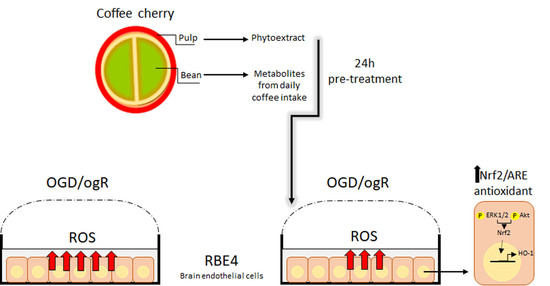
Coffee-Derived Phenolic Compounds Activate Nrf2 Antioxidant Pathway in I/R Injury In Vitro Model: A Nutritional Approach Preventing Age Related-Damages
Abstract
:1. Introduction
2. Results and Discussion
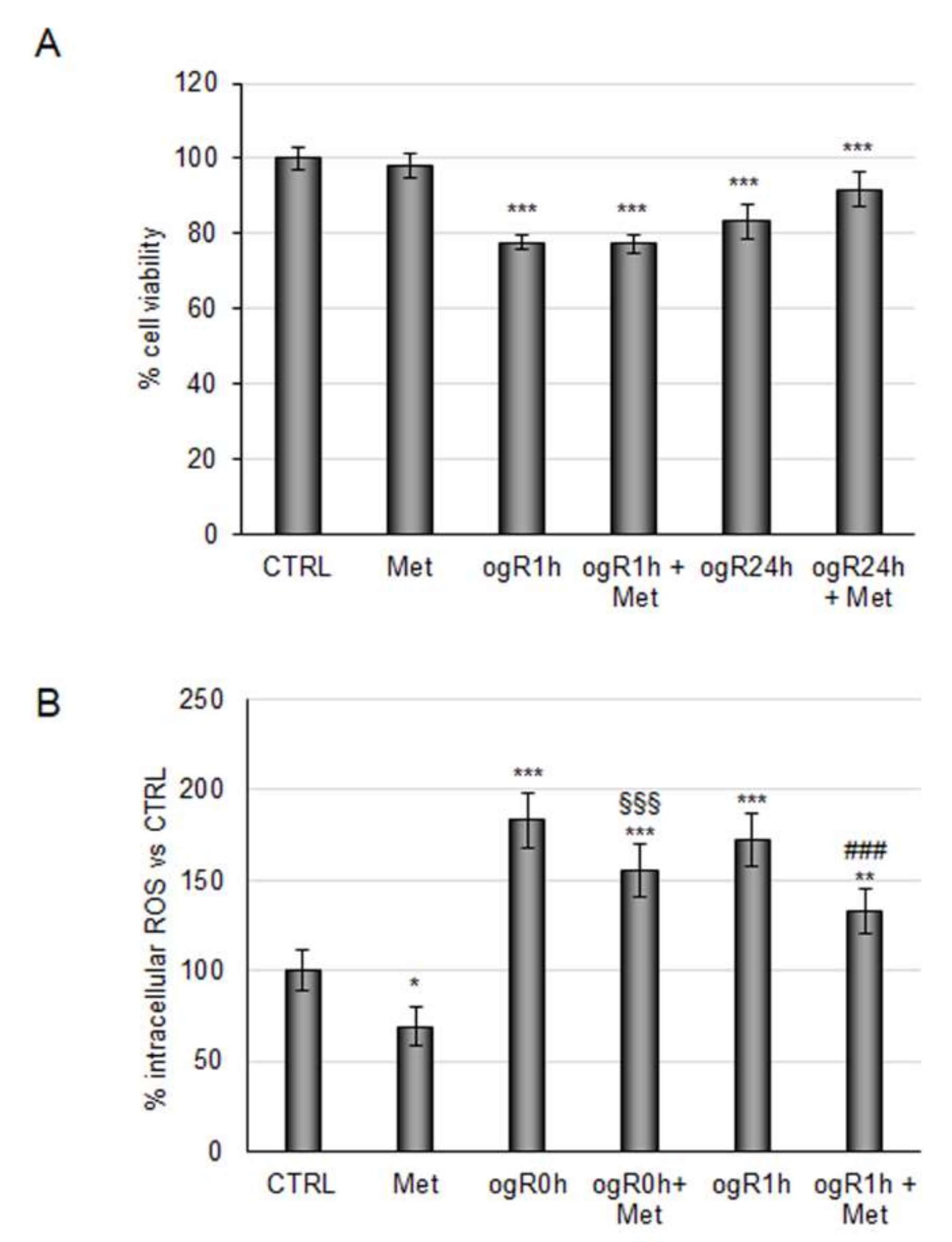
2.1. Coffee Metabolites Antioxidant Effect under Conditions Mimic Ischemia
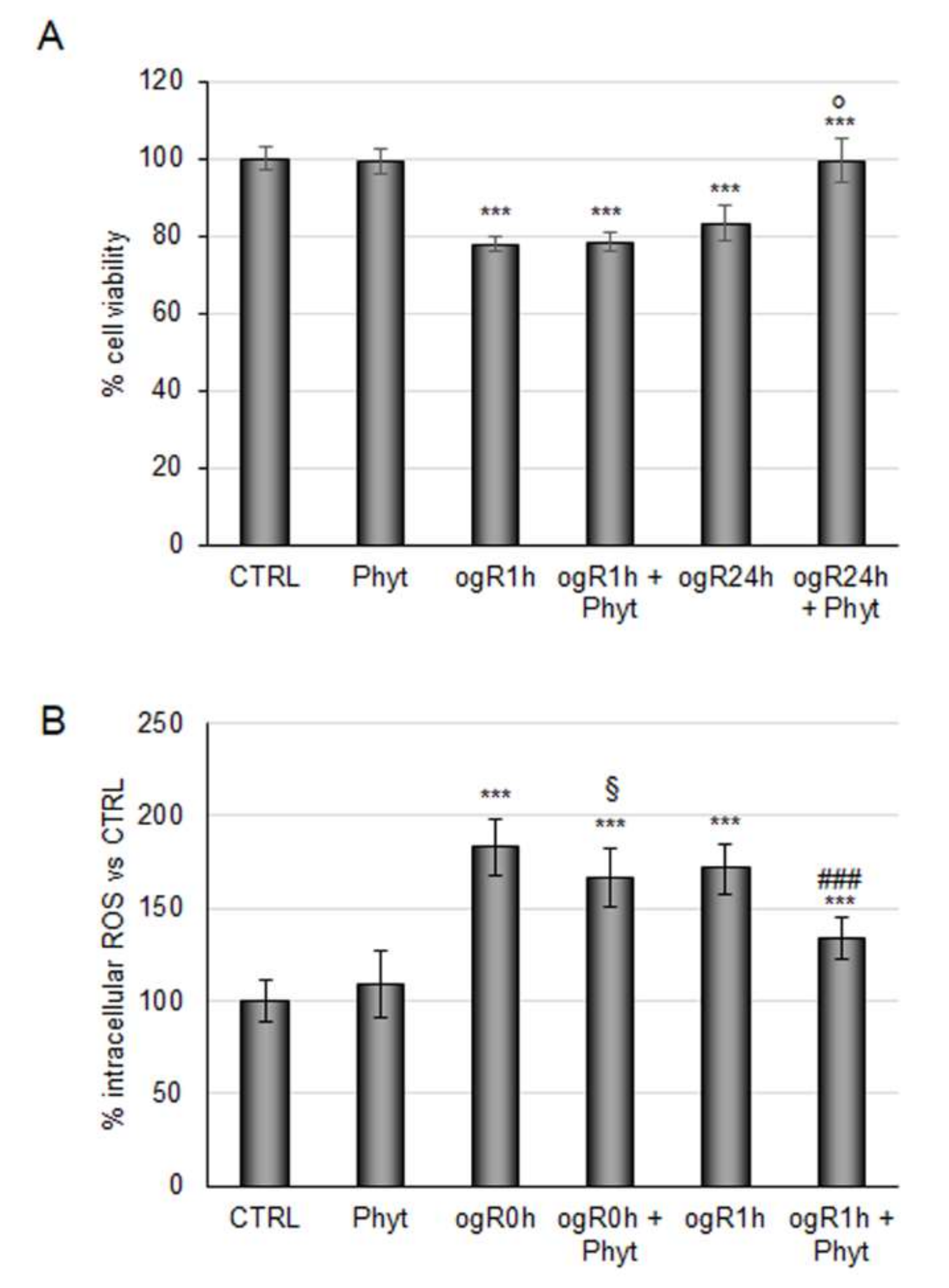
2.2. Evaluation of Antioxidant Power of Coffee Pulp Phytoextract under Conditions Mimic Ischemia
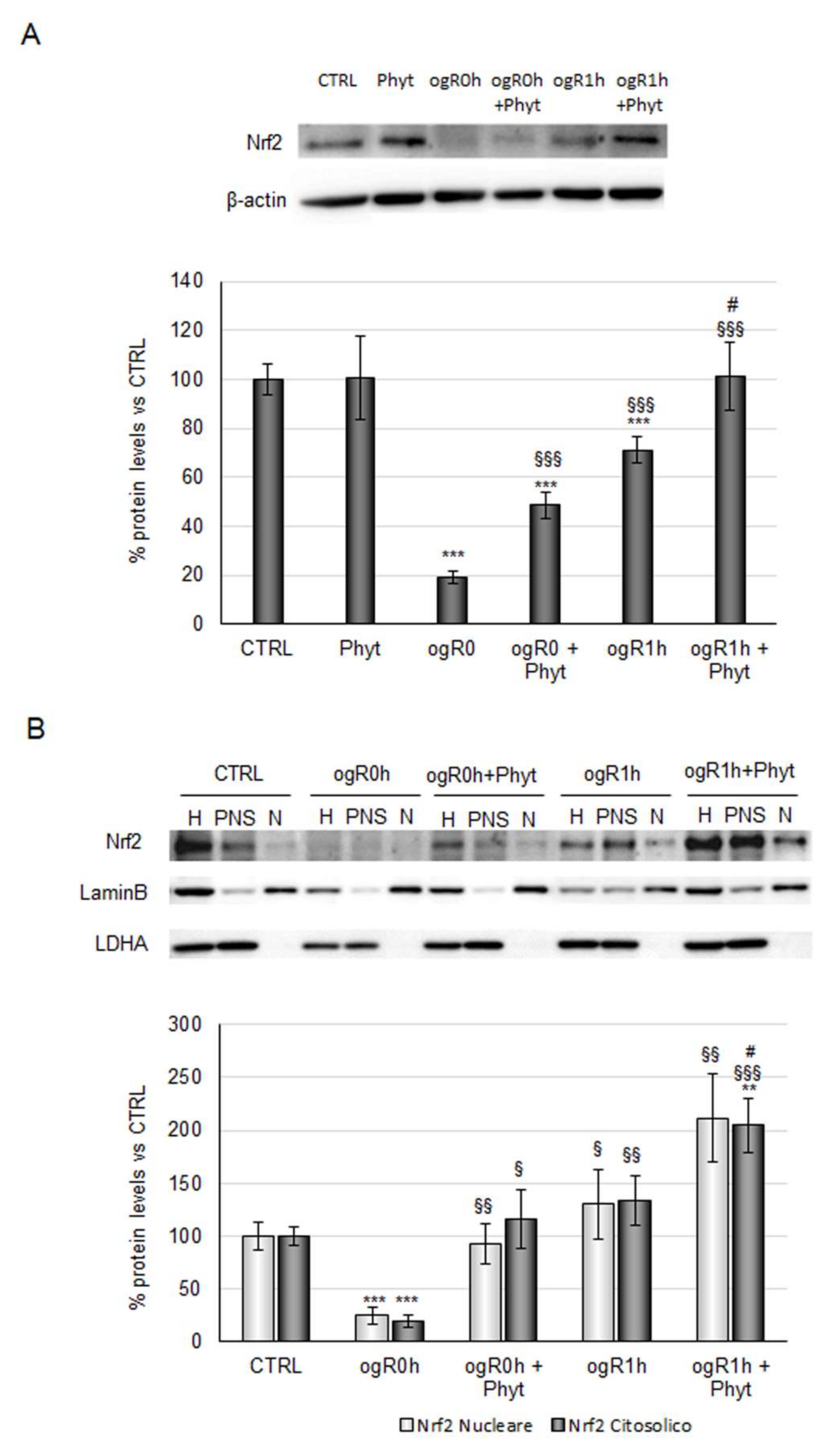
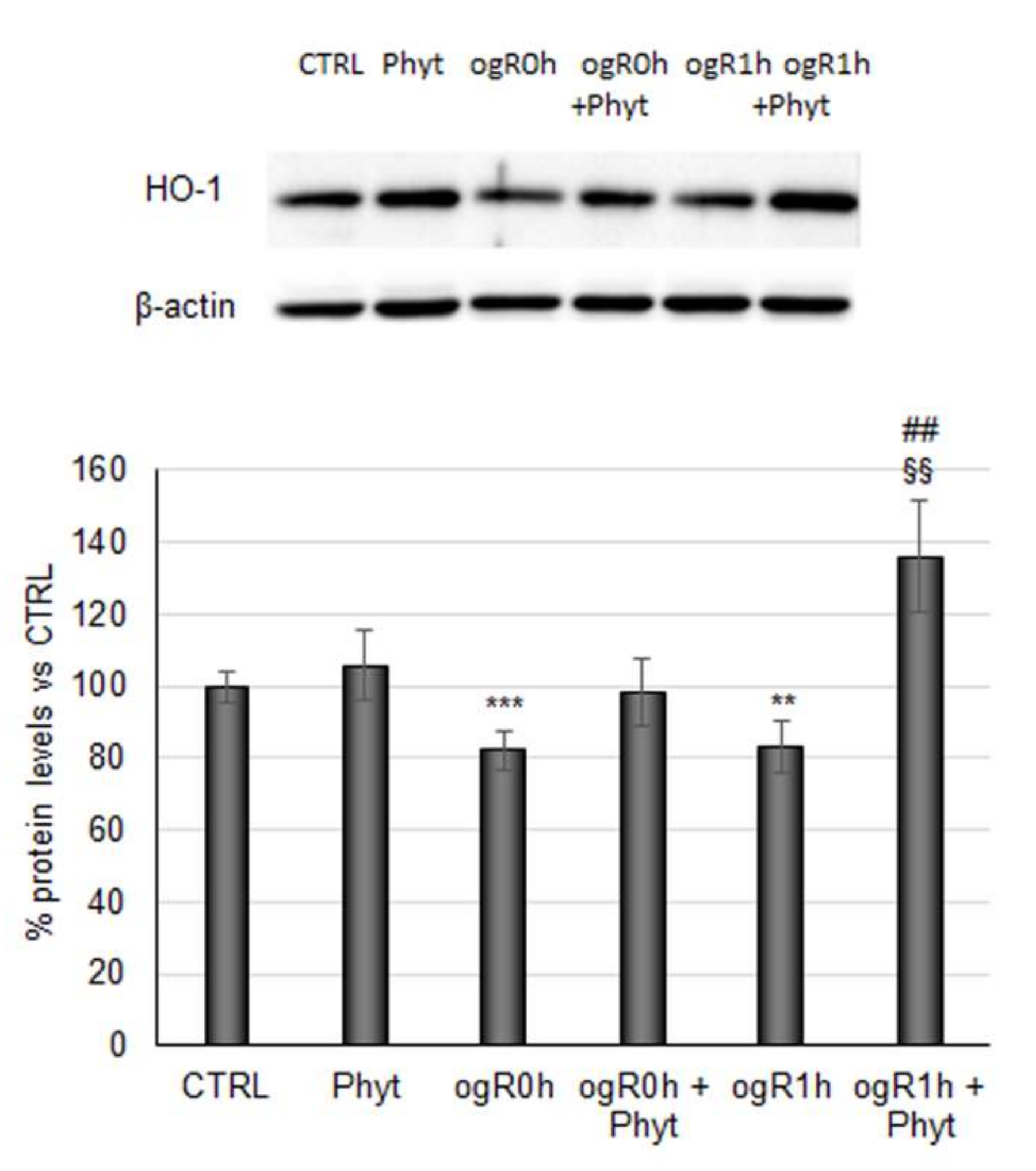
2.3. Coffee Pulp Phytoextract Induced the Nrf2 Antioxidant Pathway under OGD/ogR
3. Materials and Methods
3.1. Materials
3.2. Rat Brain Endothelial Cell Line (RBE4)
3.3. Coffee Phenolic Metabolites
3.4. Coffee Pulp Extraction Process and LC-MS Analysis
3.5. Tert-Butyl Hydroperoxide (TBHP) Treatment
3.6. Cell Viability Analysis
3.7. Determination of Intracellular Reactive Oxygen Species (ROS)
3.8. Oxygen and Glucose Deprivation (OGD) Treatment
3.9. Cell Fractionation
3.10. SDS-PAGE and Immunoblotting
3.11. Statistic Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Sies, H.; Berndt, C.; Jones, D.P. Oxidative Stress. Annu. Rev. Biochem. 2017, 86, 715–748. [Google Scholar] [CrossRef] [PubMed]
- Sies, H. Introductory Remarks. In Oxidative Stress; Sies, H., Ed.; Academic Press: London, UK, 1985; pp. 1–8. [Google Scholar]
- Cenini, G.; Lloret, A.; Cascella, R. Oxidative Stress in Neurodegenerative Diseases: From a Mitochondrial Point of View. Oxidative Med. Cell. Longev. 2019, 2019, 2105607. [Google Scholar] [CrossRef] [Green Version]
- Lin, D.; Wang, L.; Yan, S.; Zhang, Q.; Zhang, J.H.; Shao, A. The Role of Oxidative Stress in Common Risk Factors and Mechanisms of Cardio-Cerebrovascular Ischemia and Depression. Oxidative Med. Cell. Longev. 2019, 2019, 2491927. [Google Scholar] [CrossRef] [PubMed]
- Verhoeven, J.I.; Allach, Y.; Vaartjes, I.C.H.; Klijn, C.J.M.; de Leeuw, F.E. Ambient air pollution and the risk of ischaemic and haemorrhagic stroke. Lancet Planet. Health 2021, 5, e542–e552. [Google Scholar] [CrossRef]
- Guo, M.; Lu, H.; Qin, J.; Qu, S.; Wang, W.; Guo, Y.; Liao, W.; Song, M.; Chen, J.; Wang, Y. Biochanin A Provides Neuroprotection Against Cerebral Ischemia/Reperfusion Injury by Nrf2-Mediated Inhibition of Oxidative Stress and Inflammation Signaling Pathway in Rats. Med. Sci. Monit. 2019, 25, 8975–8983. [Google Scholar] [CrossRef] [PubMed]
- Panuganti, K.K.; Tadi, P.; Lui, F. Transient Ischemic Attack; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Suardi, C.; Cazzaniga, E.; Graci, S.; Dongo, D.; Palestini, P. Link between Viral Infections, Immune System, Inflammation and Diet. Int. J. Environ. Res. Public Health 2021, 18, 2455. [Google Scholar] [CrossRef]
- Yao, J.; Peng, S.; Xu, J.; Fang, J. Reversing ROS-mediated neurotoxicity by chlorogenic acid involves its direct antioxidant activity and activation of Nrf2-ARE signaling pathway. BioFactors 2019, 45, 616–626. [Google Scholar] [CrossRef]
- Ames, B.N. Prolonging healthy aging: Longevity vitamins and proteins. Proc. Natl. Acad. Sci. USA 2018, 115, 10836–10844. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pham-Huy, L.A.; He, H.; Pham-Huy, C. Free radicals, antioxidants in disease and health. Int. J. Biomed. Sci. IJBS 2008, 4, 89–96. [Google Scholar]
- Visioli, F.; De La Lastra, C.A.; Andres-Lacueva, C.; Aviram, M.; Calhau, C.; Cassano, A.; D’Archivio, M.; Faria, A.; Favé, G.; Fogliano, V.; et al. Polyphenols and human health: A prospectus. Crit. Rev. Food Sci. Nutr. 2011, 51, 524–546. [Google Scholar] [CrossRef]
- International Coffee Organization. What’s New. Available online: ico.org (accessed on 19 December 2021).
- Zamora-Ros, R.; Knaze, V.; Rothwell, J.A.; Hemon, B.; Moskal, A.; Overvad, K.; Tjonneland, A.; Kyro, C.; Fagherazzi, G.; Boutron-Ruault, M.C.; et al. Dietary polyphenol intake in Europe: The European Prospective Investigation into Cancer and Nutrition (EPIC) study. Eur. J. Nutr. 2016, 55, 1359–1375. [Google Scholar] [CrossRef] [PubMed]
- Romualdo, G.R.; Rocha, A.B.; Vinken, M.; Cogliati, B.; Moreno, F.S.; Chaves, M.A.G.; Barbisan, L.F. Drinking for protection? Epidemiological and experimental evidence on the beneficial effects of coffee or major coffee compounds against gastrointestinal and liver carcinogenesis. Food Res. Int. 2019, 123, 567–589. [Google Scholar] [CrossRef]
- Mena, P.; Bresciani, L.; Tassotti, M.; Rosi, A.; Martini, D.; Antonini, M.; Cas, A.D.; Bonadonna, R.; Brighenti, F.; Del Rio, D. Effect of different patterns of consumption of coffee and a cocoa-based product containing coffee on the nutrikinetics and urinary excretion of phenolic compounds. Am. J. Clin. Nutr. 2021, 114, 2107–2118. [Google Scholar] [CrossRef] [PubMed]
- Stalmach, A.; Mullen, W.; Barron, D.; Uchida, K.; Yokota, T.; Cavin, C.; Steiling, H.; Williamson, G.; Crozier, A. Metabolite profiling of hydroxycinnamate derivatives in plasma and urine after the ingestion of coffee by humans: Identification of biomarkers of coffee consumption. Drug Metab. Dispos. 2009, 37, 1749–1758. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mussatto, S.I.; Machado, E.M.S.; Martins, S.; Teixeira, J.A. Production, composition and application of coffee and its industrial residues. Food Bioprocess Technol. 2011, 4, 661–672. [Google Scholar] [CrossRef] [Green Version]
- Cristobal, J.; Matos, C.T.; Aurambout, J.P.; Manfredi, S.; Kavalov, B. Environmental sustainability assessment of bioeconomy value chains. Biomass Bioenergy 2016, 89, 159–171. [Google Scholar] [CrossRef]
- Da Silva, M.R.; Bragagnolo, F.S.; Carneiro, R.L.; Pereira, I.D.O.C.; Ribeiro, J.A.A.; Rodrigues, C.M.; Jelley, R.J.; Fedrizzi, B.; Funari, C.S. Metabolite characterization of fifteen by-products of the coffee production chain: From farm to factory. Food Chem. 2021, 369, 130753. [Google Scholar] [CrossRef]
- Lachenmeier, D.W.; Schwarz, S.; Rieke-Zapp, J.; Cantergiani, E.; Rawel, H.; Martín-Cabrejas, M.A.; Martuscelli, M.; Gottstein, V.; Angeloni, S. Coffee By-Products as Sustainable Novel Foods: Report of the 2nd International Electronic Conference on Foods—“Future Foods and Food Technologies for a Sustainable World”. Foods 2021, 11, 3. [Google Scholar] [CrossRef]
- Magoni, C.; Bruni, I.; Guzzetti, L.; Dell’Agli, M.; Sangiovanni, E.; Piazza, S.; Regonesi, M.E.; Maldini, M.; Spezzano, R.; Caruso, D.; et al. Valorizing coffee pulp by-products as anti-inflammatory ingredient of food supplements acting on IL-8 release. Food Res. Int. 2018, 112, 129–135. [Google Scholar] [CrossRef]
- Cao, X.; Yang, L.; Xue, Q.; Yao, F.; Sun, J.; Yang, F.; Liu, Y. Antioxidant evaluation-guided chemical profiling and structure-activity analysis of leaf extracts from five trees in Broussonetia and Morus (Moraceae). Sci. Rep. 2020, 10, 4808. [Google Scholar] [CrossRef]
- Valencia-Hernandez, L.J.; Wong-Paz, J.E.; Ascacio-Valdés, J.A.; Chávez-González, M.L.; Contreras-Esquivel, J.C.; Aguilar, C.N. Procyanidins: From Agro-Industrial Waste to Food asBioactive Molecules. Foods 2021, 10, 3152. [Google Scholar] [CrossRef] [PubMed]
- Morris, M.C.; Evans, D.A.; Tangney, C.C.; Bienias, J.L.; Wilson, R.S. Associations of vegetable and fruit consumption with age-related cognitive change. Neurology 2006, 67, 1370–1376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nooyens, A.C.; Bueno-de-Mesquita, H.B.; van Boxtel, M.P.; van Gelder, B.M.; Verhagen, H.; Verschuren, W.M. Fruit and vegetable intake and cognitive decline in middle-aged men and women: The Doetinchem Cohort Study. Br. J. Nutr. 2011, 106, 752–761. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ricci, A.; Parpinello, G.P.; Versari, A. The Nutraceutical Impact of Polyphenolic Composition in Commonly Consumed Green Tea, Green Coffee and Red Wine Beverages: A Review. Recent Adv. Food Sci. Nutr. Res. 2018, 1, 12–27. [Google Scholar]
- Adibhatla, R.M.; Dempsy, R.; Hatcher, J.F. Integration of cytokine biology and lipid metabolism in stroke. Front. Biosci. 2008, 13, 1250–1270. [Google Scholar] [CrossRef] [Green Version]
- Liang, G.; Shi, B.; Luo, W.; Yang, J. The protective effect of caffeic acid on global cerebral ischemia-reperfusion injury in rats. Behav. Brain Funct. 2015, 11, 18. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Fang, S.H.; Ye, Y.L.; Chu, L.S.; Zhang, W.P.; Wang, M.L.; Wei, E.Q. Caffeic acid ameliorates early and delayed brain injuries after focal cerebral ischemia in rats. Acta Pharmacol. Sin. 2006, 27, 1103–1110. [Google Scholar] [CrossRef] [Green Version]
- Olthof, M.R.; Hollman, P.C.; Katan, M.B. Chlorogenic acid and caffeic acid are absorbed in humans. J. Nutr. 2001, 31, 66–71. [Google Scholar] [CrossRef] [Green Version]
- Del Rio, D.; Stalmach, A.; Calani, L.; Crozier, A. Bioavailability of coffee chlorogenic acids and green tea flavan-3-ols. Nutrients 2010, 2, 820–833. [Google Scholar] [CrossRef]
- Martini, D.; Del Bo, C.; Tassotti, M.; Riso, P.; Del Rio, D.; Brighenti, F.; Porrini, M. Coffee consumption and oxidative stress: A review of human intervention studies. Molecules 2016, 21, 979. [Google Scholar] [CrossRef] [Green Version]
- Li, C.; Jackson, R.M. Reactive species mechanisms of cellular hypoxia-reoxygenation injury. Am. J. Physiol. Cell Physiol. 2002, 282, C227–C241. [Google Scholar] [CrossRef] [Green Version]
- Botto, L.; Bulbarelli, A.; Lonati, E.; Cazzaniga, E.; Tassotti, M.; Mena, P.; Del Rio, D.; Palestini, P. Study of the Antioxidant Effects of Coffee Phenolic Metabolites on C6 Glioma Cells Exposed to Diesel Exhaust Particles. Antioxidants 2021, 10, 1169. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay, R.; Mohan Rao, L.J. An outlook on chlorogenic acids-occurrence, chemistry, technology, and biological activities. Crit. Rev. Food Sci. Nutr. 2013, 53, 968–984. [Google Scholar] [CrossRef] [PubMed]
- Duangjai, A.; Suphrom, N.; Wungrath, J.; Ontawong, A.; Nuengchamnong, N.; Yosboonruang, A. Comparison of antioxidant, antimicrobial activities and chemical profiles of three coffee (Coffea arabica L.) pulp aqueous extracts. Integr. Med. Res. 2016, 5, 324–331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kamdem, J.; Waczuk, E.; Kade, I.; Wagner, C.; Boligon, A.; Athayde, M.; Souza, D. Catuaba (Trichilia catigua) prevents against oxidative damage induced by in vitro ischemia-reperfusion in rat hippocampal slices. Neurochem. Res. 2012, 37, 2826–2835. [Google Scholar] [CrossRef] [PubMed]
- Ya, B.L.; Liu, Q.; Li, H.F.; Cheng, H.J.; Yu, T.; Chen, L.; Wang, Y.; Yuan, L.L.; Li, W.J.; Liu, W.Y.; et al. Uric Acid Protects against Focal Cerebral Ischemia/Reperfusion-Induced Oxidative Stress via Activating Nrf2 and Regulating Neurotrophic Factor Expression. Oxidative Med. Cell. Longev. 2018, 2018, 6069150. [Google Scholar] [CrossRef]
- Wang, L.; Chen, Y.; Sternberg, P.; Cai, J. Essential roles of the PI3 kinase/Akt pathway in regulating Nrf2-dependent antioxidant functions in the RPE. Investig. Opthalmology Vis. Sci. 2008, 49, 1671–1678. [Google Scholar] [CrossRef] [Green Version]
- Zipper, L.M.; Mulcahy, R.T. Erk activation is required for Nrf2 nuclear localization during pyrrolidine dithiocarbamate induction of glutamate cysteine ligase modulatory gene expression in HepG2 cells. Toxicol Sci. 2003, 73, 124–134. [Google Scholar] [CrossRef] [PubMed]
- Owuor, E.D.; Kong, A.N. Antioxidants and oxidants regulated signal transduction pathways. Biochem. Pharm. 2002, 64, 765–770, Erratum in Biochem. Pharm. 2002, 64, 1547. [Google Scholar] [CrossRef]
- Soares, R.; Losada, D.; Jordani, M.; Évora, P. Ischemia/Reperfusion Injury Revisited: An Overview of the Latest Pharmacological Strategies. Int. J. Mol. Sci. 2019, 20, 5034. [Google Scholar] [CrossRef] [Green Version]
- Lonati, E.; Corsetto, P.A.; Montorfano, G.; Zava, S.; Carrozzini, T.; Brambilla, A.; Botto, L.; Palestini, P.; Rizzo, A.M.; Bulbarelli, A. Lipid reshaping and lipophagy are induced in a modeled Ischemia-Reperfusion injury of Blood Brain Barrier. Int. J. Mol. Sci. 2019, 20, 3752. [Google Scholar] [CrossRef] [Green Version]
- Buscà, R.; Pouysségur, J.; Lenormand, P. ERK1 and ERK2 Map Kinases: Specific Roles or Functional Redundancy? Front. Cell Dev. Biol. 2016, 4, 53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.K.; Jang, H.D. Nrf2-mediated HO-1 induction coupled with the ERK signaling pathway contributes to indirect antioxidant capacity of caffeic acid phenethyl ester in HepG2 cells. Int. J. Mol. Sci. 2014, 15, 12149–12165. [Google Scholar] [CrossRef] [Green Version]
- Ryter, S.W.; Choi, A.M. Heme oxygenase-1: Redox regulation of a stress protein in lung and cell culture models. Antioxid. Redox Signal. 2005, 7, 80–91. [Google Scholar] [CrossRef]
- Han, D.; Chen, W.; Gu, X.; Shan, R.; Zou, J.; Liu, G.; Shahid, M.; Gao, J.; Han, B. Cytoprotective effect of chlorogenic acid against hydrogen peroxide-induced oxidative stress in MC3T3-E1 cells through PI3K/Akt-mediated Nrf2/HO-1 signaling pathway. Oncotarget 2017, 8, 14680–14692. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, Y.; Li, Z.; Cao, G.; Huang, S.; Yang, H. Bamboo Leaf Flavonoids Extracts Alleviate Oxidative Stress in HepG2 Cells via Naturally Modulating Reactive Oxygen Species Production and Nrf2-Mediated Antioxidant Defense Responses. J. Food Sci. 2019, 84, 1609–1620. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Yang, J.H.; Cho, S.S.; Kim, J.H.; Xu, J.; Seo, K.; Ki, S.H. 5-Caffeoylquinic acid ameliorates oxidative stress-mediated cell death via Nrf2 activation in hepatocytes. Pharm. Biol. 2020, 58, 999–1005. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.Q.; Gao, H.; Yuan, R.; Han, S.; Li, X.X.; Tang, M.; Dong, B.; Li, J.X.; Zhao, L.C.; Feng, J.; et al. Procyanidin A2, a polyphenolic compound, exerts anti-inflammatory and anti-oxidative activity in lipopolysaccharide-stimulated RAW264.7 cells. PLoS ONE 2020, 15, e0237017. [Google Scholar] [CrossRef]
- Han, S.; Gao, H.; Chen, S.; Wang, Q.; Li, X.; Du, L.J.; Li, J.; Luo, Y.Y.; Li, J.X.; Zhao, L.C.; et al. Procyanidin A1 Alleviates Inflammatory Response induced by LPS through NF-κB, MAPK, and Nrf2/HO-1 Pathways in RAW264.7 cells. Sci. Rep. 2019, 9, 15087. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aschner, M.; Fitsanakis, V.A.; dos Santos, A.P.; Olivi, L.; Bressler, J.P. Blood-brain barrier and cell-cell interactions: Methods for establishing in vitro models of the blood-brain barrier and transport measurements. Methods Mol. Biol. 2006, 341, 1–15. [Google Scholar]
- Balbuena, P.; Li, W.; Ehrich, M. Assessments of tight junction proteins occludin, claudin 5 and scaffold proteins ZO1 and ZO2 in endothelial cells of the rat blood-brain barrier: Cellular responses to neurotoxicants malathion and lead acetate. Neurotoxicology 2011, 32, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Faria, A.; Pestana, D.; Teixeira, D.; Couraud, P.O.; Romero, I.; Weksler, B.; de Freitas, V.; Mateus, N.; Calhau, C. Insights into the putative catechin and epicatechin transport across blood-brain barrier. Food Funct. 2011, 2, 39–44. [Google Scholar] [CrossRef]
- Roux, F.; Couraud, P.O. Rat brain endothelial cell lines for the study of blood-brain barrier permeability and transport functions. Cell. Mol. Neurobiol. 2005, 25, 41–58. [Google Scholar] [CrossRef] [Green Version]
- Wilhelm, I.; Fazakas, C.; Krizbai, I.A. In vitro models of the blood-brain barrier. Acta Neurobiol. Exp. 2011, 71, 113–128. [Google Scholar]
- Robb, S.J.; Connor, J.R. An in vitro model for analysis of oxidative death in primary mouse astrocytes. Brain Res. 1998, 788, 125–132. [Google Scholar] [CrossRef]
- Hansen, M.B.; Nielsen, S.E.; Berg, K. Re-examination and further development of a precise and rapid dye method for measuring cell growth/cell kill. J. Immunol. Methods 1989, 119, 203–210. [Google Scholar] [CrossRef]
- Bulbarelli, A.; Lonati, E.; Brambilla, A.; Orlando, A.; Cazzaniga, E.; Piazza, F.; Ferrarese, C.; Masserini, M.; Sancini, G. Aβ42 production in brain capillary endothelial cells after oxygen and glucose deprivation. Mol. Cell. Neurosci. 2012, 49, 415–422. [Google Scholar] [CrossRef]
- Rizk, S.; Taha, H.; Abdel Moneim, A.E.; Amin, H.K. Neuroprotective effect of green and roasted coffee bean extracts on cerebral ischemia-induced injury in rats. Metab. Brain Dis. 2021, 36, 1943–1956. [Google Scholar] [CrossRef] [PubMed]



| Peak Number | Compound |
|---|---|
| 1 | Caffeoylquinic acid |
| 2 | p-coumaroylquinic acid |
| 3 | Caffeoylquinic acid |
| 4 | Caffeoylquinic acid |
| 5 | Feruloylquinic acid |
| 6 | Caffeoylquinic acid |
| 7 | p-coumaroylquinic acid |
| 8 | Feruloylquinic acid |
| 9 | Feruloyl quinic acid |
| 10 | p-coumaroylquinic acid |
| 11 | Feruloylquinic acid |
| 12 | Di-caffeoylquinic acid |
| 13 | Di-caffeoylquinic acid |
| 14 | Di-caffeoylquinic acid |
| 15 | Di-caffeoylquinic acid |
| 16 | 3-O-p-coumaroyl-4-O-caffeoylquinic acid |
| 17 | 3-O-feruloyl-4-O-caffeoylquinic acid |
| 18 | 3-O-caffeoyl-4-O-p-coumaroylquinic acid |
| 19 | 3-O-caffeoyl-4-O-feruloylquinic acid |
| 20 | 4-O-caffeoyl-5-O-p-coumaroyl quinic acid |
| 21 | 3-O-feruloyl-5-O-caffeoylquinic acid |
| 22 | 3-O-caffeoyl-5-O-feruloylquinic acid |
| 23 | 4-O-feruloyl-5-O-caffeoylquinic acid |
| 24 | 4-O-caffeoyl-5-O-feruloylquinic acid |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lonati, E.; Carrozzini, T.; Bruni, I.; Mena, P.; Botto, L.; Cazzaniga, E.; Del Rio, D.; Labra, M.; Palestini, P.; Bulbarelli, A. Coffee-Derived Phenolic Compounds Activate Nrf2 Antioxidant Pathway in I/R Injury In Vitro Model: A Nutritional Approach Preventing Age Related-Damages. Molecules 2022, 27, 1049. https://doi.org/10.3390/molecules27031049
Lonati E, Carrozzini T, Bruni I, Mena P, Botto L, Cazzaniga E, Del Rio D, Labra M, Palestini P, Bulbarelli A. Coffee-Derived Phenolic Compounds Activate Nrf2 Antioxidant Pathway in I/R Injury In Vitro Model: A Nutritional Approach Preventing Age Related-Damages. Molecules. 2022; 27(3):1049. https://doi.org/10.3390/molecules27031049
Chicago/Turabian StyleLonati, Elena, Tatiana Carrozzini, Ilaria Bruni, Pedro Mena, Laura Botto, Emanuela Cazzaniga, Daniele Del Rio, Massimo Labra, Paola Palestini, and Alessandra Bulbarelli. 2022. "Coffee-Derived Phenolic Compounds Activate Nrf2 Antioxidant Pathway in I/R Injury In Vitro Model: A Nutritional Approach Preventing Age Related-Damages" Molecules 27, no. 3: 1049. https://doi.org/10.3390/molecules27031049
APA StyleLonati, E., Carrozzini, T., Bruni, I., Mena, P., Botto, L., Cazzaniga, E., Del Rio, D., Labra, M., Palestini, P., & Bulbarelli, A. (2022). Coffee-Derived Phenolic Compounds Activate Nrf2 Antioxidant Pathway in I/R Injury In Vitro Model: A Nutritional Approach Preventing Age Related-Damages. Molecules, 27(3), 1049. https://doi.org/10.3390/molecules27031049