Synthesis and Preliminary Evaluation of the Cytotoxicity of Potential Metabolites of Quinoline Glycoconjugates
Abstract
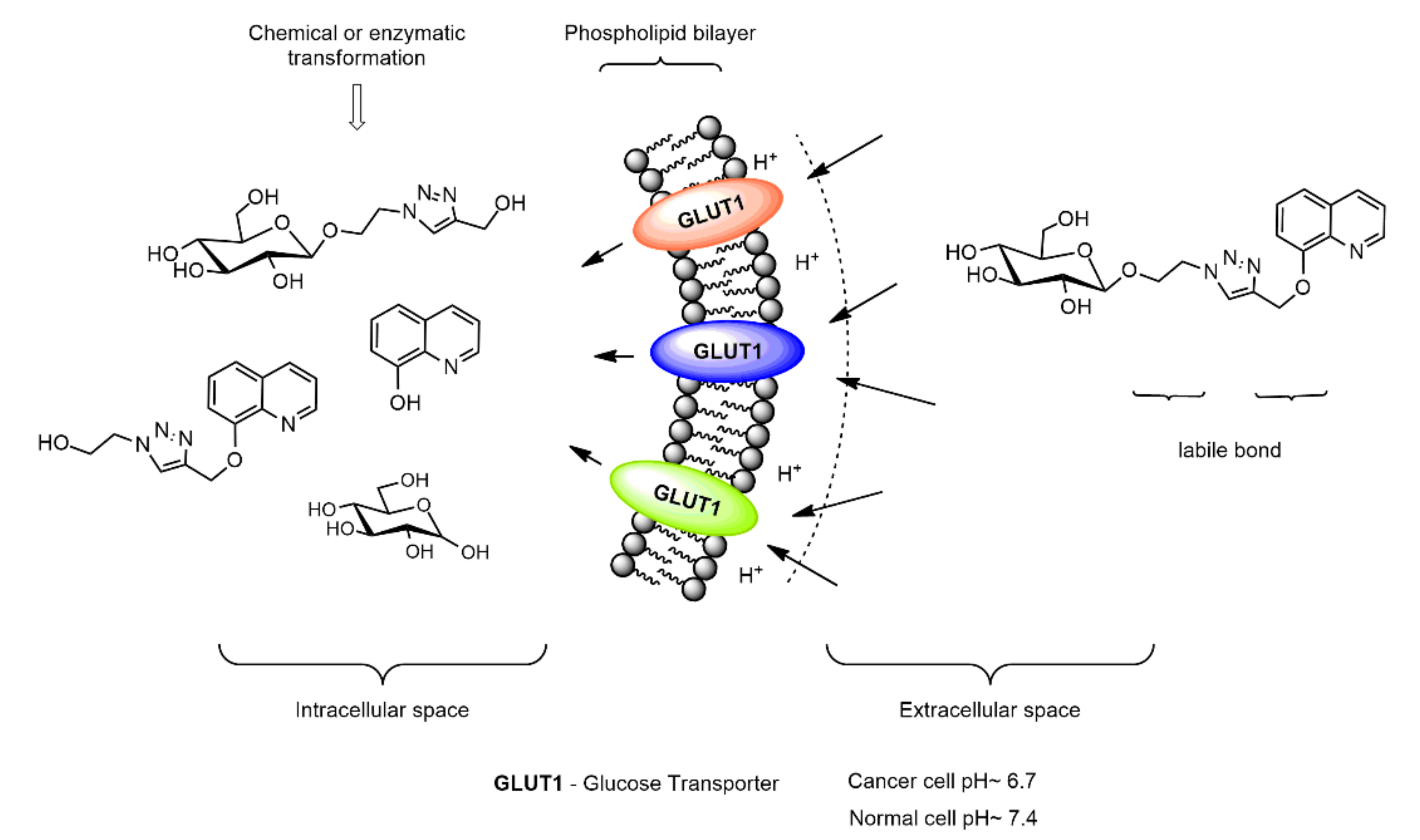
:1. Introduction
2. Results and Discussion
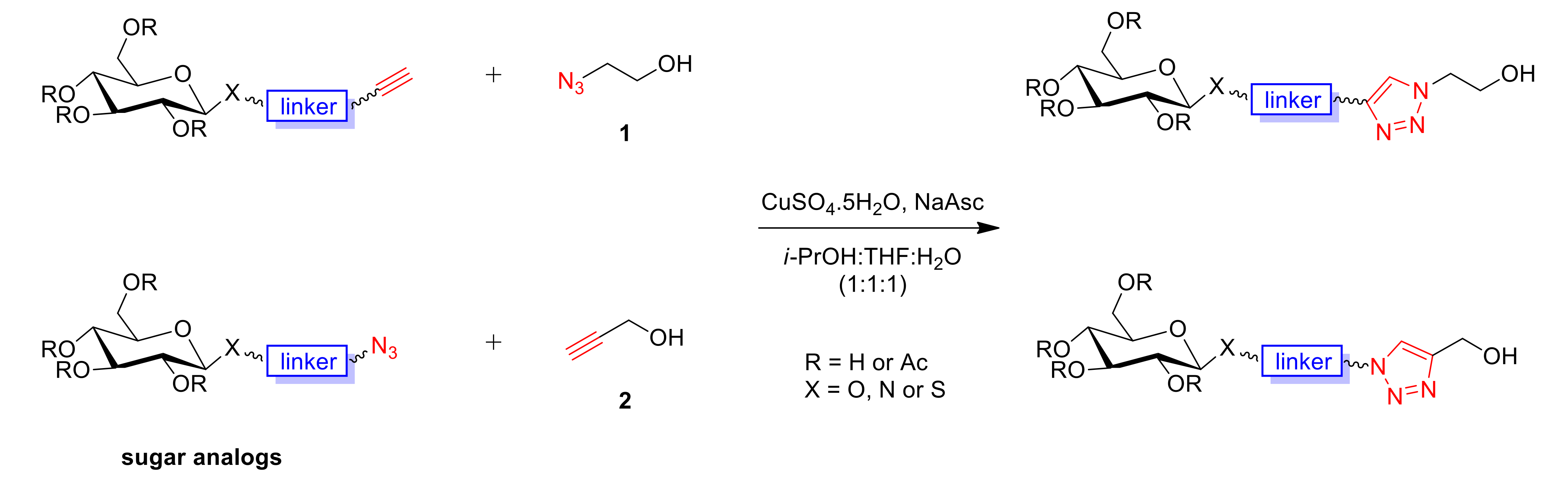
2.1. Synthesis
2.2. Cytotoxicity Studies
3. Materials and Methods
3.1. General Information
3.2. Chemistry
3.2.1. General Procedure for the Synthesis of Metabolites M1–M22
3.2.2. Preparation and Characterization of Micelles
3.3. Biological Evaluation
3.3.1. Cell Cultures
3.3.2. MTT Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Rautio, J.; Kumpulainen, H.; Heimbach, T.; Oliyai, R.; Oh, D.; Järvinen, T.; Savolainen, J. Prodrugs: Design and clinical appli-cations. Nat. Rev. Drug Discov. 2008, 7, 255–270. [Google Scholar] [CrossRef] [PubMed]
- Kratz, F.; Müller, I.A.; Ryppa, C.; Warnecke, A. Prodrug Strategies in Anticancer Chemotherapy. ChemMedChem 2008, 3, 20–53. [Google Scholar] [CrossRef] [PubMed]
- Markovic, M.; Ben-Shabat, S.; Dahan, A. Prodrugs for Improved Drug Delivery: Lessons Learned from Recently Developed and Marketed Products. Pharmaceutics 2020, 12, 1031. [Google Scholar] [CrossRef] [PubMed]
- Arpicco, S.; Dosio, F.; Stella, B.; Cattel, L. Anticancer prodrugs: An overview of major strategies and recent developments. Curr. Top. Med. Chem. 2011, 11, 2346–2381. [Google Scholar] [CrossRef]
- Mura, S.; Nicolas, J.; Couvreur, P. Stimuli-responsive nanocarriers for drug delivery. Nat. Mater. 2013, 12, 991–1003. [Google Scholar] [CrossRef]
- Danhier, F.; Feron, O.; Préat, V. To Exploit the Tumor Microenvironment: Passive and Active Tumor Targeting of Nanocarri-ers for Anti-cancer Drug Delivery. J. Control. Release 2010, 148, 135–146. [Google Scholar] [CrossRef]
- Warburg, O. On the Origin of Cancer Cells. Science 1956, 123, 309–314. [Google Scholar] [CrossRef]
- Vander Heiden, M.G.; Cantley, L.C.; Thompson, C. Understanding the Warburg Effect: The Metabolic Requirements of Cell Proliferation. Science 2009, 324, 1029–1033. [Google Scholar] [CrossRef] [Green Version]
- Barron, C.C.; Bilan, P.J.; Tsakiridis, T.; Tsiani, E. Facilitative glucose transporters: Implications for cancer detection, prognosis and treatment. Metabolism 2016, 65, 124–139. [Google Scholar] [CrossRef]
- Tanasova, M.; Fedie, J.R. Molecular Tools for Facilitative Carbohydrate Transporters (Gluts). ChemBioChem 2017, 18, 1774–1788. [Google Scholar] [CrossRef]
- Szablewski, L. Expression of glucose transporters in cancers. Biochim. Biophys. Acta 2013, 1835, 164–169. [Google Scholar] [CrossRef]
- Calvaresi, E.C.; Hergenrother, P.J. Glucose conjugation for the specific targeting and treatment of cancer. Chem. Sci. 2013, 4, 2319–2333. [Google Scholar] [CrossRef] [Green Version]
- Granchi, C.; Fortunato, S.; Minutolo, F. Anticancer agents interacting with membrane glucose transporters. MedChemComm 2016, 7, 1716–1729. [Google Scholar] [CrossRef] [Green Version]
- Ding, W.Q.; Lind, S.E. Metal Ionophores—An Emerging Class of Anticancer Drugs. IUBMB Life 2009, 61, 1013–1018. [Google Scholar] [CrossRef]
- Santini, C.; Pellei, M.; Gandin, V.; Porchia, M.; Tisato, F.; Marzano, C. Advances in Copper Complexes as Anticancer Agents. Chem. Rev. 2014, 114, 815–862. [Google Scholar] [CrossRef]
- Wang, X.; Wang, X.; Jin, S.; Muhammad, N.; Guo, Z. Stimuli-Responsive Therapeutic Metallodrugs. Chem. Rev. 2019, 119, 1138–1192. [Google Scholar] [CrossRef]
- Gupte, A.; Mumper, R.J. Elevated copper and oxidative stress in cancer cells as a target for cancer treatment. Cancer Treat. Rev. 2009, 35, 32–46. [Google Scholar] [CrossRef]
- Gaur, K.; Vázquez-Salgado, A.M.; Duran-Camacho, G.; Dominguez-Martinez, I.; Benjamín-Rivera, J.A.; Fernández-Vega, L.; Carmona Sarabia, L.; Cruz García, A.; Pérez-Deliz, F.; Méndez Román, J.A.; et al. Iron and Copper Intracellular Chelation as an Anticancer Drug Strategy. Inorganics 2018, 6, 126. [Google Scholar] [CrossRef] [Green Version]
- Denoyer, D.; Masaldan, S.; La Fontaine, S.; Cater, M.A. Targeting copper in cancer therapy: “Copper That Cancer”. Metallomics 2015, 7, 1459–1476. [Google Scholar] [CrossRef]
- Song, Y.; Xu, H.; Chen, W.; Zhan, P.; Liu, X. 8-Hydroxyquinoline: A privileged structure with a broad-ranging pharmacolog-ical potential. Med. Chem. Commun. 2015, 6, 61–74. [Google Scholar] [CrossRef]
- Prachayasittikul, V.; Prachayasittikul, S.; Ruchirawat, S. 8-Hydroxyquinolines: A review of their metal chelating properties and medicinal applications. Drug Des. Dev. Ther. 2013, 7, 1157–1178. [Google Scholar] [CrossRef] [Green Version]
- Savić-Gajić, I.M.; Savic, I.M. Drug design strategies with metal-hydroxyquinoline complexes. Expert Opin. Drug Discov. 2020, 15, 383–390. [Google Scholar] [CrossRef]
- Oliveri, V.; Vecchio, G. 8-Hydroxyquinolines in medicinal chemistry: A structural perspective. Eur. J. Med. Chem. 2016, 120, 252–274. [Google Scholar] [CrossRef]
- Ma, J.; Yang, X.; Hao, W.; Huang, Z.; Wang, X.; Wang, P.G. Mono-functionalized glycosylated platinum(IV) complexes possessed both pH and redox dual-responsive properties: Exhibited enhanced safety and preferentially accumulated in cancer cells in vitro and in vivo. Eur. J. Med. Chem. 2017, 128, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Patra, M.; Johnstone, T.C.; Suntharalingam, K.; Lippard, S.J. A Potent Glucose−Platinum Conjugate Exploits Glucose Trans-porters and Preferentially Accumulates in Cancer Cells. Angew. Chem. Int. Ed. 2016, 55, 2550–2554. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krawczyk, M.; Pastuch-Gawolek, G.; Mrozek-Wilczkiewicz, A.; Kuczak, M.; Skonieczna, M.; Musiol, R. Synthesis of 8-hydroxyquinoline glycoconjugates and preliminary assay of their β1,4-GalT inhibitory and anti-cancer properties. Bioorg. Chem. 2019, 84, 326–338. [Google Scholar] [CrossRef] [PubMed]
- Krawczyk, M.; Pastuch-Gawołek, G.; Pluta, A.; Erfurt, K.; Domiński, A.; Kurcok, P. 8-Hydroxyquinoline Glycoconjugates: Modifications in the Linker Structure and Their Effect on the Cytotoxicity of the Obtained Compounds. Molecules 2019, 24, 4181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krawczyk, M.; Pastuch-Gawołek, G.; Hadasik, A.; Erfurt, K. 8-Hydroxyquinoline Glycoconjugates Containing Sulfur at the Sugar Anomeric Position—Synthesis and Preliminary Evaluation of Their Cytotoxicity. Molecules 2020, 25, 4174. [Google Scholar] [CrossRef]
- Dheer, D.; Singh, V.; Shankar, R. Medicinal attributes of 1,2,3-triazoles: Current developments. Bioorganic Chem. 2017, 71, 30–54. [Google Scholar] [CrossRef]
- Liang, L.; Astruc, D. The copper(I)-catalyzed alkyne-azide cycloaddition (CuAAC) “click” reaction and its applications. An overview. Coord. Chem. Rev. 2011, 255, 2933–2945. [Google Scholar] [CrossRef]
- Kolb, H.C.; Finn, M.G.; Sharpless, K.B. Click Chemistry: Diverse Chemical Function from a Few Good Reactions. Angew. Chem. Int. Ed. 2001, 40, 2004–2021. [Google Scholar] [CrossRef]
- Tiwari, V.K.; Mishra, B.B.; Mishra, K.B.; Mishra, N.; Singh, A.S.; Chen, X. Cu-Catalyzed Click Reaction in Carbohydrate Chemistry. Chem. Rev. 2016, 116, 3086–3240. [Google Scholar] [CrossRef]
- Semenov, S.N.; Belding, L.; Cafferty, B.J.; Mousavi, M.P.S.; Finogenova, A.M.; Cruz, R.S.; Skorb, E.V.; Whitesides, G.M. Au-tocatalytic Cycles in a Copper-Catalyzed Azide–Alkyne Cycloaddition Reaction. J. Am. Chem. Soc. 2018, 140, 10221–10232. [Google Scholar] [CrossRef]
- Haber, R.S.; Rathan, A.; Weiser, K.R.; Pritsker, A.; Itzkowitz, S.H.; Bodian, C.; Slater, G.; Weiss, A.; Burstein, D.E. GLUT1 Glucose Transporter Expression in Colorectal Carcinoma: A marker for poor prognosis. Cancer 1998, 83, 34–40. [Google Scholar] [CrossRef]
- Brown, R.S.; Wahl, R.L. Overexpression of Glut-1 Glucose Transporter in Human Breast Cancer. Cancer 1993, 72, 2979–2985. [Google Scholar] [CrossRef]
- Gupta, S.K.; Shukla, V.K.; Vaidya, M.P.; Roy, S.K.; Gupta, S. Serum and tissue trace elements in colorectal cancer. J. Surg. Oncol. 1993, 52, 172–175. [Google Scholar] [CrossRef]
- Kuo, H.W.; Chen, S.F.; Wu, C.C.; Chen, D.R.; Lee, J.H. Serum and Tissue Trace Elements in Patients with Breast Cancer in Taiwan. Biol. Trace Element Res. 2002, 89, 1–11. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Domiński, A.; Konieczny, T.; Duale, K.; Krawczyk, M.; Pastuch-Gawołek, G.; Kurcok, P. Stimuli-Responsive Aliphatic Poly-carbonate Nanocarriers for Tumor-Targeted Drug Delivery. Polymers 2020, 12, 2890. [Google Scholar] [CrossRef]
- Kamaly, K.; Yameen, B.; Wu, J.; Farokhzad, O.C. Degradable Controlled-Release Polymers and Polymeric Nanoparticles: Mechanisms of Controlling Drug Release. Chem. Rev. 2016, 116, 2602–2663. [Google Scholar] [CrossRef] [Green Version]
- Kato, Y.; Ozawa, S.; Miyamoto, C.; Maehata, Y.; Suzuki, A.; Maeda, T.; Baba, Y. Acidic extracellular microenvironment and cancer. Cancer Cell Int. 2013, 13, 89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Domiński, A.; Krawczyk, M.; Konieczny, T.; Kasprów, M.; Foryś, A.; Pastuch-Gawołek, G.; Kurcok, P. Biodegradable pH-responsive micelles loaded with 8-hydroxyquinoline glycoconjugates for Warburg effect based tumor targeting. Eur. J. Pharm. Biopharm. 2020, 154, 317–329. [Google Scholar] [CrossRef] [PubMed]




 | ||||||||
|---|---|---|---|---|---|---|---|---|
| Compound number | Structure | Activity IC50 [µM] a | Glycoconjugate Structure d | Activity IC50 [µM] | ||||
| HCT-116b | MCF-7c | NHDFb | HCT-116 | MCF-7 | NHDF | |||
| M1 | 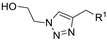 | >800 | >800 | - |  | 239.95 ± 2.27 | 105.91 ± 4.06 | 216.12 ± 9.68 |
| M2 |  | >800 | >800 | - | 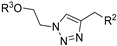 | 290.62 ± 7.02 | 135.97 ± 1.47 | 715.16 ± 10.63 |
| M3 | 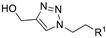 | >800 | >800 | - |  | 216.95 ± 4.73 | 196.49 ± 1.91 | 405.85 ± 5.73 |
| M4 | 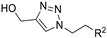 | >800 | 750.45 ± 1.07 | - | 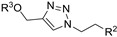 | 229.56 ± 2.59 | 375.58 ± 8.34 | - |
| M5 | 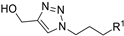 | >800 | 602.95 ± 1.95 | - | 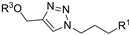 | 142.98 ± 2.30 | 200.60 ± 1.10 | 214.75 ± 6.43 |
| M6 | 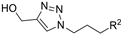 | 469.82 ± 8.61 | 194.13 ± 0.18 | 202.02 ± 3.29 | 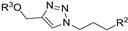 | 135.07 ± 6.98 | 221.11 ± 2.40 | 426.80 ± 3.80 |
| M7 | 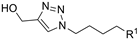 | 196.18 ± 3.55 | 155.96 ± 0.45 | 131.99 ± 1.00 | 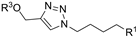 | 328.75 ± 9.02 | 254.81 ± 3.63 | - |
| M8 | 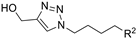 | 564.85 ± 3.59 | 286.01± 1.42 | - | 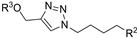 | 294.74 ± 1.79 | 214.83 ± 1.65 | >800 |
 | ||||||||
|---|---|---|---|---|---|---|---|---|
| Compound Number | Structure | Activity IC50 [µM] a | Glycoconjugate Structure d | Activity IC50 [µM] | ||||
| HCT-116 b | MCF-7 c | NHDF b | HCT-116 | MCF-7 | NHDF | |||
| M9 |  | >800 | >800 | - | 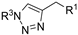 | 69.00 ± 2.53 | 57.69 ± 3.32 | 57.37 ± 3.19 |
| M10 | 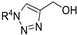 | >800 | >800 | - |  | 212.00 ± 7.71 | 185.34 ± 2.21 | 247.24 ± 11.64 |
| M11 |  | >800 | >800 | - | 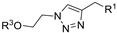 | 239.95 ± 2.27 | 105.91 ± 4.06 | 216.12 ± 9.68 |
| M12 | 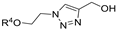 | >800 | >800 | - | 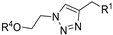 | >800 | >800 | - |
| M13 |  | >800 | >800 | - | 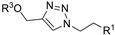 | 216.95 ± 4.73 | 196.49 ± 1.91 | 405.85 ± 5.73 |
| M14 | 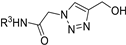 | 258.32 ± 2.06 | 428.66 ± 2.11 | 101.15 ± 4.98 | 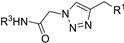 | 246.24 ± 6.19 | 192.66 ± 3.71 | 219.14 ± 2.40 |
| M15 | 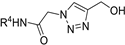 | 747.66 ± 8.29 | >800 | - | 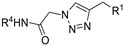 | 112.79 ± 1.58 | 87.89 ± 4.11 | 94.69 ± 0.46 |
| M16 | 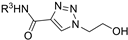 | >800 | >800 | - |  | 239.05 ± 2.97 | 203.78 ± 3.55 | 382.61 ± 2.42 |
| M17 |  | >800 | >800 | - | 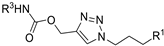 | 246.23 ± 1.31 | 176.40 ± 1.81 | 696.74 ± 1.60 |
| M18 |  | 107.24 ± 2.17 | 248.77 ± 1.58 | 89.07 ± 8.63 | 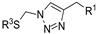 | 106.71 ± 4.10 | 59.12 ± 1.46 | 54.62 ± 0.74 |
| M19 | 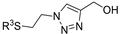 | >800 | 792.99 ± 1.30 | - | 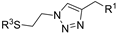 | 127.05 ± 1.75 | 76.30 ± 1.33 | 105.32 ± 3.40 |
| M20 | 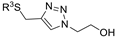 | >800 | >800 | - |  | 172.83 ± 3.48 | 153.34 ± 0.25 | 229.12 ± 2.06 |
| M21 | 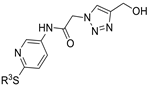 | >800 | >800 | - | 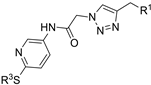 | 146.16 ± 3.49 | 69.72 ± 3.50 | 71.81 ± 6.70 |
| M22 | 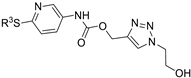 | >800 | >800 | - | 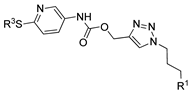 | 63.49 ± 2.37 | 67.50 ± 1.58 | 64.00 ± 5.34 |
| Compound Number | Structure | Activity IC50 [µM] a | ||
|---|---|---|---|---|
| HCT-116 b | MCF-7 b | NHDF b | ||
| M5 | 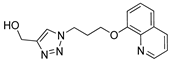 | >800 | 602.95 ± 1.95 | 509.52 ± 3.26 |
| M5-micelles | 23.59 ± 1.44 | 33.04 ± 1.73 | 59.54 ± 2.81 | |
| M7 | 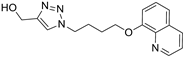 | 169.19 ± 3.90 | 155.96 ± 0.45 | 85.14 ± 4.16 |
| M7-micelles | 12.41 ± 0.41 | 4.46 ± 0.36 | 45.76 ± 1.78 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Domińska, M.; Pastuch-Gawołek, G.; Domiński, A.; Kurcok, P.; Erfurt, K. Synthesis and Preliminary Evaluation of the Cytotoxicity of Potential Metabolites of Quinoline Glycoconjugates. Molecules 2022, 27, 1040. https://doi.org/10.3390/molecules27031040
Domińska M, Pastuch-Gawołek G, Domiński A, Kurcok P, Erfurt K. Synthesis and Preliminary Evaluation of the Cytotoxicity of Potential Metabolites of Quinoline Glycoconjugates. Molecules. 2022; 27(3):1040. https://doi.org/10.3390/molecules27031040
Chicago/Turabian StyleDomińska, Monika, Gabriela Pastuch-Gawołek, Adrian Domiński, Piotr Kurcok, and Karol Erfurt. 2022. "Synthesis and Preliminary Evaluation of the Cytotoxicity of Potential Metabolites of Quinoline Glycoconjugates" Molecules 27, no. 3: 1040. https://doi.org/10.3390/molecules27031040
APA StyleDomińska, M., Pastuch-Gawołek, G., Domiński, A., Kurcok, P., & Erfurt, K. (2022). Synthesis and Preliminary Evaluation of the Cytotoxicity of Potential Metabolites of Quinoline Glycoconjugates. Molecules, 27(3), 1040. https://doi.org/10.3390/molecules27031040







