A Comprehensive Review on the Techniques for Extraction of Bioactive Compounds from Medicinal Cannabis
Abstract
:1. Introduction
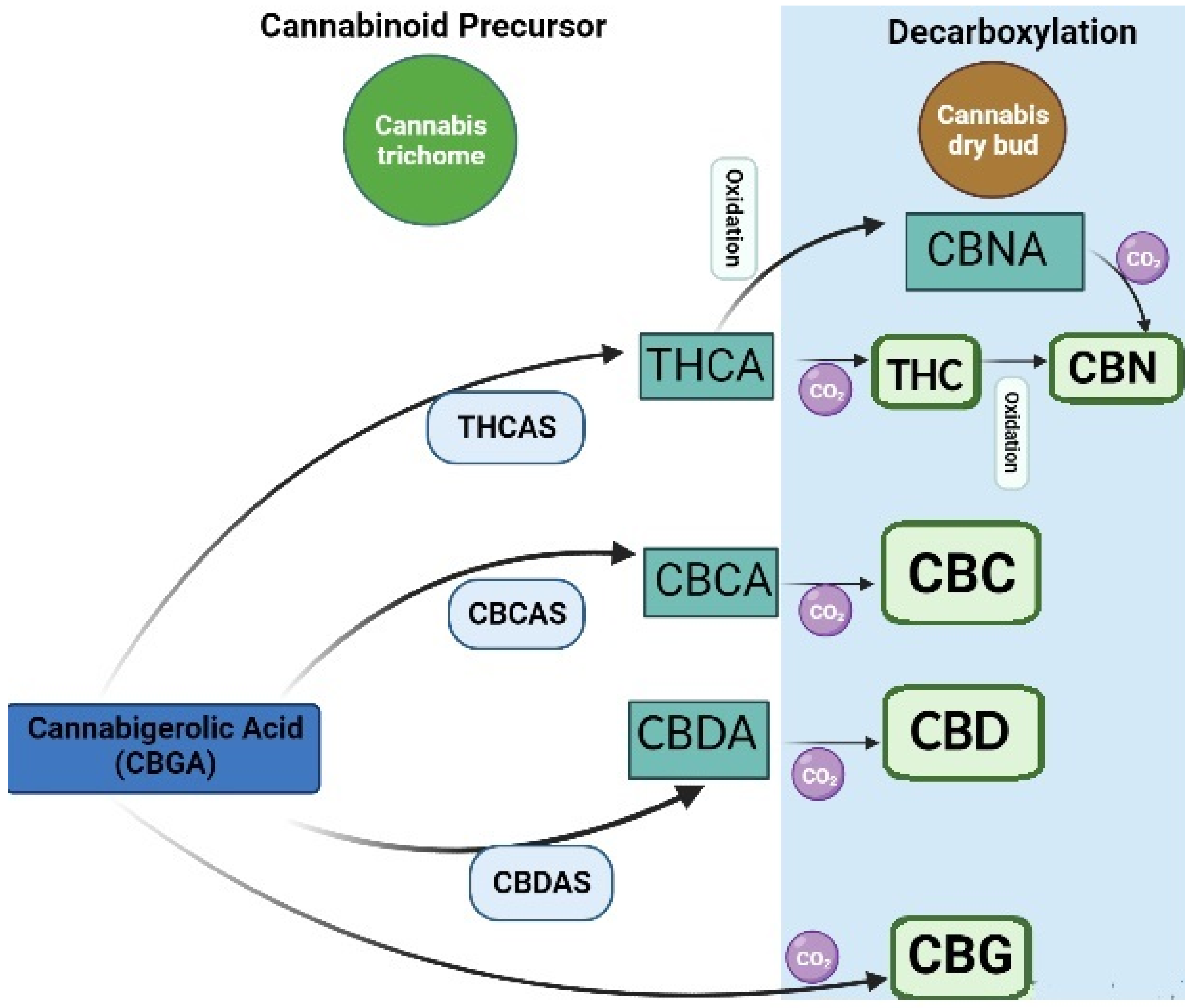
2. Major Cannabis Phytochemicals and Their Therapeutic Effects
3. Factors Affecting the Extraction Efficiency of Phytochemicals from Plant Materials
4. Current Techniques for Extraction of Phytochemicals from Cannabis
4.1. Conventional Extraction of Phytochemicals from Cannabis
4.1.1. Conventional Extraction of Cannabinoids
4.1.2. Conventional Extraction of Phenolic Compounds
4.1.3. Conventional Extraction of Terpenes
4.2. Advanced Extraction Techniques
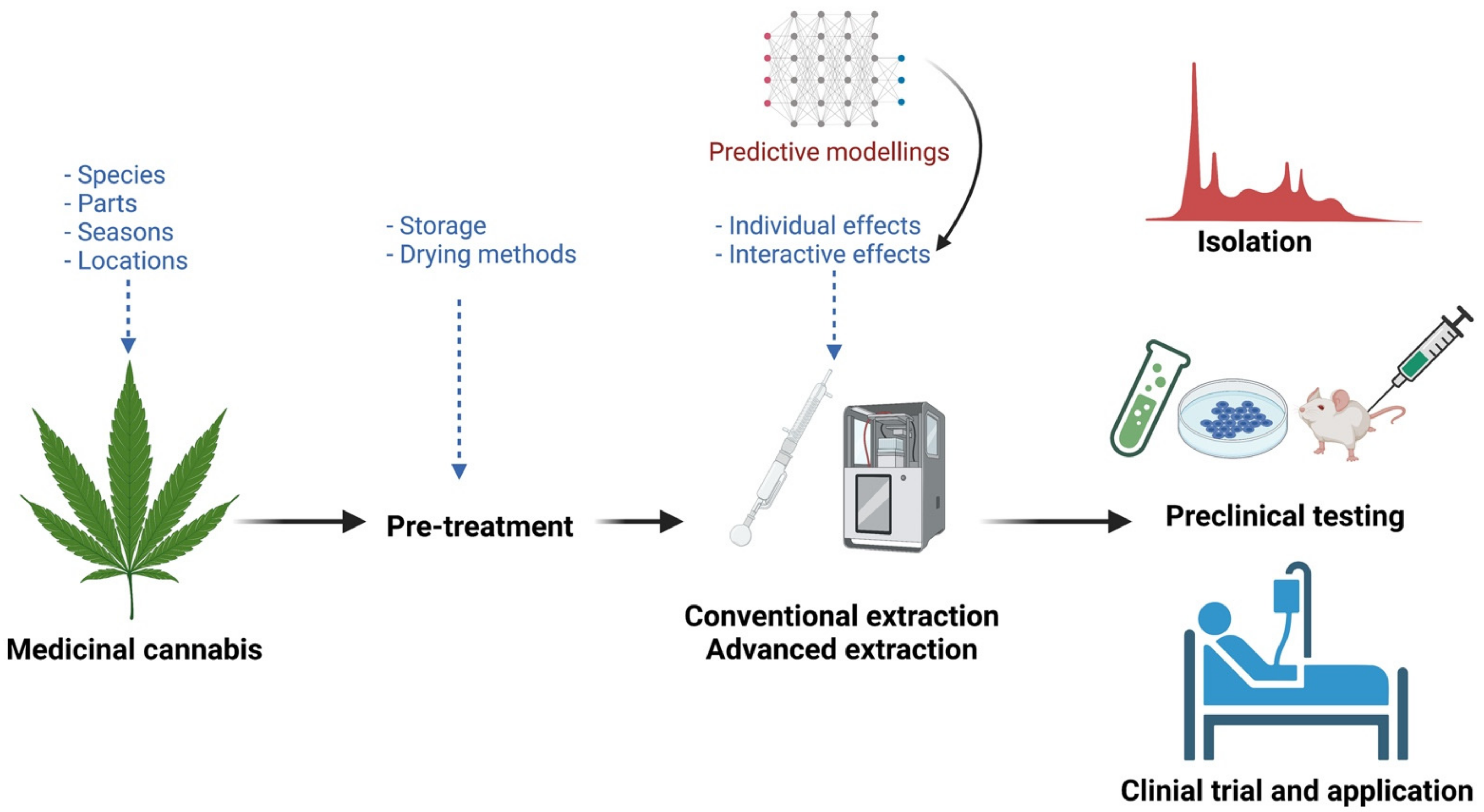
5. Future Considerations for Effective Extraction of Bioactive Compounds from Cannabis
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gloss, D. An overview of products and bias in research. Neurotherapeutics 2015, 12, 731–734. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fellermeier, M.; Eisenreich, W.; Bacher, A.; Zenk, M.H. Biosynthesis of cannabinoids. Incorporation experiments with (13)c-labeled glucoses. Eur. J. Biochem. 2001, 268, 1596–1604. [Google Scholar] [CrossRef] [PubMed]
- Taura, F.; Sirikantaramas, S.; Shoyama, Y.; Yoshikai, K.; Shoyama, Y.; Morimoto, S. Cannabidiolic-acid synthase, the chemotype-determining enzyme in the fiber-type Cannabis sativa. FEBS Lett. 2007, 581, 2929–2934. [Google Scholar] [CrossRef] [Green Version]
- Thomas, B.F.; Elsohly, M. The Analytical Chemistry of Cannabis: Quality Assessment, Assurance, and Regulation of Medicinal Marijuana and Cannabinoid Preparations; Elsevier: Amsterdam, The Netherlands, 2015. [Google Scholar]
- Abuhasira, R.; Shbiro, L.; Landschaft, Y. Medical use of cannabis and cannabinoids containing products–regulations in europe and north america. Eur. J. Intern. Med. 2018, 49, 2–6. [Google Scholar] [CrossRef] [PubMed]
- Arnold, J.C.; Nation, T.; McGregor, I.S. Prescribing medicinal cannabis. Aust. Prescr. 2020, 43, 152. [Google Scholar] [CrossRef] [PubMed]
- Radwan, M.M.; Chandra, S.; Gul, S.; ElSohly, M.A. Cannabinoids, phenolics, terpenes and alkaloids of cannabis. Molecules 2021, 26, 2774. [Google Scholar] [CrossRef] [PubMed]
- Pellati, F.; Brighenti, V.; Sperlea, J.; Marchetti, L.; Bertelli, D.; Benvenuti, S. New methods for the comprehensive analysis of bioactive compounds in Cannabis sativa L. (hemp). Molecules 2018, 23, 2639. [Google Scholar] [CrossRef] [Green Version]
- Rong, C.; Lee, Y.; Carmona, N.E.; Cha, D.S.; Ragguett, R.-M.; Rosenblat, J.D.; Mansur, R.B.; Ho, R.C.; McIntyre, R.S. Cannabidiol in medical marijuana: Research vistas and potential opportunities. Pharmacol. Res. 2017, 121, 213–218. [Google Scholar] [CrossRef] [PubMed]
- Watt, G.; Karl, T. In vivo evidence for therapeutic properties of cannabidiol (cbd) for alzheimer’s disease. Front. Pharmacol. 2017, 8, 20. [Google Scholar] [CrossRef] [Green Version]
- Bassi, M.S.; Sancesario, A.; Morace, R.; Centonze, D.; Iezzi, E. Cannabinoids in parkinson’s disease. Cannabis Cannabinoid Res. 2017, 2, 21–29. [Google Scholar] [CrossRef]
- McGuire, P.; Robson, P.; Cubala, W.J.; Vasile, D.; Morrison, P.D.; Barron, R.; Taylor, A.; Wright, S. Cannabidiol (cbd) as an adjunctive therapy in schizophrenia: A multicenter randomized controlled trial. Am. J. Psychiatry 2018, 175, 225–231. [Google Scholar] [CrossRef] [Green Version]
- Scott, K.A.; Dalgleish, A.G.; Liu, W.M. Anticancer effects of phytocannabinoids used with chemotherapy in leukaemia cells can be improved by altering the sequence of their administration. Int. J. Oncol. 2017, 51, 369–377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schley, M.; Legler, A.; Skopp, G.; Schmelz, M.; Konrad, C.; Rukwied, R. Delta-9-thc based monotherapy in fibromyalgia patients on experimentally induced pain, axon reflex flare, and pain relief. Curr. Med. Res. Opin. 2006, 22, 1269–1276. [Google Scholar] [CrossRef] [PubMed]
- Hadland, S.E.; Knight, J.R.; Harris, S.K. Medical marijuana: Review of the science and implications for developmental behavioral pediatric practice. J. Dev. Behav. Pediatr. JDBP 2015, 36, 115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeissler, M.-L.; Eastwood, J.; McCorry, K.; Hanemann, C.O.; Zajicek, J.P.; Carroll, C.B. Delta-9-tetrahydrocannabinol protects against mpp+ toxicity in sh-sy5y cells by restoring proteins involved in mitochondrial biogenesis. Oncotarget 2016, 7, 46603. [Google Scholar] [CrossRef] [Green Version]
- Tomida, I.; Pertwee, R.G.; Azuara-Blanco, A. Cannabinoids and glaucoma. Br. J. Ophthalmol. 2004, 88, 708–713. [Google Scholar] [CrossRef]
- Izzo, A.A.; Borrelli, F.; Capasso, R.; Di Marzo, V.; Mechoulam, R. Non-psychotropic plant cannabinoids: New therapeutic opportunities from an ancient herb. Trends Pharmacol. Sci. 2009, 30, 515–527. [Google Scholar] [CrossRef] [PubMed]
- Rhee, M.-H.; Vogel, Z.; Barg, J.; Bayewitch, M.; Levy, R.; Hanuš, L.; Breuer, A.; Mechoulam, R. Cannabinol derivatives: Binding to cannabinoid receptors and inhibition of adenylylcyclase. J. Med. Chem. 1997, 40, 3228–3233. [Google Scholar] [CrossRef]
- Eisohly, H.N.; Turner, C.E.; Clark, A.M.; Eisohly, M.A. Synthesis and antimicrobial activities of certain cannabichromene and cannabigerol related compounds. J. Pharm. Sci. 1982, 71, 1319–1323. [Google Scholar] [CrossRef]
- Usami, N.; Kobana, K.; Yoshida, H.; Kimura, T.; Watanabe, K.; Yoshimura, H.; Yamamoto, I. Synthesis and pharmacological activities in mice of halogenated δ9-tetrahydrocannabinol derivatives. Chem. Pharm. Bull. 1998, 46, 1462–1467. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alsherbiny, M.A.; Li, C.G. Medicinal cannabis—Potential drug interactions. Medicines 2019, 6, 3. [Google Scholar] [CrossRef] [Green Version]
- Azmir, J.; Zaidul, I.S.M.; Rahman, M.M.; Sharif, K.; Mohamed, A.; Sahena, F.; Jahurul, M.; Ghafoor, K.; Norulaini, N.; Omar, A. Techniques for extraction of bioactive compounds from plant materials: A review. J. Food Eng. 2013, 117, 426–436. [Google Scholar] [CrossRef]
- Challa, S.K.R.; Misra, N.; Martynenko, A. Drying of cannabis—State of the practices and future needs. Dry. Technol. 2020, 2055–2064. [Google Scholar] [CrossRef]
- Valizadehderakhshan, M.; Shahbazi, A.; Kazem-Rostami, M.; Todd, M.S.; Bhowmik, A.; Wang, L. Extraction of cannabinoids from Cannabis sativa L. (hemp)—Review. Agriculture 2021, 11, 384. [Google Scholar] [CrossRef]
- King, J. Cannabis Strains: Effects, Species, History and Growing. Available online: https://www.cannabisplace.com.au/learn/cannabis-strains-guide-australia#medicinal-cannabis-strains-effects-and-uses (accessed on 25 October 2021).
- Mandolino, G.; Bagatta, M.; Carboni, A.; Ranalli, P.; de Meijer, E. Qualitative and quantitative aspects of the inheritance of chemical phenotype incannabis. J. Ind. Hemp 2003, 8, 51–72. [Google Scholar] [CrossRef]
- Small, E.; Cronquist, A. A practical and natural taxonomy for cannabis. Taxon 1976, 25, 405–435. [Google Scholar] [CrossRef]
- Hillig, K.W.; Mahlberg, P.G. A chemotaxonomic analysis of cannabinoid variation in cannabis (cannabaceae). Am. J. Bot. 2004, 91, 966–975. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- White, J. Cannabis Types. Available online: https://www.cnbs.org/cannabis-types/ (accessed on 25 October 2021).
- Abd-Elsalam, W.H.; Alsherbiny, M.A.; Kung, J.Y.; Pate, D.W.; Löbenberg, R. Lc–ms/ms quantitation of phytocannabinoids and their metabolites in biological matrices. Talanta 2019, 204, 846–867. [Google Scholar] [CrossRef]
- Garrett, E.R.; Hunt, C.A. Physiochemical properties, solubility, and protein binding of delta9-tetrahydrocannabinol. J. Pharm. Sci. 1974, 63, 1056–1064. [Google Scholar] [CrossRef] [PubMed]
- Stella, B.; Baratta, F.; Della Pepa, C.; Arpicco, S.; Gastaldi, D.; Dosio, F. Cannabinoid formulations and delivery systems: Current and future options to treat pain. Drugs 2021, 81, 1513–1557. [Google Scholar] [CrossRef]
- Koch, N.; Jennotte, O.; Gasparrini, Y.; Vandenbroucke, F.; Lechanteur, A.; Evrard, B. Cannabidiol aqueous solubility enhancement: Comparison of three amorphous formulations strategies using different type of polymers. Int. J. Pharm. 2020, 589, 119812. [Google Scholar] [CrossRef] [PubMed]
- Millar, S.A.; Maguire, R.F.; Yates, A.S.; O’Sullivan, S.E. Towards better delivery of cannabidiol (cbd). Pharmaceuticals 2020, 13, 219. [Google Scholar] [CrossRef] [PubMed]
- Murty, S.B.; Murty, R.B. Oral Gastrointestinal Dosage Form Delivery System of Cannabinoids and/or Standardized Marijuana Extracts. U.S. Patent US20160184258A1, 30 June 2016. [Google Scholar]
- Bruni, N.; Della Pepa, C.; Oliaro-Bosso, S.; Pessione, E.; Gastaldi, D.; Dosio, F. Cannabinoid delivery systems for pain and inflammation treatment. Molecules 2018, 23, 2478. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tijani, A.O.; Thakur, D.; Mishra, D.; Frempong, D.; Chukwunyere, U.I.; Puri, A. Delivering therapeutic cannabinoids via skin: Current state and future perspectives. J. Control Release 2021, 334, 427–451. [Google Scholar] [CrossRef]
- Badowski, M.E. A review of oral cannabinoids and medical marijuana for the treatment of chemotherapy-induced nausea and vomiting: A focus on pharmacokinetic variability and pharmacodynamics. Cancer Chemother. Pharm. 2017, 80, 441–449. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rohleder, C.; Pahlisch, F.; Graf, R.; Endepols, H.; Leweke, F.M. Different pharmaceutical preparations of delta(9) -tetrahydrocannabinol differentially affect its behavioral effects in rats. Addict. Biol. 2020, 25, e12745. [Google Scholar] [CrossRef] [PubMed]
- Nachnani, R.; Raup-Konsavage, W.M.; Vrana, K.E. The pharmacological case for cannabigerol. J. Pharm. Exp. 2021, 376, 204–212. [Google Scholar] [CrossRef] [PubMed]
- Bonaccorso, S.; Ricciardi, A.; Zangani, C.; Chiappini, S.; Schifano, F. Cannabidiol (cbd) use in psychiatric disorders: A systematic review. Neurotoxicology 2019, 74, 282–298. [Google Scholar] [CrossRef]
- Elsaid, S.; Kloiber, S.; Le Foll, B. Effects of cannabidiol (cbd) in neuropsychiatric disorders: A review of pre-clinical and clinical findings. Prog. Mol. Biol. Transl. Sci. 2019, 167, 25–75. [Google Scholar] [PubMed]
- White, C.M. A review of human studies assessing cannabidiol’s (cbd) therapeutic actions and potential. J. Clin. Pharm. 2019, 59, 923–934. [Google Scholar] [CrossRef]
- de Almeida, D.L.; Devi, L.A. Diversity of molecular targets and signaling pathways for cbd. Pharm. Res. Perspect. 2020, 8, e00682. [Google Scholar] [CrossRef] [PubMed]
- Seltzer, E.S.; Watters, A.K.; MacKenzie, D., Jr.; Granat, L.M.; Zhang, D. Cannabidiol (cbd) as a promising anti-cancer drug. Cancers 2020, 12, 3203. [Google Scholar] [CrossRef] [PubMed]
- Urits, I.; Gress, K.; Charipova, K.; Habib, K.; Lee, D.; Lee, C.; Jung, J.W.; Kassem, H.; Cornett, E.; Paladini, A.; et al. Use of cannabidiol (cbd) for the treatment of chronic pain. Best Pract. Res. Clin. Anaesthesiol. 2020, 34, 463–477. [Google Scholar] [CrossRef] [PubMed]
- Alsherbiny, M.A.; Bhuyan, D.J.; Low, M.N.; Chang, D.; Li, C.G. Synergistic interactions of cannabidiol with chemotherapeutic drugs in mcf7 cells: Mode of interaction and proteomics analysis of mechanisms. Int. J. Mol. Sci. 2021, 22, 10103. [Google Scholar] [CrossRef] [PubMed]
- Monroe, A.Z.; Gordon, W.H.; Wood, J.S.; Martin, G.E.; Morgan, J.B.; Williamson, R.T. Structural revision of a wnt/beta-catenin modulator and confirmation of cannabielsoin constitution and configuration. Chem. Commun. 2021, 57, 5658–5661. [Google Scholar] [CrossRef]
- Stone, N.L.; Murphy, A.J.; England, T.J.; O’Sullivan, S.E. A systematic review of minor phytocannabinoids with promising neuroprotective potential. Br. J. Pharmacol. 2020, 177, 4330–4352. [Google Scholar] [CrossRef]
- Tomko, A.M.; Whynot, E.G.; Ellis, L.D.; Dupre, D.J. Anti-cancer potential of cannabinoids, terpenes, and flavonoids present in cannabis. Cancers 2020, 12, 1985. [Google Scholar] [CrossRef]
- Pollastro, F.; Minassi, A.; Fresu, L.G. Cannabis phenolics and their bioactivities. Curr. Med. Chem. 2018, 25, 1160–1185. [Google Scholar] [CrossRef]
- Bautista, J.L.; Yu, S.; Tian, L. Flavonoids in Cannabis sativa: Biosynthesis, bioactivities, and biotechnology. ACS Omega 2021, 6, 5119–5123. [Google Scholar] [CrossRef] [PubMed]
- Latter, H.L.; Abraham, D.J.; Turner, C.E.; Knapp, J.E.; Schiff, P.L.; Slatkin, D.J. Cannabisativine, a new alkaloid from Cannabis sativa L. Root. Tetrahedron Lett. 1975, 16, 2815–2818. [Google Scholar] [CrossRef]
- Elsohly, M.A.; Turner, C.E.; Phoebe, C.H., Jr.; Knapp, J.E.; Schiff, P.L., Jr.; Slatkin, D.J. Anhydrocannabisativine, a new alkaloid from Cannabis sativa L. J. Pharm. Sci. 1978, 67, 124. [Google Scholar] [CrossRef] [PubMed]
- Ryz, N.R.; Remillard, D.J.; Russo, E.B. Cannabis roots: A traditional therapy with future potential for treating inflammation and pain. Cannabis Cannabinoid Res. 2017, 2, 210–216. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, K.Q.; Scarlett, C.J.; Vuong, Q.V. Assessment and comparison of phytochemicals and antioxidant properties from various parts of the australian maroon bush (Scaevola spinescens). Heliyon 2021, 7, e06810. [Google Scholar] [CrossRef] [PubMed]
- Vuong, Q.V.; Golding, J.B.; Stathopoulos, C.E.; Nguyen, M.H.; Roach, P.D. Optimizing conditions for the extraction of catechins from green tea using hot water. J. Sep. Sci. 2011, 34, 3099–3106. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, K.Q.; Vuong, Q.V.; Nguyen, M.H.; Roach, P.D. The effects of drying conditions on bioactive compounds and antioxidant activity of the australian maroon bush, Scaevola spinescens. J. Food Processing Preserv. 2018, 42, e13711. [Google Scholar] [CrossRef]
- Dailey, A.; Vuong, Q.V. Effect of extraction solvents on recovery of bioactive compounds and antioxidant properties from macadamia (Macadamia tetraphylla) skin waste. Cogent Food Agric. 2015, 1, 1115646. [Google Scholar] [CrossRef]
- Vuong, Q.V.; Golding, J.B.; Stathopoulos, C.E.; Roach, P.D. Effects of aqueous brewing solution ph on the extraction of the major green tea constituents. Food Res. Int. 2013, 53, 713–719. [Google Scholar] [CrossRef]
- Ahmed, M.; Ramachandraiah, K.; Jiang, G.-H.; Eun, J.B. Effects of ultra-sonication and agitation on bioactive compounds and structure of amaranth extract. Foods 2020, 9, 1116. [Google Scholar] [CrossRef]
- Xi, J. Ultrahigh pressure extraction of bioactive compounds from plants—A review. Crit. Rev. Food Sci. Nutr. 2017, 57, 1097–1106. [Google Scholar] [CrossRef]
- Saifullah, M.; McCullum, R.; McCluskey, A.; Vuong, Q.V. Comparison of conventional extraction technique with ultrasound assisted extraction on recovery of phenolic compounds from lemon scented tea tree (Leptospermum petersonii) leaves. Heliyon 2020, 6, e03666. [Google Scholar] [CrossRef]
- Bhuyan, D.J.; Vuong, Q.V.; Chalmers, A.C.; van Altena, I.A.; Bowyer, M.C.; Scarlett, C.J. Development of the ultrasonic conditions as an advanced technique for extraction of phenolic compounds from eucalyptus robusta. Sep. Sci. Technol. 2017, 52, 100–112. [Google Scholar] [CrossRef]
- Fischedick, J.T.; Hazekamp, A.; Erkelens, T.; Choi, Y.H.; Verpoorte, R. Metabolic fingerprinting of Cannabis sativa L., cannabinoids and terpenoids for chemotaxonomic and drug standardization purposes. Phytochemistry 2010, 71, 2058–2073. [Google Scholar] [CrossRef]
- Namdar, D.; Mazuz, M.; Ion, A.; Koltai, H. Variation in the compositions of cannabinoid and terpenoids in Cannabis sativa derived from inflorescence position along the stem and extraction methods. Ind. Crops Prod. 2018, 113, 376–382. [Google Scholar] [CrossRef]
- Ramirez, C.L.; Fanovich, M.A.; Churio, M.S. Chapter 4—cannabinoids: Extraction methods, analysis, and physicochemical characterization. In Studies in Natural Products Chemistry; Attaur, R., Ed.; Elsevier: Amsterdam, The Netherlands, 2019; Volume 61, pp. 143–173. [Google Scholar]
- Dussy, F.E.; Hamberg, C.; Luginbühl, M.; Schwerzmann, T.; Briellmann, T.A. Isolation of δ9-thca-a from hemp and analytical aspects concerning the determination of δ9-thc in cannabis products. Forensic Sci. Int. 2005, 149, 3–10. [Google Scholar] [CrossRef]
- Wianowska, D.; Dawidowicz, A.L.; Kowalczyk, M. Transformations of tetrahydrocannabinol, tetrahydrocannabinolic acid and cannabinol during their extraction from Cannabis sativa L. J. Anal. Chem. 2015, 70, 920–925. [Google Scholar] [CrossRef]
- Lehmann, T.; Brenneisen, R. A new chromatographic method for the isolation of (−)-δ9-(trans)-tetrahydrocannabinolic acid a. Phytochem. Anal. 1992, 3, 88–90. [Google Scholar] [CrossRef]
- Ibrahim, E.A.; Wang, M.; Radwan, M.M.; Wanas, A.S.; Majumdar, C.G.; Avula, B.; Wang, Y.-H.; Khan, I.A.; Chandra, S.; Lata, H. Analysis of terpenes in Cannabis sativa L. Using gc/ms: Method development, validation, and application. Planta Med. 2019, 85, 431–438. [Google Scholar] [CrossRef] [PubMed]
- Brighenti, V.; Pellati, F.; Steinbach, M.; Maran, D.; Benvenuti, S. Development of a new extraction technique and hplc method for the analysis of non-psychoactive cannabinoids in fibre-type Cannabis sativa L. (hemp). J. Pharm. Biomed. Anal. 2017, 143, 228–236. [Google Scholar] [CrossRef]
- Haji-Moradkhani, A.; Rezaei, R.; Moghimi, M. Optimization of pulsed electric field-assisted oil extraction from cannabis seeds. J. Food Process. Eng. 2019, 42, e13028. [Google Scholar] [CrossRef]
- Isidore, E.; Karim, H.; Ioannou, I. Extraction of phenolic compounds and terpenes from Cannabis sativa L. By-products: From conventional to intensified processes. Antioxidants 2021, 10, 942. [Google Scholar] [CrossRef]
- Addo, P.W.; Brousseau, V.D.; Morello, V.; MacPherson, S.; Paris, M.; Lefsrud, M. Cannabis chemistry, post-harvest processing methods and secondary metabolite profiling: A review. Ind. Crops Prod. 2021, 170, 113743. [Google Scholar] [CrossRef]
- Baranauskaite, J.; Marksa, M.; Ivanauskas, L.; Vitkevicius, K.; Liaudanskas, M.; Skyrius, V.; Baranauskas, A. Development of extraction technique and gc/fid method for the analysis of cannabinoids in Cannabis sativa L. spp. santicha (hemp). Phytochem. Anal. 2020, 31, 516–521. [Google Scholar] [CrossRef] [PubMed]
- Drinić, Z.; Vladic, J.; Koren, A.; Zeremski, T.; Stojanov, N.; Tomić, M.; Vidović, S. Application of conventional and high-pressure extraction techniques for the isolation of bioactive compounds from the aerial part of hemp (Cannabis sativa L.) assortment helena. Ind. Crops Prod. 2021, 171, 113908. [Google Scholar] [CrossRef]
- Fathordoobady, F.; Singh, A.; Kitts, D.D.; Singh, A.P. Hemp (Cannabis sativa L.) extract: Anti-microbial properties, methods of extraction, and potential oral delivery. Food Rev. Int. 2019, 35, 664–684. [Google Scholar] [CrossRef]
- Lazarjani, M.P.; Young, O.; Kebede, L.; Seyfoddin, A. Processing and extraction methods of medicinal cannabis: A narrative review. J. Cannabis Res. 2021, 3, 32. [Google Scholar] [CrossRef] [PubMed]
- Marzorati, S.; Friscione, D.; Picchi, E.; Verotta, L. Cannabidiol from inflorescences of Cannabis sativa L.: Green extraction and purification processes. Ind. Crops Prod. 2020, 155, 112816. [Google Scholar] [CrossRef]
- Micalizzi, G.; Vento, F.; Alibrando, F.; Donnarumma, D.; Dugo, P.; Mondello, L. Cannabis sativa L.: A comprehensive review on the analytical methodologies for cannabinoids and terpenes characterization. J. Chromatogr. 2021, 1637, 461864. [Google Scholar] [CrossRef] [PubMed]
- Nuapia, Y.; Tutu, H.; Chimuka, L.; Cukrowska, E. Selective extraction of cannabinoid compounds from cannabis seed using pressurized hot water extraction. Molecules 2020, 25, 1335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Radoiu, M.; Kaur, H.; Bakowska-Barczak, A.; Splinter, S. Microwave-assisted industrial scale cannabis extraction. Technologies 2020, 8, 45. [Google Scholar] [CrossRef]
- Sirikantaramas, S.; Taura, F. Cannabinoids: Biosynthesis and biotechnological applications. In Cannabis sativa L.—Botany and Biotechnology; Springer: Berlin/Heidelberg, Germany, 2017; pp. 183–206. [Google Scholar]
- Lewis-Bakker, M.M.; Yang, Y.; Vyawahare, R.; Kotra, L.P. Extractions of medical cannabis cultivars and the role of decarboxylation in optimal receptor responses. Cannabis Cannabinoid Res. 2019, 4, 183–194. [Google Scholar] [CrossRef] [Green Version]
- Ciolino, L.A.; Ranieri, T.L.; Taylor, A.M. Commercial cannabis consumer products part 1: Gc–ms qualitative analysis of cannabis cannabinoids. Forensic Sci. Int. 2018, 289, 429–437. [Google Scholar] [CrossRef]
- Hazekamp, A.; Fischedick, J.T. Cannabis from cultivar to chemovar. Drug Test. Anal. 2012, 4, 660–667. [Google Scholar] [CrossRef] [PubMed]
- Stefanidou, M.; Athanaselis, S.; Alevisopoulos, G.; Papoutsis, J.; Koutselinis, A. Δ9-tetrahydrocannabinol content in cannabis plants of greek origin. Chem. Pharm. Bull. 2000, 48, 743–745. [Google Scholar] [CrossRef] [Green Version]
- Namdar, D.; Charuvi, D.; Ajjampura, V.; Mazuz, M.; Ion, A.; Kamara, I.; Koltai, H. Led lighting affects the composition and biological activity of Cannabis sativa secondary metabolites. Ind. Crops Prod. 2019, 132, 177–185. [Google Scholar] [CrossRef]
- Hazekamp, A. Cannabis: Extracting the Medicine; Leiden University: Leiden, The Netherlands, 2007; pp. 1–188. [Google Scholar]
- Romano, L.L.; Hazekamp, A. Cannabis oil: Chemical evaluation of an upcoming cannabis-based medicine. Cannabinoids 2013, 1, 1–11. [Google Scholar]
- Citti, C.; Ciccarella, G.; Braghiroli, D.; Parenti, C.; Vandelli, M.A.; Cannazza, G. Medicinal cannabis: Principal cannabinoids concentration and their stability evaluated by a high performance liquid chromatography coupled to diode array and quadrupole time of flight mass spectrometry method. J. Pharm. Biomed. Anal. 2016, 128, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Izzo, L.; Castaldo, L.; Narváez, A.; Graziani, G.; Gaspari, A.; Rodríguez-Carrasco, Y.; Ritieni, A. Analysis of phenolic compounds in commercial Cannabis sativa L. Inflorescences using uhplc-q-orbitrap hrms. Molecules 2020, 25, 631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allegrone, G.; Pollastro, F.; Magagnini, G.; Taglialatela-Scafati, O.; Seegers, J.; Koeberle, A.; Werz, O.; Appendino, G. The bibenzyl canniprene inhibits the production of pro-inflammatory eicosanoids and selectively accumulates in some Cannabis sativa strains. J. Nat. Prod. 2017, 80, 731–734. [Google Scholar] [CrossRef] [PubMed]
- Babiker, E.E.; Uslu, N.; Al Juhaimi, F.; Mohamed Ahmed, I.A.; Ghafoor, K.; Özcan, M.M.; Almusallam, I.A. Effect of roasting on antioxidative properties, polyphenol profile and fatty acids composition of hemp (Cannabis sativa L.) seeds. LWT 2021, 139, 110537. [Google Scholar] [CrossRef]
- Palmieri, S.; Pellegrini, M.; Ricci, A.; Compagnone, D.; Lo Sterzo, C. Chemical composition and antioxidant activity of thyme, hemp and coriander extracts: A comparison study of maceration, soxhlet, uae and rslde techniques. Foods 2020, 9, 1221. [Google Scholar] [CrossRef]
- Teh, S.-S.; Bekhit, A.E.-D.; Birch, J. Antioxidative polyphenols from defatted oilseed cakes: Effect of solvents. Antioxidants 2014, 3, 67–80. [Google Scholar] [CrossRef]
- Ahmed, M.; Ji, M.; Qin, P.; Gu, Z.; Liu, Y.; Sikandar, A.; Iqbal, M.; Javeed, A. Phytochemical screening, total phenolic and flavonoids contents and antioxidant activities of Citrullus colocynthis L. and Cannabis sativa L. Appl. Ecol. Environ. Res. 2019, 17, 6961–6979. [Google Scholar] [CrossRef]
- Drinić, Z.; Vidović, S.; Vladić, J.; Koren, A.; Kiprovski, B.; Sikora, V. Effect of extraction solvent on total polyphenols content and antioxidant activity of Cannabis sativa L. Lek. Sirovine 2018, 38, 17–21. [Google Scholar] [CrossRef]
- Lesma, G.; Consonni, R.; Gambaro, V.; Remuzzi, C.; Roda, G.; Silvani, A.; Vece, V.; Visconti, G.L. Cannabinoid-free Cannabis sativa L. Grown in the po valley: Evaluation of fatty acid profile, antioxidant capacity and metabolic content. Nat. Prod. Res. 2014, 28, 1801–1807. [Google Scholar] [CrossRef]
- Mkpenie, V.; Essien, E.; Udoh, I. Effect of extraction conditions on total polyphenol contents, antioxidant and antimicrobial activities of Cannabis sativa L. Electron. J. Environ. Agric. Food Chem. 2012, 11, 300–307. [Google Scholar]
- Mourtzinos, I.; Menexis, N.; Iakovidis, D.; Makris, D.P.; Goula, A. A green extraction process to recover polyphenols from byproducts of hemp oil processing. Recycling 2018, 3, 15. [Google Scholar] [CrossRef] [Green Version]
- Fiorini, D.; Molle, A.; Nabissi, M.; Santini, G.; Benelli, G.; Maggi, F. Valorizing industrial hemp (Cannabis sativa L.) by-products: Cannabidiol enrichment in the inflorescence essential oil optimizing sample pre-treatment prior to distillation. Ind. Crops Prod. 2019, 128, 581–589. [Google Scholar] [CrossRef]
- Naz, S.; Hanif, M.A.; Bhatti, H.N.; Ansari, T.M. Impact of supercritical fluid extraction and traditional distillation on the isolation of aromatic compounds from cannabis indica and Cannabis sativa. J. Essent. Oil Bear. Plants 2017, 20, 175–184. [Google Scholar] [CrossRef]
- Zheljazkov, V.D.; Sikora, V.; Semerdjieva, I.B.; Kačániová, M.; Astatkie, T.; Dincheva, I. Grinding and fractionation during distillation alter hemp essential oil profile and its antimicrobial activity. Molecules 2020, 25, 3943. [Google Scholar] [CrossRef] [PubMed]
- Booth, J.K.; Page, J.E.; Bohlmann, J. Terpene synthases from Cannabis sativa. PLoS ONE 2017, 12, e0173911. [Google Scholar] [CrossRef] [Green Version]
- Casano, S.; Grassi, G.; Martini, V.; Michelozzi, M. Variations in Terpene Profiles of Different Strains of Cannabis sativa L. In Proceedings of the XXVIII International Horticultural Congress on Science and Horticulture for People (IHC2010), A New Look at Medicinal and 925, Lisbon, Portugal, 22–27 August 2010; pp. 115–121. [Google Scholar]
- Ternelli, M.; Brighenti, V.; Anceschi, L.; Poto, M.; Bertelli, D.; Licata, M.; Pellati, F. Innovative methods for the preparation of medical cannabis oils with a high content of both cannabinoids and terpenes. J. Pharm. Biomed. Anal. 2020, 186, 113296. [Google Scholar] [CrossRef]
- Rosenthal, E.; Bho, G. Butane hash oil-shatter, wax, sauce and beyond, quick american. In Beyond Buds: Next Generation-marijuana Extracts and Cannabis Infusions; Blythe, D.T., Ed.; Quick American: Piedmont, CA, USA, 2018; pp. 87–115. [Google Scholar]
- Pubchem Compound Summary for Cid 7843, Butane. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Butane (accessed on 1 January 2022).
- Stogner, J.M.; Miller, B.L.J.S. The dabbing dilemma: A call for research on butane hash oil and other alternate forms of cannabis use. Subst. Abus. 2015, 36, 393–395. [Google Scholar] [CrossRef]
- Gegax, K. Gegax Hemp Financial Research; California State University San Marcos: San Marcos, CA, USA, 2020. [Google Scholar]
- Mustafa, A.; Turner, C. Pressurized liquid extraction as a green approach in food and herbal plants extraction: A review. Anal. Chim. Acta 2011, 703, 8–18. [Google Scholar] [CrossRef]
- Rosenthal, E.; Zeman, G. CO2 extraction. In Beyond Buds: Next Generation-Marijuana Extracts and Cannabis Infusions; Rolph Blythe, D.T., Ed.; Quick American: Piedmont, CA, USA, 2018; pp. 149–167. [Google Scholar]
- Perrotin-Brunel, H. Sustainable Production of Cannabinoids with Supercritical Carbon Dioxide Technologies; University of Technology of Compiègne Geboren te Rouen: Rouen, France, 2011. [Google Scholar]
- Hanuš, L.O.; Meyer, S.M.; Muñoz, E.; Taglialatela-Scafati, O.; Appendino, G. Phytocannabinoids: A unified critical inventory. Nat. Prod. Rep. 2016, 33, 1357–1392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cascio, M.G.; Pertwee, R.G. Known pharmacological actions of nine nonpsychotropic phytocannabinoids. In Handbook of Cannabis; Pertwee, R., Ed.; Oxford University Press: Oxford, UK, 2014; Volume 137, pp. 137–156. [Google Scholar]
- Lucas, P.G. Regulating compassion: An overview of Canada’s federal medical cannabis policy and practice. Harm Reduct. J. 2008, 5, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Da Porto, C.; Decorti, D.; Natolino, A. Separation of aroma compounds from industrial hemp inflorescences (Cannabis sativa L.) by supercritical CO2 extraction and on-line fractionation. Ind. Crops Prod. 2014, 58, 99–103. [Google Scholar] [CrossRef] [Green Version]
- Cravotto, G.; Boffa, L.; Mantegna, S.; Perego, P.; Avogadro, M.; Cintas, P. Improved extraction of vegetable oils under high-intensity ultrasound and/or microwaves. Ultrason. Sonochem. 2008, 15, 898–902. [Google Scholar] [CrossRef] [PubMed]
- Syed, Q.A.; Ishaq, A.; Rahman, U.; Aslam, S.; Shukat, R. Pulsed electric field technology in food preservation: A review. J. Nutr. Health Food Eng. 2017, 6, 168–172. [Google Scholar]
- Boussetta, N.; Soichi, E.; Lanoisellé, J.-L.; Vorobiev, E. Valorization of oilseed residues: Extraction of polyphenols from flaxseed hulls by pulsed electric fields. Ind. Crops Prod. 2014, 52, 347–353. [Google Scholar] [CrossRef]
- Sarkis, J.; Vorobiev, E. Application of pulsed electric energies for oil and polyphenol extraction from sesame cake and sesame seeds. In Handbook of Electroporation; Miklavcic, D., Ed.; Springer: Cham, Switzerland, 2019; pp. 1–13. [Google Scholar]
- Yu, X.; Bals, O.; Grimi, N.; Vorobiev, E. A new way for the oil plant biomass valorization: Polyphenols and proteins extraction from rapeseed stems and leaves assisted by pulsed electric fields. Ind. Crops Prod. 2015, 74, 309–318. [Google Scholar] [CrossRef]
- Rosenthal, A.; Pyle, D.; Niranjan, K. Aqueous and enzymatic processes for edible oil extraction. Enzym. Microb. Technol. 1996, 19, 402–420. [Google Scholar] [CrossRef]
- Kitrytė, V.; Bagdonaitė, D.; Venskutonis, P.R. Biorefining of industrial hemp (Cannabis sativa L.) threshing residues into cannabinoid and antioxidant fractions by supercritical carbon dioxide, pressurized liquid and enzyme-assisted extractions. Food Chem. 2018, 267, 420–429. [Google Scholar] [CrossRef] [PubMed]
- Latif, S.; Anwar, F. Physicochemical studies of hemp (Cannabis sativa) seed oil using enzyme-assisted cold-pressing. Eur. J. Lipid Sci. 2009, 111, 1042–1048. [Google Scholar] [CrossRef]


| Extraction Technique | Extraction Conditions/Procedures | Advantages and Limitations | References |
|---|---|---|---|
| Solvent extraction | The plant materials (0.9–1.1 g) were crushed and extracted in 45-mL ethanol for 15 min with the agitation of 400 rpm. Extracts were centrifuged briefly for 30 s at 2000 rpm. The supernatant was collected and filtered. | A simple technique, but not very efficient | [66] |
| Solvent extraction | Samples were extracted in hexane and ethanol mixture at 7:3 (v/v) and shaken for 45 min at 225 rpm in a TU-400 orbital shaker incubator at room temperature to obtain the extract. | A simple technique, but not very effective | [67] |
| Solvent extraction | Samples were extracted in ethanol at room temperature for 45 min to obtain the extract. | A simple technique, but not very effective | [68] |
| Solvent extraction | The plant material (100 g) was Pulverised and extracted with 500-mL petrol ether acidified with acetic acid (0.5-mL CH3COOH in 500-mL PE). The filtrated extract was re-extracted 3 times with 400 mL of NaOH and Na2SO3 (2% each). These combined extracts were acidified with 500 mL of 5% sulfuric acid until pH reached 3 and immediately extracted 3 times with 400-mL TBME. These combined organic extracts were dried with Na2SO4, filtrated, and concentrated in a rotary evaporator at 25–30 °C with cryostatic cooling of the vapours. The concentrate was dried overnight at vacuum conditions, yielding 1.71-g brown amorphous material. | A simple technique, but not effective and difficult for commercial production | [69] |
| Soxhlet extraction | Ground dried samples (2 g) were extracted using Soxhlet extractor for 1, 2, or 3 h with 75 mL of n-hexane or methanol then cooled to room temperature to obtain the extract. | A simple technique, but not effective | [70] |
| Sonication | The dried and pulverised plant material (50 g) was extracted by sonication and periodic shaking (30 min) with 250-mL petroleum ether, which was acidified with 0.5-mL concentrated acetic acid. The extract was further extracted 3 times with 200 mL of an aqueous solution (2% w/v each) of sodium hydroxide and sodium sulphite. The combined and cooled water phases were acidified with about 250-mL cooled sulphuric acid to pH 3 and immediately extracted 5 times with 200-mL diethyl ether. The combined organic phases were dried with sodium sulphate and evaporated to dryness. | Quite effective advanced technique, but it is challenging to apply on a commercial scale | [71] |
| Sonication | Samples (1 g) were extracted with 10 mL of the extraction solution (100 μg/mL of n-Tridecane in ethyl acetate) by sonication for 15 min to obtain the extract. | An advanced technique, but not under optimal conditions | [72] |
| Ultrasound-assisted extraction (UAE) | A small amount (0.25 g) of the sample was mixed with 10 mL of ethanol and was then extracted 3 times using UAE at 40 °C for 15 min. The solution was then filtered through a paper filter to obtain the final extract. | An advanced technique, but not under optimal conditions | [73] |
| Pressurized liquid extraction (PLE) | Samples of Cannabis (0.3 g) were mixed with sand and then placed into a 22-mL stainless-steel extraction cell with a cellulose filter. The sample cells were then closed and placed into the carousel of the ASE 200 system. Methanol or n-hexane was used as extraction solvents. Extractions were carried out at 25, 50, 75, 100, 125, and 150 °C at a pressure of 40 bar. Extractions were performed for 5, 10, 15, or 20 min. After the extraction process, the extraction cell content was flushed with the same solvent in the amount equal to 60% of the extraction cell volume and purged for 60 s by applying pressurized nitrogen (at 150 psi) to obtain the final extracts. | An advanced technique, but it has not been operated under optimal conditions. | [70] |
| Ultrasound-assisted extraction (UAE) | A small amount (0.25 g) of sample was mixed with 10 mL of ethanol and was then extracted 3 times using UAE at 40 °C for 15 min. The solution was then filtered through a paper filter to obtain the final extract. | An advanced technique, but not under optimal conditions | [73] |
| Pulse electric field extraction (PEF) | The seeds were treated by the PEF process (0, 3, and 6 kV/cm). The PEF process was conducted with a capacity of a process chamber of 4 L. Maximum voltage of the instrument was 7 Kv, and its capacitance was 8 μF. | An advanced technique, but not under optimal conditions | [74] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
AL Ubeed, H.M.S.; Bhuyan, D.J.; Alsherbiny, M.A.; Basu, A.; Vuong, Q.V. A Comprehensive Review on the Techniques for Extraction of Bioactive Compounds from Medicinal Cannabis. Molecules 2022, 27, 604. https://doi.org/10.3390/molecules27030604
AL Ubeed HMS, Bhuyan DJ, Alsherbiny MA, Basu A, Vuong QV. A Comprehensive Review on the Techniques for Extraction of Bioactive Compounds from Medicinal Cannabis. Molecules. 2022; 27(3):604. https://doi.org/10.3390/molecules27030604
Chicago/Turabian StyleAL Ubeed, Hebah Muhsien Sabiah, Deep Jyoti Bhuyan, Muhammad A. Alsherbiny, Amrita Basu, and Quan V. Vuong. 2022. "A Comprehensive Review on the Techniques for Extraction of Bioactive Compounds from Medicinal Cannabis" Molecules 27, no. 3: 604. https://doi.org/10.3390/molecules27030604
APA StyleAL Ubeed, H. M. S., Bhuyan, D. J., Alsherbiny, M. A., Basu, A., & Vuong, Q. V. (2022). A Comprehensive Review on the Techniques for Extraction of Bioactive Compounds from Medicinal Cannabis. Molecules, 27(3), 604. https://doi.org/10.3390/molecules27030604










