Chemical Constituents and Anti-Angiogenic Principles from a Marine Algicolous Penicillium sumatraense SC29
Abstract
:1. Introduction
2. Results and Discussion
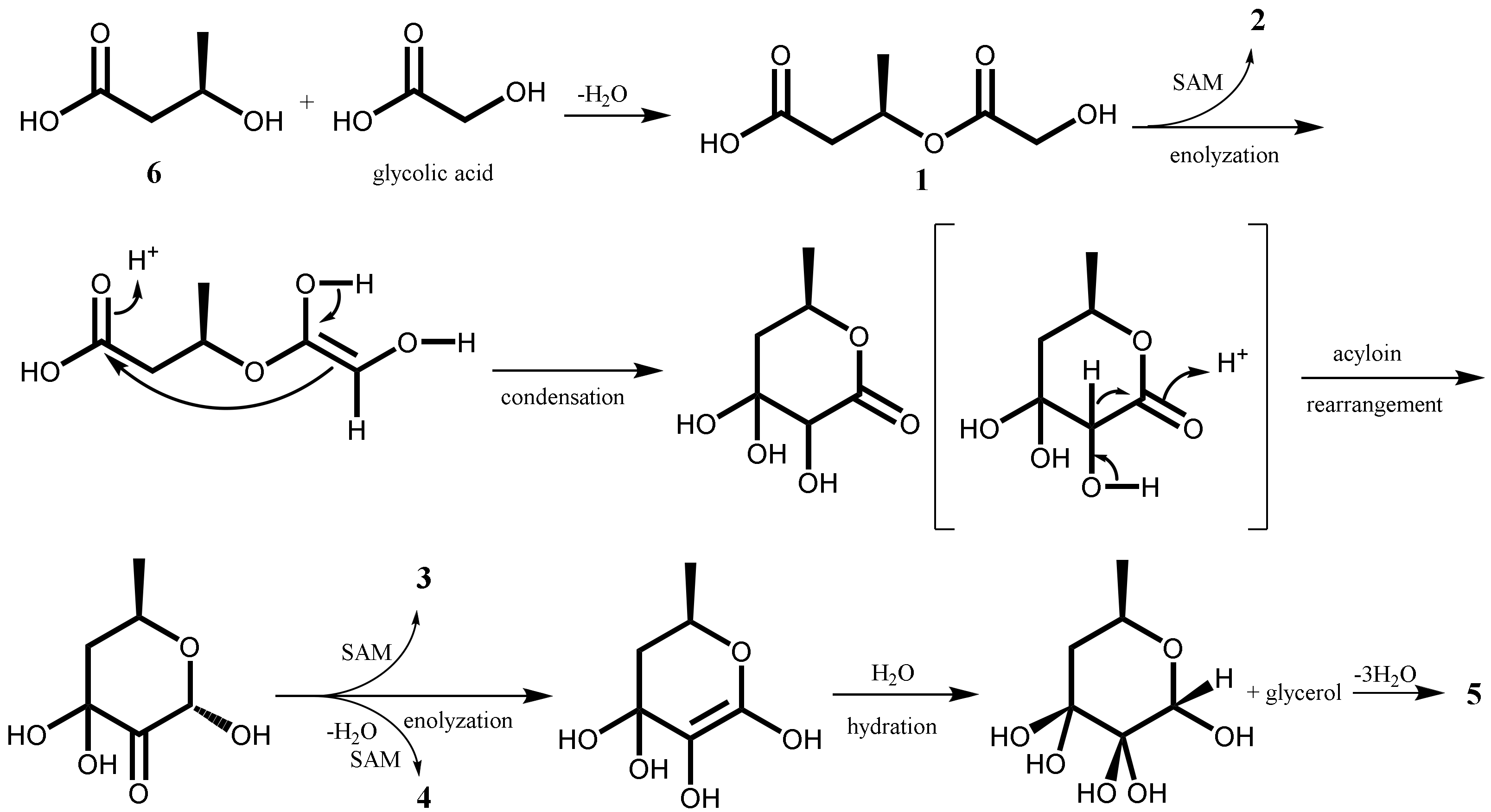
2.1. Isolation and Characterization of Secondary Metabolites
2.2. Anti-Angiogenesis Activities in Human Endothelial Progenitor Cells
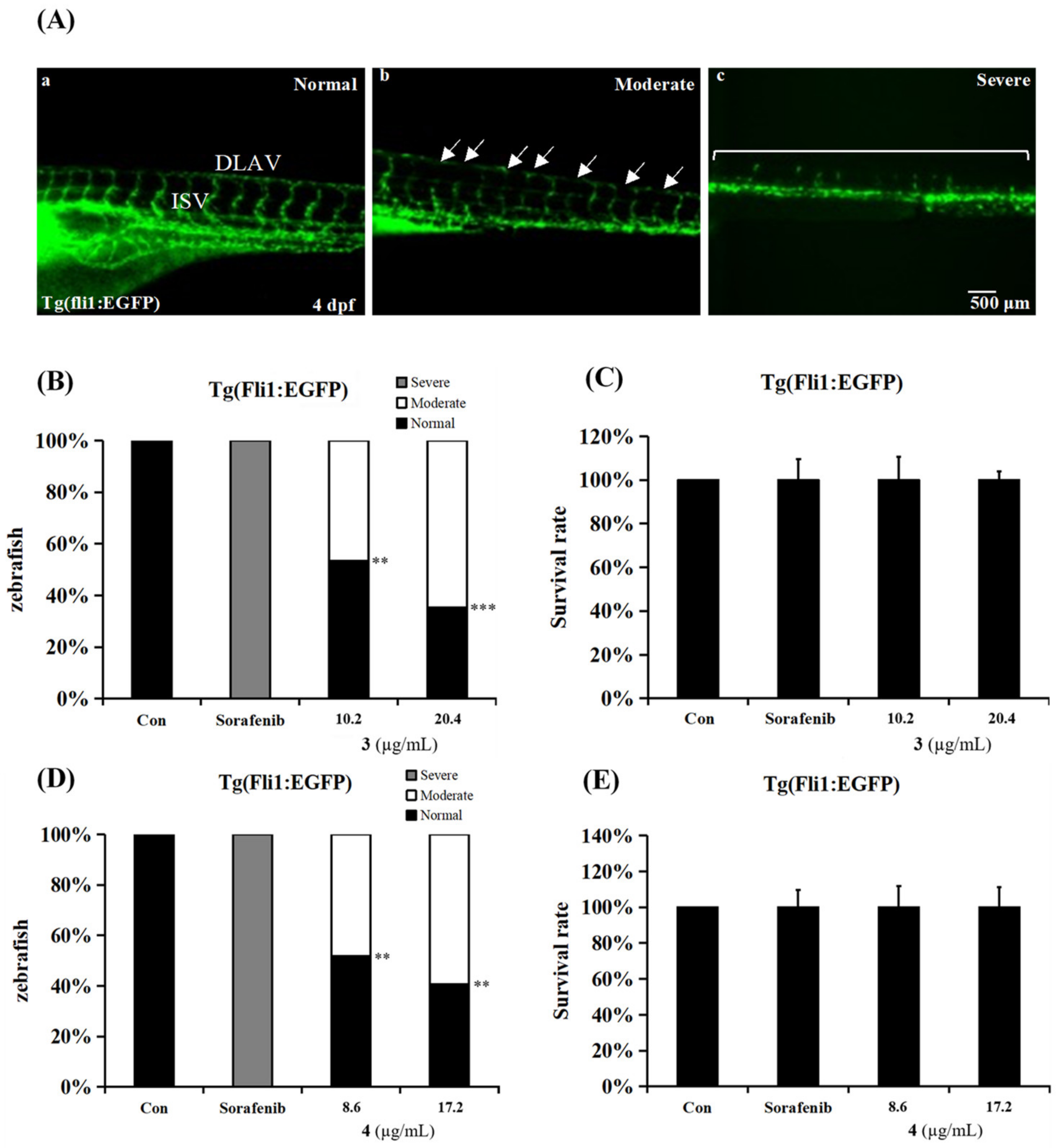
2.3. Anti-Angiogenesis Activities in an In Vivo Zebrafish Model
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Algal Material
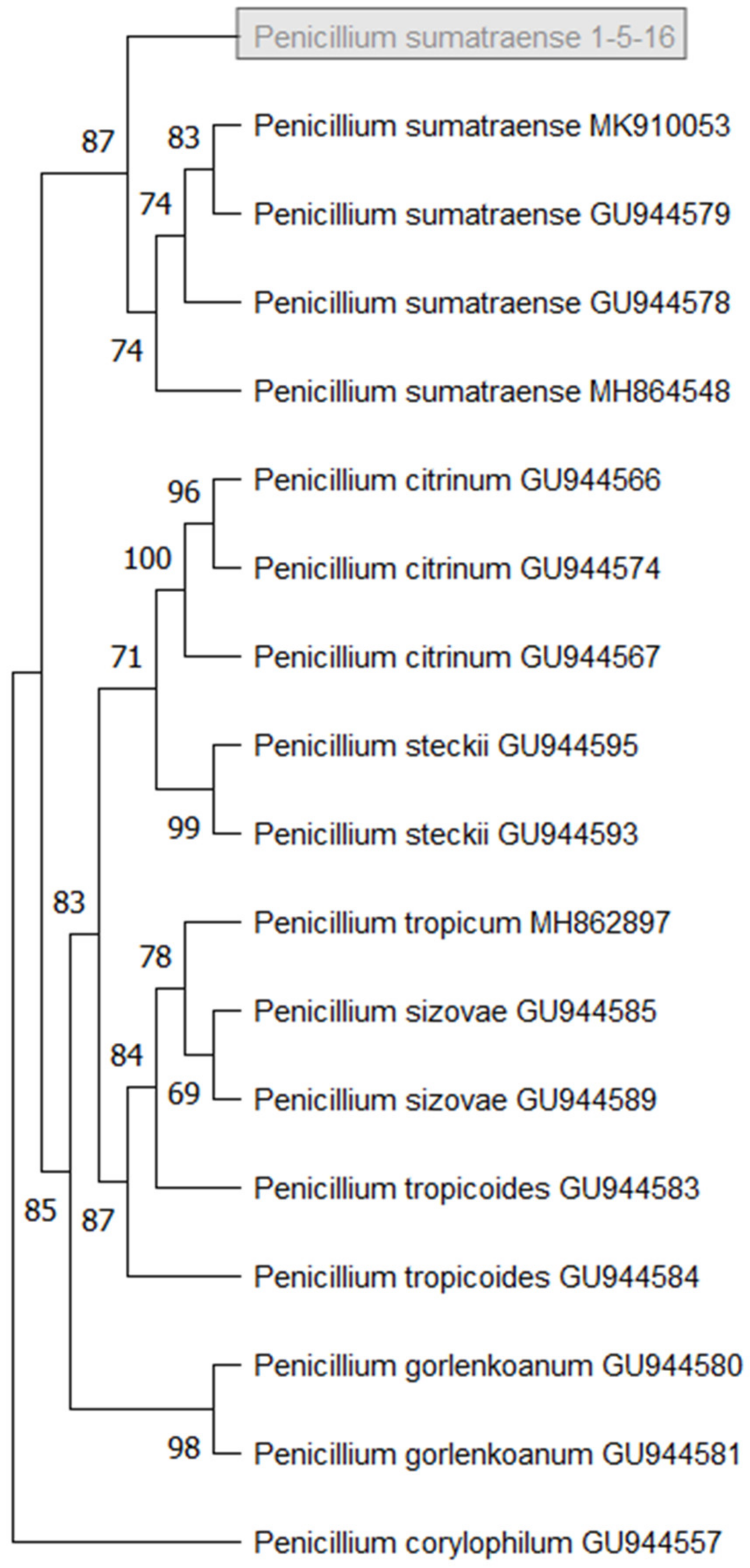
3.3. Isolation and Identification of Fungal Stain
3.4. Extraction and Isolation of Secondary Metabolites
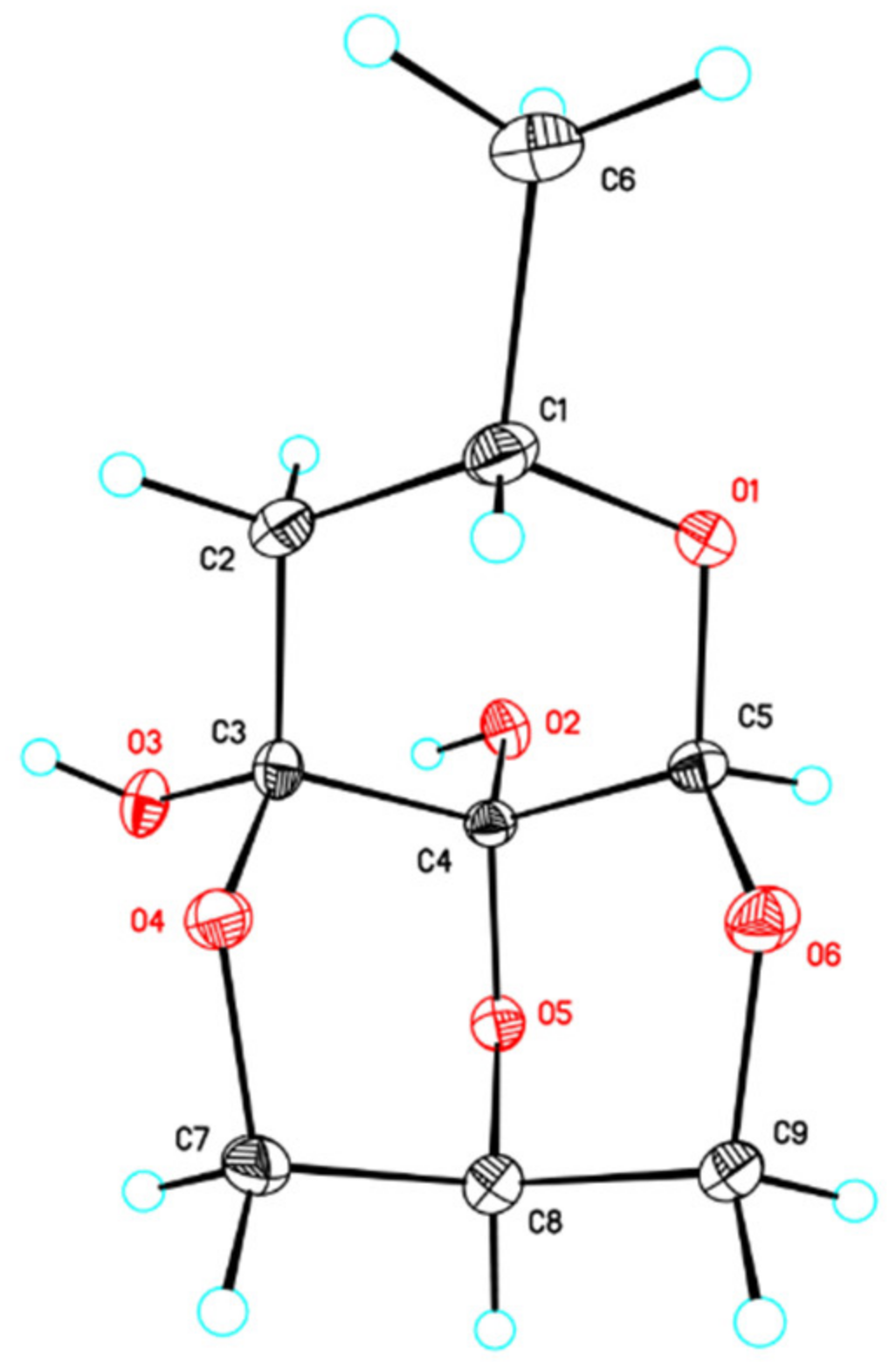
3.5. X-ray Diffraction Analysis
3.6. Alkaline Hydrolysis of 1
3.7. Isolation and Cultivation of Human EPCs
3.8. Cell Growth Assay
3.9. Capillary Tube Formation Assay
3.10. Cell Migration Assay
3.11. Cytotoxicity Assay
3.12. Zebrafish
3.13. Transgenic Zebrafish Lines
3.14. Embryo Collection
3.15. Angiogenesis Inhibition Drug Screening Platform
3.16. Survival Test
3.17. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Liu, L.; Heinrich, M.; Myers, S.; Dworjanyn, S.A. Towards a better understanding of medicinal uses of the brown seaweed Sargassum in Traditional Chinese Medicine: A phytochemical and pharmacological review. J. Ethnopharmacol. 2012, 142, 591–619. [Google Scholar] [CrossRef]
- Sun, S.; Hu, S.; Zhang, B.; Sun, X.; Xu, N. Allelopathic effects and potential allelochemical of Sargassum fusiforme on red tide microalgae Heterosigma akashiwo. Mar. Pollut. Bull. 2021, 170, 112673. [Google Scholar] [CrossRef] [PubMed]
- Puspo Edi Giriwono, S.; Iskandriati, D.; Tan, C.P.; Andarwulan, N. Sargassum seaweed as a source of anti-inflammatory substances and the potential insight of the tropical species: A review. Mar. Drugs 2019, 17, 590. [Google Scholar]
- Yip, Z.T.; Quck, R.Z.B. Historical biogeography of the widespread macroalga Sargassum (Fucales, Phaeophyceae). J. Phycol. 2020, 56, 300–309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsiao, G.; Wang, S.-W.; Chiang, Y.-R.; Chi, W.-C.; Kuo, Y.-H.; Phong, D.A.; Chen, C.-Y.; Lee, T.-H. Anti-inflammatory effects of peptides from a marine algicolous fungus Acremonium sp. NTU492 in BV-2 microglial cells. J. Food Drug Anal. 2020, 28, 89–97. [Google Scholar] [CrossRef]
- Yang, C.-Y.; Chen, C.; Lin, C.-Y.; Chen, Y.-H.; Lin, C.-Y.; Chi, C.-W.; Chen, Y.-J.; Liu, S.-C.; Chang, T.-K.; Tang, C.-H. Garcimultiflorone K inhibits angiogenesis through Akt/eNOS-and mTOR-dependent pathways in human endothelial progenitor cells. Phytomedicine 2019, 64, 152911. [Google Scholar] [CrossRef]
- Flamini, V.; Jiang, W.G.; Lane, J.; Cui, Y.-X. Significance and therapeutic implications of endothelial progenitor cells in angiogenic-mediated tumour metastasis. Crit. Rev. Oncol. Hematol. 2016, 100, 177–189. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.-R.; Wang, S.-W.; Lin, Y.-C.; Yu, C.-L.; Yen, J.-Y.; Chen, Y.-F.; Cheng, Y.-B. Additional alkaloids from Zoanthus vietnamensis with neuroprotective and anti-angiogenic effects. Bioorg. Chem. 2021, 109, 104700. [Google Scholar] [CrossRef]
- Mimeault, M.; Batra, S.K. Emergence of zebrafish models in oncology for validating novel anticancer drug targets and nanomaterials. Drug Discov. Today 2013, 18, 128–140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Howe, K.; Clark, M.D.; Torroja, C.F.; Torrance, J.; Berthelot, C.; Muffato, M.; Collins, J.E.; Humphray, S.; McLaren, K.; Matthews, L.; et al. The zebrafish reference genome sequence and its relationship to the human genome. Nature 2013, 496, 498–503. [Google Scholar] [CrossRef] [Green Version]
- Lin, C.-Y.; Chiang, C.-Y.; Tsai, H.-J. Zebrafish and Medaka: New model organisms for modern biomedical research. J. Biomed. Sci. 2016, 23, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.L.; Liu, S.L.; Lin, Y.B. 225 Seaweeds of Kenting National Park; Kenting National Park Headquarters: Pingtung, Taiwan, 2020; pp. 207–214. [Google Scholar]
- Hsiao, W.-C.; Hong, Y.-H.; Tsai, Y.-H.; Lee, Y.-C.; Patel, A.K.; Guo, H.-R.; Kuo, C.-H.; Huang, C.-Y. Extraction, biochemical characterization, and health effects of native and degraded fucoidans from Sargassum crispifolium. Polymers 2022, 14, 1812. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.-H.; Huang, C.-Y.; Chen, C.-Y.; Chang, C.-C.; Huang, C.-Y.; Dong, C.-D.; Chang, J.-S. Structure and biological activity analysis of fucoidan isolated from Sargassum siliquosum. ACS Omega 2020, 5, 32447–32455. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.-R.; Li, W.-M.; Li, T.-L.; Chan, Y.-L.; Wu, C.-J. Fucoidan from Sargassum hemiphyllum inhibits infection and inflammation of Helicobacter pylori. Sci. Rep. 2022, 12, 429. [Google Scholar] [CrossRef]
- Seebach, D.; Albert, M.; Arvidsson, P.I.; Rueping, M.; Schreiber, J.V. From the biopolymer PHB to biological investigations of unnatural beta- and gamma-peptides. Chimia 2001, 55, 345–353. [Google Scholar] [CrossRef]
- Tokiwa, Y.; Ugwu, C.U. Biotechnological production of (R)-hydroxybutyric acid monomer. J. Biotechnol. 2007, 132, 264–272. [Google Scholar] [CrossRef]
- Wu, Z.; Liu, D.; Huang, J.; Proksch, P.; Zhu, K.; Lin, W. Hansforesters A–M, polyesters from sponge-associated fungus Hansfordia sinuosea with antibacterial activities. RSC Adv. 2018, 8, 39756–39768. [Google Scholar] [CrossRef] [Green Version]
- Matsumoto, S.; Matsuo, M.; Iitaka, Y. Oxygenations of vitamin E (α-tocopherol) and its model compound, 2, 2, 5, 7, 8-pentamethylchroman-6-ol, in the presence of potassium superoxide suspended in tetrahydrofuran, and unusual acyloin rearrangements. J. Org. Chem. 1986, 51, 1435–1440. [Google Scholar] [CrossRef]
- Childs, S.; Chen, J.-N.; Garrity, D.M.; Fishman, M.C. Patterning of angiogenesis in the zebrafish embryo. Development 2002, 129, 973–982. [Google Scholar] [CrossRef]
- Bowley, G.; Kugler, E.; Wilkinson, R.; Lawrie, A.; van Eeden, F.; Chico, T.J.; Evans, P.C.; Noël, E.S.; Serbanovic-Canic, J. Zebrafish as a tractable model of human cardiovascular disease. Br. J. Pharmacol. 2022, 179, 900–917. [Google Scholar] [CrossRef]
- Felsenstein, J. Confidence limits on phylogenies: An approach using the bootstrap. Evolution 1985, 39, 783–791. [Google Scholar] [PubMed]
- Nei, M.; Kumar, S. Molecular Evolution and Phylogenetics; Oxford University Press: New York, NY, USA, 2000. [Google Scholar]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA 11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.-C.; Chung, C.-H.; Lu, Y.-C.; Wu, M.-H.; Chou, P.-H.; Yen, J.-Y.; Lai, Y.-W.; Wang, G.-S.; Liu, S.-C.; Cheng, J.-K. BMP-2 induces angiogenesis by provoking integrin α6 expression in human endothelial progenitor cells. Biochem. Pharmacol. 2018, 150, 256–266. [Google Scholar] [CrossRef]
- Wang, G.-S.; Shen, Y.-S.; Chou, W.-Y.; Tang, C.-H.; Yeh, H.-I.; Wang, L.-Y.; Yen, J.-Y.; Huang, T.-Y.; Liu, S.-C.; Yang, C.-Y. Senescence Induces Dysfunctions in Endothelial Progenitor Cells and Osteoblasts by Interfering Translational Machinery and Bioenergetic Homeostasis. Int. J. Mol. Sci. 2018, 19, 1997. [Google Scholar] [CrossRef] [Green Version]
- Lawson, N.D.; Weinstein, B.M. In vivo imaging of embryonic vascular development using transgenic zebrafish. Dev. Biol. 2002, 248, 307–318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vogt, A.; Codore, H.; Day, B.W.; Hukriede, N.A.; Tsang, M. Development of automated imaging and analysis for zebrafish chemical screens. J. Vis. Exp. 2010, 40, e1900. [Google Scholar] [CrossRef]
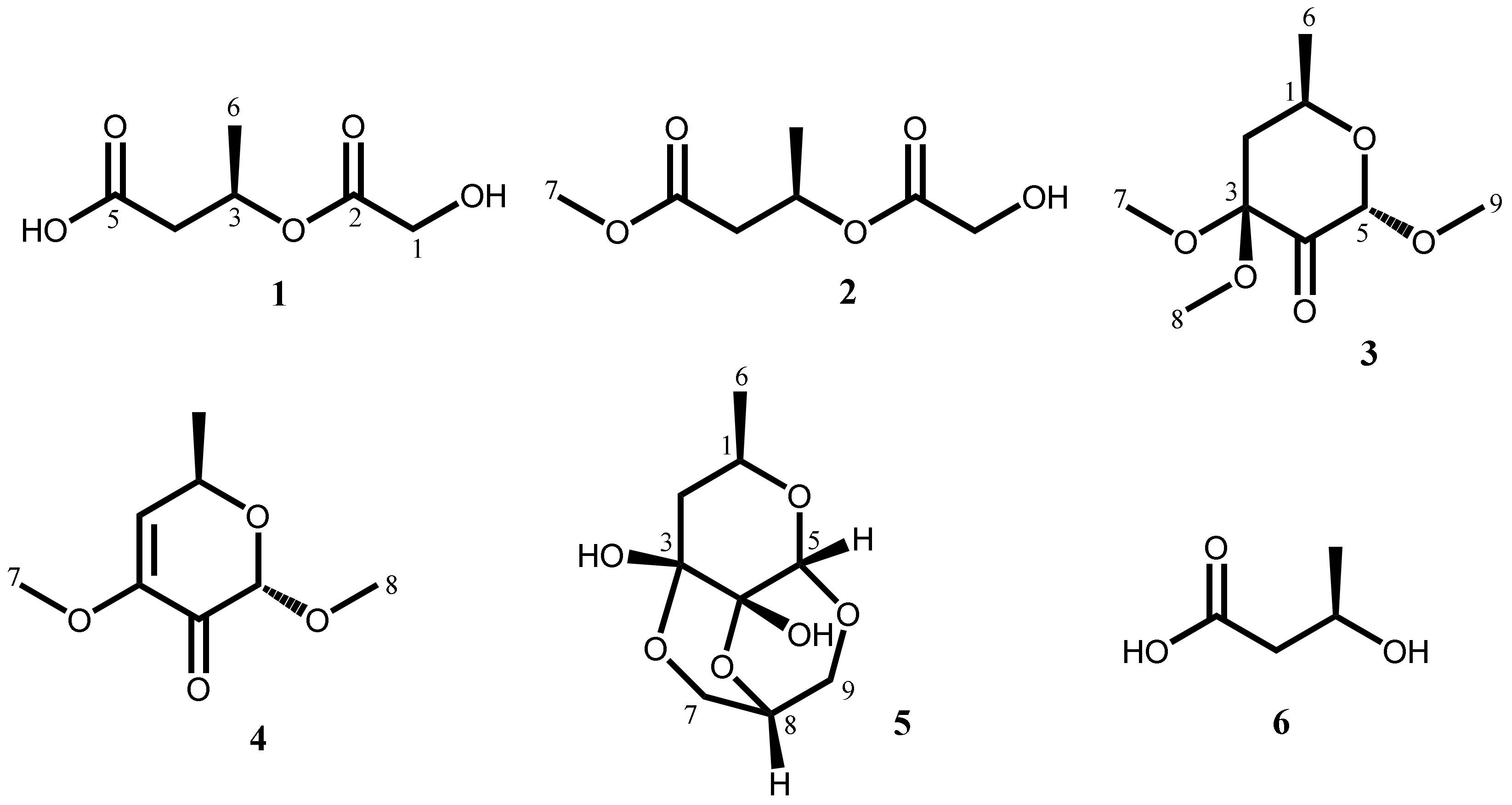


| No. | 1 a,b | 2 a,b | 3 a,b | 4 a,b | 5 a,b |
|---|---|---|---|---|---|
| 1 | 61.2, CH2 | 61.2, CH2 | 67.4, CH | 66.3, CH | 66.6, CH |
| 2 | 173.8, C | 172.6, C | 48.4, CH2 | 121.2, CH | 44.1, CH2 |
| 3 | 69.6, CH | 69.4, CH | 99.4, C | 148.2, C | 89.4, C |
| 4 | 41.4, CH2 | 41.3, CH2 | 202.9, C | 186.7, C | 93.1, C |
| 5 | 174.1, C | 173.8, C | 102.1, CH | 100.7, CH | 98.8, CH |
| 6 | 20.1, CH3 | 20.2, CH3 | 21.6, CH3 | 22.0, CH3 | 20.8, CH3 |
| 7 | 52.4, CH3 | 50.7, CH3 | 55.5, CH3 | 63.6, CH2 | |
| 8 | 48.4, CH3 | 57.1, CH3 | 72.5, CH | ||
| 9 | 55.4, CH3 | 69.2, CH2 |
| No. | 1 a | 2 a | 3 a | 4 a | 5 a |
|---|---|---|---|---|---|
| 1 | 4.07, brs | 4.07, brs | 4.11, dqd (11.4, 6.6, 3.0) | 4.82, qd (6.6, 1.8) | 4.38, dqd (11.4, 6.6, 2.4) |
| 2a | 2.36, dd (13.5, 3.0) | 6.02, d (1.8) | 1.67, dd (13.5, 2.4) | ||
| 2b | 2.65, dd (13.5, 11.4) | 1.79, dd (13.5, 11.4) | |||
| 3 | 5.31, dqd (8.4, 6.6, 5.4) | 5.31, dqd (7.8, 6.6, 5.4) | |||
| 4a | 2.56, dd (16.2, 5.4) | 2.60, dd (15.9, 5.4) | |||
| 4b | 2.63, dd (16.2, 8.4) | 2.66, dd (15.9, 7.8) | |||
| 5 | 4.94, s | 4.74, s | 4.62, s | ||
| 6 | 1.31, d (6.6) | 1.31, d (6.6) | 1.31, d (6.6) | 1.38, d (6.6) | 1.14, d (6.6) |
| 7a | 3.67, s | 3.18, s | 3.61, s | 3.72, d (12.0) | |
| 7b | 4.34, dt (12.0, 3.0) | ||||
| 8 | 3.31, s | 3.50, s | 3.77, t (3.0) | ||
| 9a | 3.39, s | 4.03, d (12.0) | |||
| 9b | 4.07, dt (12.0, 3.0) |
| Compound a (µg/mL) | EPCs Growth | |
|---|---|---|
| 20 | 40 | |
| 1 | >100% | >100% |
| 2 | >100% | >100% |
| 3 | 99.3 ± 1% | >100% |
| 4 | 65 ± 5% | 34 ± 1% |
| 5 | >100% | >100% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsi, H.-Y.; Wang, S.-W.; Cheng, C.-H.; Pang, K.-L.; Leu, J.-Y.; Chang, S.-H.; Lee, Y.-T.; Kuo, Y.-H.; Huang, C.-Y.; Lee, T.-H. Chemical Constituents and Anti-Angiogenic Principles from a Marine Algicolous Penicillium sumatraense SC29. Molecules 2022, 27, 8940. https://doi.org/10.3390/molecules27248940
Hsi H-Y, Wang S-W, Cheng C-H, Pang K-L, Leu J-Y, Chang S-H, Lee Y-T, Kuo Y-H, Huang C-Y, Lee T-H. Chemical Constituents and Anti-Angiogenic Principles from a Marine Algicolous Penicillium sumatraense SC29. Molecules. 2022; 27(24):8940. https://doi.org/10.3390/molecules27248940
Chicago/Turabian StyleHsi, Hsiao-Yang, Shih-Wei Wang, Chia-Hsiung Cheng, Ka-Lai Pang, Jyh-Yih Leu, Szu-Hsing Chang, Yen-Tung Lee, Yueh-Hsiung Kuo, Chia-Ying Huang, and Tzong-Huei Lee. 2022. "Chemical Constituents and Anti-Angiogenic Principles from a Marine Algicolous Penicillium sumatraense SC29" Molecules 27, no. 24: 8940. https://doi.org/10.3390/molecules27248940






