Dimerization of Acetylene to Monovinylacetylene (MVA) by Bimetallic Zr/Cu Catalyst in Nieuwland Catalytic System
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Catalyst Preparation
2.3. Catalytic Activity Analysis and Evaluation
2.4. Catalyst Characterization
3. Results and Discussion
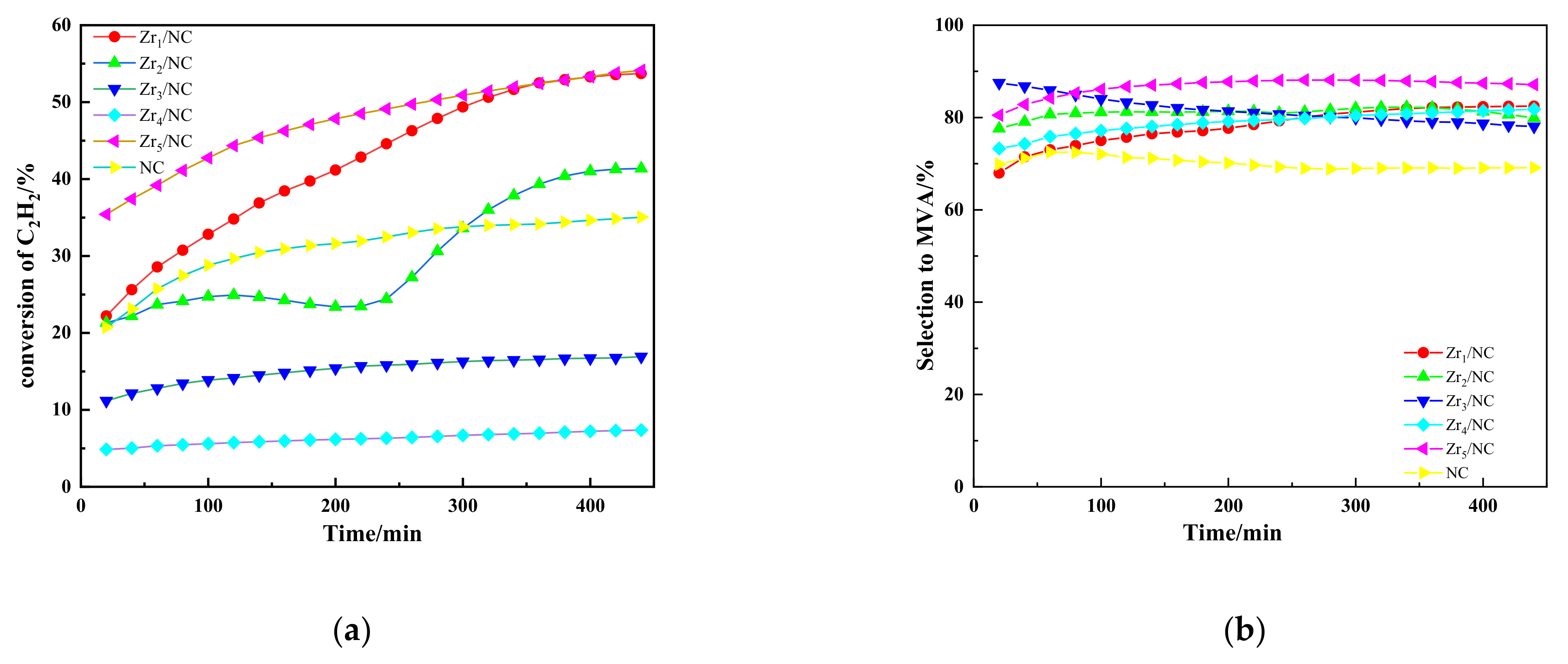
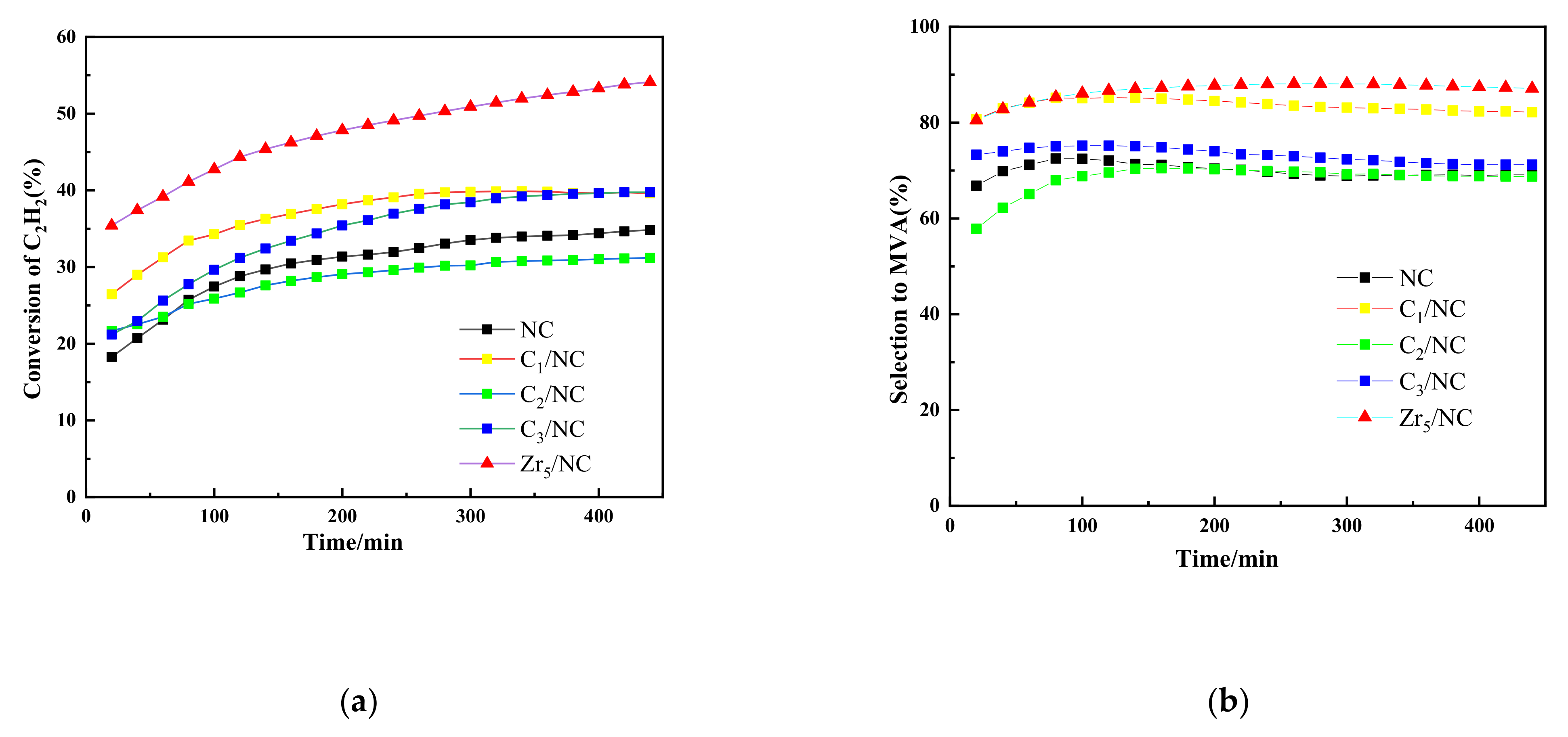
3.1. Results
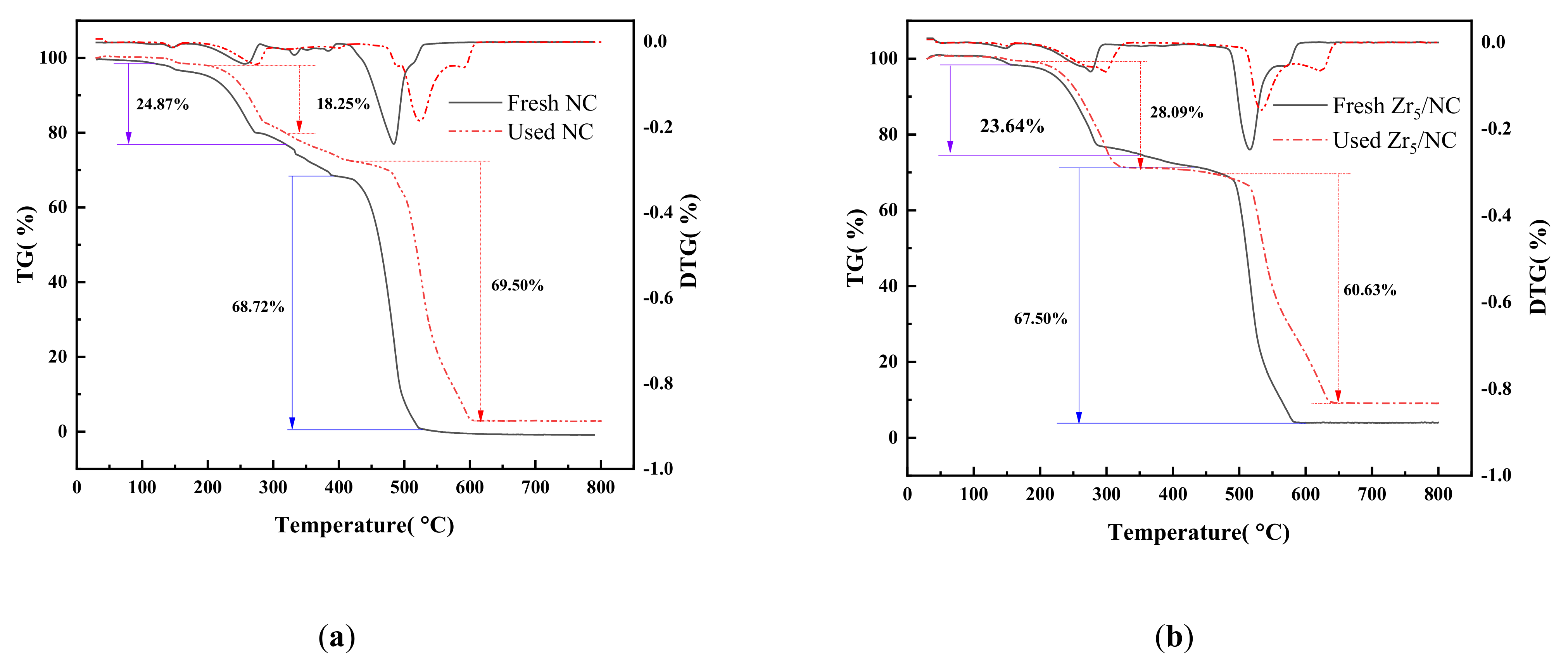
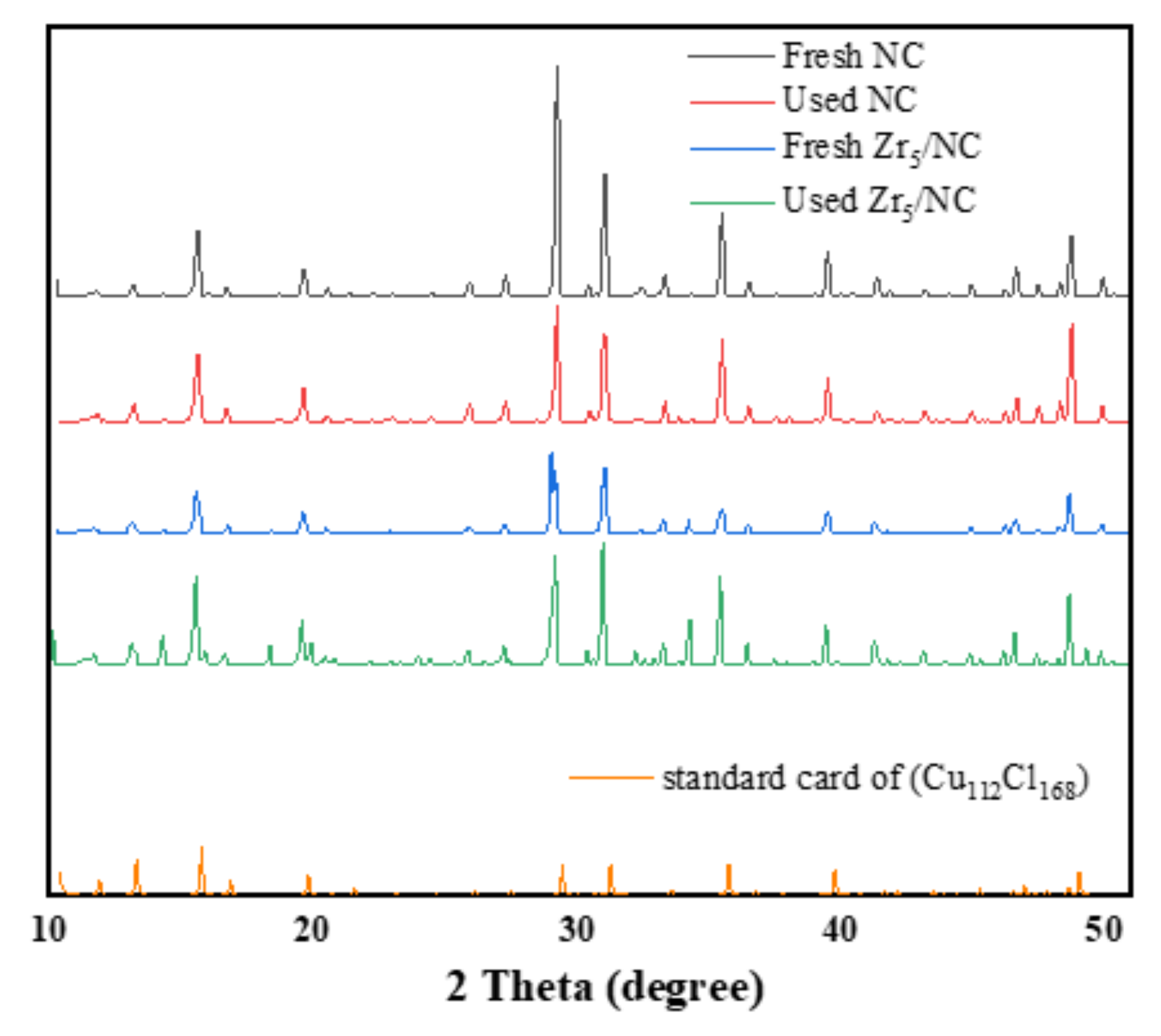
3.2. Characterization and Analysis of Catalyst
3.2.1. Composition of Catalyst
3.2.2. Effects of Additional Components on Catalytic Performance of NC Catalyst
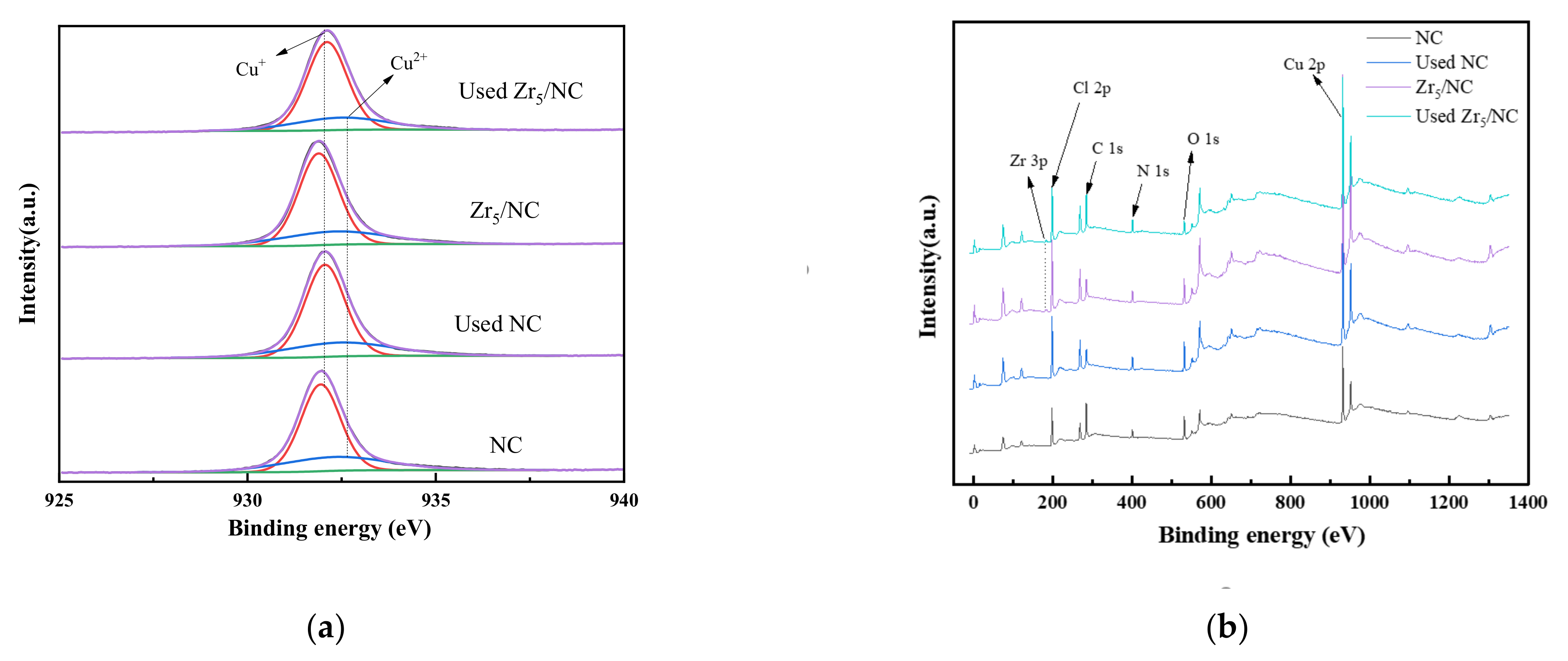
3.2.3. Change in Valence of Active Component during Reaction
3.2.4. Morphology and Structure of Catalysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Bridgewater, E. Neoprene, The Chloroprene Rubber. Ind. Eng. Chem. 1940, 32, 1155–1156. [Google Scholar] [CrossRef]
- Cui, X. Production and consumption of neoprene at home and abroad. Rubber Sci. Technol. 2012, 12, 5. [Google Scholar] [CrossRef]
- Vasil’eva, V.G.; Klebanskii, A.L. The Structure of Synthetic Types of Rubber. Polychloroprenes. Rubber Chem. Technol. 1937, 10, 126–134. [Google Scholar] [CrossRef]
- Ismail, H.; Leong, H.C. Curing characteristics and mechanical properties of natural rubber/chloroprene rubber and epoxidized natural rubber/chloroprene rubber blends. Polym. Test. 2001, 20, 509–516. [Google Scholar] [CrossRef]
- Nieuwland, J.A.; Calcott, W.S.; Downing, F.B.; Carter, A.S. Acetylene Polymers and Their Derivatives. I. The Controlled Polymerization of Acetylene. J. Am. Chem. Soc. 1931, 53, 4197–4202. [Google Scholar] [CrossRef]
- Carothers, W.H.; Williams, I.; Collins, A.M.; Kirby, J.E. Acetylene Polymers and Their Derivatives. Ⅱ. A New Synthetic Rubber: Chloroprene and Its Polymers. J. Am. Chem. Soc. 1931, 53, 4203–4225. [Google Scholar] [CrossRef]
- Cummings, C. Neoprene and nylon stockings: The legacy of Wallace Hume Carothers. J. Chem. Educ. 1984, 61, 241–242. [Google Scholar] [CrossRef]
- Maggio, G.; Cacciola, G. A variant of the Hubbert curve for world oil production forecasts. Energy Policy 2009, 37, 4761–4770. [Google Scholar] [CrossRef]
- Tachiyama, T.; Yoshida, M.; Aoyagi, T.; Fukuzumi, S. Deuterium kinetic isotope effects and H/D exchange in dimerization of acetylene with a Nieuwland catalyst in aqueous media. J. Phys. Org. Chem. 2008, 21, 510–515. [Google Scholar] [CrossRef]
- Tokita, Y.; Okamoto, A.; Nishiwaki, K.; Kobayashi, M.; Nakamura, E. Kinetics of Copper(I)-Catalyzed Dimerization and Hydration of Acetylene in Water. Bull. Chem. Soc. Jpn. 2004, 77, 1395–1399. [Google Scholar] [CrossRef]
- Changyuan, T.; Rong, H.; Zuohua, L.; Jun, D.; Xing, F.; Xiaoxia, Z.; Renlong, L.; Jinjing, T.; Zhaoming, X.; Dagui, S. Method for Preparing Vinyl Acetylene by Strengthening Acetylene Dimerization by Electric Field. Patent CN 102267856 A, 7 December 2011. [Google Scholar]
- Zhang, Q.; You, Y.; Xie, J.; Dai, B.; Zhang, J. Effect of high-speed shear reactor in liquid phase catalytic reaction of acetylene dimerization. J. Shihezi Univ. (Nat. Sci.) 2019, 37, 6. [Google Scholar] [CrossRef]
- Changyuan, T.; Lijuan, Y.; Jun, D.; Xing, F.; Zuohua, L.; Dagui, S.; Renlong, L.; Zhaohong, Z.; Jin-jing, T.; Xiaoxia, Z. Graphene-Enhanced Acetylene Dimerization Catalyst and Application Method. Patent CN103691470 A, 2 April 2014. [Google Scholar]
- Lu, J.; Xie, J.; Liu, H.; Liu, P.; Liu, Z.; Dai, B. Strontium Chloride Modified Nieuwland Catalyst in the Dimerization of Acetylene to Monovinylacetylene. Asian J. Chem. 2014, 26, 4. [Google Scholar] [CrossRef]
- Lu, J.; Liu, H.; Xie, J.; Liu, P.; Liu, Z. Study on Catalytic Activity Catalysis in of Zinc(II)-Copper(I) Acetylene Dimerization Collaborative Bimetallic l action. J. Shihezi Univ. (Nat. Sci.) 2014, 32, 4. [Google Scholar] [CrossRef]
- Liu, H.; Xie, J.; Liu, P.; Dai, B. Effect of Cu+/Cu2+ Ratio on the Catalytic Behavior of Anhydrous Nieuwland Catalyst during Dimerization of Acetylene. Catalysts 2016, 6, 120. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.; Li, C.; Luo, J.; Xie, J.; Zhang, J.; Dai, B. Hydrazinylbenzenesulfonic Acid-Modified Nieuwland Catalyst for Acetylene Dimerization Reaction. Catal. Lett. 2020, 150, 1766–1773. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, C.; Luo, J.; Xie, J.; Zhang, J.; Dai, B. A novel risedronic acid-modified Nieuwland catalyst for acetylene dimerization. Catal. Commun. 2020, 136, 5. [Google Scholar] [CrossRef]
- Lu, J.; Liu, H.; Xie, J.; Liu, P.; Dai, B.; Liu, Z. Effect of polyethylene glycol/Nieuwland catalyst on acetylene dimerization re-action. Chem. Eng. 2015, 43, 5. [Google Scholar] [CrossRef]
- You, Y.; Luo, J.; Xie, J.; Dai, B. Effect of Iminodiacetic Acid-Modified Nieuwland Catalyst on the Acetylene Dimerization Reaction. Catalysts 2017, 7, 394. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Han, M.; Wang, Z. Effect of solvent on catalytic performance of anhydrous catalyst in acetylene dimerization to monovinylacetylene. J. Energy Chem. 2013, 22, 599–604. [Google Scholar] [CrossRef]
- Liu, J.; Han, M.; Wang, Z. Studies on the Catalytic Performance of the Nieuwland Catalyst and Anhydrous Catalyst in the Dimerization of Acetylene to Monovinylacetylene. Adv. Mater. Res. 2012, 550–553, 312–316. [Google Scholar] [CrossRef]
- Li, C.; Luo, J.; Zhang, Q.; Xie, J.; Zhang, J.; Dai, B. Gas–solid acetylene dimerization over copper-based catalysts. New J. Chem. 2019, 43, 13608–13615. [Google Scholar] [CrossRef]
- Zeng, Q.; Xu, G.; Zhang, L.; Lin, H.; Lv, Y.; Jia, D. Porous CuO nanofibers derived from a Cu-based coordination polymer as a photocatalyst for the degradation of rhodamine B. New J. Chem. 2018, 42, 7016–7024. [Google Scholar] [CrossRef]
- Li, C.; Xie, J.; Zhang, J.; Dai, B. Nitrogen-Modified Activated Carbon Supported Cu(II)Cu(I)/NAC Catalysts for Gas–Solid Acetylene Dimerization. Catal. Lett. 2021, 151, 2990–2995. [Google Scholar] [CrossRef]
- Reddy Kannapu, H.P.; Mullen, C.A.; Elkasabi, Y.; Boateng, A.A. Catalytic transfer hydrogenation for stabilization of bio-oil oxygenates: Reduction of p-cresol and furfural over bimetallic Ni–Cu catalysts using isopropanol. Fuel Process. Technol. 2015, 137, 220–228. [Google Scholar] [CrossRef]
- Lin, C.; Li, R. Study on the temperature-programmed reduction of CuCl2/NaY system heating the monolayer dispersion state. J. Shenyang Normal Univ. (Nat. Sci. Ed.) 2002, 20, 202–205. [Google Scholar] [CrossRef]
- Deng, G.; Li, J.; Chen, R. Dimerization kinetics and reaction mechanism of acetylene in the catalytic system of CuCl-KCl-HCl-H2O: The relationship between reaction rate and catalyst acidity. China Synth. Rubber Ind. 1983, 12, 3. [Google Scholar]
- You, Y.; Luo, J.; Xie, J.; Zhang, J.; Dai, B. Effects of Coordination Ability of Nitrogen-Containing Carboxylic Acid Ligands on Nieuwland Catalyst. Catalysts 2018, 8, 337. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.; Liu, X.; Luo, J.; Ma, C.; Xu, C. Complexation effect of copper(ii) with HEDP supported by activated carbon and influence on acetylene hydration. New J. Chem. 2021, 45, 1712–1720. [Google Scholar] [CrossRef]


| Sample | pH | |||||||
|---|---|---|---|---|---|---|---|---|
| 1 h | 2 h | 3 h | 4 h | 5 h | 6 h | 7 h | 8 h | |
| NC | 1.2 | 1.2 | 1.3 | 1.2 | 1.2 | 1.2 | 1.3 | 1.3 |
| Zr5/NC | 1.1 | 1.1 | 1.2 | 1.2 | 1.2 | 1.3 | 1.2 | 1.3 |
| Sample | Area% (Cu) a | Cu% (Metal Ion Content) b | Cu Loss (%) b | |
|---|---|---|---|---|
| Cu+ | Cu2+ | |||
| Fresh NC | 76.27 | 26.73 | 40.77 | |
| Used NC | 72.78 | 27.22 | 40.35 | 1.03 |
| Fresh Zr5/NC | 71.20 | 28.80 | 38.46 | |
| Used Zr5/NC | 68.64 | 31.36 | 37.19 | 3.30 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, L.; Lin, R.; Luo, D.; Guo, L.; Zhang, J. Dimerization of Acetylene to Monovinylacetylene (MVA) by Bimetallic Zr/Cu Catalyst in Nieuwland Catalytic System. Molecules 2022, 27, 602. https://doi.org/10.3390/molecules27030602
Zheng L, Lin R, Luo D, Guo L, Zhang J. Dimerization of Acetylene to Monovinylacetylene (MVA) by Bimetallic Zr/Cu Catalyst in Nieuwland Catalytic System. Molecules. 2022; 27(3):602. https://doi.org/10.3390/molecules27030602
Chicago/Turabian StyleZheng, Leng, Ruolin Lin, Dingjie Luo, Liang Guo, and Jinli Zhang. 2022. "Dimerization of Acetylene to Monovinylacetylene (MVA) by Bimetallic Zr/Cu Catalyst in Nieuwland Catalytic System" Molecules 27, no. 3: 602. https://doi.org/10.3390/molecules27030602
APA StyleZheng, L., Lin, R., Luo, D., Guo, L., & Zhang, J. (2022). Dimerization of Acetylene to Monovinylacetylene (MVA) by Bimetallic Zr/Cu Catalyst in Nieuwland Catalytic System. Molecules, 27(3), 602. https://doi.org/10.3390/molecules27030602






