Zn Doping Effect on the Performance of Fe-Based Catalysts for the Hydrogenation of CO2 to Light Hydrocarbons
Abstract
:1. Introduction
2. Results
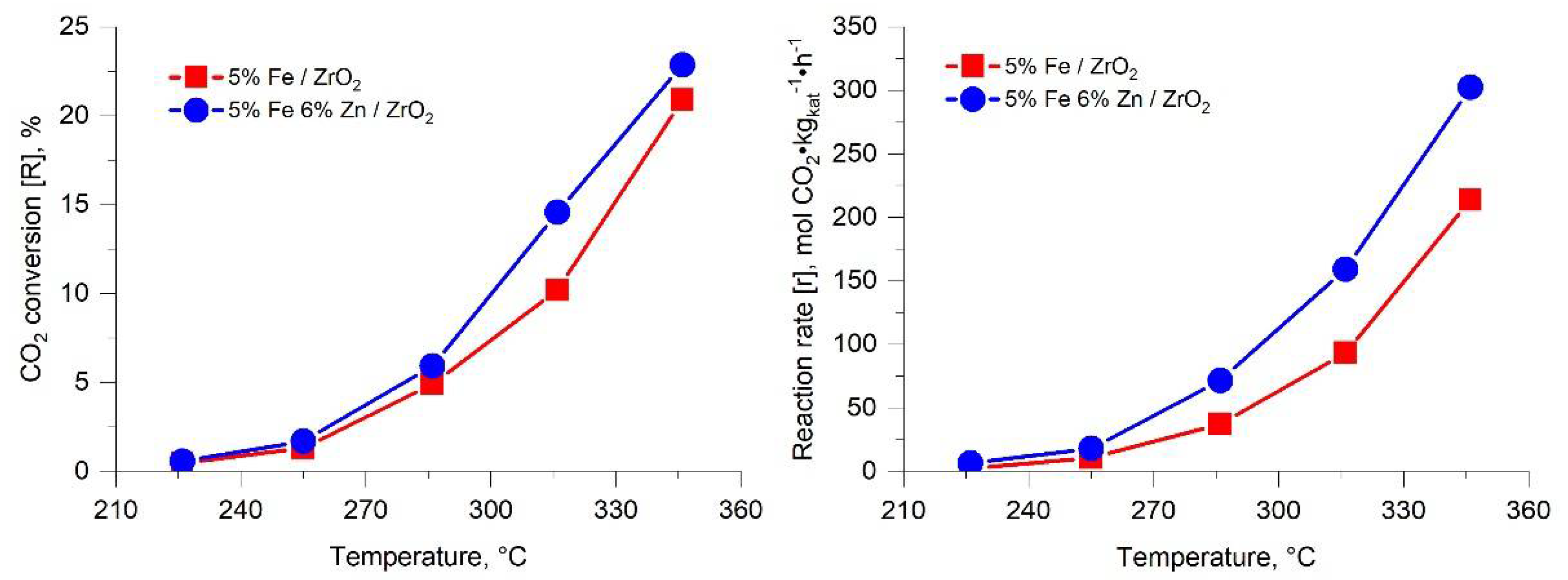
2.1. CO2 Hydrogenation
2.2. Raman Spectroscopy
2.3. DRIFT-CO
2.4. TPR-H2
2.5. XPS
2.6. UV/VIS
3. Discussion
4. Materials and Methods
4.1. Catalyst Preparation
4.2. CO2 Hydrogenation
4.3. Raman Spectroscopy
4.4. Diffuse Reflectance IR Spectroscopy
4.5. Thermoprogrammed Reduction with Hydrogen
4.6. X-ray Photoelectron Spectroscopy
4.7. Diffuse Reflectance UV/VIS Spectroscopy
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Cui, Y.; Schubert, B.A.; Jahren, A.H. A 23 m.y. record of low atmospheric CO2. Geology 2020, 48, 888–892. [Google Scholar] [CrossRef]
- Xiaoding, X.; Moulijn, J.A. Mitigation of CO2 by chemical conversion: Plausible chemical reactions and promising products. Energy Fuels 1996, 10, 305–325. [Google Scholar] [CrossRef]
- Sun, Y.; Lin, Z.; Peng, S.H.; Sage, V.; Sun, Z. A Critical Perspective on CO2 Conversions into Chemicals and Fuels. J. Nanosci. Nanotechnol. 2019, 19, 3097–3109. [Google Scholar] [CrossRef] [PubMed]
- Vu, T.T.N.; Desgagnés, A.; Iliuta, M.C. Efficient approaches to overcome challenges in material development for conventional and intensified CO2 catalytic hydrogenation to CO, methanol, and DME. Appl. Catal. A Gen. 2021, 617, 118119. [Google Scholar] [CrossRef]
- Tedeeva, M.A.; Kustov, A.L.; Pribytkov, P.V.; Evdokimenko, N.D.; Sarkar, B.; Kustov, L.M. Dehydrogenation of propane in the presence of CO2 on Cr(3%)/SiO2 catalyst under supercritical conditions. Mendeleev Commun. 2020, 30, 195–197. [Google Scholar] [CrossRef]
- Müller, T.E.; Leitner, W.; Markewitz, P.; Kuckshinrichs, W. Opportunities for utilizing and recycling CO2. In Carbon Capture, Storage and Use: Technical, Economic, Environmental and Societal Perspectives; Springer International Publishing: Berlin/Heidelberg, Germany, 2015; pp. 67–100. ISBN 9783319119434. [Google Scholar]
- Wang, W.; Wang, S.; Ma, X.; Gong, J. Recent advances in catalytic hydrogenation of carbon dioxide. Chem. Soc. Rev. 2011, 40, 3703–3727. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, X.; Qin, W.; Guan, Q.; Li, W. The Synergistic Effect of CuZnCeOx in Controlling the Formation of Methanol and CO from CO2 Hydrogenation. ChemCatChem 2018, 10, 4438–4449. [Google Scholar] [CrossRef]
- Kirchner, J.; Baysal, Z.; Kureti, S. Activity and Structural Changes of Fe-based Catalysts during CO2 Hydrogenation towards CH4—A Mini Review. ChemCatChem 2020, 12, 981–988. [Google Scholar] [CrossRef] [Green Version]
- Evdokimenko, N.D.; Kustov, A.L.; Kim, K.O.; Mishin, I.V.; Nissenbaum, V.D.; Kapustin, G.I.; Aymaletdinov, T.R.; Kustov, L.M. Ce–Zr materials with a high surface area as catalyst supports for hydrogenation of CO2. Funct. Mater. Lett. 2020, 13, 2040004. [Google Scholar] [CrossRef]
- Bogdan, V.I.; Koklin, A.E.; Kustov, A.L.; Pokusaeva, Y.A.; Bogdan, T.V.; Kustov, L.M. Carbon dioxide reduction with hydrogen on Fe, Co supported alumina and carbon catalysts under supercritical conditions. Molecules 2021, 26, 2883. [Google Scholar] [CrossRef]
- Ramirez, A.; Gevers, L.; Bavykina, A.; Ould-Chikh, S.; Gascon, J. Metal Organic Framework-Derived Iron Catalysts for the Direct Hydrogenation of CO2 to Short Chain Olefins. ACS Catal. 2018, 8, 9174–9182. [Google Scholar] [CrossRef]
- Saeidi, S.; Amin, N.A.S.; Rahimpour, M.R. Hydrogenation of CO2 to value-added products—A review and potential future developments. J. CO2 Util. 2014, 5, 66–81. [Google Scholar] [CrossRef]
- Nam, S.S.; Kim, H.; Kishan, G.; Choi, M.J.; Lee, K.W. Catalytic conversion of carbon dioxide into hydrocarbons over iron supported on alkali ion-exchanged Y-zeolite catalysts. Appl. Catal. A Gen. 1999, 179, 155–163. [Google Scholar] [CrossRef]
- Schulz, H. Short history and present trends of Fischer-Tropsch synthesis. Appl. Catal. A Gen. 1999, 186, 3–12. [Google Scholar] [CrossRef]
- Dry, M.E. Practical and theoretical aspects of the catalytic Fischer-Tropsch process. Appl. Catal. A Gen. 1996, 138, 319–344. [Google Scholar] [CrossRef]
- Shesterkina, A.A.; Kirichenko, O.A.; Kozlova, L.M.; Kapustin, G.I.; Mishin, I.V.; Strelkova, A.A.; Kustov, L.M. Liquid-phase hydrogenation of phenylacetylene to styrene on silica-supported Pd–Fe nanoparticles. Mendeleev Commun. 2016, 26, 228–230. [Google Scholar] [CrossRef]
- Kirichenko, O.A.; Strekalova, A.A.; Kapustin, G.I.; Shesterkina, A.A. A New Redox Method for Depositing FeO x on the Surface of Pd(0)/SiO2 Nanoparticles—Catalysts for Selective Phenylacetylene Hydrogenation. Russ. J. Phys. Chem. A 2018, 92, 2396–2398. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, G.; Jiang, X.; Wang, J.; Song, C.; Guo, X. Insight into the role of Fe5C2 in CO2 catalytic hydrogenation to hydrocarbons. Catal. Today 2021, 371, 162–170. [Google Scholar] [CrossRef]
- Bradley, M.J.; Ananth, R.; Willauer, H.D.; Baldwin, J.W.; Hardy, D.R.; Williams, F.W. The Effect of Copper Addition on the Activity and Stability of Iron-Based CO₂ Hydrogenation Catalysts. Molecules 2017, 22, 1579. [Google Scholar] [CrossRef]
- Zhang, C.; Xu, M.; Yang, Z.; Zhu, M.; Gao, J.; Han, Y.F. Uncovering the electronic effects of zinc on the structure of Fe5C2–ZnO catalysts for CO2 hydrogenation to linear α-olefins. Appl. Catal. B Environ. 2021, 295, 120287. [Google Scholar] [CrossRef]
- Wang, J.; You, Z.; Zhang, Q.; Deng, W.; Wang, Y. Synthesis of lower olefins by hydrogenation of carbon dioxide over supported iron catalysts. Catal. Today 2013, 215, 186–193. [Google Scholar] [CrossRef]
- Satthawong, R.; Koizumi, N.; Song, C.; Prasassarakich, P. Bimetallic Fe–Co catalysts for CO2 hydrogenation to higher hydrocarbons. J. CO2 Util. 2013, 3–4, 102–106. [Google Scholar] [CrossRef]
- Li, S.; Li, A.; Krishnamoorthy, S.; Iglesia, E. Effects of Zn, Cu, and K promoters on the structure and on the reduction, carburization, and catalytic behavior of iron-based Fischer-Tropsch synthesis catalysts. Catal. Lett. 2001, 77, 197–205. [Google Scholar] [CrossRef]
- Malhi, H.S.; Sun, C.; Zhang, Z.; Liu, Y.; Liu, W.; Ren, P.; Tu, W.; Han, Y.F. Catalytic consequences of the decoration of sodium and zinc atoms during CO2 hydrogenation to olefins over iron-based catalyst. Catal. Today 2021, in press. [Google Scholar] [CrossRef]
- Wang, H.; Yang, Y.; Xu, J.; Wang, H.; Ding, M.; Li, Y. Study of bimetallic interactions and promoter effects of FeZn, FeMn and FeCr Fischer-Tropsch synthesis catalysts. J. Mol. Catal. A Chem. 2010, 326, 29–40. [Google Scholar] [CrossRef]
- Chaipraditgul, N.; Numpilai, T.; Kui Cheng, C.; Siri-Nguan, N.; Sornchamni, T.; Wattanakit, C.; Limtrakul, J.; Witoon, T. Tuning interaction of surface-adsorbed species over Fe/K–Al2O3 modified with transition metals (Cu, Mn, V, Zn or Co) on light olefins production from CO2 hydrogenation. Fuel 2021, 283, 119248. [Google Scholar] [CrossRef]
- Liu, X.M.; Lu, G.Q.; Yan, Z.F.; Beltramini, J. Recent Advances in Catalysts for Methanol Synthesis via Hydrogenation of CO and CO2. Ind. Eng. Chem. Res. 2003, 42, 6518–6530. [Google Scholar] [CrossRef]
- Shido, T.; Iwasawa, Y. Reactant-promoted reaction mechanism for water-gas shift reaction on ZnO, as the genesis of surface catalysis. J. Catal. 1991, 129, 343–355. [Google Scholar] [CrossRef]
- Saito, M.; Wu, J.; Tomoda, K.; Takahara, I.; Murata, K. Effects of ZnO contained in supported Cu-based catalysts on their activities for several reactions. Catal. Lett. 2002, 83, 1–4. [Google Scholar] [CrossRef]
- Zhang, J.; Lu, S.; Su, X.; Fan, S.; Ma, Q.; Zhao, T. Selective formation of light olefins from CO2 hydrogenation over Fe–Zn–K catalysts. J. CO2 Util. 2015, 12, 95–100. [Google Scholar] [CrossRef]
- Zhai, P.; Xu, C.; Gao, R.; Liu, X.; Li, M.; Li, W.; Fu, X.; Jia, C.; Xie, J.; Zhao, M.; et al. Highly Tunable Selectivity for Syngas-Derived Alkenes over Zinc and Sodium-Modulated Fe5C2 Catalyst. Angew. Chem. 2016, 128, 10056–10061. [Google Scholar] [CrossRef]
- Sai Prasad, P.S.; Bae, J.W.; Jun, K.W.; Lee, K.W. Fischer-Tropsch synthesis by carbon dioxide hydrogenation on Fe-based catalysts. Catal. Surv. Asia 2008, 12, 170–183. [Google Scholar] [CrossRef]
- Choi, Y.H.; Ra, E.C.; Kim, E.H.; Kim, K.Y.; Jang, Y.J.; Kang, K.N.; Choi, S.H.; Jang, J.H.; Lee, J.S. Sodium-Containing Spinel Zinc Ferrite as a Catalyst Precursor for the Selective Synthesis of Liquid Hydrocarbon Fuels. ChemSusChem 2017, 10, 4764–4770. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Yin, H.; Yu, G.; He, S.; Kang, J.; Liu, Z.; Cheng, K.; Zhang, Q.; Wang, Y. Selective hydrogenation of CO2 and CO into olefins over Sodium- and Zinc-Promoted iron carbide catalysts. J. Catal. 2021, 395, 350–361. [Google Scholar] [CrossRef]
- Witoon, T.; Chaipraditgul, N.; Numpilai, T.; Lapkeatseree, V.; Ayodele, B.V.; Cheng, C.K.; Siri-Nguan, N.; Sornchamni, T.; Limtrakul, J. Highly active Fe–Co–Zn/K–Al2O3 catalysts for CO2 hydrogenation to light olefins. Chem. Eng. Sci. 2021, 233, 116428. [Google Scholar] [CrossRef]
- Kim, K.O.; Evdokimenko, N.D.; Pribytkov, P.V.; Tedeeva, M.A.; Borkov, S.A.; Kustov, A.L. Synthesis of Methanol from CO2 on Cu–Zn/xAl2O3–(1 – x)SiO2 Catalysts. Effect of Support Composition. Russ. J. Phys. Chem. A 2021, 95, 2422–2425. [Google Scholar] [CrossRef]
- Murthy, P.S.; Liang, W.; Jiang, Y.; Huang, J. Cu-Based Nanocatalysts for CO2 Hydrogenation to Methanol. Energy Fuels 2021, 35, 8558–8584. [Google Scholar] [CrossRef]
- Zeng, F.; Mebrahtu, C.; Xi, X.; Liao, L.; Ren, J.; Xie, J.; Heeres, H.J.; Palkovits, R. Catalysts design for higher alcohols synthesis by CO2 hydrogenation: Trends and future perspectives. Appl. Catal. B Environ. 2021, 291, 120073. [Google Scholar] [CrossRef]
- Zhang, S.; Wu, Z.; Liu, X.; Hua, K.; Shao, Z.; Wei, B.; Huang, C.; Wang, H.; Sun, Y. A Short Review of Recent Advances in Direct CO2 Hydrogenation to Alcohols. Top. Catal. 2021, 64, 371–394. [Google Scholar] [CrossRef]
- Borovinskaya, E.S.; Oswald, S.; Reschetilowski, W. Effects of promoter on structural and surface properties of zirconium oxide-based catalyst materials. Molecules 2020, 25, 2619. [Google Scholar] [CrossRef]
- Guo, L.; Sun, J.; Ge, Q.; Tsubaki, N. Recent advances in direct catalytic hydrogenation of carbon dioxide to valuable C2+ hydrocarbons. J. Mater. Chem. A 2018, 6, 23244–23262. [Google Scholar] [CrossRef]
- Yang, H.; Zhang, C.; Gao, P.; Wang, H.; Li, X.; Zhong, L.; Wei, W.; Sun, Y. A review of the catalytic hydrogenation of carbon dioxide into value-added hydrocarbons. Catal. Sci. Technol. 2017, 7, 4580–4598. [Google Scholar] [CrossRef]
- Cornell, R.M.; Schwertmann, U. Also of Interest Iron Oxides in the Laboratory; Wiley: Weinheim, Germany, 2003; ISBN 3527302743. [Google Scholar]
- Visser, T. Molecular Spectroscopy of Oxide Catalyst Surfaces; John Wiley & Sons: Chichester, UK, 2003; Volume 33, ISBN 047198731X. [Google Scholar]
- Aribi, K.; Ghelamallah, M.; Bellifa, A.; Granger, P.; Choukchou-Braham, A. Structural and textural modifications of ZrO2 induced by La2O3 addition, thermal treatment and reducing process. J. Struct. Chem. 2018, 59, 486. [Google Scholar] [CrossRef]
- Niu, L.; Liu, X.; Wen, X.; Yang, Y.; Xu, J.; Li, Y. Effect of potassium promoter on phase transformation during H2 pretreatment of a Fe2O3 Fischer Tropsch synthesis catalyst precursor. Catal. Today 2020, 343, 101–111. [Google Scholar] [CrossRef]
- Zieliński, J.; Zglinicka, I.; Znak, L.; Kaszkur, Z. Reduction of Fe2O3 with hydrogen. Appl. Catal. A Gen. 2010, 381, 191–196. [Google Scholar] [CrossRef]
- Lin, H.Y.; Chen, Y.W.; Li, C. The mechanism of reduction of iron oxide by hydrogen. Thermochim. Acta 2003, 400, 61–67. [Google Scholar] [CrossRef]
- Jozwiak, W.K.; Kaczmarek, E.; Maniecki, T.P.; Ignaczak, W.; Maniukiewicz, W. Reduction behavior of iron oxides in hydrogen and carbon monoxide atmospheres. Appl. Catal. A Gen. 2007, 326, 17–27. [Google Scholar] [CrossRef]
- Kangvansura, P.; Chew, L.M.; Kongmark, C.; Santawaja, P.; Ruland, H.; Xia, W.; Schulz, H.; Worayingyong, A.; Muhler, M. Effects of Potassium and Manganese Promoters on Nitrogen-Doped Carbon Nanotube-Supported Iron Catalysts for CO2 Hydrogenation. Engineering 2017, 3, 385–392. [Google Scholar] [CrossRef]
- Grosvenor, A.P.; Kobe, B.A.; Biesinger, M.C.; McIntyre, N.S. Investigation of multiplet splitting of Fe 2p XPS spectra and bonding in iron compounds. Surf. Interface Anal. 2004, 36, 1564–1574. [Google Scholar] [CrossRef]






| Sample | Selectivity, % | Reaction Rate, mol CO2×kgkat−1×h−1 | |
|---|---|---|---|
| CO | HC | ||
| 5%Fe/ZrO2 (La) | 95 | 5 | 8.0 |
| 5%Zn/ZrO2 (La) | 98 | 2 | 0.2 |
| Co-impregnation | 91 | 9 | 16.6 |
| Imp. Fe → 500 °C 4 h on air → Imp. Zn | 96 | 4 | 3.2 |
| Imp. Zn → 500 °C 4 h on air → Imp. Fe | 93 | 7 | 5.7 |
| Zn Content (XZn), Mass. % | Selectivity, % | Reaction Rate, mol CO2×kgkat−1×h−1 | |
|---|---|---|---|
| CO | HC | ||
| 0 | 95 | 5 | 8.0 |
| 1 | 99 | 1 | 9.2 |
| 3 | 97 | 3 | 12.6 |
| 5 | 91 | 9 | 16.6 |
| 7 | 91 | 9 | 16.0 |
| 9 | 92 | 8 | 12.0 |
| Sample | Specific Absorption of Hydrogen, mol H2/g |
|---|---|
| Carrier ZrO2 | 2.12 × 10−4 |
| 5%Fe/ZrO2 | 1.28 × 10−3 |
| 5%Fe6%Zn/ZrO2 | 1.31 × 10−3 |
| Sample | Element Content on the Sample Surface,% wt. | |||||
|---|---|---|---|---|---|---|
| C | O | Zr | Fe | La | Zn | |
| 5%Fe/ZrO2 (La) | 12.3 | 60.8 | 22.3 | 2.1 | 2.6 | - |
| 5%Fe6%Zn/ZrO2 (La) | 13.3 | 60.8 | 18.3 | 1.8 | 2.4 | 3.4 |
| Sample | Element Content on the Sample Surface,% wt. | ||
|---|---|---|---|
| Fe0 | Fe2+ | Fe3+ | |
| 5%Fe/ZrO2(La) | - | 48 | 52 |
| 5%Fe6%Zn/ZrO2 (La) | 3 | 47 | 50 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Evdokimenko, N.D.; Kapustin, G.I.; Tkachenko, O.P.; Kalmykov, K.B.; Kustov, A.L. Zn Doping Effect on the Performance of Fe-Based Catalysts for the Hydrogenation of CO2 to Light Hydrocarbons. Molecules 2022, 27, 1065. https://doi.org/10.3390/molecules27031065
Evdokimenko ND, Kapustin GI, Tkachenko OP, Kalmykov KB, Kustov AL. Zn Doping Effect on the Performance of Fe-Based Catalysts for the Hydrogenation of CO2 to Light Hydrocarbons. Molecules. 2022; 27(3):1065. https://doi.org/10.3390/molecules27031065
Chicago/Turabian StyleEvdokimenko, Nikolay Dmitrievich, Gennady Ivanovich Kapustin, Olga Petrovna Tkachenko, Konstantin Borisovich Kalmykov, and Alexander Leonidovich Kustov. 2022. "Zn Doping Effect on the Performance of Fe-Based Catalysts for the Hydrogenation of CO2 to Light Hydrocarbons" Molecules 27, no. 3: 1065. https://doi.org/10.3390/molecules27031065
APA StyleEvdokimenko, N. D., Kapustin, G. I., Tkachenko, O. P., Kalmykov, K. B., & Kustov, A. L. (2022). Zn Doping Effect on the Performance of Fe-Based Catalysts for the Hydrogenation of CO2 to Light Hydrocarbons. Molecules, 27(3), 1065. https://doi.org/10.3390/molecules27031065






