Simple Detection of Pigment Red 53 as a Hazardous Substance in Cosmetic Preparation Using a Polymer Combination of Polystyrene (PS) and Polymethylmethacrylate (PMMA)
Abstract
1. Introduction
2. Results and Discussions
2.1. Sample Collection and Preparation
2.2. Selection of Specific Chemical Reagent for Pigment Red 53
2.3. Development of Indicator Strip from PMMA and A Mixture of PS and PMMA Using a Reagent Blending Method
2.4. Performance Test of the Indicator Strip
2.4.1. Sensitivity and Stability Test
2.4.2. Accuracy Test
2.4.3. Selectivity Test
2.5. Characterization of Indicator Strip
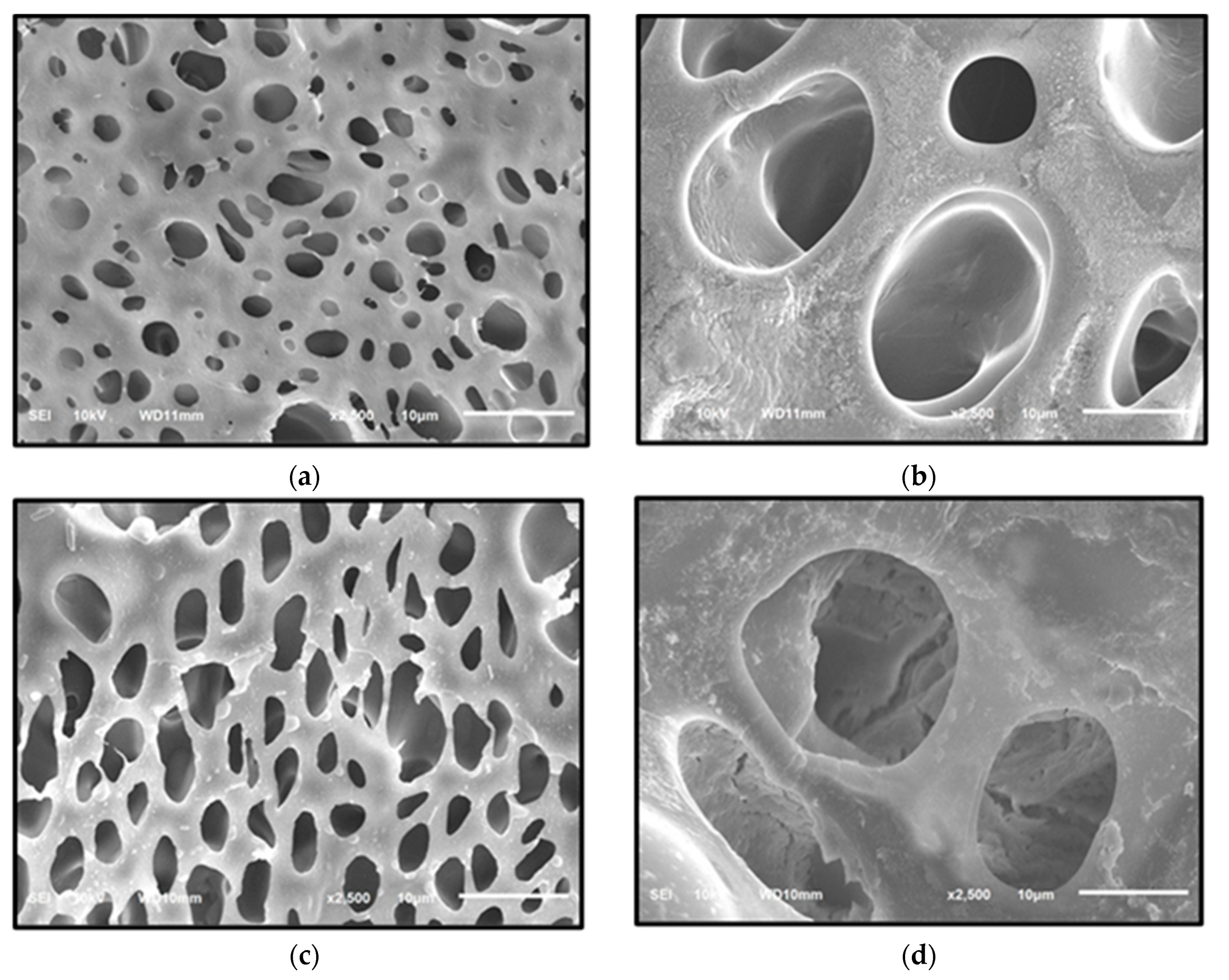
2.5.1. Scanning Electron Microscope-Energy Dispersive X-ray Spectroscopy (SEM-EDX)
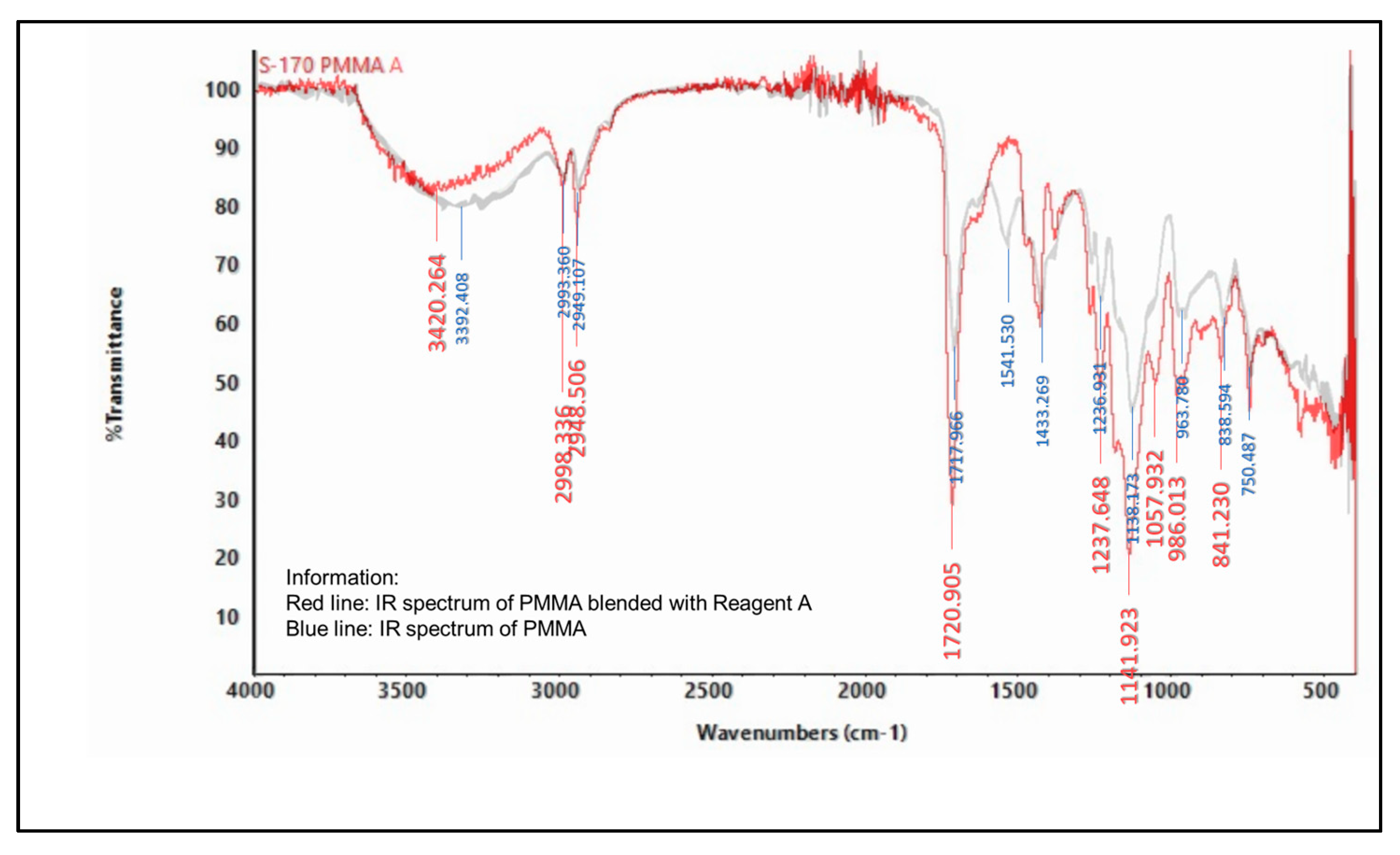
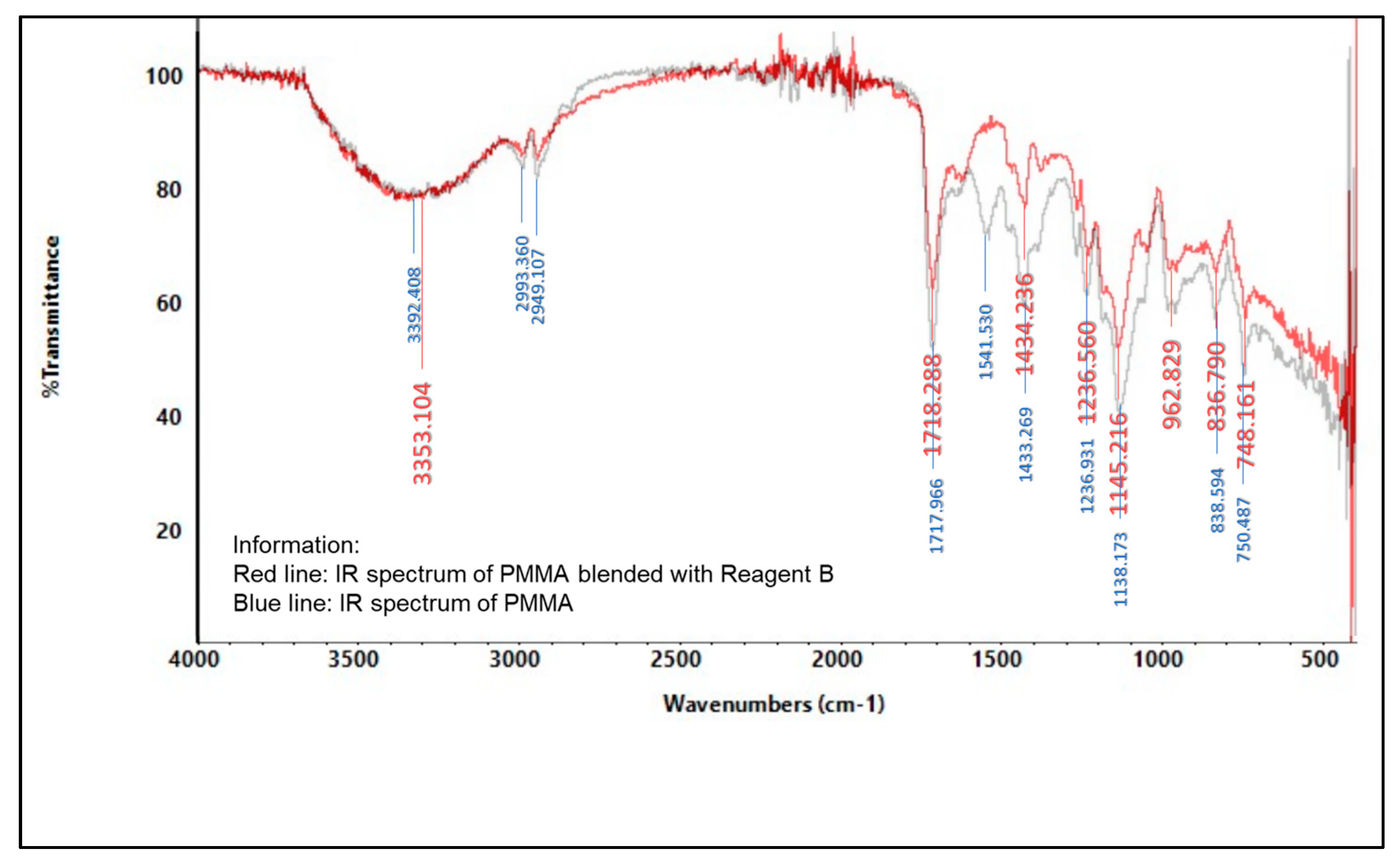
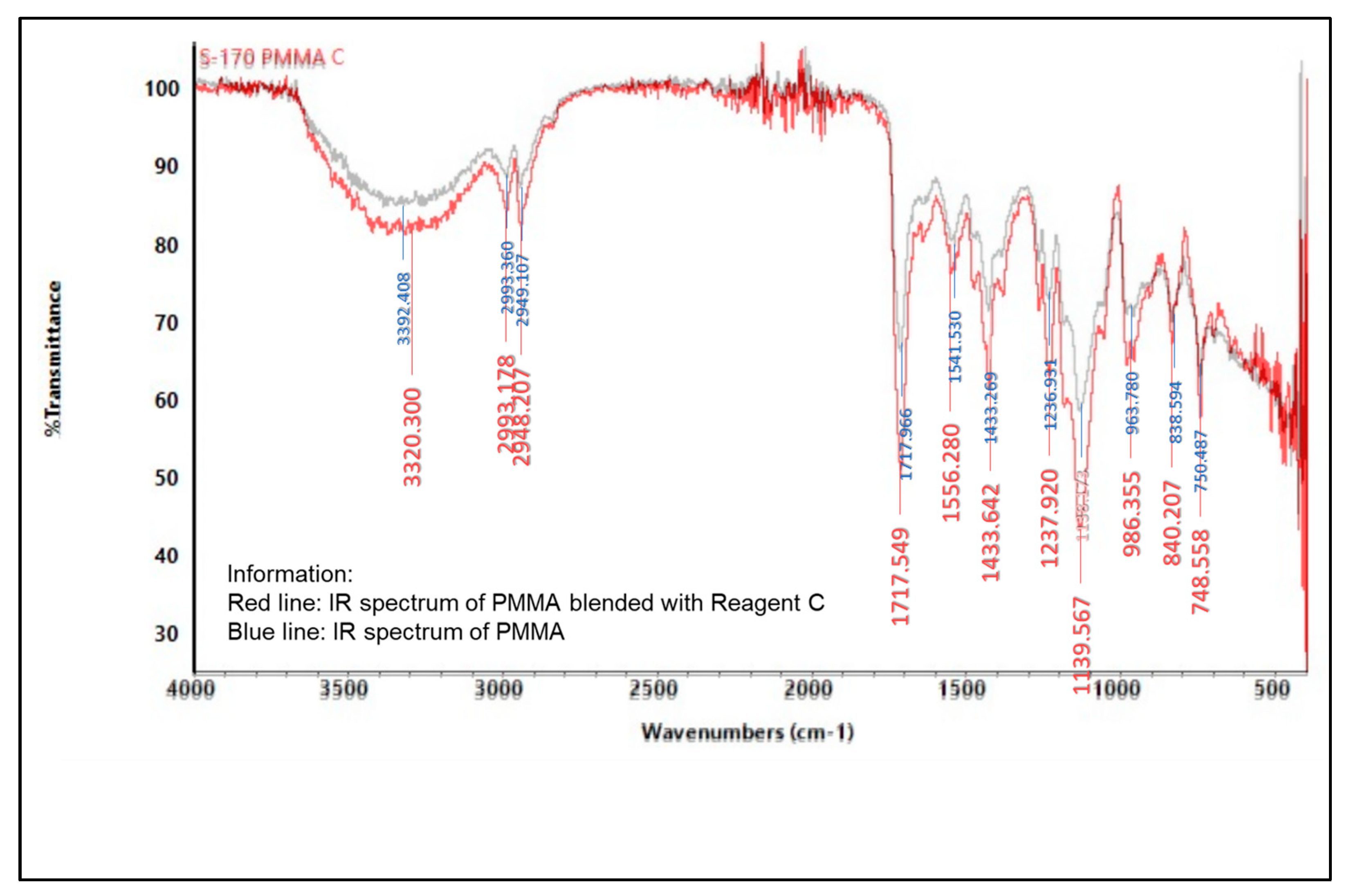
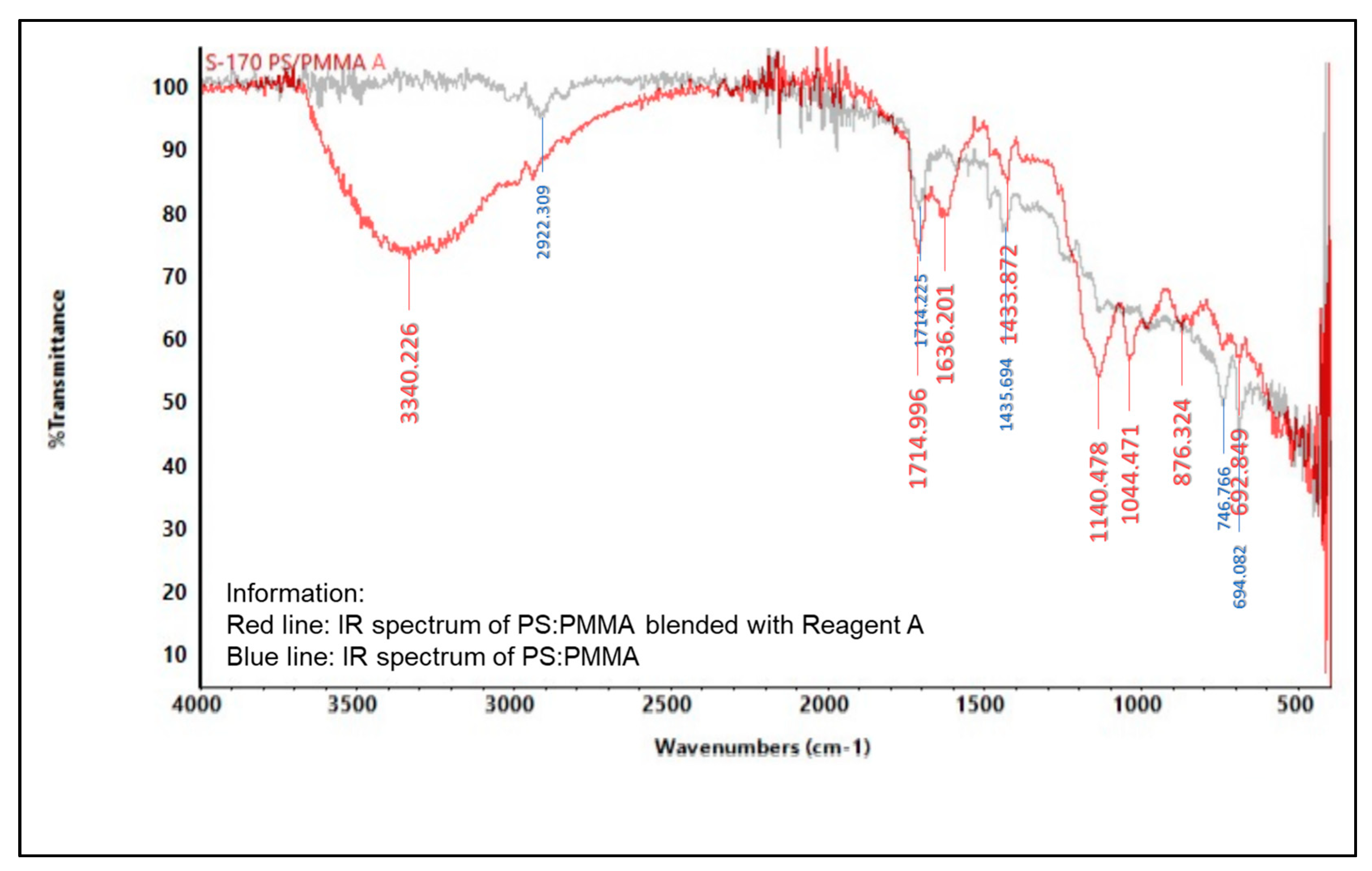
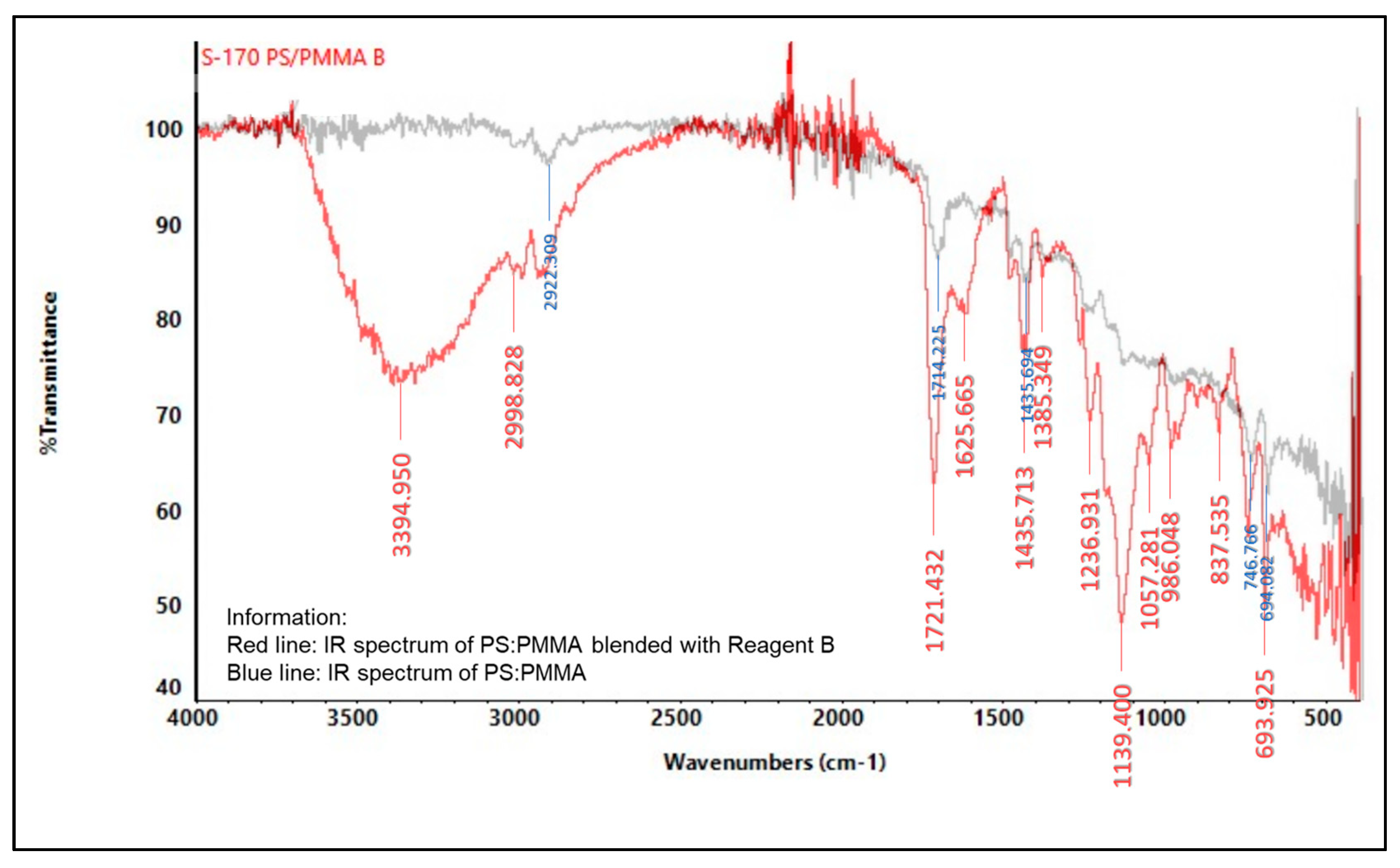
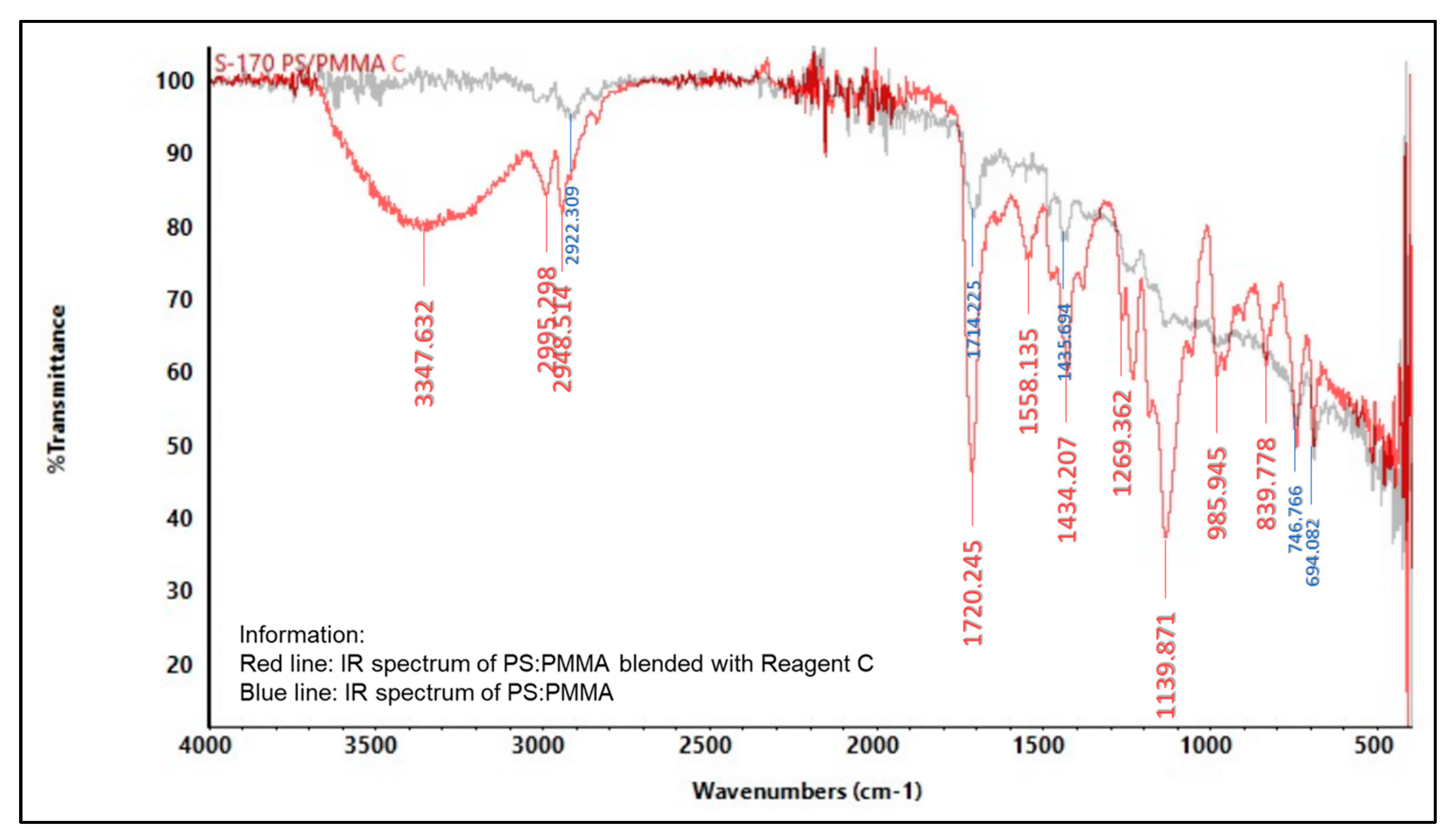
2.5.2. Infrared (IR) Spectroscopy
3. Materials and Methods
3.1. Chemicals and Materials
3.2. Sample Collection and Preparation
3.3. Selection of Specific Chemical Reagent for Pigment Red 53
3.4. Fabrication of Indicator Strip from PMMA and a Mixture of PS and PMMA Using the Reagent Blending Method
3.5. Performance Test of the Indicator Strip
3.5.1. Sensitivity Test
3.5.2. Accuracy Test
3.5.3. Stability Test
3.5.4. Selectivity Test
3.6. Characterization of Indicator Strip
3.6.1. Scanning Electron Microscope-Energy Dispersive X-ray Spectroscopy (SEM-EDX)
3.6.2. Spectrophotometer Infrared (IR)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pandey, A.; Jatana, G.K.; Sonthalia, S. Cosmeceuticals; StartPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Ferdinand, M.; Ciptono, W.S. Indonesia’s Cosmetics Industry Attractiveness, Competitiveness and Critical Success Factor Analysis. J. Manaj. Teor. Terap. 2022, 15, 209–223. [Google Scholar] [CrossRef]
- Affat, S.S. Classifications, Advantages, Disadvantages, Toxicity Effects of Natural and Synthetic Dyes: A Review. Univ. Thi-Qar J. Sci. 2021, 8, 130–135. [Google Scholar]
- Musnaini; Wijoyo, H. Impact of Variety Seeking, and Electronic Word of Mouth of Cosmetic Brand Switching. J. Ekon. 2021, 3, 23–32. [Google Scholar]
- BPOM. Temuan Kosmetik Ilegal dan mengandung Bahan Dilarang/Bahan Berbahaya Serta Obat Tradisional Ilegal dan Mengandung Bahan Kimia Obat; Badan Pengawas Obat dan Makanan: Jakarta, Indonesia, 2018; p. 23. [Google Scholar]
- Manafe, D. BPOM: Nilai Temuan Kosmetik Ilegal Meningkat Drastis. Available online: https://www.pom.go.id/new/view/more/pers/286/WASPADA-KOSMETIKA-MENGANDUNG-BAHAN-BERBAHAYA-----Teliti-Sebelum-Memilih-Kosmetika----.html (accessed on 27 July 2021).
- Yunianto, E.P.; Anggoro, Y. Understanding Illegal Cosmetic Circulation in Indonesian Online Marketplace through Problem Analysis Triangle. J. Int. Conf. Proc. 2021, 4, 34–41. [Google Scholar] [CrossRef]
- PubChem. Brilliant Red. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Brilliant-Red (accessed on 23 July 2021).
- Keck-Wilhelm, A.; Kratz, E.; Mildau, G.; Ilse, M.; Schlee, C.; Lachenmeier, D.W. Chemical analysis and risk assessment of prohibited colouring agents in face paint with special regard to CI 15585 (D&C Red No. 9, Pigment Red 53:1). Int. J. Cosmet. Sci. 2015, 37, 187–195. [Google Scholar] [PubMed]
- ZeyaChem. Pigment Red 53:1-Corimax Red CNS. Available online: https://www.zeyachem.net/pigment-red-531.html (accessed on 23 July 2021).
- Dalli, I.; Ramdhani, D.; Hasanah, A.N. Design of indicator strip using polystyrene (PS) and polymethylmethacrylate (PMMA) for detection of diclofenac sodium in traditional pain relief herbal medicines. Indones. J. Chem. 2017, 17, 71–78. [Google Scholar] [CrossRef]
- Grosso, C.; Valentão, P.; Ferreres, F.; Andrade, P.B. Alternative and efficient extraction methods for marine-derived compounds. Mar. Drugs 2015, 13, 3182–3230. [Google Scholar] [CrossRef] [PubMed]
- Herlina, P.; Feshirawan; Isnaini, F. Identifikasi Pewarna Berbahaya (Rhodamin B, Methanyl Yellow, Merah K3, Jingga K1) pada Pemerah Pipi (Blush On). J. Ilm. Pharm. 2011, 5, 82–89. [Google Scholar]
- Gao, G.Q.; Xu, A.W. Efficient catalytic reduction of azo dyes by N,N-dimethylformamide mediated by viologen. New J. Chem. 2014, 38, 4661–4665. [Google Scholar] [CrossRef]
- BPOM. Peraturan Kepala BPOM RI No. HK.03.1.23.08.11.07331 Tentang Metode Analisa Kosmetika; BPOM RI: Jakarta, Indonesia, 2011. [Google Scholar]
- Azizahwati; Kurniadi, M.; Hidayati, H. Analisis Zat Warna Sintetik. Maj. Ilmu Kefarmasian 2007, 4, 7–25. [Google Scholar]
- Mathpati, S. Qualitative analysis of organic mixture. Available online: https://www.researchgate.net/publication/332029217_Qualitative_analysis_of_organic_mixture_Binary_and_Ternary_chart_for_MSc_organic_students (accessed on 26 August 2021).
- Clark, J. Acidity of Phenols. Available online: https://chem.libretexts.org/Bookshelves/Organic_Chemistry/Supplemental_Modules_(Organic_Chemistry)/Phenols/Properties_of_Phenols/Acidity_of_Phenols (accessed on 26 August 2021).
- Vedantu. Phenol Reactions with NaOH. Available online: https://www.vedantu.com/question-answer/happens-when-phenol-reactswith-naoh-class-11-chemistry-cbse-60d4d59d993a2c20d5443b9a (accessed on 26 August 2021).
- Nesmeyanov, A.N.; Perevaloval, E.G.; Nikitina, T.V.; Kuznetsova, N.I. Action of Hydrochloric Acid on Azo Derivatives of Ferrocene. Russ. Chem. Bull. 1965, 14, 2094–2097. [Google Scholar] [CrossRef]
- Svehla, G. Macro and Semmicro Qualitative Inorganic Analysis; Kalman Media Pustaka: Jakarta, Indonesia, 1985. [Google Scholar]
- Sinha, N.; Rangarajan, R.; Machanda, R.P.; Gupta, R.K.; Kumar, R. Lab Manual Chemistry; New Saraswati House: New Delhi, India, 2018. [Google Scholar]
- Technologies, H. Barium with Ammonium Hydroxide Reaction. Available online: https://www.toppr.com/ask/question/barium-on-reacting-with-ammonium-hydroxide-gives/ (accessed on 5 February 2022).
- Sirumpea, L. Sintesis dan Karakterisasi Membran Polistirena (PS)-Poli(metilmetakrilat) (PMMA) untuk Pemisahan Penisilin G. Master’s Thesis, Institut Teknologi Bandung, Bandung, Indonesia, 2019; pp. 12–39. [Google Scholar]
- Roughton, B.C.; White, J.; Camarda, K.V.; Gani, R. Simultaneous Design of Ionic Liquids and Azeotropic Separation Processes; Elsevier, B.V.: Amsterdam, The Netherlands, 2011. [Google Scholar]
- Burke, J. Solubility Parameters: Theory and Application; Book and Paper Group: Washington, DC, USA, 2015; pp. 1–35. [Google Scholar]
- Satiti, R. Analisis Rhodamin B dengan Penyangga Polimer Sintetik dan Alam. Undergraduate Thesis, Universitas Padjadjaran, Jatinangor, Indonesia, 2012. [Google Scholar]
- Agustin, R.; Oktaviantari, D.E.; Feladita, N. Identifikasi Hidrokuinon dalam Sabun Pemutih Pembersih Wajah di Tiga Klinik Kecantikan dengan Metode Kromatografi Lapis Tipis dan Spektrofotometri UV-Vis. J. Anal. Farm. 2021, 6, 95–101. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information. Rhodamine B. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Rhodamine-B (accessed on 7 February 2022).
- Pan, Y.; Liu, C.; Zhao, L.; Li, F.; Liu, Y.; Liu, P. Study and application of a new blockage remover in polymer injection wells. Petroleum 2018, 4, 329–336. [Google Scholar] [CrossRef]
- Vashishtha, S.R.; Chand, N.; Hashmi, S.A.R. Morphology of PS/PMMA blends and their solution rheology. Indian J. Chem. Technol. 2022, 9, 316–323. [Google Scholar]
- Kaniappan, K.; Latha, S. Certain investigations on the formulation and characterization of polystyrene/poly(methyl methacrylate) blends. Int. J. ChemTech Res. 2011, 3, 708–715. [Google Scholar]
- Lumongga, S. Analisis Rhodamin B dengan Penyangga Polimer dan Silika pada Produk Makanan dan Kosmetik. Undergraduate Thesis, Universitas Padjadjaran, Jatinangor, Indonesia, 2012. [Google Scholar]


| Reagent | Reagent Strength | Documentation | Result |
|---|---|---|---|
| A | Concentrated H2SO4 |  | + |
| B | Concentrated HCl |  | + |
| C | 10% NaOH |  | + |
| D | 10% Ammonia |  | − |
| Polymer | Solvent and Reagent Ratio | Solvent | Reagent | Result | Time Reaction to Color Change (Second) |
|---|---|---|---|---|---|
| PMMA 5% | 60:40 | Ethyl acetate | A | − | − |
| 80:20 | − | − | |||
| 90:10 | + | 25.41 | |||
| 60:40 | B | − | − | ||
| 80:20 | + | 10.33 | |||
| 60:40 | C | + | 22.48 | ||
| PS:PMMA (1:2) | 90:10 | Ethyl acetate | A | + | 32.13 |
| 80:20 | B | + | 22.69 | ||
| 60:40 | C | + | 18.36 | ||
| PS:PMMA (1:3) | 90:10 | Ethyl acetate | A | + | 31.42 |
| 80:20 | B | + | 17.41 | ||
| 60:40 | C | + | 16.51 | ||
| PS:PMMA (1:4) | 90:10 | Ethyl acetate | A | + | 30.78 |
| 80:20 | B | + | 10.14 | ||
| 60:40 | C | + | 17.26 |
| Indicator Strip Material | Reagent | Dye | |
|---|---|---|---|
| Pigment Red 53 | Rhodamine B | ||
| PMMA 5% | A | + | − |
| B | + | − | |
| C | + | − | |
| PS:PMMA (1:4) | A | + | − |
| B | + | − | |
| C | + | − | |
| Indicator Strip | Element | %Mass |
|---|---|---|
| PMMA 5% | Carbon | 67.24 |
| Oxygen | 32.76 | |
| PMMA-H2SO4 90:10 | Carbon | 41.73 |
| Oxygen | 39.31 | |
| Sulfur | 18.96 | |
| PMMA-HCl 80:20 | Carbon | 61.99 |
| Oxygen | 36.04 | |
| Chlorine | 1.97 | |
| PMMA-10% NaOH 60:40 | Carbon | 62.02 |
| Oxygen | 36.46 | |
| Sodium | 1.52 | |
| PS:PMMA (1:4) 5% | Carbon | 71.20 |
| Oxygen | 28.80 | |
| PS:PMMA-H2SO4 90:10 | Carbon | 40.59 |
| Oxygen | 42.45 | |
| Sulfur | 16.96 | |
| PS:PMMA-HCl 80:20 | Carbon | 67.61 |
| Oxygen | 28.62 | |
| Chlorine | 3.77 | |
| PS:PMMA-10% NaOH 60:40 | Carbon | 66.45 |
| Oxygen | 29.79 | |
| Sodium | 3.76 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pratiwi, R.; Amanda, S.; Hasanah, A.N. Simple Detection of Pigment Red 53 as a Hazardous Substance in Cosmetic Preparation Using a Polymer Combination of Polystyrene (PS) and Polymethylmethacrylate (PMMA). Molecules 2022, 27, 9016. https://doi.org/10.3390/molecules27249016
Pratiwi R, Amanda S, Hasanah AN. Simple Detection of Pigment Red 53 as a Hazardous Substance in Cosmetic Preparation Using a Polymer Combination of Polystyrene (PS) and Polymethylmethacrylate (PMMA). Molecules. 2022; 27(24):9016. https://doi.org/10.3390/molecules27249016
Chicago/Turabian StylePratiwi, Rimadani, Syifa Amanda, and Aliya Nur Hasanah. 2022. "Simple Detection of Pigment Red 53 as a Hazardous Substance in Cosmetic Preparation Using a Polymer Combination of Polystyrene (PS) and Polymethylmethacrylate (PMMA)" Molecules 27, no. 24: 9016. https://doi.org/10.3390/molecules27249016
APA StylePratiwi, R., Amanda, S., & Hasanah, A. N. (2022). Simple Detection of Pigment Red 53 as a Hazardous Substance in Cosmetic Preparation Using a Polymer Combination of Polystyrene (PS) and Polymethylmethacrylate (PMMA). Molecules, 27(24), 9016. https://doi.org/10.3390/molecules27249016






