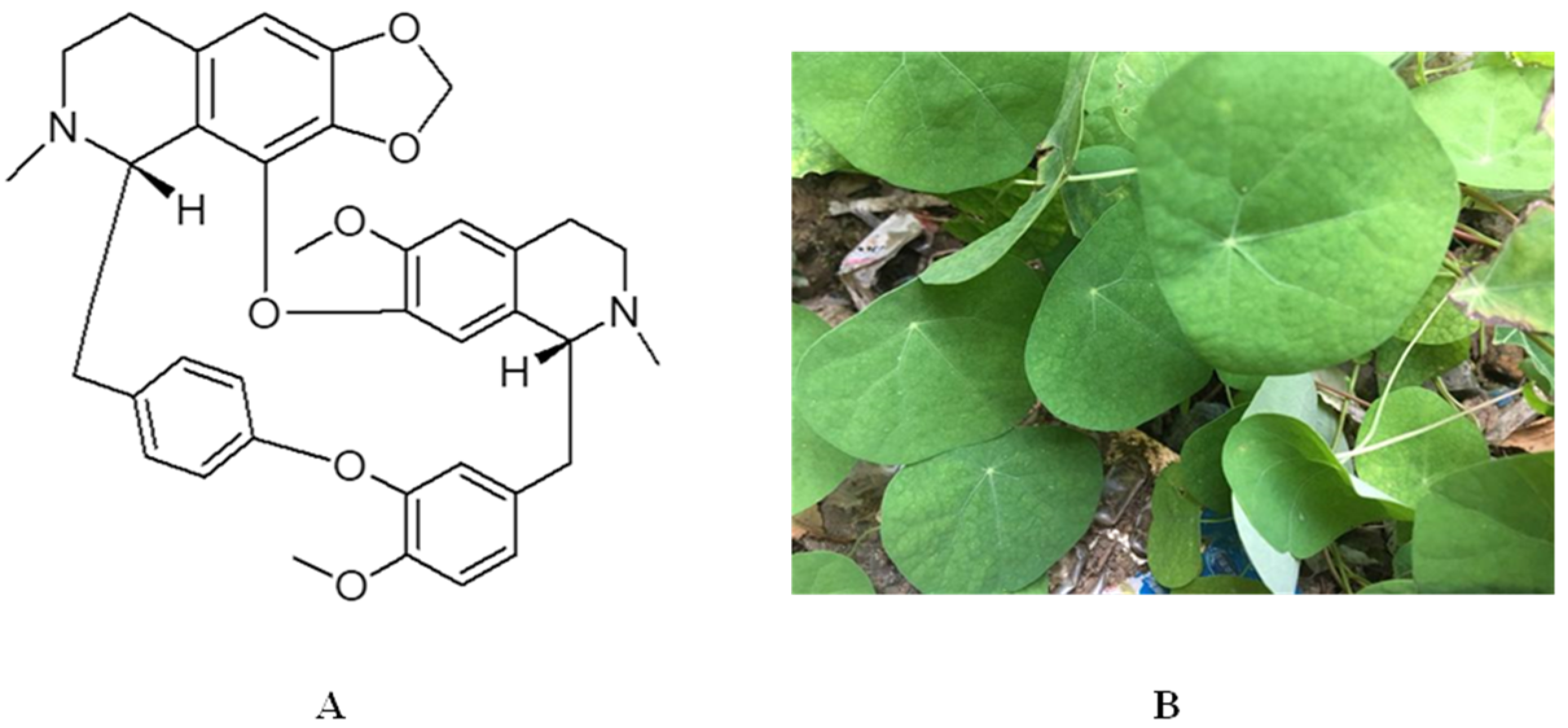
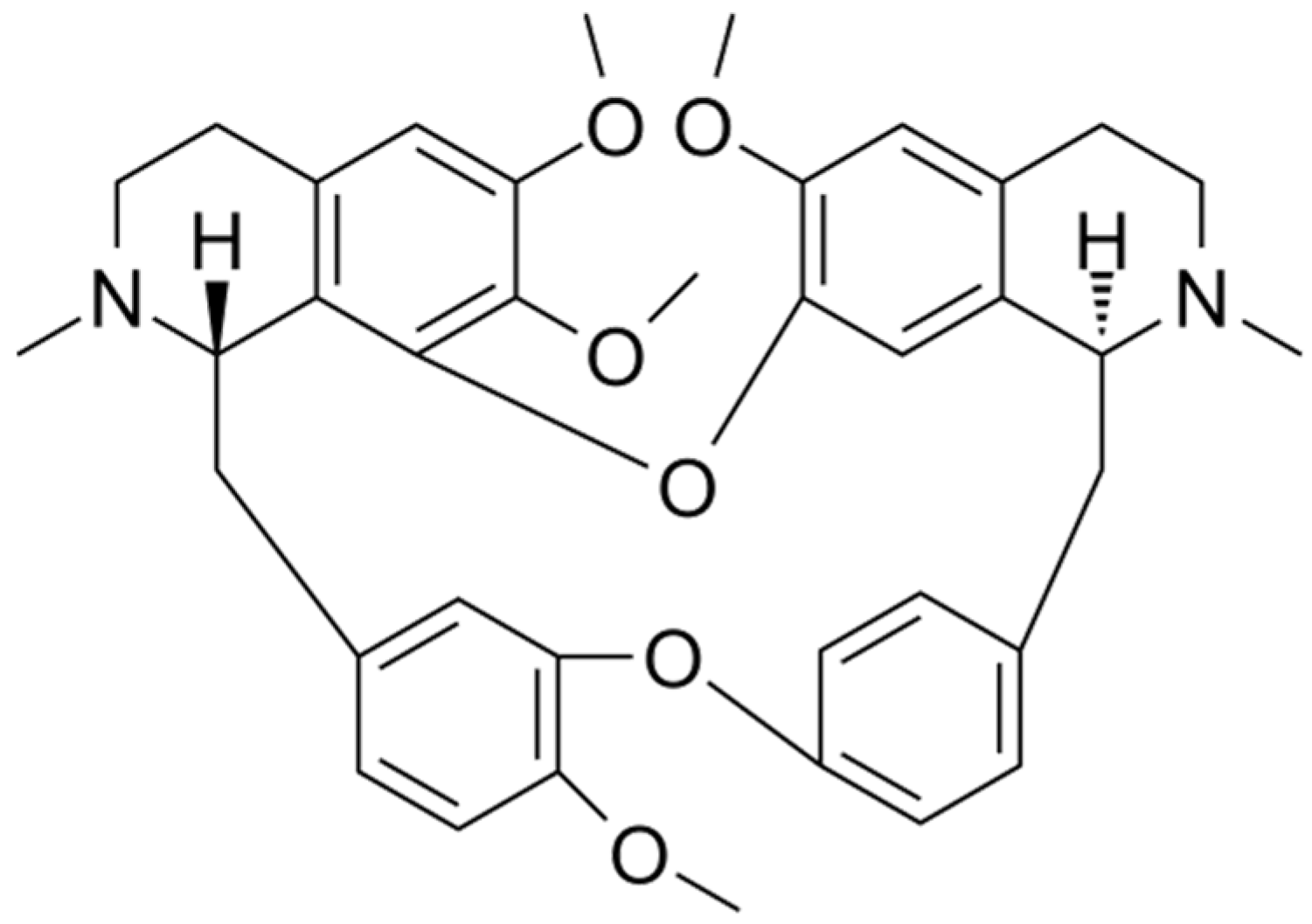
| Drugs | Conventional Use | Dosage | Administration Routes |
|---|---|---|---|
| Interferon-α | Hepatitis, pneumonia | 5 million U (2 mL of sterile water for injection) each time, 2 times/day | Vapor inhalation, intramuscular injection |
| Lopinavir–ritonavir | Human immunodeficiency virus | 400 mg/100 mg each time, 2 times/day | Oral |
| Ribavirin | Viral infection | 500 mg each time, 2–3 times/day in combination with IFN-α or lopinavir/ritonavir | Intravenous infusion |
| Chloroquine phosphate | Malaria | 500 mg (300 mg for chloroquine) each time, 2 times/day | Oral |
| Arbidol | Infection of the upper respiratory tract | 200 mg each time, 3 times/day | Oral |
| Favipiravir | Flu virus | On day 1, 800 mg each time; on days 2–5, 300 mg each time, 2 times/day | Oral |
| Remdesivir | Respiratory virus, hepatitis C | On day 1, 200 mg/day; on days 2–9, 100 mg/day | Intravenous infusion |
| Property | Mechanism of Action | References |
|---|---|---|
| Antiviral effects |
| [15] |
| [16,17,18] | |
| [13,26,27] | |
| Prevention of leukopenia |
| [30] |
| Antitumor effects |
| [32,33,35,52] |
| Anti-inflammatory effects |
| [41] |
| Immunomodulation |
| [43,46] |
| Targets | Detailed Molecular Mechanisms |
|---|---|
| NF-κB |
|
| Apoptosis |
|
| Cell cycle control |
|
| MAPK |
|
| PI3K/Akt/mTOR |
|
| P-glycoprotein |
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, D.; Li, Q.; Du, L.; Dou, G. Pharmacological Effects and Clinical Prospects of Cepharanthine. Molecules 2022, 27, 8933. https://doi.org/10.3390/molecules27248933
Liang D, Li Q, Du L, Dou G. Pharmacological Effects and Clinical Prospects of Cepharanthine. Molecules. 2022; 27(24):8933. https://doi.org/10.3390/molecules27248933
Chicago/Turabian StyleLiang, Di, Qi Li, Lina Du, and Guifang Dou. 2022. "Pharmacological Effects and Clinical Prospects of Cepharanthine" Molecules 27, no. 24: 8933. https://doi.org/10.3390/molecules27248933
APA StyleLiang, D., Li, Q., Du, L., & Dou, G. (2022). Pharmacological Effects and Clinical Prospects of Cepharanthine. Molecules, 27(24), 8933. https://doi.org/10.3390/molecules27248933






