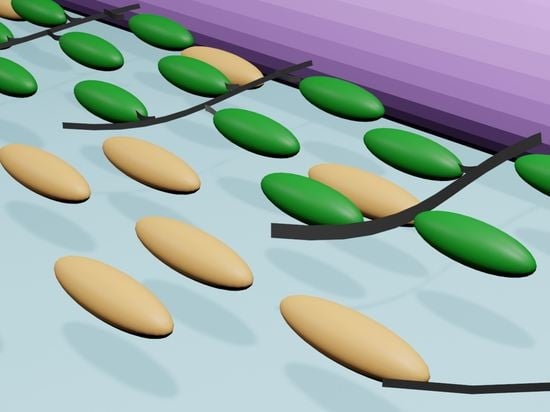
Unidirectional Alignment of Surface-Grafted ZnO Nanorods in Micrometer-Thick Cells Using Low-Molecular-Weight Liquid Crystals
Abstract
:1. Introduction
2. Results and Discussion
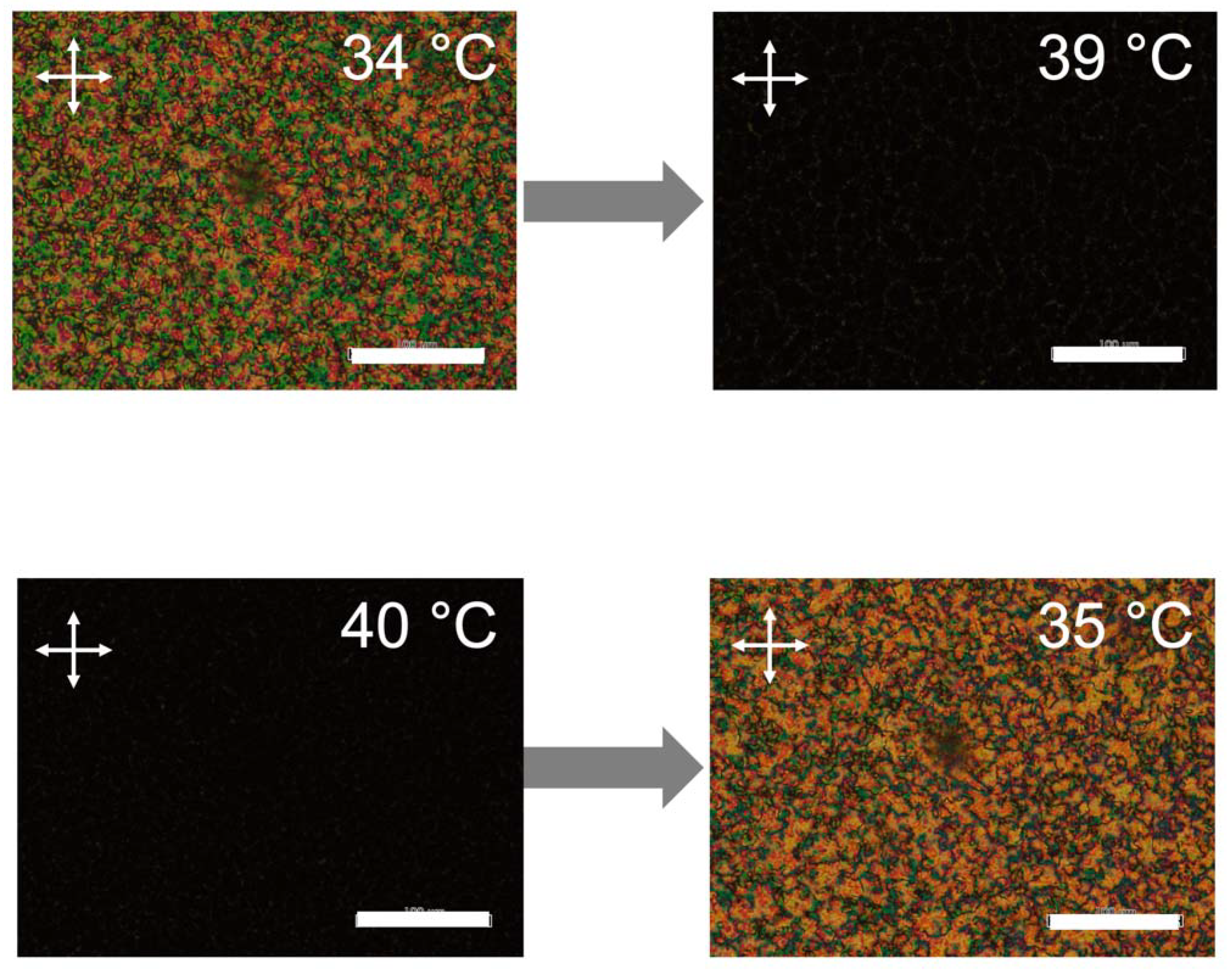
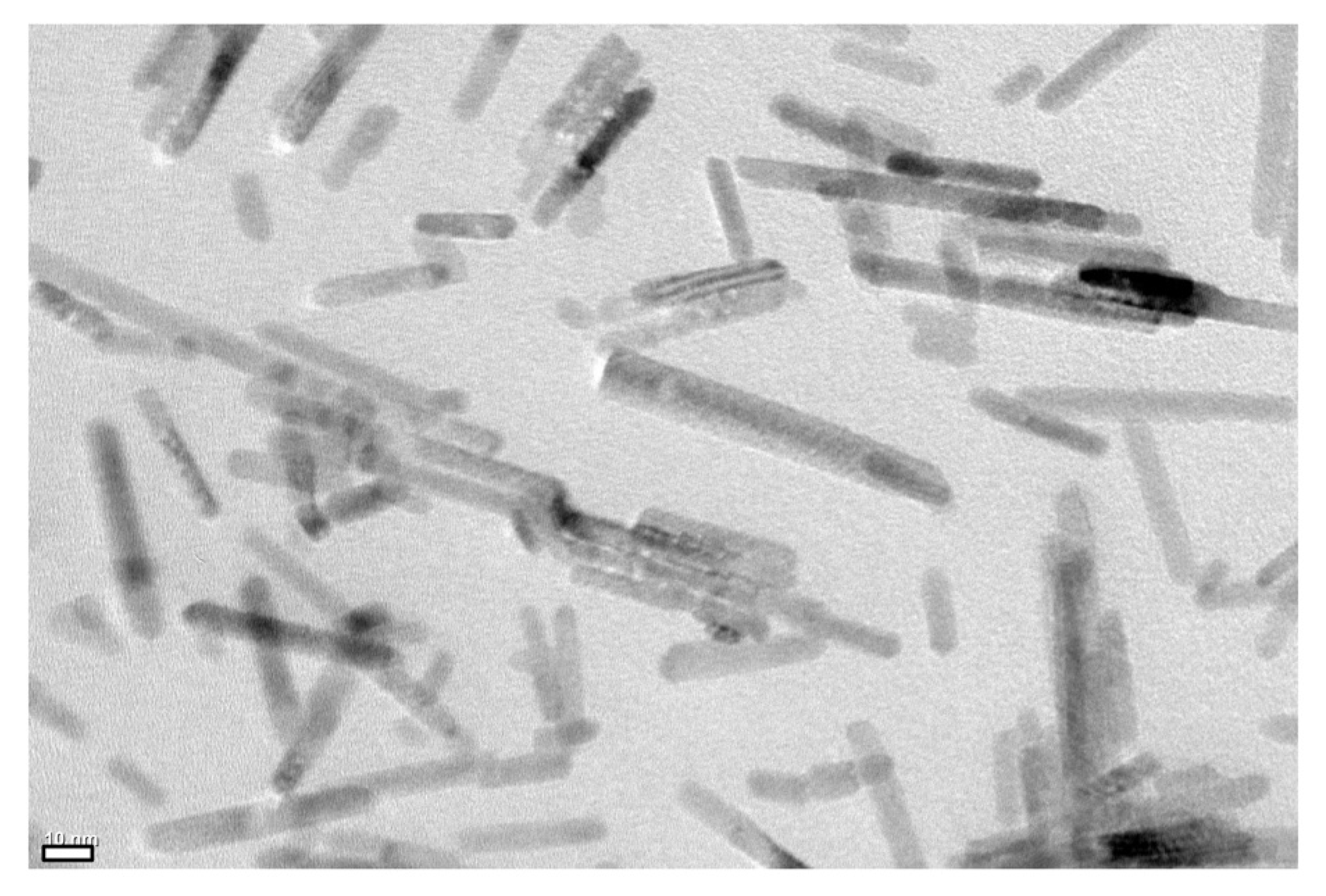
2.1. Liquid Crystalline Behavior of the Nematic LC Host Dispersed with LC Polymer-Grafted Nanorods
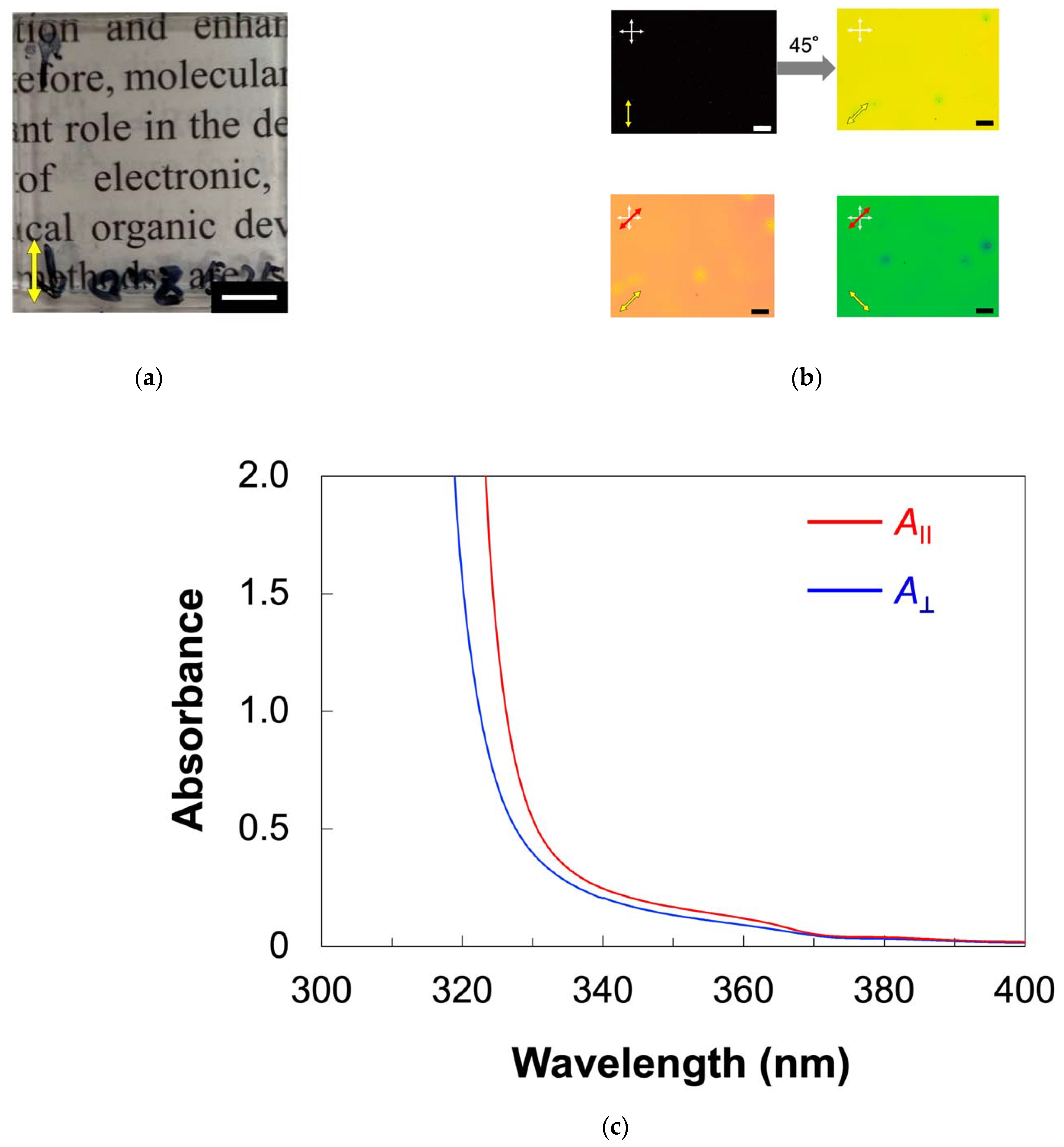
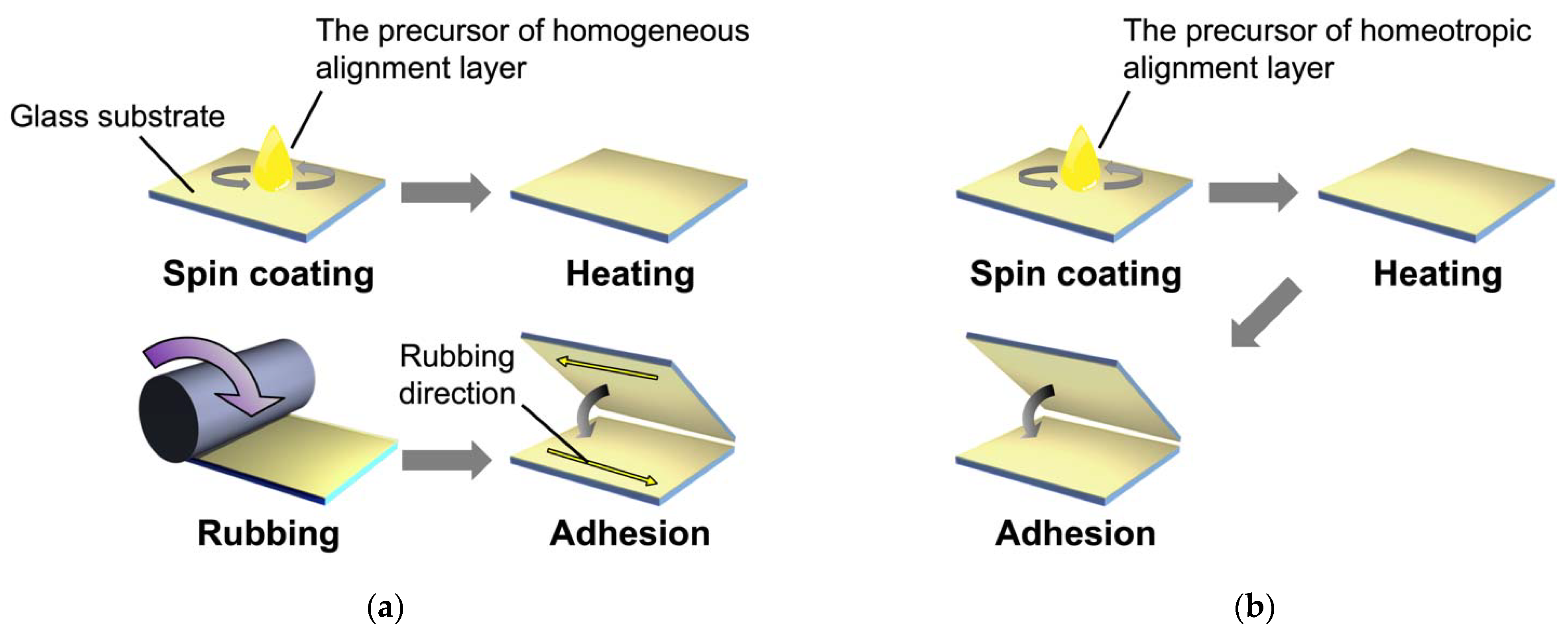
2.2. Homogeneous Alignment of ZnO Nanorods in Host LC
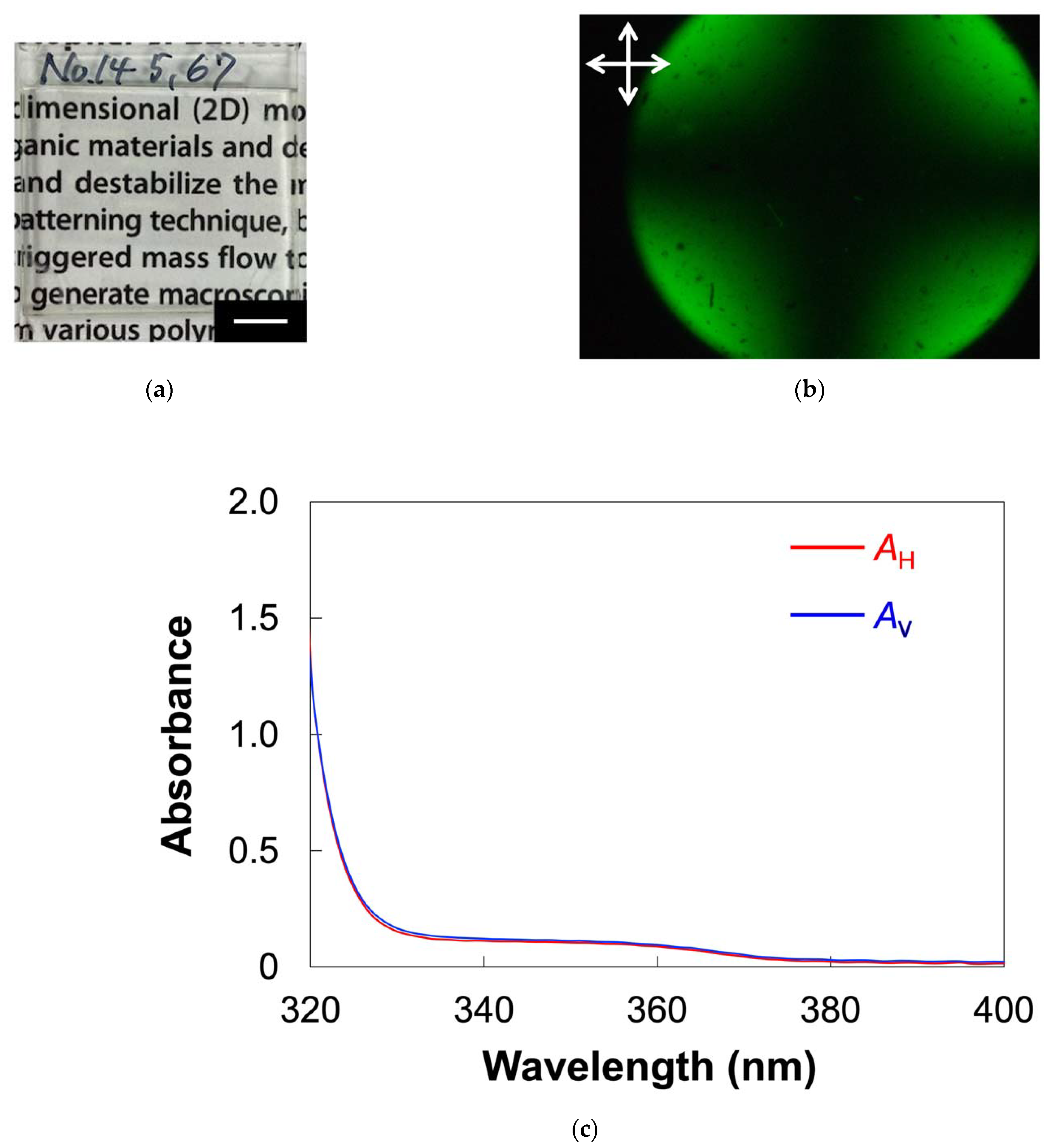
2.3. Homeotropic Alignment of ZnO Nanorods in the Host LC
3. Materials and Methods
3.1. Materials
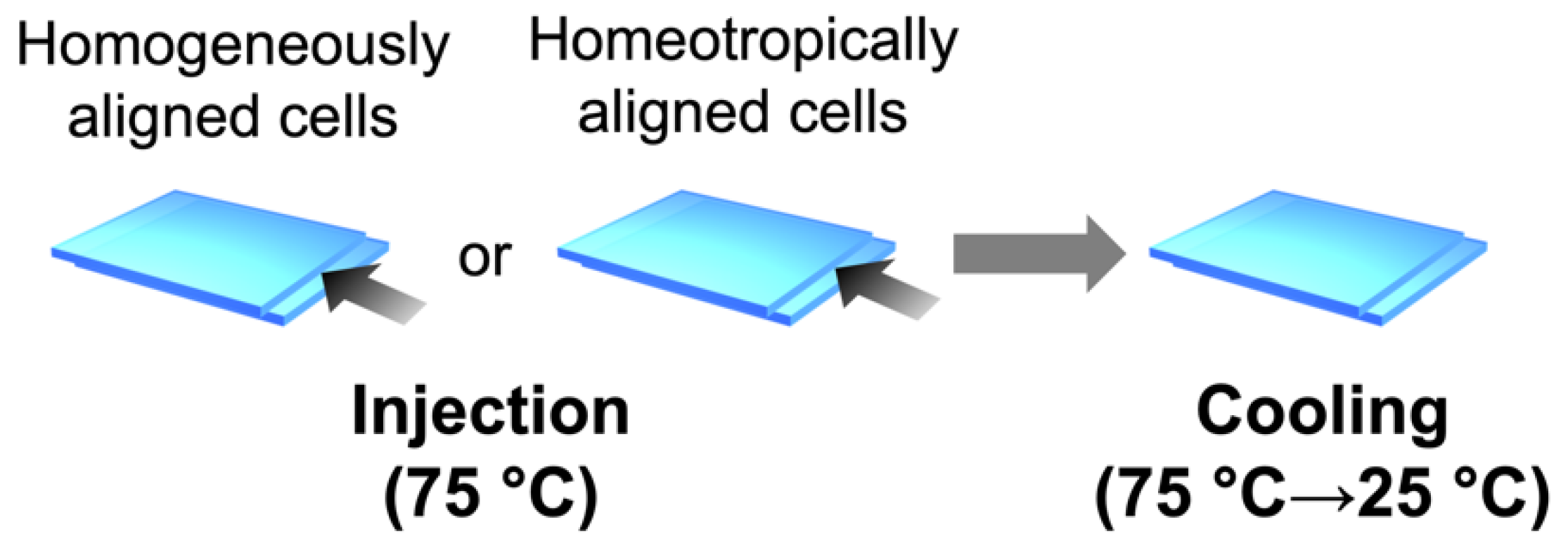
3.2. Sample Preparation
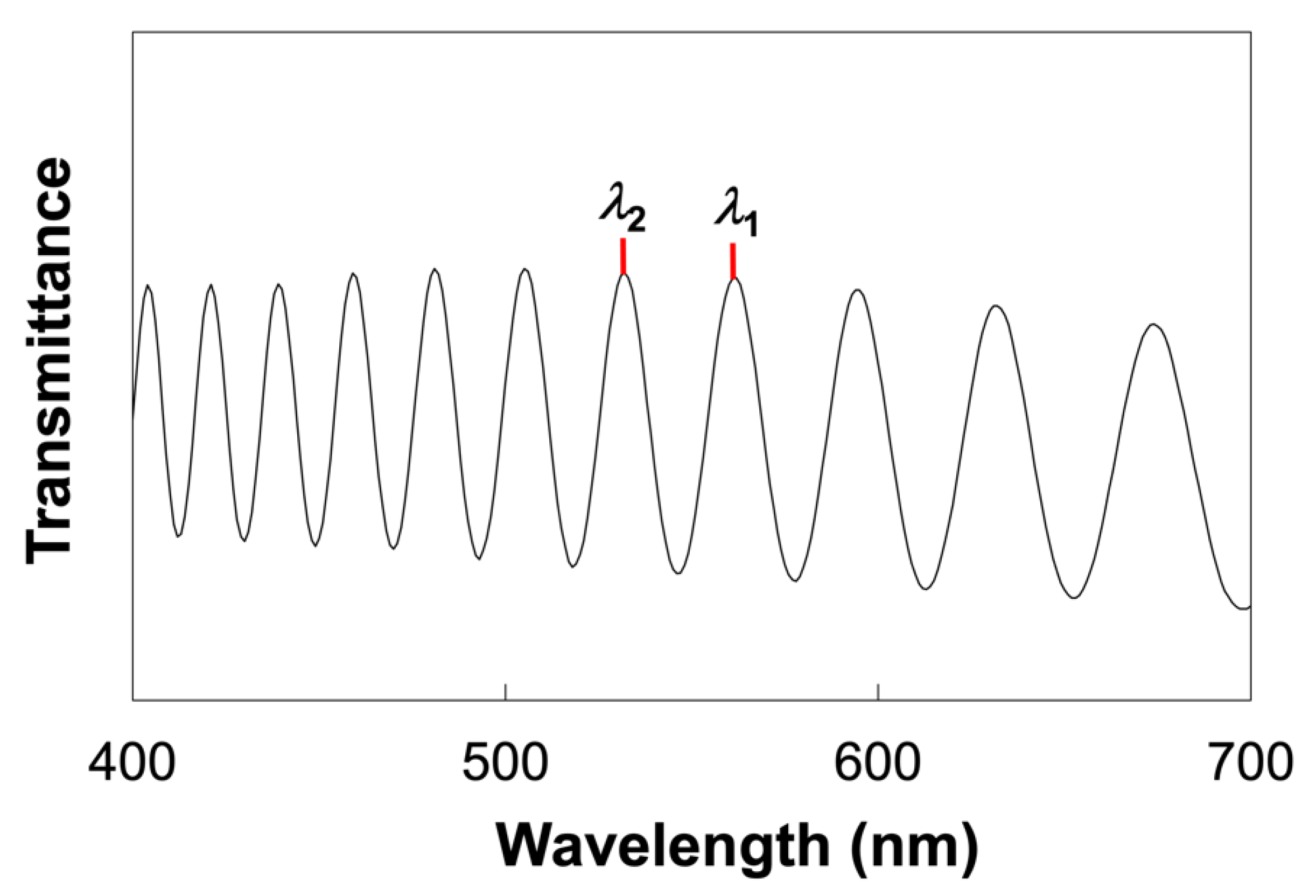
3.3. Characterization Equipment
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
Appendix A

References
- Dayal, S.; Reese, M.O.; Ferguson, A.J.; Ginley, D.S.; Rumbles, C.; Kopidakis, N. The effect of nanoparticle shape on the photocarrier dynamics and photovoltaic device performance of poly(3-hexylthiophene): CdSe nanoparticle bulk heterojunction solar cells. Adv. Funct. Mater. 2010, 20, 2629–2635. [Google Scholar] [CrossRef]
- Liu, J.; Wang, S.; Bian, Z.; Shan, M.; Huang, C. Organic/inorganic hybrid solar cells with vertically oriented ZnO nanowires. Appl. Phys. Lett. 2009, 94, 173107. [Google Scholar] [CrossRef]
- Schneider, J.; Zhang, W.; Srivastava, A.K.; Chigrinov, V.G.; Kwok, H.S.; Rogach, A.L. Photoinduced micropattern alignment of semiconductor nanorods with polarized emission in a liquid crystal polymer matrix. Nano Lett. 2017, 17, 3133–3138. [Google Scholar] [CrossRef]
- Cunningham, P.D.; Souza, J.B.; Fedin, I.; She, C.; Lee, B.; Talapin, D.V. Assessment of anisotropic semiconductor nanorod and nanoplatelet heterostructures with polarized emission for liquid crystal display technology. ACS Nano 2016, 10, 5769–5781. [Google Scholar] [CrossRef]
- Huang, H.; Liu, C.; Wu, Y.; Fan, S. Aligned carbon nanotube composite films for thermal management. Adv. Mater. 2005, 17, 1652–1656. [Google Scholar] [CrossRef]
- Das, R.; Alonso, J.; Porshokouh, Z.N.; Kalappattil, V.; Torres, D.; Phan, M.H.; Garaio, E.; García, J.Á.; Llamazares, J.L.S.; Srikanth, H. Tunable high aspect ratio iron oxide nanorods for enhanced hyperthermia. J. Phys. Chem. C 2016, 120, 10086–10093. [Google Scholar] [CrossRef]
- Lee, M.; Chen, C.Y.; Wang, S.; Cha, S.N.; Park, Y.J.; Kim, J.M.; Chou, L.J.; Wang, Z.L. A hybrid piezoelectric structure for wearable nanogenerators. Adv. Mater. 2012, 24, 1759–1764. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; McAlpine, M.C. Nanotechnology-enabled flexible and biocompatible energy harvesting. Energy Environ. Sci. 2010, 3, 1275–1285. [Google Scholar] [CrossRef]
- Ta, N.; Liu, J.Y.; Shen, W.J. Tuning the shape of ceria nanomaterials for catalytic applications. Chin. J. Catal. 2013, 34, 838–850. [Google Scholar] [CrossRef]
- Beek, W.J.E.; Wienk, M.M.; Janssen, R.A.J. Efficient hybrid solar cells from zinc oxide nanoparticles and a conjugated polymer. Adv. Mater. 2004, 16, 1009–1013. [Google Scholar] [CrossRef]
- Whittaker-Brooks, L.; McClain, W.E.; Schwartz, J.; Loo, Y.L. Donor-acceptor interfacial interactions dominate device performance in hybrid P3HT-ZnO nanowire-array solar cells. Adv. Energy Mater. 2014, 4, 1400585. [Google Scholar] [CrossRef]
- Casse, B.D.F.; Lu, W.T.; Huang, Y.J.; Gultepe, E.; Menon, L.; Sridhar, S. Super-resolution imaging using a three-dimensional metamaterials nanolens. Appl. Phys. Lett. 2010, 96, 023114. [Google Scholar] [CrossRef] [Green Version]
- Sun, M.; Zhang, Q.F.; Sun, H.; Zhang, J.; Wu, J.L. Enhanced ultraviolet electroluminescence from p-Si/n-ZnO nanorod array heterojunction. J. Vac. Sci. Technol. B 2009, 27, 618–621. [Google Scholar] [CrossRef]
- Zhang, Y.; Yao, Y.; Sendeku, M.G.; Yin, L.; Zhan, X.; Wang, F.; Wang, Z.; He, J. Recent progress in CVD growth of 2D transition metal dichalcogenides and related heterostructures. Adv. Mater. 2019, 31, 1901694. [Google Scholar] [CrossRef]
- Yang, Z.; Hao, J. Progress in pulsed laser deposited two-dimensional layered materials for device applications. J. Mater. Chem. C 2016, 4, 8859–8878. [Google Scholar] [CrossRef] [Green Version]
- Liu, B.; Aydil, E.S. Growth of oriented single-crystalline rutile TiO2 nanorods on transparent conducting substrates for dye-sensitized solar cells. J. Am. Chem. Soc. 2009, 131, 3985–3990. [Google Scholar] [CrossRef]
- Pawar, S.M.; Pawar, B.S.; Kim, J.H.; Joo, O.S.; Lokhande, C.D. Recent status of chemical bath deposited metal chalcogenide and metal oxide thin films. Curr. Appl. Phys. 2011, 11, 117–161. [Google Scholar] [CrossRef]
- Dev, A.; Panda, S.K.; Kar, S.; Chakrabarti, S.; Chaudhuri, S. Surfactant-assisted route to synthesize well-aligned ZnO nanorod arrays on sol-gel-derived ZnO thin films. J. Phys. Chem. B 2006, 110, 14266–14272. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Singh, A.; Ong, G.K.; Jones, M.R.; Nordlund, D.; Bustillo, K.; Ciston, J.; Alivisatos, A.P.; Milliron, D.J. Dopant mediated assembly of Cu2ZnSnS4 nanorods into atomically coupled 2D sheets in solution. Nano Lett. 2017, 17, 3421–3428. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harnack, O.; Pacholski, C.; Weller, H.; Yasuda, A.; Wessels, J.M. Rectifying behavior of electrically aligned ZnO nanorods. Nano Lett. 2003, 3, 1097–1101. [Google Scholar] [CrossRef]
- Ryan, K.M.; Mastroianni, A.; Stancil, K.A.; Liu, H.; Alivisatos, A.P. Electric-field-assisted assembly of perpendicularly oriented nanorod superlattices. Nano Lett. 2006, 6, 1479–1482. [Google Scholar] [CrossRef] [Green Version]
- Pelligra, C.I.; Majewski, P.W.; Osuji, C.O. Large area vertical alignment of ZnO nanowires in semiconducting polymer thin films directed by magnetic fields. Nanoscale 2013, 5, 10511–10517. [Google Scholar] [CrossRef]
- Hartmann, L.; Djurado, D.; Florea, I.; Legrand, J.F.; Fiore, A.; Reiss, P.; Doyle, S.; Vorobiev, A.; Pouget, S.; Chandezon, F.; et al. Large-scale simultaneous orientation of CdSe nanorods and regioregular poly(3-hexylthiophene) by mechanical rubbing. Macromolecules 2013, 46, 6177–6186. [Google Scholar] [CrossRef]
- Ploshnik, E.; Salant, A.; Banin, U.; Shenhar, R. Hierarchical surface patterns of nanorods obtained by Co-assembly with block copolymers in ultrathin films. Adv. Mater. 2010, 22, 2774–2779. [Google Scholar] [CrossRef] [PubMed]
- Thorkelsson, K.; Nelson, J.H.; Alivisatos, A.P.; Xu, T. End-to-end alignment of nanorods in thin films. Nano Lett. 2013, 13, 4908–4913. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thorkelsson, K.; Mastroianni, A.J.; Ercius, P.; Xu, T. Direct nanorod assembly using block copolymer-based supramolecules. Nano Lett. 2012, 12, 498–504. [Google Scholar] [CrossRef]
- Feng, X.; Sosa-Vargas, L.; Umadevi, S.; Mori, T.; Shimizu, Y.; Hegmann, T. Discotic liquid crystal-functionalized gold nanorods: 2- and 3D self-assembly and macroscopic alignment as well as increased charge carrier mobility in hexagonal columnar liquid crystal hosts affected by molecular packing and π-π interactions. Adv. Funct. Mater. 2015, 25, 1180–1192. [Google Scholar] [CrossRef]
- Nemati, A.; Shadpour, S.; Querciagrossa, L.; Li, L.; Mori, T.; Gao, M.; Zannoni, C.; Hegmann, T. Chirality amplification by desymmetrization of chiral ligand-capped nanoparticles to nanorods quantified in soft condensed matter. Nat. Commun. 2018, 9, 3908. [Google Scholar] [CrossRef] [Green Version]
- Umadevi, S.; Feng, X.; Hegmann, T. Large area self-assembly of nematic liquid-crystal-functionalized gold nanorods. Adv. Funct. Mater. 2013, 23, 1393–1403. [Google Scholar] [CrossRef]
- Kanie, K.; Muramatsu, A. Organic-inorganic hybrid liquid crystals: Thermotropic mesophases formed by hybridization of liquid-crystalline phosphates and monodispersed α-Fe2O3 particles. J. Am. Chem. Soc. 2005, 127, 11578–11579. [Google Scholar] [CrossRef]
- Liu, X.; Qi, G.; Park, A.M.G.; Rodriguez-Gonzalez, A.; Enotiadis, A.; Pan, W.; Kosma, V.; Fuchs, G.D.; Kirby, B.J.; Giannelis, E.P. Scalable synthesis of switchable assemblies of gold nanorod lyotropic liquid crystal nanocomposites. Small 2019, 15, 1901666. [Google Scholar] [CrossRef] [PubMed]
- Klöckner, B.; Daniel, P.; Brehmer, M.; Tremel, W.; Zentel, R. Liquid crystalline phases from polymer functionalized ferri-magnetic Fe3O4 nanorods. J. Mater. Chem. C 2017, 5, 6688–6696. [Google Scholar] [CrossRef]
- Zorn, M.; Tahir, M.N.; Bergmann, B.; Tremel, W.; Grigoriadis, C.; Floudas, G.; Zentel, R. Orientation and dynamics of ZnO nanorod liquid crystals in electric fields. Macromol. Rapid Commun. 2010, 31, 1101–1107. [Google Scholar] [CrossRef] [PubMed]
- Kubo, S.; Taguchi, R.; Hadano, S.; Narita, M.; Watanabe, O.; Iyoda, T.; Nakagawa, M. Surface-assisted unidirectional orientation of ZnO nanorods hybridized with nematic liquid crystals. ACS Appl. Mater. Interfaces 2014, 6, 811–818. [Google Scholar] [CrossRef]
- Blinov, L.M. Electro-Optical and Magneto-Optical Properties of Liquid Crystals; John Wiley & Sons Ltd.: New York, NY, USA, 1983; pp. 19–22. [Google Scholar]
- Jiang, P.; Bertone, J.F.; Hwang, K.S.; Colvin, V.L. Single-crystal colloidal multilayers of controlled thickness. Chem. Mater. 1999, 11, 2132–2140. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ogata, K.; Matsumoto, K.; Kobayashi, Y.; Kubo, S.; Shishido, A. Unidirectional Alignment of Surface-Grafted ZnO Nanorods in Micrometer-Thick Cells Using Low-Molecular-Weight Liquid Crystals. Molecules 2022, 27, 689. https://doi.org/10.3390/molecules27030689
Ogata K, Matsumoto K, Kobayashi Y, Kubo S, Shishido A. Unidirectional Alignment of Surface-Grafted ZnO Nanorods in Micrometer-Thick Cells Using Low-Molecular-Weight Liquid Crystals. Molecules. 2022; 27(3):689. https://doi.org/10.3390/molecules27030689
Chicago/Turabian StyleOgata, Kaho, Kohsuke Matsumoto, Yoshiaki Kobayashi, Shoichi Kubo, and Atsushi Shishido. 2022. "Unidirectional Alignment of Surface-Grafted ZnO Nanorods in Micrometer-Thick Cells Using Low-Molecular-Weight Liquid Crystals" Molecules 27, no. 3: 689. https://doi.org/10.3390/molecules27030689
APA StyleOgata, K., Matsumoto, K., Kobayashi, Y., Kubo, S., & Shishido, A. (2022). Unidirectional Alignment of Surface-Grafted ZnO Nanorods in Micrometer-Thick Cells Using Low-Molecular-Weight Liquid Crystals. Molecules, 27(3), 689. https://doi.org/10.3390/molecules27030689