Analytical Evaluation of Carbamate and Organophosphate Pesticides in Human and Environmental Matrices: A Review
Abstract
:1. Introduction
2. Physicochemical Properties and Applications of Carbamate and Organophosphate Pesticides
2.1. Physicochemical Properties
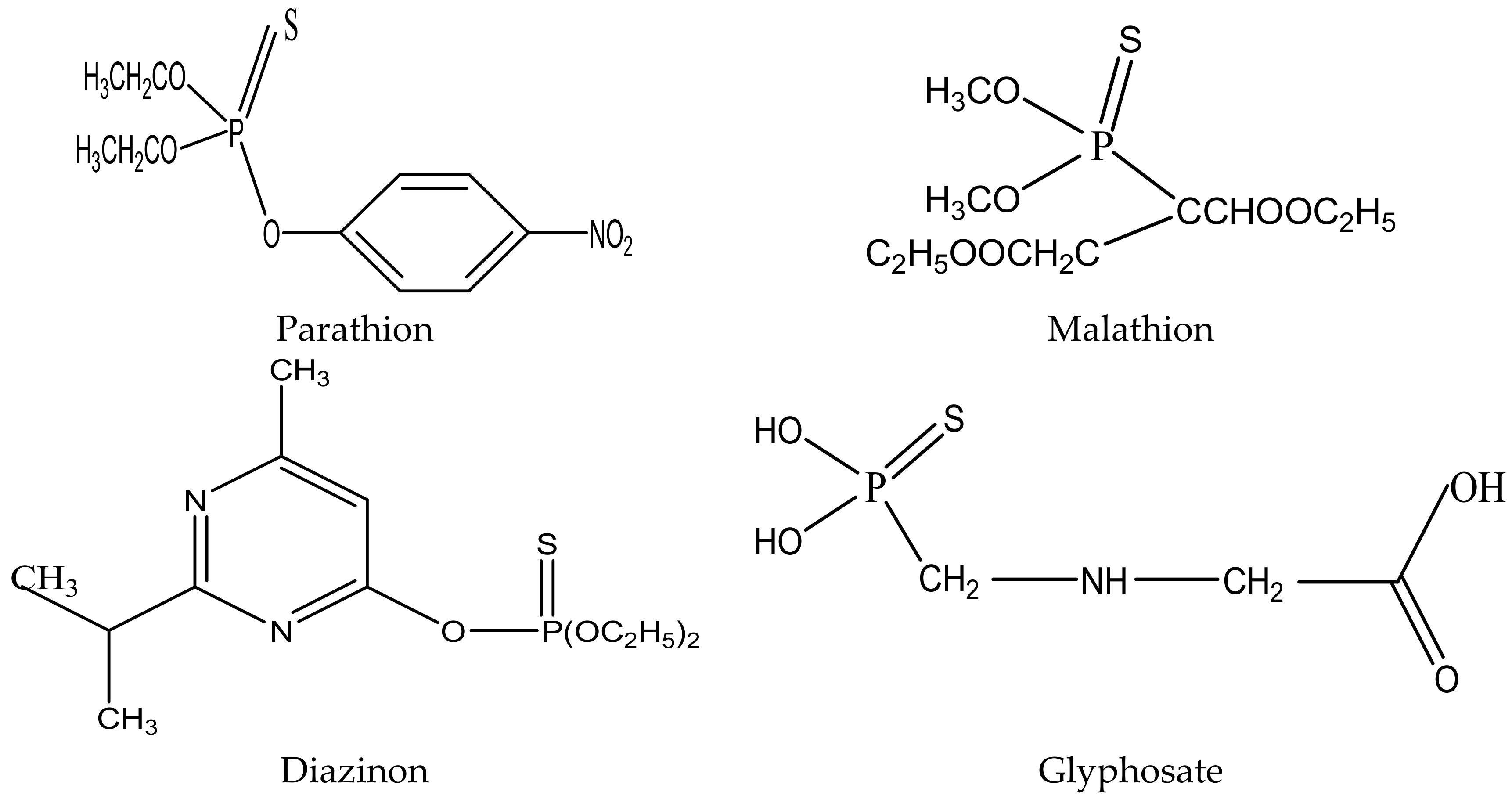
2.1.1. Organophosphate Pesticides
2.1.2. Carbamate Pesticides
3. Sources of CM and OPs in the Environment
4. Toxicity of CM and OPs and Risk of Exposure
5. Sample Collection and Preservation
5.1. Aqueous Samples
5.2. Solid, Semi-Solid, Mixed-Phase, and Oily Samples
5.3. Fish and Other Tissue Samples
6. Extraction Methods for CM and OPs in Water and Sediment
7. Analytical Methods for CMs and OPs in Water and Sediment
7.1. Electrochemical Methods
7.2. Spectroscopy
7.3. Chromatographic or Mass-Spectrometric Techniques
7.4. Fluorescence Techniques
7.5. Spectrometric Techniques
8. Levels of OP and CM Pesticides in the Environment
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Vlček, V.; Pohanka, M. Carbamate insecticides in the czech republic: Health and environmental impacts. Mil. Med. Sci. Lett. 2012, 81, 2–8. [Google Scholar] [CrossRef] [Green Version]
- Van Dyk, J.S.; Pletschke, B. Review on the use of enzymes for the detection of organochlorine, organophosphate and carbamate pesticides in the environment. Chemosphere 2011, 82, 291–307. [Google Scholar] [CrossRef] [PubMed]
- Marican, A.; Durán-Lara, E.F. A review on pesticide removal through different processes. Environ. Sci. Pollut. Res. 2017, 25, 2051–2064. [Google Scholar] [CrossRef] [PubMed]
- Silberman, J.; Taylor, A. Carbamate Toxicity; StatPearls Publishing: Treasure Island, FL, USA, 2020. [Google Scholar]
- Zacharia, J.T. Identity, physical and chemical properties of pesticides. Pestic. Mod. World-Trends Pestic. Anal. 2011, 1, 1–18. [Google Scholar]
- World Health Organization. Carbamate Pesticides: A General Introduction; No. 64 Environmental Health Criteria; WHO: Geneva, Switzerland, 1986. [Google Scholar]
- Tanen, D.A. Organophosphorus and Carbamate Insecticides, Poisoning & Drug Overdose, 5th ed.; McGraw-Hill: New York, NY, USA, 2007; pp. 291–295. [Google Scholar]
- Bird, S.; Traub, S.J.; Grayzel, J. Organophosphate and carbamate poisoning. UpToDate 2014, 14, 339. [Google Scholar]
- Friend, M. Chemical Toxins (Field Manual of Wildlife Diseases). 1999. Available online: https://digitalcommons.unl.edu/zoonoticspub/17/ (accessed on 4 December 2021).
- World Health Organization. Public Health Impact of Pesticides Used in Agriculture; World Health Organization: Geneva, Switzerland, 1990. [Google Scholar]
- Raţă, G.; Perković, A. The Vocabulary of Pesticides: A Terminological Approach. In Proceedings of the 43rd Croatian and 3rd International Symposium on Agriculture, Opatija, Croatia, 18–21 February 2008; Volume 725, p. 729. [Google Scholar]
- Petrovic, G.M.; Stojanovic, G.S.; Jovanovic, O.P.; Dordevic, A.S.; Palic, I.R.; Sovilj, S.V. Inclusion complexes of pesticides in aqueous solutions of methylated [beta]-cyclodextrin/Inkluzioni kompleksi pesticida u vodenom rastvoru metilovanog [beta]-ciklodekstrina. Hem. Ind. 2013, 6, 231–238. [Google Scholar] [CrossRef]
- Marrazza, G. Piezoelectric Biosensors for Organophosphate and Carbamate Pesticides: A Review. Biosensors 2014, 4, 301–317. [Google Scholar] [CrossRef] [Green Version]
- Amdany, R. Passive Samplers: Development and Application in Monitoring Organic Micropollutants in South African Water Bodies and Wastewater. Ph.D. Thesis, Dedan Kimathi University of Technology, Nyeri, Kenya, 2014. [Google Scholar]
- Brown, T.M.; Macdonald, R.W.; Muir, D.C.; Letcher, R.J. The distribution and trends of persistent organic pollutants and mercury in marine mammals from Canada’s Eastern Arctic. Sci. Total Environ. 2018, 618, 500–517. [Google Scholar] [CrossRef]
- Bejarano, A.C.; Widenfalk, A.; Decho, A.W.; Chandler, G.T. Bioavailability of the organophosphorous insecticide chlorpyrifos to the suspension-feeding bivalve, Mercenaria mercenaria, following exposure to dis-solved and particulate matter. Environ. Toxicol. Chem. Int. J. 2003, 22, 2100–2105. [Google Scholar] [CrossRef]
- Giesy, J.P.; Solomon, K.R. Ecological Risk Assessment for Chlorpyrifos in Terrestrial and Aquatic Systems in North America; Springer: Berlin/Heidelberg, Germany, 2014. [Google Scholar]
- Research Council. Bioavailability of Contaminants in Soils and Sediments: Processes, Tools, and Applications; National Academies Press: Washington, DC, USA, 2003. [Google Scholar] [CrossRef]
- Sud, D.; Kaur, P. Heterogeneous Photocatalytic Degradation of Selected Organophosphate Pesticides: A Review. Crit. Rev. Environ. Sci. Technol. 2012, 42, 2365–2407. [Google Scholar] [CrossRef]
- Downing, E.; Environmental fate of Acephate. Environmental Monitoring and Pest Management. Department of Pesticide Regulation. Available online: http://citeseerx.ist.psu.edu/viewdoc/download?doi=10.1.1.639.9028&rep=rep1&type=pdf (accessed on 4 December 2021).
- Oerke, E.C. Crop losses to pests. J. Agric. Sci. 2006, 144, 31–43. [Google Scholar] [CrossRef]
- Freed, V.H.; Haque, R.; Schmedding, D.; Kohnert, R. Physicochemical properties of some organophosphates in relation to their chronic toxicity. Environ. Health Perspect. 1976, 13, 77–81. [Google Scholar] [CrossRef] [PubMed]
- Kubiak, R.; Burkle, L.; Cousins, I.; Hourdakis, A.; Jarvis, T.; Jene, B.; Koch, W.; Kreuger, J.; Maier, W.; Millet, M.; et al. Pesticides in air: Considerations for exposure assessment. 2008. Available online: https://esdac.jrc.ec.europa.eu/public_path/projects_data/focus/air/docs/FOCUS_AIR_GROUP_REPORT-FINAL.pdf (accessed on 4 December 2021).
- National Center for Biotechnology Information. PubChem Compound Summary for CID 276, Carbamate. 2022. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Carbamate (accessed on 4 December 2021).
- Székács, A. Herbicide mode of action. Herbicides; Elsevier: Amsterdam, The Netherlands, 2021; pp. 41–86. [Google Scholar]
- Hilal, S.H.; Karickhoff, S.W.; Carreira, L.A. Prediction of Chemical Reactivity Parameters and Physical Properties of Organic Compounds from Molecular Structure Using SPARC.; US Environmental Protection Agency: Washington, DC, USA, 2003. [Google Scholar]
- National Center for Biotechnology Information. PubChem Compound Summary for CID 2314, Bendiocarb. 2022. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Bendiocarb (accessed on 4 December 2021).
- Buckley-Golder, D.; Coleman, P.; Davies, M.; King, K.; Petersen, A.; Watterson, J.; Fiedler, H.; Hanberg, A. Compilation of EU dioxin exposure and health data. Organohalogen Compd. 1999, 44, 79–82. [Google Scholar]
- National Research Council. Physicochemical Properties and Environmental Fate. A Framework to Guide Selection of Chemical Alternatives; National Academies Press: Washington, DC, USA, 2014. [Google Scholar]
- Anderson, B.; Bailey, T.; Garber, K.; Hetrick, J.; Hoffman, M.; Kahn, F.; Odenkirchen, E.; Parker, R.; Ruhman, M.; Sappington, K.; et al. White Paper on Methods for Assessing Ecological Risk of Pesticides with Persistent, Bioaccumulative and Toxic Characteristics; Office of Prevention, Pesticides and Toxic Substances, Office of Pesticide Programs, US EPA: Washington, DC, USA, 2008; p. 221. [Google Scholar]
- Murphy, B.L.; Morrison, R.D. (Eds.) Introduction to Environmental Forensics; Academic Press: San Diego, CA, USA, 2014. [Google Scholar]
- Vallero, D.A. Environmental Biotechnology: A Biosystems Approach; Academic Press: Cambridge, MA, USA, 2015. [Google Scholar]
- Shiu, W.Y.; Ma, K.C.; Mackay, D.; Seiber, J.N.; Wauchope, R.D. Solubilities of Pesticide Chemicals in Water Part I: Environmental Physical Chemistry. In Reviews of Environmental Contamination and Toxicology; Springer: New York, NY, USA, 1990; Volume 116, pp. 1–13. [Google Scholar] [CrossRef]
- Vermeire, T.; McPhail, R.; Waters, M.D. Organophosphorous pesticides in the environment. In Report of the International Workshop on Approaches to Integrated Risk Assessment; World Health Organization: Geneva, Switzerland, 2001; pp. 1–18. [Google Scholar]
- Samuels, T.A.; Obare, S.O. Advances in Analytical Methods for Organophosphorus Pesticide Detection. Pestic. Mod. World–Trends Pestic. Anal. 2011, 1, 93. [Google Scholar]
- Abdul-Rahman, A.M. Subsidence of Acute Poisoning in Children Using Pesticide Accidentally in Agriculture Region and Study Their Response to Atropine. Int. J. Med. Pharm. Sci. 2016, 30, 29–38. [Google Scholar]
- Ogah, C.O.; Coker, H.B. Quantification of organophosphate and carbamate pesticide residues in maize. J. Appl. Pharm. Sci. 2012, 2, 93. [Google Scholar] [CrossRef] [Green Version]
- Moore, D.R.; Teed, R.S. Risks of carbamate and organophosphate pesticide mixtures to salmon in the Pacific Northwest. Integr. Environ. Assess. Manag. 2012, 9, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Erhirhie, E.O.; Ihekwereme, C.P.; Ilodigwe, E.E. Advances in acute toxicity testing: Strengths, weaknesses and regulatory acceptance. Interdiscip. Toxicol. 2018, 11, 5–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Than, K. Organophosphates: A common but deadly pesticide. Available online: https://www.nationalgeographic.com/culture/article/130718-organophosphates-pesticides-indian-food-poisoning (accessed on 4 December 2021).
- Toxic Substances and Disease Registry (ATSDR). Public Health Assessment Guidance Manual (Update) Agency for Toxic Substances and Disease Registry. Available online: https://www.atsdr.cdc.gov/hac/phamanual/pdfs/phagm_final1-27-05.pdf (accessed on 4 December 2021).
- U.S. EPA. Environmental Protection Agency. Method 1699: Pesticides in water, Soil, Sediment, Biosolids, and Tissue by HRGC/HRMS; U.S. Environmental Protection Agency, Office of Water, Office of Science and Technology Engineering and Analysis Division: Washington, DC, USA, 2007. [Google Scholar]
- US. EPA. Methods for Collection, Storage and Manipulation of Sediments for Chemical and Toxicological Analyses: Technical Manual; EPA 823-B-01-002; U.S. Environmental Protection Agency, Office of Water: Washington, DC, USA, 2001. [Google Scholar]
- Moldoveanu, S.; David, V. Chapter 7—Solid-phase extraction. In Modern Sample Preparation for Chromatography, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 281–421. [Google Scholar] [CrossRef]
- Filik, H.; Çekiç, S.D. Cloud point extraction of pesticide residues. In Pesticides in the Modern World-Trends in Pesticides Analysis; IntechOpen: London, UK, 2011. [Google Scholar]
- Masiá, A.; Vásquez, K.; Campo, J.; Picó, Y. Assessment of two extraction methods to determine pesticides in soils, sediments and sludges. Application to the Túria River Basin. J. Chromatogr. A 2015, 1378, 19–31. [Google Scholar] [CrossRef]
- De Castro, M.L.; Priego-Capote, F. Soxhlet extraction: Past and present panacea. J. Chromatogr. A 2010, 1217, 2383–2389. [Google Scholar] [CrossRef]
- Fakirov, S. Modified Soxhlet apparatus for high-temperature extraction. J. Appl. Polym. Sci. 2006, 102, 2013–2014. [Google Scholar] [CrossRef]
- Santos, D.T.; Meireles, A.A. Extraction of volatile oils by supercritical fluid extraction: Patent survey. Recent Pat. Eng. 2011, 5, 17–22. [Google Scholar] [CrossRef]
- Andreu, V.; Picó, Y. Pressurized liquid extraction of organic contaminants in environmental and food samples. TrAC Trends Anal. Chem. 2019, 118, 709–721. [Google Scholar] [CrossRef]
- Liang, H.; Bilon, N.; Hay, M.T. Analytical Methods for Pesticide Residues in the Water Environment. Water Environ. Res. 2015, 87, 1923–1937. [Google Scholar] [CrossRef]
- Del Carlo, M.; Mascini, M.; Pepe, A.; Diletti, G.; Compagnone, D. Screening of food samples for carbamate and organophosphate pesticides using an electrochemical bioassay. Food Chem. 2004, 84, 651–656. [Google Scholar] [CrossRef]
- Garrido, E.M.; Delerue-Matos, C.; Lima, J.L.F.C.; Brett, A.O. Electrochemical methods in pesticides control. Anal. Lett. 2004, 37, 1755–1791. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Shen, L.; Gong, Z.; Pan, J.; Zheng, X.; Xue, J. Analytical methods to analyze pesticides and herbicides. Water Environ. Res. 2019, 91, 1009–1024. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Ye, B.; Wan, C.; Hao, Y.; Lan, Y.; Ouyang, A. Rapid Quantitative Analysis of Dimethoate Pesticide Using Surface–Enhanced Raman Spectroscopy. Trans. Asabe. 2013, 56, 1043–1049. [Google Scholar]
- Shayeghi, M.; Dehghani, M.; Alimohammadi, M.; Goodini, K. Using Ultraviolet Irradiation for Removal of Malathion Pesticide in Water. J. Arthropod-Borne Dis. 2012, 6, 45–53. [Google Scholar] [PubMed]
- Acharya, U.K.; Subedi, P.P.; Walsh, K.B. Evaluation of a dry extract system involving NIR spectros-copy (DESIR) for rapid assessment of pesticide contamination of fruit surfaces. Am. J. Anal. Chem. 2012, 3, 524–533. [Google Scholar] [CrossRef] [Green Version]
- US EPA (United States Environmental Protection Agency). Method 622: The Determination of Organ-Ophosphorus Pesticides in Municipal and Industrial Wastewater. 1992; p. 22. Available online: https://www.epa.gov/sites/default/files/2015-10/documents/method_622_1992.pdf (accessed on 4 December 2021).
- US EPA (United States Environmental Protection Agency). Method 632: The Determination of Carba-Mate and Urea Pesticides in Municipal and Industrial Wastewater. 1992; p. 20. Available online: https://www.epa.gov/sites/default/files/2015-10/documents/method_632_1992.pdf (accessed on 4 December 2021).
- Passeport, E.; Guenne, A.; Culhaoglu, T.; Moreau, S.; Bouyé, J.-M.; Tournebize, J. Design of experiments and detailed uncertainty analysis to develop and validate a solid-phase microextraction/gas chromatography–mass spectrometry method for the simultaneous analysis of 16 pesticides in water. J. Chromatogr. A 2010, 1217, 5317–5327. [Google Scholar] [CrossRef] [PubMed]
- De Souza Pinheiro, A.; Da Rocha, G.O.; De Andrade, J.B. A SDME/GC–MS methodology for determination of organophosphate and pyrethroid pesticides in water. Microchem. J. 2011, 99, 303–308. [Google Scholar] [CrossRef]
- Sharma, V.K.; Jadhav, R.K.; Rao, G.J.; Saraf, A.K.; Chandra, H. High performance liquid chromato-graphic method for the analysis of organophosphorus and carbamate pesticides. Forensic Sci. Int. 1990, 48, 21–25. [Google Scholar] [CrossRef]
- Liu, M.; Hashi, Y.; Song, Y.; Lin, J.M. Simultaneous determination of carbamate and organophosphorus pesticides in fruits and vegetables by liquid chromatography–mass spectrometry. J. Chromatogr. A 2005, 1097, 183–187. [Google Scholar] [CrossRef]
- Chen, H.; Chen, R.; Feng, R.; Li, S. Simultaneous analysis of carbamate and organophosphorus pesticides in water by single-drop microextraction coupled with GC–MS. Chromatographia 2009, 70, 165–172. [Google Scholar] [CrossRef]
- Bontoyan, W. Use of Thin Layer Chromatography for Detection of Contaminating Pesticides in Com-mercial Pesticide Formulations. J. Assoc. Off. Anal. Chem. 1966, 49, 1169–1174. [Google Scholar]
- Akkad, R. Determination of Organophosphorus and Carbamate Insecticides in Food Samples by High-Performance Thin-Layer Chromatography Multi-Enzyme Inhibition Assay. Ph.D. Thesis, Institute of Food Chemistry, University of Hohenheim, Stuttgart, Germany, 2011. [Google Scholar]
- Zhang, W.; Yang, F.; Zhang, Y.; Zhou, K. Simultaneous Determination of Seven Carbamate Pesticide Residues in Vegetable by Capillary Electrophoresis with Solid Phase Microextraction. Int. J. Electrochem. Sci. 2021, 16, 1–14. [Google Scholar] [CrossRef]
- Guo, J.J.; Luo, Y.L.; Li, H.K.; Liu, X.; Bie, J.X.; Zhang, M.W.; Cao, X.Y.; Shen, F.; Sun, C.Y.; Liu, J.B. Sensitive Fluorescent Detection of Carbamate Pesticides Represented by Methomyl Based on the Inner Filter Effect of Au Nanoparticles on the Fluorescence of CdTe Quantum Dots. Anal. Meth. 2013, 5, 6830–6838. [Google Scholar] [CrossRef]
- Jin, S.; Xu, Z.; Chen, J.; Liang, X.; Wu, Y.; Qian, X. Determination of organophosphate and carbamate pesticides based on enzyme inhibition using a pH-sensitive fluorescence probe. Anal. Chim. Acta 2004, 523, 117–123. [Google Scholar] [CrossRef]
- Ke, G.A.I.; Wang, S.Y.; Qi, H.L.; Feng, Y.X. Determination of Carbamate Pesticide Residues in Agricultural Products by Fluorescence Spectrometry. In DEStech Transactions on Social Science, Education and Human Science; DEStech Publishing Inc.: Lancaster, PA, USA, 2018. [Google Scholar]
- Wei, J.-C.; Wei, B.; Yang, W.; He, C.-W.; Su, H.; Wan, J.-B.; Li, P.; Wang, Y.-T. Trace determination of carbamate pesticides in medicinal plants by a fluorescent technique. Food Chem. Toxicol. 2018, 119, 430–437. [Google Scholar] [CrossRef]
- Zhu, R.; Yan, L.; Guo-Hui, L.; Yong-Liang, Y.; Xiao-Chun, W.; Yi, H.; Ran, G. Determination of Carbamate and Triazol Pesticides in Soil Using QuEChERS with Liquid Chromatography-Tandem Mass Spectrometry. Asian J. Chem. 2014, 26, 6456. [Google Scholar] [CrossRef]
- Hoyos-Arbeláez, J.; Vázquez, M.; Contreras-Calderón, J. Electrochemical methods as a tool for determining the antioxidant capacity of food and beverages: A review. Food Chem. 2017, 221, 1371–1381. [Google Scholar] [CrossRef]
- Fang, L.; Liao, X.; Jia, B.; Shi, L.; Kang, L.; Zhou, L.; Kong, W. Recent progress in immunosensors for pesticides. Biosens. Bioelectron. 2020, 164, 112255. [Google Scholar] [CrossRef]
- Pico, Y.; Fernández, M.; Ruiz, M.J.; Font, G. Current trends in solid-phase-based extraction techniques for the determination of pesticides in food and environment. J. Biochem. Biophys. Methods 2007, 70, 117–131. [Google Scholar] [CrossRef]
- Murray, R.A. Limitations to the use of solid-phase microextraction for quantitation of mixtures of volatile organic sulfur compounds. Anal. Chem. 2001, 73, 1646–1649. [Google Scholar] [CrossRef] [PubMed]
- Mitrani, E.; Perdum, E.; Iordache, O.G.; Dumitrescu, I. Advantages and disadvantages of pesticide analysis methods used in agricultural samples. Sci. Pap.-Ser. B-Hortic. 2018, 62, 709–714. [Google Scholar]
- Colby, J.M.; Thoren, K.L.; Lynch, K.L. Optimization and validation of high-resolution mass spectrometry data analysis parameters. J. Anal. Toxicol. 2017, 41, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Amvrazi, E.G.; Martini, M.A.; Tsiropoulos, N.G. Headspace single-drop microextraction of common pesticide contaminants in honey–method development and comparison with other extraction methods. Int. J. Environ. Anal. Chem. 2012, 92, 450–465. [Google Scholar] [CrossRef]
- Fuchs, B.; Süß, R.; Teuber, K.; Eibisch, M.; Schiller, J. Lipid analysis by thin-layer chromatography—A review of the current state. J. Chromatogr. A 2011, 1218, 2754–2774. [Google Scholar] [CrossRef]
- Ongley, E.D. Control of Water Pollution from Agriculture; Food & Agriculture Organization: Washington, DC, USA, 1996; Volume 55. [Google Scholar]
- World Health Organization. Global situation of Pesticide Management in Agriculture and Public Health: Report of A 2018 WHO–FAO Survey; WHO: Geneva, Switzerland, 2019. [Google Scholar]
- Sereda, B.L.; Meinhardt, H.R. Insecticide Contamination of the Water Environment in Malaria Endemic Areas of KwaZulu-Natal (South Africa); Water Research Commission: Pretoria, South Africa, 2003. [Google Scholar]
- Sumon, K.A.; Rashid, H.; Peeters, E.T.; Bosma, R.H.; Brink, P.J.V.D. Environmental monitoring and risk assessment of organophosphate pesticides in aquatic ecosystems of north-west Bangladesh. Chemosphere 2018, 206, 92–100. [Google Scholar] [CrossRef]
- Bocquené, G.; Franco, A. Pesticide contamination of the coastline of Martinique. Mar. Pollut. Bull. 2005, 51, 612–619. [Google Scholar] [CrossRef] [Green Version]
- Stehle, S.; Bub, S.; Schulz, R. Compilation and analysis of global surface water concentrations for individual insecticide compounds. Sci. Total Environ. 2018, 639, 516–525. [Google Scholar] [CrossRef]
- Li, F.; Yuan, Y.; Meng, P.; Wu, M.; Li, S.; Chen, B. Probabilistic acute risk assessment of cumulative exposure to organophosphorus and carbamate pesticides from dietary vegetables and fruits in Shanghai populations. Food Addit. Contam. Part A 2017, 34, 819–831. [Google Scholar] [CrossRef]
- Akoto, O.; Andoh, H.; Darko, G.; Eshun, K.; Osei-Fosu, P. Health risk assessment of pesticides residue in maize and cowpea from Ejura, Ghana. Chemosphere 2013, 92, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Jabeen, F.; Chaudhry, A.S.; Manzoor, S.; Shaheen, T. Examining pyrethroids, carbamates and neonicotenoids in fish, water and sediments from the Indus River for potential health risks. Environ. Monit. Assess. 2015, 187, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Van Toan, P.; Sebesvari, Z.; Bläsing, M.; Rosendahl, I.; Renaud, F. Pesticide management and their residues in sediments and surface and drinking water in the Mekong Delta, Vietnam. Sci. Total Environ. 2013, 452, 28–39. [Google Scholar] [CrossRef] [PubMed]
- Machekano, H.; Masamba, W.; Mvumi, B.M.; Nyamukondiwa, C. Cabbage or ‘pesticide’ on the platter? Chemical analysis reveals multiple and excessive residues in African vegetable markets. Int. J. Food Contam. 2019, 6, 2. [Google Scholar] [CrossRef] [Green Version]
- Fatunsin, O.T.; Oyeyiola, A.O.; Moshood, M.O.; Akanbi, L.M.; Fadahunsi, D.E. Dietary Risk Assessment of Organophosphate and Carbamate Pesticide Residues in Commonly Eaten Food Crops. Sci. Afr. 2020, 8, e00442. [Google Scholar] [CrossRef]
- Pan, L.; Sun, J.; Li, Z.; Zhan, Y.; Xu, S.; Zhu, L. Organophosphate pesticide in agricultural soils from the Yangtze River Delta of China: Concentration, distribution, and risk assessment. Environ. Sci. Pollut. Res. 2016, 25, 4–11. [Google Scholar] [CrossRef]
- Kouzayha, A.; Al Ashi, A.; Al Akoum, R.; Al Iskandarani, M.; Budzinski, H.; Jaber, F. Occurrence of Pesticide Residues in Lebanon’s Water Resources. Bull. Environ. Contam. Toxicol. 2013, 91, 503–509. [Google Scholar] [CrossRef]
- Forde, M.S.; Robertson, L.; Sidi, E.A.L.; Côté, S.; Gaudreau, E.; Drescher, O.; Ayotte, P. Evaluation of exposure to organophosphate, carbamate, phenoxy acid, and chlorophenol pesticides in pregnant women from 10 Caribbean countries. Environ. Sci. Process. Impacts 2015, 17, 1661–1671. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Lai, K.; Rasco, B.A.; Huang, Y. Determination of carbaryl pesticide in Fuji apples using sur-face-enhanced Raman spectroscopy coupled with multivariate analysis. LWT-Food Sci. Technol. 2015, 60, 352–357. [Google Scholar] [CrossRef]
- Chowdhury, M.; Zaman, A.; Banik, S.; Uddin, B.; Moniruzzaman, M.; Karim, N.; Gan, S.H. Organo-phosphorus and carbamate pesticide residues detected in water samples collected from paddy and vegetable fields of the Savar and Dhamrai Upazilas in Bangladesh. Int. J. Environ. Res. Public Health 2012, 9, 3318–3329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nataraj, M.B.R.; Krishnamurthy, S.V.B. Individual and combined effects of organophosphate and carbamate pesticides on the cricket frog Fejervarya limnocharis. Environ. Geochem. Heal. 2019, 42, 1767–1774. [Google Scholar] [CrossRef] [PubMed]
- Székács, A.; Mörtl, M.; Darvas, B. Monitoring Pesticide Residues in Surface and Ground Water in Hungary: Surveys in 1990–2015. J. Chem. 2015, 2015, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Youssef, L.; Younes, G.; Kouzayha, A.; Jaber, F. Occurrence and levels of pesticides in South Lebanon water. Chem. Speciat. Bioavailab. 2015, 27, 62–70. [Google Scholar] [CrossRef] [Green Version]



| Target Pest/Organism | Type of Pesticide |
|---|---|
| larvae plants egg of insect/mites | larvicides herbicides ovicides |
| insects | insecticides |
| bacteria | bactericides |
| virus | virucides |
| ticks, mites | miticides, acaricides |
| molluscs | molluscicides |
| rodents | rodenticides |
| algae | algicides |
| fungi | fungicides |
| bird pests | avicides |
| Crop | Percentage of Estimated Losses | |||
|---|---|---|---|---|
| Weeds | Diseases | Insects | Total | |
| rice | - | - | 37 | 37 |
| maize | - | - | 31 | 31 |
| wheat | 9.8 | 9.1 | 5.0 | 23.9 |
| millet | 17.8 | 10.6 | 9.6 | 38.0 |
| potatoes | - | - | 40 | 40 |
| cassava | 9.2 | 16.6 | 7.7 | 33.5 |
| soybeans | 13.5 | 11.1 | 4.5 | 29.1 |
| peanuts | 11.8 | 11.3 | 17.1 | 40.4 |
| sugarcane | 25.1 | 10.7 | 9.2 | 45.0 |
| Pesticide | Koc (cm3/g) | Solubility (20–25 °C) (mg/L) | Vp (Pa) (20–25 °C) | Half-Life T1/2 (Days) | Log Kow |
|---|---|---|---|---|---|
| acephate | 0.88 | 650 | 2.26 × 10−4 1.7 × 10−6 (23–25 °C) | 13 | −1.87 |
| azinphos-methyl | 1465 | 44 | 1.8 × 10−4 | 52 | 2.7 |
| chlorfenvinphos | - | 145 | 1.0 × 10−3 | - | 3.8 |
| chlorpyriphos | - | 1.4 | 2.7 × 10−3 | 94 | 4.96 |
| diazinon | 4981 | 60 | 1.2 × 10−2 | 23 | 3.3 |
| dichlorvos | 272 | 18,000 | 2.1 | - | 1.9 |
| dimethoate | 20 | 23 | 1.1 × 10−3 | 7 | 0.7 |
| ethyl-parathion | 5000 | 11 | 8.9 × 10−4 | 14 | 3.83 |
| fenamiphos | 267 | 700 | 0.12 × 10−3 | 16 | 3.3 |
| fenitrothion | - | 30 | 18 × 10−3 | - | - |
| fenthion | 15,000 | 4.2 | 7.4 × 10−4 | 34 | 4.84 |
| malathion | 1800 | 145 | 5.3 × 10−3 | 1 | 2.75 |
| methamidophos | 1.7 | 90,000 | 2.3 × 10−3 | ˃2.6 | 0.8 |
| mevinphos | 44 | Miscible | 1.7 × 10−3 | 3 | 0.13 |
| monocrotophos | 1 | Miscible | 2.9 × 10−4 | 30 | −0.22 |
| parathion-methyl | 236 | 55 | 0.2 × 10−3 | 18.5 | 3.0 |
| phorate | 1000 | 50 | 8.5 × 10−3 | 60 | 3.9 |
| pirimiphos-methyl | 1000 | 9.9 | 2.0 × 10−3 | 10 | 10 |
| terbufos | 500 | 4.5 | 3.46 × 10−2 | 5 | 5 |
| triazophos | - | 30 | 0.39 × 10−3 | - | 3.3 |
| trichlorfon | 29 | 120,000 | 2.1 × 10−4 | 29 | 0.43 |
| Chemical | Half-Life, h |
|---|---|
| Malathion Dursban parathion | 24 2256 43.0 |
| dicapthon | 6.4 |
| dichlorofenthion | 19 |
| leptophos | 48 |
| ronnel | 10.5 |
| fenitronthion | 11.2 |
| Pesticidal Activity | Common or Other Names | Chemical Structure |
|---|---|---|
| Herbicide | barban, chlorbufam, desmedipham, phenmedipham, swep, carbetamide, dichlormate, Asulam, karbutilate, terbucarb |   |
| herbicides and sprout inhibitors | Chlorpropham |  |
| propham | 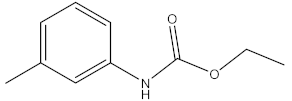 | |
| Fungicide | Benomyl, thiophanate-methyl, thiophanate ethyl, carbendazim |  |
| Insecticide | aldoxycarb, aminocarb, BPMC, bendiocarb, butacarb, carbanolate, carbaryl, bufencarb, carbofuran, cloethocarb, dimetilan, methiocarb | 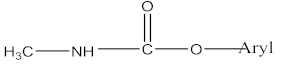 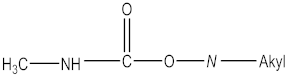 |
| aldicarb | 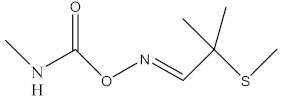 |
| Name | EPA Toxicity Classification | Kow | MW | Koc | Water Solubility | Vp |
|---|---|---|---|---|---|---|
| Bendiocarb/ ficam | Class II | 50 | 223.23 g/mol | 570 | 40 mg/L at 20 °C 260 mg/L at 25 °C | 5 × 10−6 mm Hg at 25 °C |
| methomyl | Class I | 3.98 | 162.210 g/mol | 51.72, 160 | 10 g/L at 25 °C | 5.0 × 10−5 mm Hg at 25 °C |
| aprocarb/ propoxur | Class II for oral exposures and Class III for dermal and inhalation exposures | 1.4 | 209.245 g/mol | 30 | 1750 mg/L at 25 °C | 3 × 10−6 mm Hg at 20 °C 1 × 10−2 mHg at 12 °C |
| Extraction Technique | Cost, T, and P | Solvent Type/Solvent Consumption/Extraction Time | Advantages | Disadvantages | References |
|---|---|---|---|---|---|
| Soxhlet | low cost boiling point of solvent atm. pressure | organic solvent 60–500 mL 6–24 h | It does not require filtration; samples in large amounts; easy to operate; does not depend on the matrix | Extraction time is long; large consumption of solvents; sample must be preconcentrated after extraction | [47,48] |
| supercritical fluid extraction (SFE) | high cost 70–150 °C 15–50 MPa | CO2 10–40 mL 30–60 min | Friendly to the environment because it is not toxic; extraction is fast; uses little solvent; does not require filtration | Sample size limited; dependent on the matrix and analyte | [49] |
| ultrasonic-assisted extraction (UAE) | low cost 30–35 °C Atm. pressure | organic solvent 30–100 mL 30–60 min | Fast and easy to operate; large amount of sample; does not depend on the matrix | Risk of being exposed to the solvent vapour; large amount of solvent, labour intensive; requires filter | [45,46] |
| microwave-assisted extraction (MAE) | moderate cost 100–150 °C Atm. pressure | organic solvent 10–40 mL 20–30 min | Uses small solvent and is fast full control of extraction parameters | Filtration required; solvent must be polar; exhaustive extraction | [48] |
| Pressurized liquid extraction (PLE) | high cost 100–150 °C 7–15 MPa | organic solvent 10–60 mL 10–60 min | Uses small solvent and is fast; does not require filtration and is easy to use | Extraction efficiency dependent on matrix | [45,50] |
| subcritical water extraction (SWE) | moderate cost 200–300 °C 5 MPa | water 30–60 mL 30–60 min | Uses water, which is non-toxic, fast, friendly to the environment; uses little solvent | Optimization of operating conditions required | [45] |
| Analytical Methods | Advantages | Disadvantages | References |
|---|---|---|---|
| electrochemical | Quick and simple measurements Good detection limits Easy sample preparation Small amount of sample (up to 50 μL using screen printed electrodes) | Total reducing power Not selective to a family of molecules unless the electrode is modified | [73] |
| surface-enhanced Raman Spectroscopy (SERS) | High sensitivity, simple and rapid, label free | Lack of active substrates, poor portability, poor reproducibility, limitations on batch fabrication, high cost | [74] |
| solid-phase microextraction (SPME) | Allows attainment of satisfactory LODs and cleaner chromatograms for volatile analytes SPME in combination with GC/MS or LC is a solvent-free or almost solvent-free procedure, obviating the need for further preparation steps | SPME fibres are not uniformly sensitive to all compounds | [75,76] |
| GC–MS | Very good recovery value Sensitive method | Not capable of directly analysing compounds that are nonvolatile, polar, or thermally labile | [77,78] |
| GC–µECD | Very good for determination of organophosphorus pesticides Highly sensitive Low detection limit | Only volatile compounds can be analysed | [79] |
| thin-layer chromatography (TLC) | Equipment needed is inexpensive Convenient and simple to use Consumes smaller amounts of solvents | Preparative applications are limited. Oxidation may occur if the TLC plate is stored for a while since a large surface is exposed to atmospheric oxygen | [80] |
| high-performance liquid chromatography (HPLC) | High quality separations are achievable Coupling with MS is well established | More time-consuming and expensive | [80] |
| Sample Source | Matrices | Concentrations Reported | Analytical Method | References |
|---|---|---|---|---|
| Martinique Island in the French West Indies | sediments water | 44 µg/kg (chlordecone) 0.083 µg/L (aldicarb sulfone) | HPLC | [85] |
| Pakistan, Indus River | sediments | 0.069 ± 0.0023 μg/g WW (carbofuran) | HPLC | [89] |
| northwest Bangladesh | water sediments | chlorpyrifos 9.1 μg/L 51 μg/kg | GC–MS | [84] |
| Shanghai China | leafy vegetables | 22.20 μg/kg | GC/FTD | [87] |
| North America | water | 9000 μg/L | [86] | |
| Capot River in France | water | 0.043 and 0.052 μg/L | HPLC | [85] |
| Galion River in France | 0.083 and 0.032 μg/L | |||
| Mekong Delta, Vietnam | surface water soils and sediments | (fenobucarb) 0.11 μg/L 1.7 and 4.3 μg/kg | [90] | |
| Botswana (Africa) | cabbage | methamidophos 0.0262 mg/kg Methomyl 0.0140 mg/kg | LC–MS/MS | [91] |
| Lagos, Nigeria | sorghum and beans fruits and vegetables | Dichlorvos 2.00 ng/g Chlorpyrifos 0.002 and 60 ng/g Methiocarb 30 ng/g and 70 ng/g | GC–MS | [92] |
| Ejura, Ghana | maize cowpea | organophosphates 0.002–0.019 mg/kg 0.002–0.015 mg/kg | GC–ECD GC–PFPD | [88] |
| Zhejiang, China | soil | Parathion 43.3 ng/g | GC–MS | [93] |
| Lebanon | ground water drinking water ground water surface water rain water | diazinon 4.2 ng/L 2.2 ng/L 7.49 ng/L 15.8 ng/L | GC–MS | [94] |
| Indus River, Punjab, Pakistan, | channa Marulius muscles sediments | carbofuran 0.613–0.946 μg/g 0.069–0.081 μg/g | HPLC | [89] |
| Jamaica | maternal urine samples | diethylphosphate 29.0 μg/L | GC–MS | [95] |
| Shanghai, China | Fuji apples | carbaryl 0.5 μg/g | GC–MS | [96] |
| Bangladesh | water | diazinon 0.9 μg/L carbofuran 198.7 μg/L | HPLC | [97] |
| India (Western Ghats) | fejervarya limnocharis | carbaryl 50 µg/L malathion 500 µg/L | HPLC | [98] |
| Hungary | water | 10–100 ng/L | GC–MS HPLC | [99] |
| South Litani region in South Lebanon | ground water | pirimiphos-methyl 300.87 ng/L | GC–MS | [100] |
| KwaZulu–Natal (Ubombo and Ingwavuma districts) | sediment water sediment water sediment water | carbaryl 0.0010 μg/Kg 0.30 μg/L carbofuran 800 × 10−6 μg/Kg 250 × 10−3 μg/L carbosulfan 300 × 10−6 μg/kg 80 × 10−3 μg/L | GC–NPD GC–FID | [83] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mdeni, N.L.; Adeniji, A.O.; Okoh, A.I.; Okoh, O.O. Analytical Evaluation of Carbamate and Organophosphate Pesticides in Human and Environmental Matrices: A Review. Molecules 2022, 27, 618. https://doi.org/10.3390/molecules27030618
Mdeni NL, Adeniji AO, Okoh AI, Okoh OO. Analytical Evaluation of Carbamate and Organophosphate Pesticides in Human and Environmental Matrices: A Review. Molecules. 2022; 27(3):618. https://doi.org/10.3390/molecules27030618
Chicago/Turabian StyleMdeni, Nonkululeko Landy, Abiodun Olagoke Adeniji, Anthony Ifeanyi Okoh, and Omobola Oluranti Okoh. 2022. "Analytical Evaluation of Carbamate and Organophosphate Pesticides in Human and Environmental Matrices: A Review" Molecules 27, no. 3: 618. https://doi.org/10.3390/molecules27030618
APA StyleMdeni, N. L., Adeniji, A. O., Okoh, A. I., & Okoh, O. O. (2022). Analytical Evaluation of Carbamate and Organophosphate Pesticides in Human and Environmental Matrices: A Review. Molecules, 27(3), 618. https://doi.org/10.3390/molecules27030618








