Developing Novel Fabrication and Optimisation Strategies on Aggregation-Induced Emission Nanoprobe/Polyvinyl Alcohol Hydrogels for Bio-Applications
Abstract
:1. Introduction
2. Results and Discussion
2.1. Nanoprobes Characterization
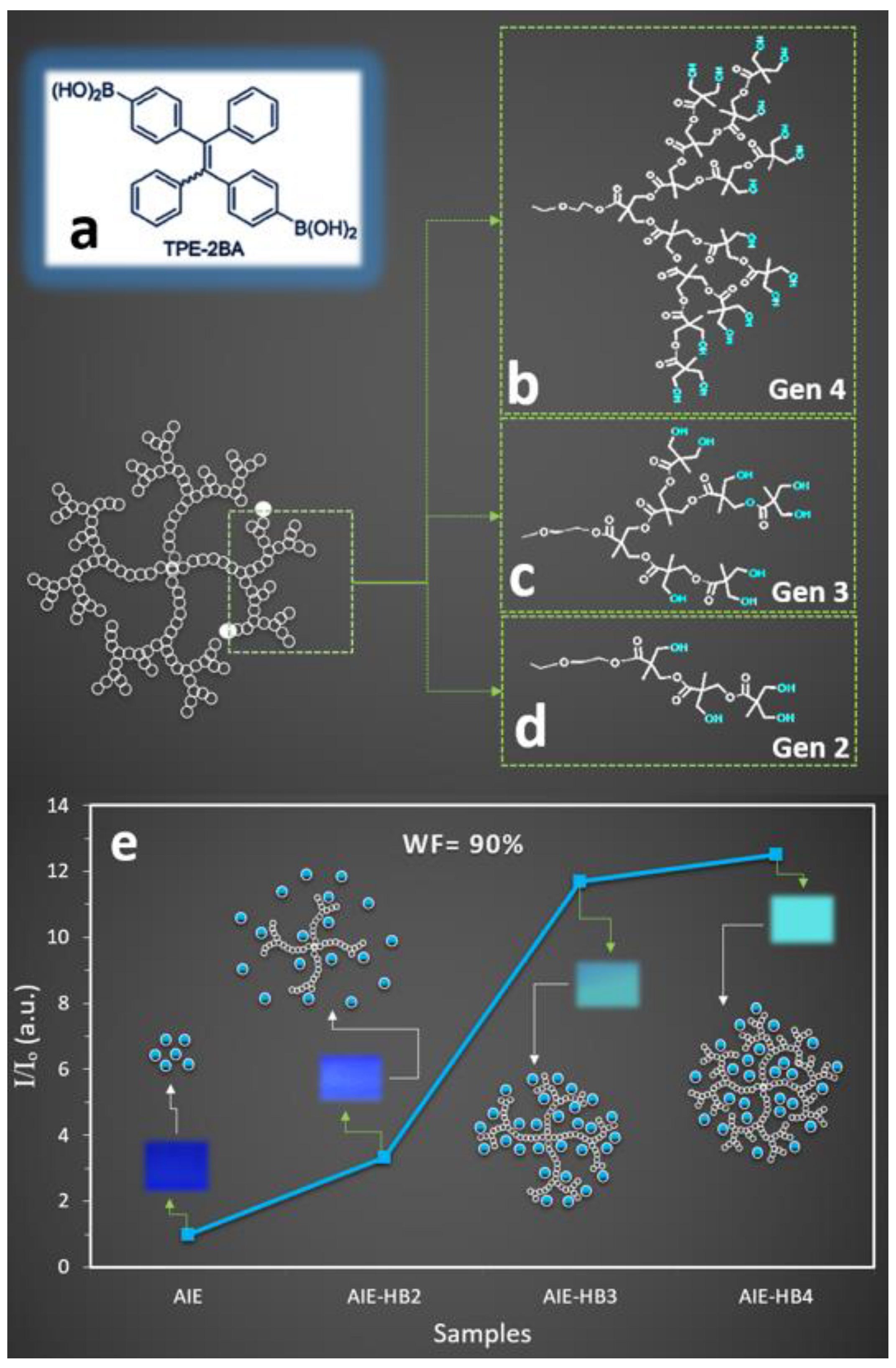
2.1.1. The Generation of HBs Affected the Fluorescent Property of the AIEgen
2.1.2. VFD Enhanced the Fluorescent Property of the AIE-HB Nanoprobes
2.2. Fluorescent Nanoprobe-Based Polymeric Hydrogel Fabrication and Characterization
2.2.1. Case Study 1: Injectable Bioadhesive Fluorescent Gel
2.2.2. Case Study 2: Fluorescent Microchip for Cell Culture
3. Experimental Section
3.1. Materials
3.2. AIEgen-Hyperbranched Polymer (AIE-HB) Preparation and Characterization
3.3. Fluorescent Gel Preparation and Characterization
3.4. Fabrication and Characterization of Fluorescent Microchip
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ullah, F.; Othman, M.B.H.; Javed, F.; Ahmad, Z.; Akil, H.M. Classification, processing and application of hydrogels: A review. Mater. Sci. Eng. C 2015, 57, 414–433. [Google Scholar] [CrossRef] [PubMed]
- Tavakoli, J.; Mirzaei, S.; Tang, Y. Cost-effective double-layer hydrogel composites for wound dressing applications. Polymers 2018, 10, 305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nele, V.; Wojciechowski, J.P.; Armstrong, J.P.; Stevens, M.M. Tailoring gelation mechanisms for advanced hydrogel applications. Adv. Funct. Mater. 2020, 30, 2002759. [Google Scholar] [CrossRef]
- Hanson Shepherd, J.N.; Parker, S.T.; Shepherd, R.F.; Gillette, M.U.; Lewis, J.A.; Nuzzo, R.G. 3D microperiodic hydrogel scaffolds for robust neuronal cultures. Adv. Funct. Mater. 2011, 21, 47–54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, Y.; Wang, Y.; Xia, S.; Gao, G. An environment-stable hydrogel with skin-matchable performance for human-machine interface. Sci. China Mater. 2021, 64, 2313–2324. [Google Scholar] [CrossRef]
- Wang, J.; Chen, G.; Zhao, Z.; Sun, L.; Zou, M.; Ren, J.A.; Zhao, Y. Responsive graphene oxide hydrogel microcarriers for controllable cell capture and release. Sci. China Mater. 2018, 61, 1314–1324. [Google Scholar] [CrossRef] [Green Version]
- Hu, J.; Zhang, C.; Zhou, L.; Hu, Q.; Kong, Y.; Song, D.; Cheng, Y.; Zhang, Y. A smart hydrogel for on-demand delivery of antibiotics and efficient eradication of biofilms. Sci. China Mater. 2021, 64, 1035–1046. [Google Scholar] [CrossRef]
- Jabbari, E.; Tavakoli, J.; Sarvestani, A.S. Swelling characteristics of acrylic acid polyelectrolyte hydrogel in a dc electric field. Smart Mater. Struct. 2007, 16, 1614. [Google Scholar] [CrossRef]
- Noh, M.; Choi, Y.H.; An, Y.-H.; Tahk, D.; Cho, S.; Yoon, J.W.; Jeon, N.L.; Park, T.H.; Kim, J.; Hwang, N.S. Magnetic Nanoparticle-Embedded Hydrogel Sheet with a Groove Pattern for Wound Healing Application. ACS Biomater. Sci. Eng. 2019, 5, 3909–3921. [Google Scholar] [CrossRef]
- Tavakoli, J.; Tang, Y. Hydrogel based sensors for biomedical applications: An updated review. Polymers 2017, 9, 364. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Y.; Zhang, J.; Song, J.; Yang, J.; Du, Z.; Zhao, W.; Guo, H.; Wen, C.; Li, Q.; Sui, X. A Multifunctional Pro-Healing Zwitterionic Hydrogel for Simultaneous Optical Monitoring of pH and Glucose in Diabetic Wound Treatment. Adv. Funct. Mater. 2020, 30, 1905493. [Google Scholar] [CrossRef]
- Zhang, K.; Zhao, Z.; Huang, J.; Zhao, T.; Fang, R.; Liu, M. Self-recoverable semi-crystalline hydrogels with thermomechanics and shape memory performance. Sci. China Mater. 2019, 62, 586–596. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Xi, Y.; Ke, Y.; Li, W.; Long, Y.; Li, J.; Wang, L.-N.; Zhang, X. A skin-like stretchable colorimetric temperature sensor. Sci. China Mater. 2018, 61, 969–976. [Google Scholar] [CrossRef]
- Dehbari, N.; Tavakoli, J.; Khatrao, S.S.; Tang, Y. In situ polymerized hyperbranched polymer reinforced poly (acrylic acid) hydrogels. Mater. Chem. Front. 2017, 1, 1995–2004. [Google Scholar] [CrossRef] [Green Version]
- Li, P.; Wang, Z.; Lin, X.; Wang, X.; Guo, H. Muscle-inspired ion-sensitive hydrogels with highly tunable mechanical performance for versatile industrial applications. Sci. China Mater. 2021, 65, 229–236. [Google Scholar] [CrossRef]
- Tang, P.; Yan, H.; Chen, L.; Wu, Q.; Zhao, T.; Li, S.; Gao, H.; Liu, M. Anisotropic nanocomposite hydrogels with enhanced actuating performance through aligned polymer networks. Sci. China Mater. 2020, 63, 832–841. [Google Scholar] [CrossRef] [Green Version]
- Le, X.-X.; Lu, W.; He, J.; Serpe, M.J.; Zhang, J.-W.; Chen, T. Ionoprinting controlled information storage of fluorescent hydrogel for hierarchical and multi-dimensional decryption. Sci. China Mater. 2019, 62, 831–839. [Google Scholar] [CrossRef] [Green Version]
- Griffith, A.; Bandy, T.J.; Light, M.; Stulz, E. Fluorescent hydrogel formation from carboxyphenyl-terpyridine. Chem. Commun. 2013, 49, 731–733. [Google Scholar] [CrossRef]
- Wu, B.Y.; Le, X.X.; Jian, Y.K.; Lu, W.; Yang, Z.Y.; Zheng, Z.K.; Théato, P.; Zhang, J.W.; Zhang, A.; Chen, T. pH and Thermo Dual-Responsive Fluorescent Hydrogel Actuator. Macromol. Rapid Commun. 2019, 40, 1800648. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, H.; Chen, B.; Liu, H.; Zhao, Y. Emerging barcode particles for multiplex bioassays. Sci. China Mater. 2019, 62, 289–324. [Google Scholar] [CrossRef]
- Jiang, N.; Shen, T.; Sun, J.Z.; Tang, B.Z. Aggregation-induced emission: Right there shining. Sci. China Mater. 2019, 62, 1227–1235. [Google Scholar] [CrossRef] [Green Version]
- Tang, Y.; Tang, B.Z. Principles and Applications of Aggregation-Induced Emission; Springer: Berlin/Heidelberg, Germany, 2018. [Google Scholar]
- Tian, R.; Zhong, J.; Lu, C.; Duan, X. Hydroxyl-triggered fluorescence for location of inorganic materials in polymer-matrix composites. Chem. Sci. 2018, 9, 218–222. [Google Scholar] [CrossRef] [Green Version]
- Park, H.-I.; Park, S.-Y. Smart fluorescent hydrogel glucose biosensing microdroplets with dual-mode fluorescence quenching and size reduction. ACS Appl. Mater. Interfaces 2018, 10, 30172–30179. [Google Scholar] [CrossRef] [PubMed]
- Qin, A.; Jim, C.K.; Tang, Y.; Lam, J.W.; Liu, J.; Mahtab, F.; Gao, P.; Tang, B.Z. Aggregation-enhanced emissions of intramolecular excimers in disubstituted polyacetylenes. J. Phys. Chem. B 2008, 112, 9281–9288. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Lam, J.W.; Tang, B.Z. Self-assembly of organic luminophores with gelation-enhanced emission characteristics. Soft Matter. 2013, 9, 4564–4579. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Ji, X.; Xie, H.; Tang, B.Z. Aggregation-Induced Emission-Active Gels: Fabrications, Functions, and Applications. Adv. Mater. 2021, 33, 2100021. [Google Scholar] [CrossRef]
- Tavakoli, J.; Gascooke, J.; Xie, N.; Tang, B.Z.; Tang, Y. Enlightening Freeze–Thaw Process of Physically Cross-Linked Poly (vinyl alcohol) Hydrogels by Aggregation-Induced Emission Fluorogens. ACS Appl. Polym. Mater. 2019, 1, 1390–1398. [Google Scholar] [CrossRef]
- Tavakoli, J.; Zhang, H.-p.; Tang, B.Z.; Tang, Y. Aggregation-induced emission lights up the swelling process: A new technique for swelling characterisation of hydrogels. Mater. Chem. Front. 2019, 3, 664–667. [Google Scholar] [CrossRef]
- Tavakoli, J.; Laisak, E.; Gao, M.; Tang, Y. AIEgen quantitatively monitoring the release of Ca2+ during swelling and degradation process in alginate hydrogels. Mater. Sci. Eng. C 2019, 104, 109951. [Google Scholar] [CrossRef]
- Xia, Y.; Xue, B.; Qin, M.; Cao, Y.; Li, Y.; Wang, W. Printable Fluorescent Hydrogels Based on Self-Assembling Peptides. Sci. Rep. 2017, 7, 9691. [Google Scholar] [CrossRef]
- Solheim, T.E.; Salvemini, F.; Dalziel, S.B.; Raston, C.L. Neutron imaging and modelling inclined vortex driven thin films. Sci. Rep. 2019, 9, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Britton, J.; Raston, C.L. Multi-step continuous-flow synthesis. Chem. Soc. Rev. 2017, 46, 1250–1271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tavakoli, J.; Joseph, N.; Chuah, C.; Raston, C.L.; Tang, Y. Vortex fluidic enabling and significantly boosting light intensity of graphene oxide with aggregation induced emission luminogen. Mater. Chem. Front. 2020, 4, 2126–2130. [Google Scholar] [CrossRef]
- Luo, X.; Al-Antaki, A.H.M.; Harvey, D.P.; Ruan, Y.; He, S.; Zhang, W.; Raston, C.L. Vortex fluidic mediated synthesis of macroporous bovine serum albumin-based microspheres. ACS Appl. Mater. Interfaces 2018, 10, 27224–27232. [Google Scholar] [CrossRef]
- Vimalanathan, K.; Suarez-Martinez, I.; Peiris, M.C.R.; Antonio, J.; De Tomas, C.; Zou, Y.; Zou, J.; Duan, X.; Lamb, R.N.; Harvey, D.P. Vortex fluidic mediated transformation of graphite into highly conducting graphene scrolls. Nanoscale Adv. 2019, 1, 2495–2501. [Google Scholar] [CrossRef] [Green Version]
- Luo, X.; Mohammed Al-Antaki, A.H.; Igder, A.; Stubbs, K.A.; Su, P.; Zhang, W.; Weiss, G.A.; Raston, C.L. Vortex Fluidic-Mediated Fabrication of Fast Gelated Silica Hydrogels with Embedded Laccase Nanoflowers for Real-Time Biosensing under Flow. ACS Appl. Mater. Interfaces 2020, 12, 51999–52007. [Google Scholar] [CrossRef]
- He, S.; Joseph, N.; Mirzamani, M.; Pye, S.J.; Al-anataki, A.H.M.; Whitten, A.E.; Chen, Y.; Kumari, H.; Raston, C.L. Vortex fluidic mediated encapsulation of functional fish oil featuring in situ probed small angle neutron scattering. NPJ Sci. Food 2020, 4, 12. [Google Scholar] [CrossRef]
- Mathew, M.; Rad, M.; Mata, J.; Mahmodi, H.; Kabakova, I.; Raston, C.; Tang, Y.; Tipper, J.; Tavakoli, J. Hyperbranched polymers tune the physicochemical, mechanical, and biomedical properties of alginate hydrogels. Mater. Today Chem. 2022, 23, 100656. [Google Scholar] [CrossRef]
- Tavakoli, J.; Pye, S.; Reza, A.M.; Xie, N.; Qin, J.; Raston, C.L.; Tang, B.Z.; Tang, Y. Tuning aggregation-induced emission nanoparticle properties under thin film formation. Mater. Chem. Front. 2020, 4, 537–545. [Google Scholar] [CrossRef]
- Tavakoli, J.; Joseph, N.; Raston, C.L.; Tang, Y. A hyper-branched polymer tunes the size and enhances the fluorescent properties of aggregation-induced emission nanoparticles. Nanoscale Adv. 2020, 2, 633–641. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Zhang, W.; Li, J.; Fu, J. A novel LEuH/PVA luminescent hydrogel with ammonia response and self-recovery luminescence behavior. New J. Chem. 2019, 43, 5133–5138. [Google Scholar] [CrossRef]
- Zhi, H.; Fei, X.; Tian, J.; Zhao, L.; Zhang, H.; Jing, M.; Xu, L.; Wang, Y.; Li, Y. A novel high-strength photoluminescent hydrogel for tissue engineering. Biomater. Sci. 2018, 6, 2320–2326. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Qiu, Y.; Chen, G.; Chu, Z.; Shadike, A.; Chen, C.; Chen, C.; Zhu, Z. Self-healable poly (vinyl alcohol) photonic crystal hydrogel. ACS Appl. Polym. Mater. 2020, 2, 2086–2092. [Google Scholar] [CrossRef]
- Yang, Y.; Li, Q.; Zhang, H.; Liu, H.; Ji, X.; Tang, B.Z. Codes in Code: AIE Supramolecular Adhesive Hydrogels Store Huge Amounts of Information. Adv. Mater. 2021, 33, 2105418. [Google Scholar] [CrossRef]
- Tavakoli, J.; Tang, Y. Honey/PVA hybrid wound dressings with controlled release of antibiotics: Structural, physico-mechanical and in-vitro biomedical studies. Mater. Sci. Eng. C 2017, 77, 318–325. [Google Scholar] [CrossRef]
- Yang, F.; Zhao, J.; Koshut, W.J.; Watt, J.; Riboh, J.C.; Gall, K.; Wiley, B.J. A synthetic hydrogel composite with the mechanical behavior and durability of cartilage. Adv. Funct. Mater. 2020, 30, 2003451. [Google Scholar] [CrossRef]
- Tavakoli, J. Physico-mechanical, morphological and biomedical properties of a novel natural wound dressing material. J. Mech. Behav. Biomed. Mater. 2017, 65, 373–382. [Google Scholar] [CrossRef]
- Helminger, M.; Wu, B.; Kollmann, T.; Benke, D.; Schwahn, D.; Pipich, V.; Faivre, D.; Zahn, D.; Cölfen, H. Synthesis and Characterization of Gelatin-Based Magnetic Hydrogels. Adv. Funct. Mater. 2014, 24, 3187–3196. [Google Scholar] [CrossRef] [Green Version]
- Spoljaric, S.; Salminen, A.; Luong, N.D.; Seppälä, J. Stable, self-healing hydrogels from nanofibrillated cellulose, poly (vinyl alcohol) and borax via reversible crosslinking. Eur. Polym. J. 2014, 56, 105–117. [Google Scholar] [CrossRef] [Green Version]
- Zhao, D.; Huang, J.; Zhong, Y.; Li, K.; Zhang, L.; Cai, J. High-strength and high-toughness double-cross-linked cellulose hydrogels: A new strategy using sequential chemical and physical cross-linking. Adv. Funct. Mater. 2016, 26, 6279–6287. [Google Scholar] [CrossRef]
- Thombare, N.; Jha, U.; Mishra, S.; Siddiqui, M.Z. Borax cross-linked guar gum hydrogels as potential adsorbents for water purification. Carbohydr. Polym. 2017, 168, 274–281. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Wang, L.; Zheng, W.; Yang, G.; Jiang, X. Rapid Fabrication of Self-Healing, Conductive, and Injectable Gel as Dressings for Healing Wounds in Stretchable Parts of the Body. Adv. Funct. Mater. 2020, 30, 2002370. [Google Scholar] [CrossRef]
- Luo, X.; Su, P.; Zhang, W.; Raston, C.L. Microfluidic Devices in Fabricating Nano or Micromaterials for Biomedical Applications. Adv. Mater. Technol. 2019, 4, 1900488. [Google Scholar] [CrossRef]
- Britton, J.; Castle, J.W.; Weiss, G.A.; Raston, C.L. Harnessing Thin-Film Continuous-Flow Assembly Lines. Chem. A Eur. J. 2016, 22, 10773–10776. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vimalanathan, K.; Chen, X.; Raston, C.L. Shear induced fabrication of intertwined single walled carbon nanotube rings. Chem. Commun. 2014, 50, 11295–11298. [Google Scholar] [CrossRef] [PubMed]
- Britton, J.; Dyer, R.P.; Majumdar, S.; Raston, C.L.; Weiss, G.A. Ten-Minute Protein Purification and Surface Tethering for Continuous-Flow Biocatalysis. Angew. Chem. Int. Ed. 2017, 56, 2296–2301. [Google Scholar] [CrossRef]
- Tavakoli, J.; Raston, C.L.; Ma, Y.; Tang, Y. Vortex fluidic mediated one-step fabrication of polyvinyl alcohol hydrogel films with tunable surface morphologies and enhanced self-healing properties. Sci. China Mater. 2020, 63, 1310–1317. [Google Scholar] [CrossRef] [Green Version]
- Heo, Y.J.; Shibata, H.; Okitsu, T.; Kawanishi, T.; Takeuchi, S. Long-term in vivo glucose monitoring using fluorescent hydrogel fibers. Proc. Natl. Acad. Sci. USA 2011, 108, 13399–13403. [Google Scholar] [CrossRef] [Green Version]
- Liu, K.; Xing, R.; Zou, Q.; Ma, G.; Möhwald, H.; Yan, X. Simple peptide-tuned self-assembly of photosensitizers towards anticancer photodynamic therapy. Angew. Chem. 2016, 128, 3088–3091. [Google Scholar] [CrossRef]
- Sun, P.; Wang, Z.; Bi, Y.; Sun, D.; Zhao, T.; Zhao, F.; Wang, W.; Xin, X. Self-Assembly-Driven Aggregation-Induced Emission of Silver Nanoclusters for Light Conversion and Temperature Sensing. ACS Appl. Nano Mater. 2020, 3, 2038–2046. [Google Scholar] [CrossRef]
- Cheng, X.; Sun, P.; Zhang, N.; Zhou, S.; Xin, X. Self-assembly of silver nanoclusters and phthalic acid into hollow tubes as a superior sensor for Fe3+. J. Mol. Liq. 2021, 323, 115032. [Google Scholar] [CrossRef]
- Liu, Y.; Deng, C.; Tang, L.; Qin, A.; Hu, R.; Sun, J.Z.; Tang, B.Z. Specific detection of D-glucose by a tetraphenylethene-based fluorescent sensor. J. Am. Chem. Soc. 2011, 133, 660–663. [Google Scholar] [CrossRef] [PubMed]
- Zhao, E.; Hong, Y.; Chen, S.; Leung, C.W.; Chan, C.Y.; Kwok, R.T.; Lam, J.W.; Tang, B.Z. Highly fluorescent and photostable probe for long-term bacterial viability assay based on aggregation-induced emission. Adv. Healthc. Mater. 2014, 3, 88–96. [Google Scholar] [CrossRef] [PubMed]
- He, T.; Ou, W.; Tang, B.Z.; Qin, J.; Tang, Y. In vivo Visualization of the Process of Hg2+ Bioaccumulation in Water Flea Daphnia carinata by a Novel Aggregation-Induced Emission Fluorogen. Chem. Asian J. 2019, 14, 796–801. [Google Scholar] [CrossRef] [Green Version]
- Qin, A.; Lam, J.W.; Tang, B.Z. Luminogenic polymers with aggregation-induced emission characteristics. Prog. Polym. Sci. 2012, 37, 182–209. [Google Scholar] [CrossRef] [Green Version]
- Shaha, R.K.; Jiang, Z.; Frick, C.P.; Oakey, J. Cell-Laden Particulate-Composite Hydrogels with Tunable Mechanical Properties Constructed with Gradient-Interface Hydrogel Particles. ACS Appl. Polym. Mater. 2019, 1, 2571–2576. [Google Scholar] [CrossRef]
- Qiu, Z.; Liu, X.; Lam, J.W.; Tang, B.Z. The Marriage of Aggregation-Induced Emission with Polymer Science. Macromol. Rapid Commun. 2019, 40, 1800568. [Google Scholar] [CrossRef]
- Huang, Y.; Chen, P.; Wei, B.; Hu, R.; Tang, B.Z. Aggregation-induced emission-active hyperbranched poly (tetrahydropyrimidine) s synthesized from multicomponent tandem polymerization. Chin. J. Polym. Sci. 2019, 37, 428–436. [Google Scholar] [CrossRef]
- Zheng, W.; Yang, G.; Jiang, S.-T.; Shao, N.; Yin, G.-Q.; Xu, L.; Li, X.; Chen, G.; Yang, H.-B. A tetraphenylethylene (TPE)-based supra-amphiphilic organoplatinum (ii) metallacycle and its self-assembly behaviour. Mater. Chem. Front. 2017, 1, 1823–1828. [Google Scholar] [CrossRef]








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tavakoli, J.; Shrestha, J.; Bazaz, S.R.; Rad, M.A.; Warkiani, M.E.; Raston, C.L.; Tipper, J.L.; Tang, Y. Developing Novel Fabrication and Optimisation Strategies on Aggregation-Induced Emission Nanoprobe/Polyvinyl Alcohol Hydrogels for Bio-Applications. Molecules 2022, 27, 1002. https://doi.org/10.3390/molecules27031002
Tavakoli J, Shrestha J, Bazaz SR, Rad MA, Warkiani ME, Raston CL, Tipper JL, Tang Y. Developing Novel Fabrication and Optimisation Strategies on Aggregation-Induced Emission Nanoprobe/Polyvinyl Alcohol Hydrogels for Bio-Applications. Molecules. 2022; 27(3):1002. https://doi.org/10.3390/molecules27031002
Chicago/Turabian StyleTavakoli, Javad, Jesus Shrestha, Sajad R. Bazaz, Maryam A. Rad, Majid E. Warkiani, Colin L. Raston, Joanne L. Tipper, and Youhong Tang. 2022. "Developing Novel Fabrication and Optimisation Strategies on Aggregation-Induced Emission Nanoprobe/Polyvinyl Alcohol Hydrogels for Bio-Applications" Molecules 27, no. 3: 1002. https://doi.org/10.3390/molecules27031002
APA StyleTavakoli, J., Shrestha, J., Bazaz, S. R., Rad, M. A., Warkiani, M. E., Raston, C. L., Tipper, J. L., & Tang, Y. (2022). Developing Novel Fabrication and Optimisation Strategies on Aggregation-Induced Emission Nanoprobe/Polyvinyl Alcohol Hydrogels for Bio-Applications. Molecules, 27(3), 1002. https://doi.org/10.3390/molecules27031002











