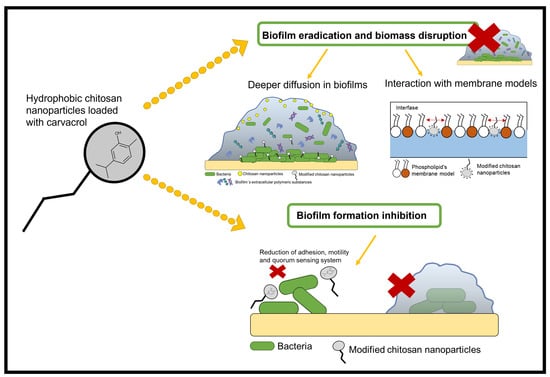
Hydrophobic Chitosan Nanoparticles Loaded with Carvacrol against Pseudomonas aeruginosa Biofilms
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chitosan Modification
2.2. Characterization of Nanoparticles
2.3. Antibacterial Activity of Nanoparticles against Planktonic P. aeruginosa
2.4. Activity of Nanoparticles against Preformed Biofilms
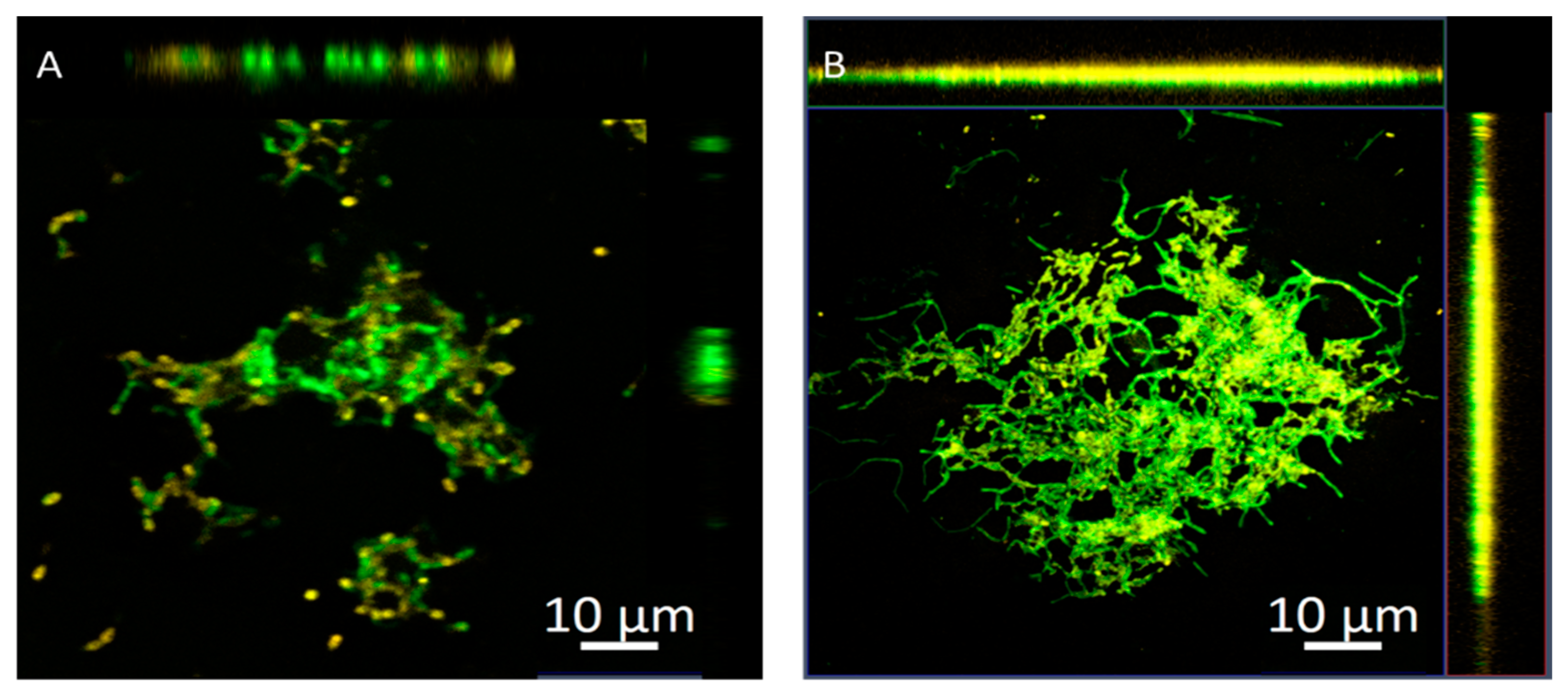
2.5. Penetration of Nanoparticles into Biofilms Measured by Confocal Microscopy
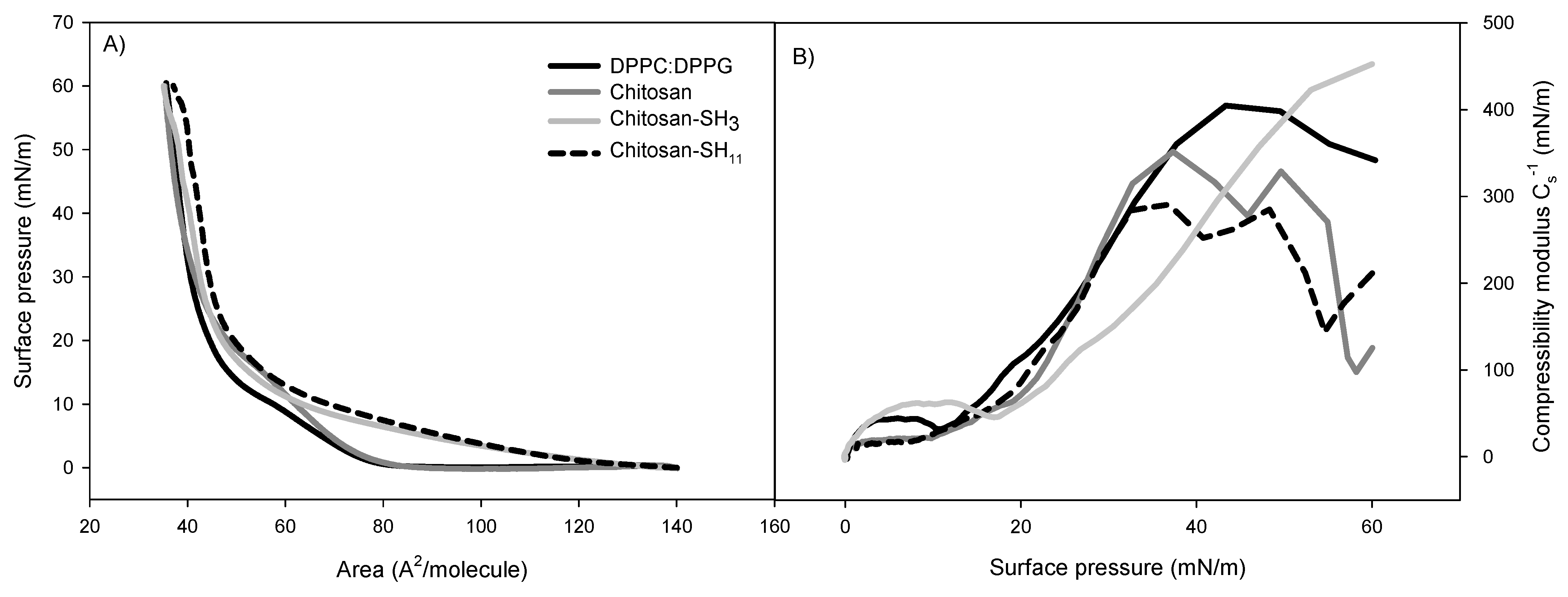
2.6. Phospholipid Monolayer Interaction with the Formulated Nanoparticles
2.7. Antibiofilm Activity
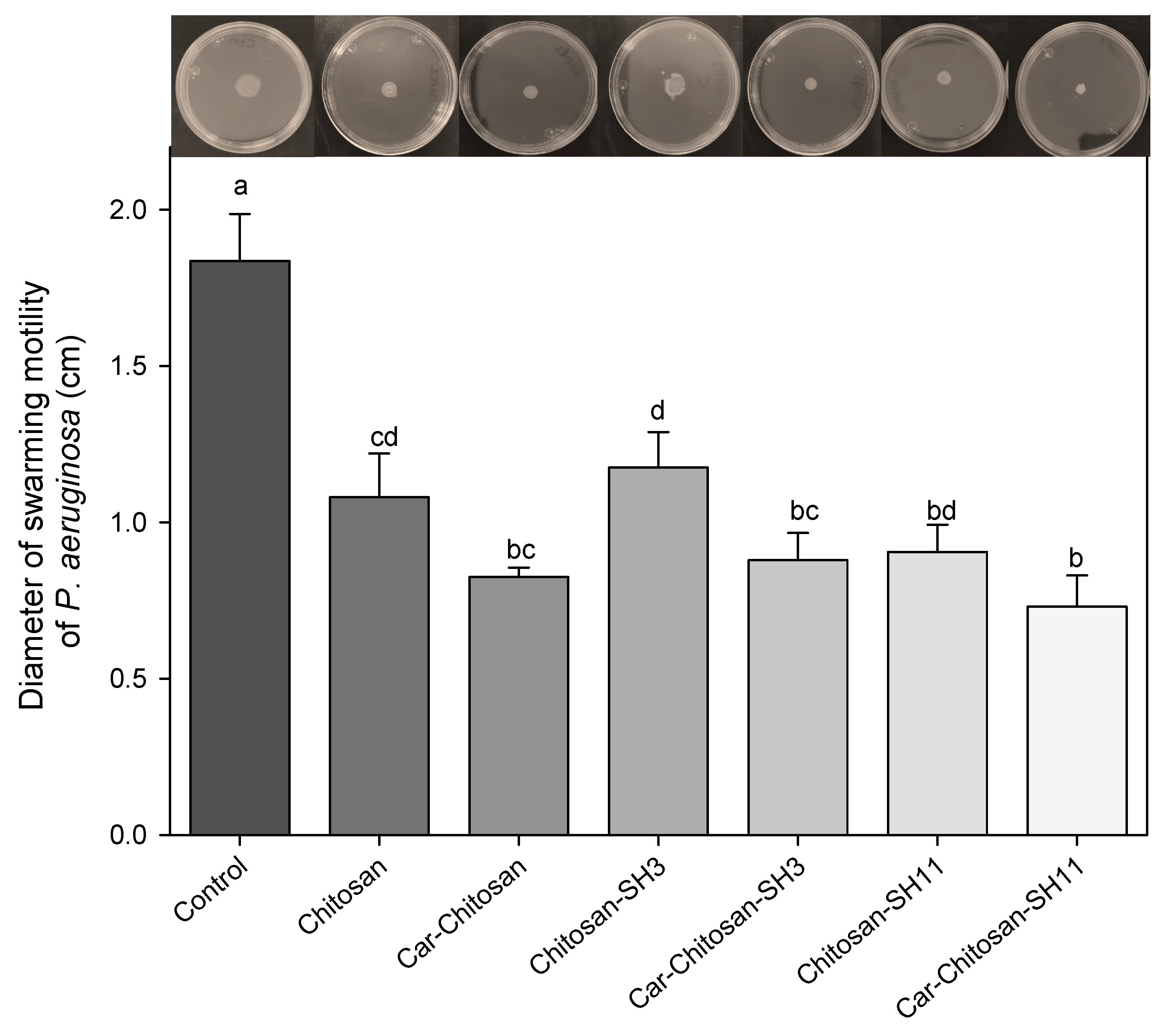
2.8. Antimotility Effect
2.9. Anti-Quorum-Sensing Effect
3. Materials and Methods
3.1. Chitosan Modification
3.2. Fourier-Transform Infrared Spectroscopy (FT-IR)
3.3. Synthesis of Nanoparticles
3.4. Carvacrol Content in the Chitosan Nanoparticles
3.5. Zeta Potential, Size, and Morphology of the Nanoparticles
3.6. Antibacterial Activity of the Carvacrol–Chitosan Nanoparticles
3.7. Biofilm Eradication Activity of Nanoparticles
3.8. Confocal Laser Scanning Microscopy (CLSM)
3.9. Isotherms of Phospholipids Monolayer with Nanoparticles
3.10. Penetration of Nanoparticles into the Phospholipid Monolayers
3.11. Antibiofilm Activity of Carvacrol–Chitosan Nanoparticles
3.12. Motility of P. aeruginosa Exposed to Nanoparticles
3.13. Anti-Quorum-Sensing Effect of Carvacrol Nanoparticles
3.14. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Ciofu, O.; Tolker-Nielsen, T. Tolerance and resistance of Pseudomonas aeruginosa biofilms to antimicrobial agents—How P. aeruginosa can escape antibiotics. Front. Microbiol. 2019, 10, 913. [Google Scholar] [CrossRef] [Green Version]
- Pachori, P.; Gothalwal, R.; Gandhi, P. Emergence of antibiotic resistance Pseudomonas aeruginosa in intensive care unit; a critical review. Genes Dis. 2019, 6, 109. [Google Scholar] [CrossRef]
- Pang, Z.; Raudonis, R.; Glick, B.R.; Lin, T.-J.; Cheng, Z. Antibiotic resistance in Pseudomonas aeruginosa: Mechanisms and alternative therapeutic strategies. Biotechnol. Adv. 2019, 37, 177–192. [Google Scholar] [CrossRef]
- Hirsch, E.B.; Tam, V.H. Impact of multidrug-resistant Pseudomonas aeruginosa infection on patient outcomes. Expert. Rev. Pharmacoecon. Outcomes Res. 2010, 10, 441–451. [Google Scholar] [CrossRef] [Green Version]
- Maurice, N.M.; Bedi, B.; Sadikot, R.T. Pseudomonas aeruginosa biofilms: Host response and clinical implications in lung infections. Am. J. Respir. Cell Mol. Biol. 2018, 58, 428–439. [Google Scholar] [CrossRef]
- Jakobsen, T.H.; Bjarnsholt, T.; Jensen, P.Ø.; Givskov, M.; Høiby, N. Targeting quorum sensing in Pseudomonas aeruginosa biofilms: Current and emerging inhibitors. Future Microbiol. 2013, 8, 901–921. [Google Scholar] [CrossRef] [PubMed]
- Mauch, R.M.; Jensen, P.Ø.; Moser, C.; Levy, C.E.; Høiby, N. Mechanisms of humoral immune response against Pseudomonas aeruginosa biofilm infection in cystic fibrosis. J. Cyst. Fibros. 2018, 17, 143–152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ciofu, O.; Tolker-Nielsen, T.; Jensen, P.Ø.; Wang, H.; Høiby, N. Antimicrobial resistance, respiratory tract infections and role of biofilms in lung infections in cystic fibrosis patients. Adv. Drug Deliv. Rev. 2015, 85, 7–23. [Google Scholar] [CrossRef]
- Maura, D.; Ballok, A.E.; Rahme, L.G. Considerations and caveats in anti-virulence drug development. Curr. Opin. Microbiol. 2016, 33, 41–46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suntres, Z.E.; Coccimiglio, J.; Alipour, M. The bioactivity and toxicological actions of carvacrol. Crit. Rev. Food Sci. Nut. 2015, 55, 304–318. [Google Scholar] [CrossRef] [PubMed]
- Cacciatore, F.A.; Dalmás, M.; Maders, C.; Isaía, H.A.; Brandelli, A.; da Silva Malheiros, P. Carvacrol encapsulation into nanostructures: Characterization and antimicrobial activity against foodborne pathogens adhered to stainless steel. Food Res. Int. 2020, 1, 109143. [Google Scholar] [CrossRef]
- Giovagnoni, G.; Rossi, B.; Tugnoli, B.; Ghiselli, F.; Bonetti, A.; Piva, A.; Grilli, E. Thymol and carvacrol downregulate the expression of Salmonella typhimurium virulence genes during an in vitro infection on caco-2 cells. Microorganisms 2020, 8, 862. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez-Pacheco, M.M.; Gonzalez-Aguilar, G.A.; Martinez-Tellez, M.A.; Lizardi-Mendoza, J.; Madera-Santana, T.J.; Bernal-Mercado, A.T.; Vazquez-Armenta, F.J.; Ayala-Zavala, J.F. Carvacrol inhibits biofilm formation and production of extracellular polymeric substances of Pectobacterium carotovorum subsp. carotovorum. Food Control 2018, 89, 210–218. [Google Scholar] [CrossRef]
- Niza, E.; Božik, M.; Bravo, I.; Clemente-Casares, P.; Lara-Sanchez, A.; Juan, A.; Klouček, P.; Alonso-Moreno, C. PEI-coated PLA nanoparticles to enhance the antimicrobial activity of carvacrol. Food Chem 2020, 328, 127131. [Google Scholar] [CrossRef] [PubMed]
- Tapia-Rodriguez, M.R.; Bernal-Mercado, A.T.; Gutierrez-Pacheco, M.M.; Vazquez-Armenta, F.J.; Hernandez-Mendoza, A.; Gonzalez-Aguilar, G.A.; Martinez-Tellez, M.A.; Nazzaro, F.; Ayala-Zavala, J.F. Virulence of Pseudomonas aeruginosa exposed to carvacrol: Alterations of the Quorum sensing at enzymatic and gene levels. J. Cell Commun. Signal. 2019, 13, 531–537. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.T.; Khan, M.; Ahmad, J.; Wahab, R.; Abd-Elkader, O.H.; Musarrat, J.; Alkhathlan, H.Z.; Al-Kedhairy, A.A. Thymol and carvacrol induce autolysis, stress, growth inhibition and reduce the biofilm formation by Streptococcus mutans. AMB Express 2017, 7, 49. [Google Scholar] [CrossRef] [Green Version]
- Koraichi Saad, I.; Hassan, L.; Ghizlane, Z.; Hind, M.; Adnane, R. Carvacrol and thymol components inhibiting Pseudomonas aeruginosa adherence and biofilm formation. Afr. J. Microbiol. Res. 2011, 5, 3229–3232. [Google Scholar]
- Khan, I.; Bahuguna, A.; Kumar, P.; Bajpai, V.K.; Kang, S.C. Antimicrobial Potential of Carvacrol against Uropathogenic Escherichia coli via Membrane Disruption, Depolarization, and Reactive Oxygen Species Generation. Front. Microbiol. 2017, 8, 2421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shinde, P.; Agraval, H.; Srivastav, A.K.; Yadav, U.C.; Kumar, U. Physico-chemical characterization of carvacrol loaded zein nanoparticles for enhanced anticancer activity and investigation of molecular interactions between them by molecular docking. Int. J. Pharm. 2020, 588, 119795. [Google Scholar] [CrossRef] [PubMed]
- Ilk, S.; Sağlam, N.; Özgen, M.; Korkusuz, F. Chitosan nanoparticles enhances the anti-quorum sensing activity of kaempferol. Int. J. Biol. Macromol. 2017, 94, 653–662. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Shi, L.; Su, L.; van der Mei, H.C.; Jutte, P.C.; Ren, Y.; Busscher, H.J. Nanotechnology-based antimicrobials and delivery systems for biofilm-infection control. Chem. Soc. Rev. 2019, 48, 428–446. [Google Scholar] [CrossRef]
- Mu, H.; Tang, J.; Liu, Q.; Sun, C.; Wang, T.; Duan, J. Potent antibacterial nanoparticles against biofilm and intracellular bacteria. Sci. Rep. 2016, 6, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feyzioglu, G.C.; Tornuk, F. Development of chitosan nanoparticles loaded with summer savory (Satureja hortensis L.) essential oil for antimicrobial and antioxidant delivery applications. LWT 2016, 70, 104–110. [Google Scholar] [CrossRef]
- Tran, T.-T.; Hadinoto, K. A Potential Quorum-sensing inhibitor for bronchiectasis therapy: Quercetin–chitosan nanoparticle complex exhibiting superior inhibition of biofilm formation and swimming motility of Pseudomonas aeruginosa to the native quercetin. Int. J. Mol. Sci. 2021, 22, 1541. [Google Scholar] [CrossRef]
- Li, X.; Yeh, Y.-C.; Giri, K.; Mout, R.; Landis, R.F.; Prakash, Y.; Rotello, V.M. Control of nanoparticle penetration into biofilms through surface design. Chem. Commun. 2015, 51, 282–285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shebl, R.I.; Farouk, F.; Azzazy, H.M.E.-S. Effect of surface charge and hydrophobicity modulation on the antibacterial and antibiofilm potential of magnetic iron nanoparticles. J. Nanomater. 2017, 2017, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Philippova, O.; Korchagina, E. Chitosan and its hydrophobic derivatives: Preparation and aggregation in dilute aqueous solutions. Polym. Sci. Ser. A 2012, 54, 552–572. [Google Scholar] [CrossRef] [Green Version]
- Zhu, X.; Su, M.; Tang, S.; Wang, L.; Liang, X.; Meng, F.; Hong, Y.; Xu, Z. Synthesis of thiolated chitosan and preparation nanoparticles with sodium alginate for ocular drug delivery. Mol. Vis. 2012, 18, 1973. [Google Scholar] [PubMed]
- Tapia-Rodriguez, M.R.; Hernandez-Mendoza, A.; Gonzalez-Aguilar, G.A.; Martinez-Tellez, M.A.; Martins, C.M.; Ayala-Zavala, J.F. Carvacrol as potential quorum sensing inhibitor of Pseudomonas aeruginosa and biofilm production on stainless steel surfaces. Food Control 2017, 75, 255–261. [Google Scholar] [CrossRef]
- Qu, L.; She, P.; Wang, Y.; Liu, F.; Zhang, D.; Chen, L.; Luo, Z.; Xu, H.; Qi, Y.; Wu, Y. Effects of norspermidine on Pseudomonas aeruginosa biofilm formation and eradication. Microbiologyopen 2016, 5, 402–412. [Google Scholar] [CrossRef] [Green Version]
- Nafee, N.; Husari, A.; Maurer, C.K.; Lu, C.; de Rossi, C.; Steinbach, A.; Hartmann, R.W.; Lehr, C.-M.; Schneider, M. Antibiotic-free nanotherapeutics: Ultra-small, mucus-penetrating solid lipid nanoparticles enhance the pulmonary delivery and anti-virulence efficacy of novel quorum sensing inhibitors. J. Control Release 2014, 192, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Ma, S.; Leonhard, M.; Moser, D.; Haselmann, G.M.; Wang, J.; Eder, D.; Schneider-Stickler, B. Enhancing antibiofilm activity with functional chitosan nanoparticles targeting biofilm cells and biofilm matrix. Carbohydr. Polym. 2018, 200, 35–42. [Google Scholar] [CrossRef]
- López-Oyama, A.B.; Taboada, P.; Burboa, M.G.; Rodríguez, E.; Mosquera, V.; Valdez, M.A. Interaction of the cationic peptide bactenecin with mixed phospholipid monolayers at the air–water interface. J. Colloid Interface Sci. 2011, 359, 279–288. [Google Scholar] [CrossRef] [PubMed]
- Freudenthal, O. Study of the Action of Antimicrobial Peptides by Spectroscopic Methods: From Model Membrane to Bacterial Biofilm; Université de Lorraine: Lorraine, France, 2016. [Google Scholar]
- Nowotarska, S.W.; Nowotarski, K.J.; Friedman, M.; Situ, C. Effect of structure on the interactions between five natural antimicrobial compounds and phospholipids of bacterial cell membrane on model monolayers. Molecules 2014, 19, 7497–7515. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bernal-Mercado, A.T.; Gutierrez-Pacheco, M.M.; Encinas-Basurto, D.; Mata-Haro, V.; Lopez-Zavala, A.A.; Islas-Osuna, M.A.; Gonzalez-Aguilar, G.A.; Ayala-Zavala, J.F. Synergistic mode of action of catechin, vanillic and protocatechuic acids to inhibit the adhesion of uropathogenic Escherichia coli on silicone surfaces. J. Appl. Microbiol. 2020, 128, 387–400. [Google Scholar] [CrossRef]
- Giri, K.; Yepes, L.R.; Duncan, B.; Parameswaran, P.K.; Yan, B.; Jiang, Y.; Bilska, M.; Moyano, D.F.; Thompson, M.A.; Rotello, V.M. Targeting bacterial biofilms via surface engineering of gold nanoparticles. RSC Adv. 2015, 5, 105551–105559. [Google Scholar] [CrossRef] [Green Version]
- Goodman, C.M.; McCusker, C.D.; Yilmaz, T.; Rotello, V.M. Toxicity of gold nanoparticles functionalized with cationic and anionic side chains. Bioconjug. Chem. 2004, 15, 897–900. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Landis, R.F.; Rotello, V.M. Nanoparticle-based antimicrobials: Surface functionality is critical. F1000Res. 2016, 5, 364. [Google Scholar] [CrossRef]
- Peulen, T.-O.; Wilkinson, K. Diffusion of nanoparticles in a biofilm. Environ. Sci. Technol. 2011, 45, 3367–3373. [Google Scholar] [CrossRef]
- Upadhyay, A.; Arsi, K.; Wagle, B.R.; Upadhyaya, I.; Shrestha, S.; Donoghue, A.M.; Donoghue, D.J. Trans-cinnamaldehyde, carvacrol, and eugenol reduce Campylobacter jejuni colonization factors and expression of virulence genes in vitro. Front. Microbiol. 2017, 8, 713. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, Y.; Leonhard, M.; Moser, D.; Ma, S.; Schneider-Stickler, B. Long-term antibiofilm activity of carboxymethyl chitosan on mixed biofilm on silicone. Laryngoscope 2016, 126, E404–E408. [Google Scholar] [CrossRef]
- Kilmury, S.L.; Burrows, L.L. The Pseudomonas aeruginosa PilSR two-component system regulates both twitching and swimming motilities. Mbio 2018, 9, e01310–e01318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leja, K.; Drożdżyńska, A.; Majcher, M.; Kowalczewski, P.Ł.; Czaczyk, K. Influence of sub-inhibitory concentration of selected plant essential oils on the physical and biochemical properties of Pseudomonas orientalis. Open Chem. 2019, 17, 492–505. [Google Scholar] [CrossRef]
- Van Alphen, L.B.; Burt, S.A.; Veenendaal, A.K.; Bleumink-Pluym, N.M.; Van Putten, J.P. The natural antimicrobial carvacrol inhibits Campylobacter jejuni motility and infection of epithelial cells. PLoS ONE 2012, 7, e45343. [Google Scholar]
- Mohammed, H.B.; Rayyif, S.M.I.; Curutiu, C.; Birca, A.C.; Oprea, O.-C.; Grumezescu, A.M.; Ditu, L.-M.; Gheorghe, I.; Chifiriuc, M.C.; Mihaescu, G. Eugenol-functionalized magnetite nanoparticles modulate virulence and persistence in Pseudomonas aeruginosa clinical strains. Molecules 2021, 26, 2189. [Google Scholar] [CrossRef]
- Bose, S.K.; Nirbhavane, P.; Batra, M.; Chhibber, S.; Harjai, K. Nanolipoidal α-terpineol modulates quorum sensing regulated virulence and biofilm formation in Pseudomonas aeruginosa. Nanomedicine 2020, 15, 1743–1760. [Google Scholar] [CrossRef]
- Subhaswaraj, P.; Barik, S.; Macha, C.; Chiranjeevi, P.V.; Siddhardha, B. Anti quorum sensing and anti biofilm efficacy of cinnamaldehyde encapsulated chitosan nanoparticles against Pseudomonas aeruginosa PAO1. LWT 2018, 97, 752–759. [Google Scholar] [CrossRef]
- Luna, M.; Beltran, O.; Encinas-Basurto, D.A.; Ballesteros-Monrreal, M.G.; Topete, A.; Hassan, N.; López-Mata, M.A.; Reyes-Márquez, V.; Valdez, M.A.; Juarez, J. High antibacterial performance of hydrophobic chitosan-based nanoparticles loaded with carvacrol. Colloids Surf. B. 2022, 209, 112191. [Google Scholar] [CrossRef]
- Robles, E.; Juárez, J.; Burboa, M.G.; Gutiérrez, L.E.; Taboada, P.; Mosquera, V.; Valdez, M.A. Properties of insulin–chitosan complexes obtained by an alkylation reaction on chitosan. J. Appl. Polym. Sci. 2014, 131, 1–10. [Google Scholar] [CrossRef]
- Almada, M.; Burboa, G.; Robles, E.; Gutiérrez, L.; Valdés, M.; Juárez, J. Interaction and cytotoxic effects of hydrophobized chitosan nanoparticles on MDA-MB-231, HeLa and Arpe-19 cell lines. Curr. Top. Med. Chem. 2014, 14, 692–701. [Google Scholar] [CrossRef]
- Hajimehdipoor, H.; Shekarchi, M.; Khanavi, M.; Adib, N.; Amri, M. A validated high performance liquid chromatography method for the analysis of thymol and carvacrol in Thymus vulgaris L. volatile oil. Pharmacogn. Mag. 2010, 6, 154. [Google Scholar] [CrossRef] [Green Version]
- Alvarez, M.V.; Ortega-Ramirez, L.A.; Gutierrez-Pacheco, M.M.; Bernal-Mercado, A.T.; Rodriguez-Garcia, I.; Gonzalez-Aguilar, G.A.; Ponce, A.; Moreira, M.d.R.; Roura, S.I.; Ayala-Zavala, J.F. Oregano essential oil-pectin edible films as anti-quorum sensing and food antimicrobial agents. Front. Microbiol. 2014, 5, 699. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bernal-Mercado, A.T.; Vazquez-Armenta, F.J.; Tapia-Rodriguez, M.R.; Islas-Osuna, M.A.; Mata-Haro, V.; Gonzalez-Aguilar, G.A.; Lopez-Zavala, A.A.; Ayala-Zavala, J.F. Comparison of single and combined use of catechin, protocatechuic, and vanillic acids as antioxidant and antibacterial agents against uropathogenic Escherichia coli at planktonic and biofilm levels. Molecules 2018, 23, 2813. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, H.; Yu, X.; Su, C.; Shi, Y.; Zhao, L. Chitosan nanoparticles triggered the induction of ROS-mediated cytoprotective autophagy in cancer cells. Artif. Cells Nanomed. Biotechnol. 2018, 46, 293–301. [Google Scholar] [CrossRef]
- Reynolds, F.; O’loughlin, T.; Weissleder, R.; Josephson, L. Method of determining nanoparticle core weight. Anal. Chem. 2005, 77, 814–817. [Google Scholar] [CrossRef] [PubMed]
- Broniatowski, M.; Macho, I.S.; Dynarowicz-Łątka, P. Study of perfluorooctyl-n-alkanes monolayers at the air–water interface. Thin Solid Film. 2005, 493, 249–257. [Google Scholar] [CrossRef]

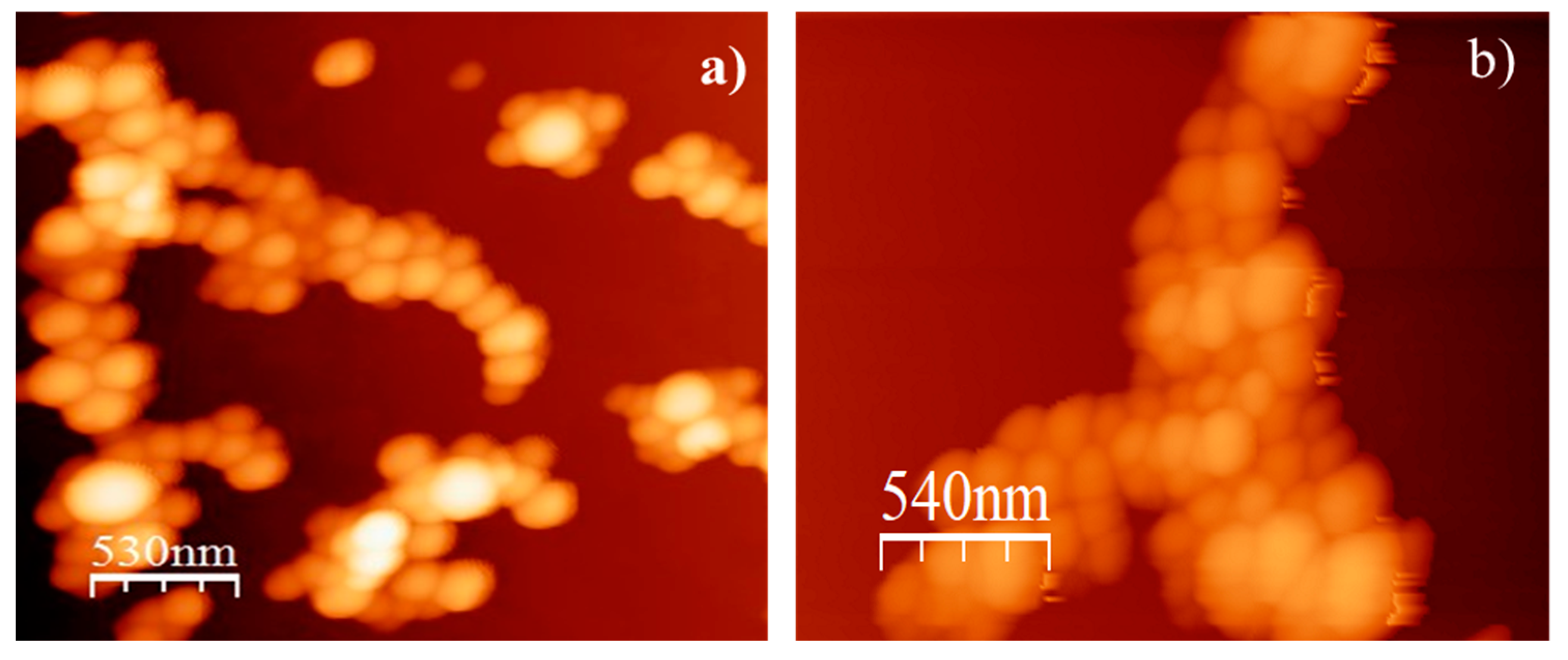


| Formulation | Size (nm) | PDI * | ζ Potential (mV) | % Carvacrol EE * |
|---|---|---|---|---|
| Chitosan | 140.3 ± 1.3 | 0.2 | 14.4 | 50.7 ± 1 |
| Chitosan–SH3 | 166.6 ± 5.1 | 0.1 | 10.5 | 25.1 ± 4.7 |
| Chitosan–SH11 | 152.1 ± 2.1 | 0.3 | 11.2 | 68.8 ± 3.2 |
| Nanoparticles Treatments | Bacterial Adhered Cells in Polystyrene Surface (log CFU·cm−2) | Percentage of Biofilm Biomass (%) | Viable Bacteria Cells in Preformed Biofilms (log CFU·cm−2) |
|---|---|---|---|
| Control | 7.82 ± 0.01 a | 100 | 7.09 ± 0.03 a |
| Chitosan | 7.04 ± 0.03 bc | 59.91 ± 0.40 a | 6.60 ± 0.05 b |
| Carvacrol–chitosan | 6.03 ± 0.07 d | 53.08 ± 0.51 bc | 5.92 ± 0.02 de |
| Chitosan–SH3 | 7.16 ± 0.02 b | 63.76 ± 1.76 d | 6.39 ± 0.09 bc |
| Carvacrol–chitosan–SH3 | 6.09 ± 0.05 d | 53.30 ± 0.71 c | 5.81 ± 0.07 e |
| Chitosan–SH11 | 6.89 ± 0.07 c | 61.62 ± 1.60 d | 6.16 ± 0.15 cd |
| Carvacrol–chitosan–SH11 | 5.82 ± 0.06 e | 46.37 ± 1.23 b | 5.37 ± 0.19 f |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bernal-Mercado, A.T.; Juarez, J.; Valdez, M.A.; Ayala-Zavala, J.F.; Del-Toro-Sánchez, C.L.; Encinas-Basurto, D. Hydrophobic Chitosan Nanoparticles Loaded with Carvacrol against Pseudomonas aeruginosa Biofilms. Molecules 2022, 27, 699. https://doi.org/10.3390/molecules27030699
Bernal-Mercado AT, Juarez J, Valdez MA, Ayala-Zavala JF, Del-Toro-Sánchez CL, Encinas-Basurto D. Hydrophobic Chitosan Nanoparticles Loaded with Carvacrol against Pseudomonas aeruginosa Biofilms. Molecules. 2022; 27(3):699. https://doi.org/10.3390/molecules27030699
Chicago/Turabian StyleBernal-Mercado, Ariadna Thalia, Josué Juarez, Miguel Angel Valdez, Jesus Fernando Ayala-Zavala, Carmen Lizette Del-Toro-Sánchez, and David Encinas-Basurto. 2022. "Hydrophobic Chitosan Nanoparticles Loaded with Carvacrol against Pseudomonas aeruginosa Biofilms" Molecules 27, no. 3: 699. https://doi.org/10.3390/molecules27030699