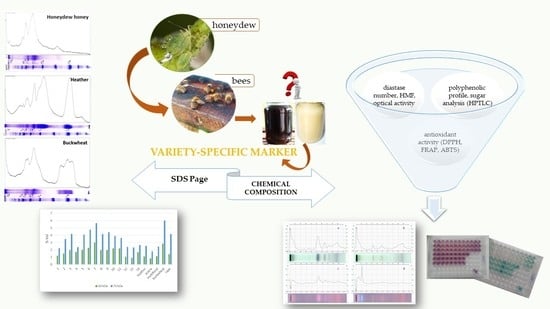
The Use of HPTLC and SDS-PAGE Methods for Coniferous Honeydew Honey Fingerprinting Compiled with Mineral Content and Antioxidant Activity
Abstract
:1. Introduction
2. Results and Discussion
2.1. Physicochemical Parameters
2.2. Mineral Composition
2.3. Antioxidant Activity
2.4. HPTLC Fingerprinting
2.4.1. Sugar Analysis
2.4.2. Polyphenolic Profile
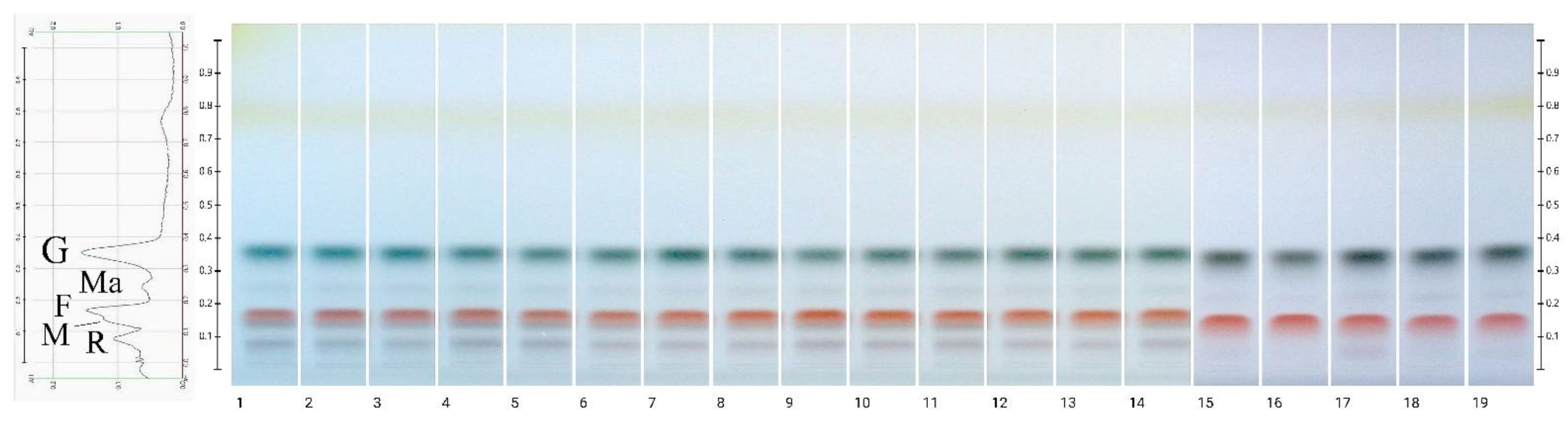
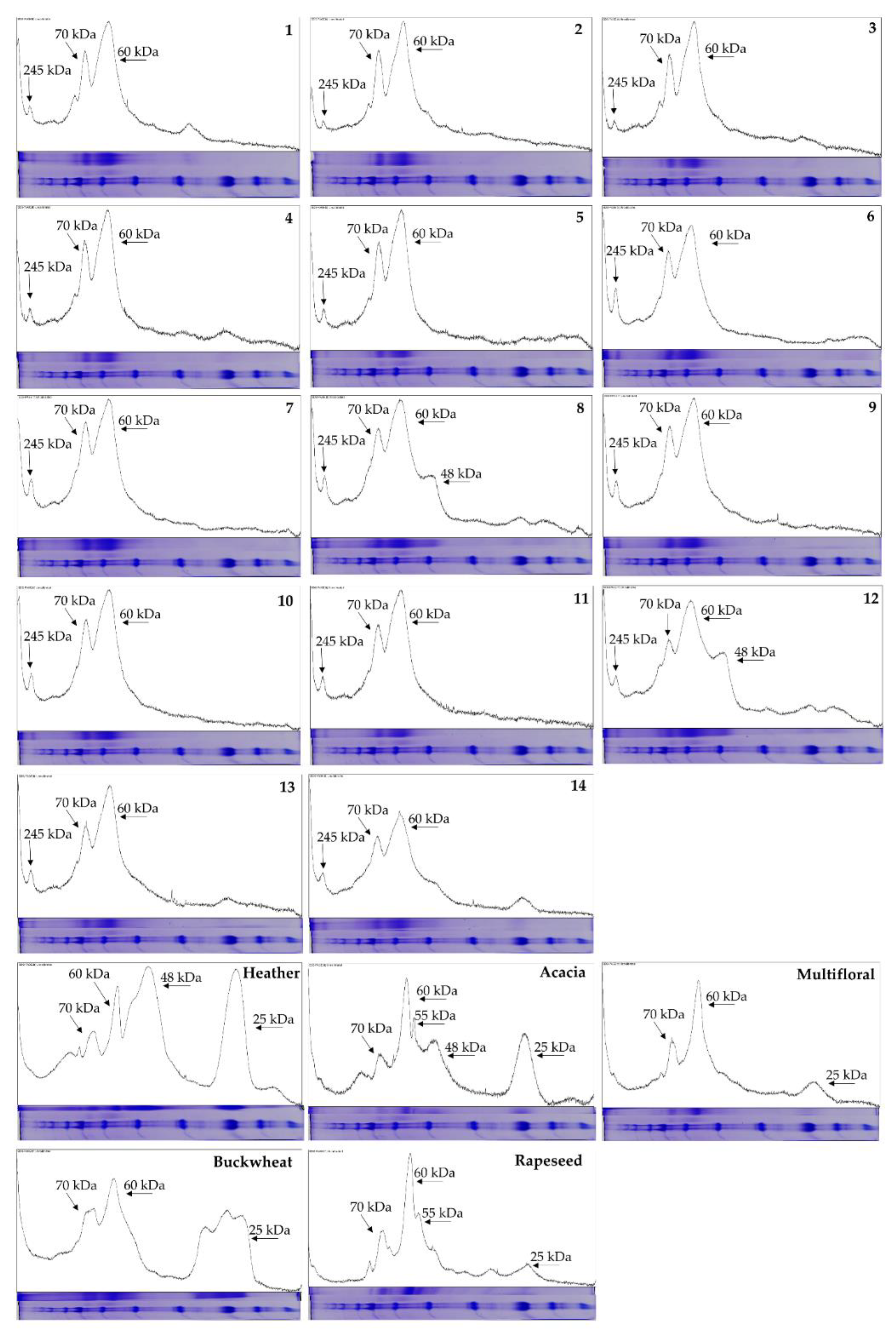
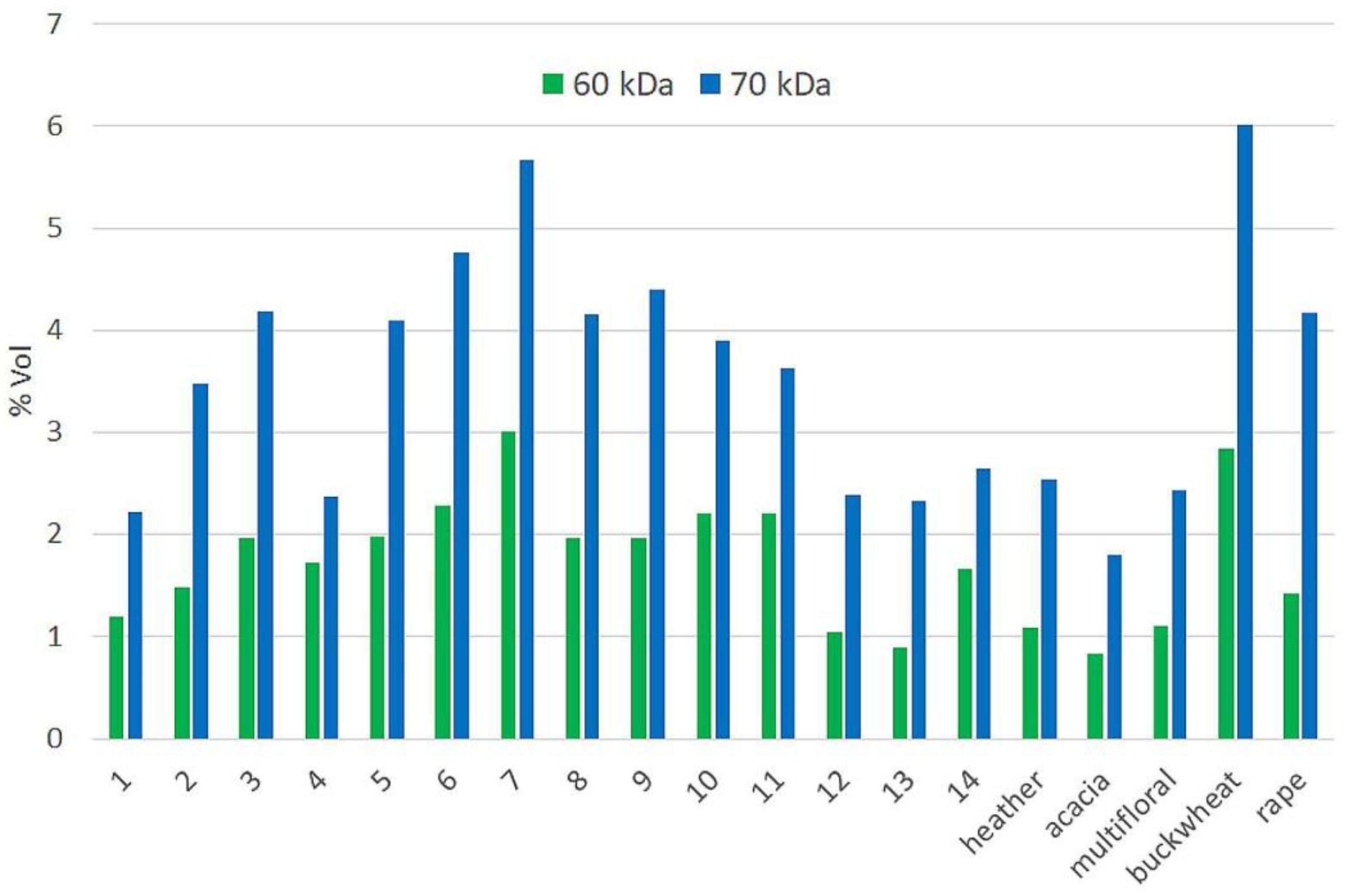
2.5. SDS-PAGE Fingerprinting
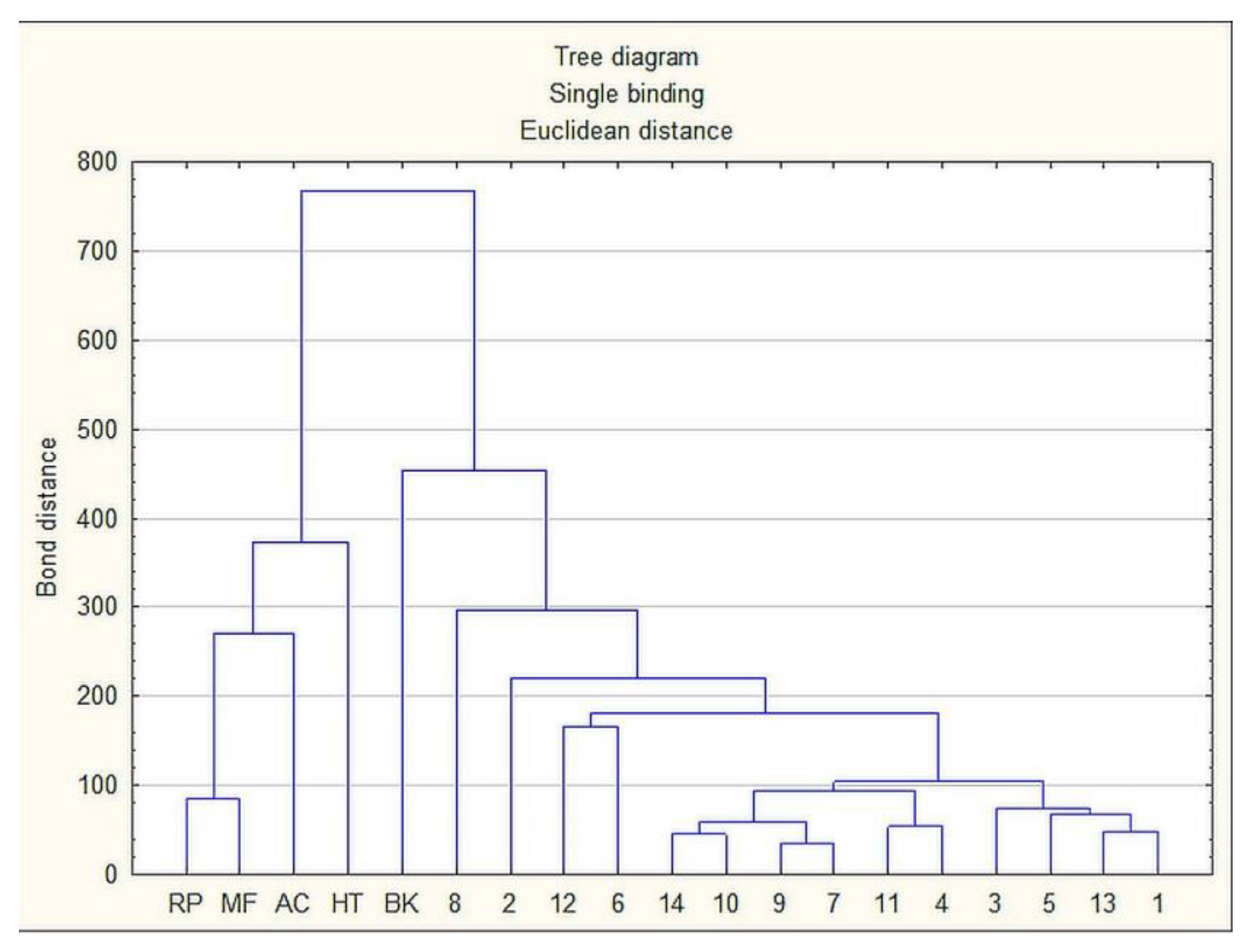
2.6. Statistical Analysis
3. Materials and Methods
3.1. Honey Samples
3.2. Physicochemical Properties
3.3. Specific Optical Rotation
3.4. Diastase Number Determination
3.5. Mineral Composition of Bioelements Using the ICP-OES Method
3.6. Antioxidant Properties
3.6.1. DPPH Test
3.6.2. ABTS Test
3.6.3. FRAP Test
3.6.4. Total Phenolic Content (TPC) Determination
3.7. High-Performance Thin Layer Chromatography (HPTLC) Implementation
3.7.1. Sugar Analysis
3.7.2. Polyphenolic Profile Analysis
3.7.3. Protein Profiling by SDS-PAGE
3.8. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Directive 2014/63/EU of the European Parliament and of the Council amending Council Directive 2001/110/EC relating to honey. Off. J. Eur. Communities 2014, 164, 1–5.
- Sanz, M.L.; Gonzalez, M.; Lorenzo, C.; Sanz, J.; Martinez-Castro, I. A contribution to the differentiation between nectar honey and honeydew honey. Food Chem. 2005, 91, 313–317. [Google Scholar] [CrossRef]
- Shaaban, B.; Seeburger, V.; Schroeder, A.; Lohaus, G. Suitability of sugar, amino acid, and inorganic ion compositions to distinguish fir and spruce honey. Eur. Food Res. Tech. 2021, 247, 879–888. [Google Scholar] [CrossRef]
- Fischer, M.K.; Shingleton, A.W. Host plant and ants influence the honeydew sugar composition of aphids. Funct. Ecol. 2001, 15, 544–550. [Google Scholar] [CrossRef]
- Bogdanov, S.; Ruoff, K.; Persano-Oddo, L. Physico-chemical methods for the characterization of unifloral honeys: A review. Apidologie 2004, 35, S4–S17. [Google Scholar] [CrossRef] [Green Version]
- Manzanares, A.B.; García, Z.H.; Galdón, B.R.; Rodríguez, E.R.; Romero, C.D. Differentiation of blossom and honeydew honeys using multivariate analysis on the physicochemical parameters and sugar composition. Food Chem. 2011, 126, 664–672. [Google Scholar] [CrossRef]
- Pita-Calvo, C.; Vazquez, M. Differences between honeydew and blossom honeys: A review. Trends Food Sci. Technol. 2018, 59, 79–87. [Google Scholar] [CrossRef]
- Seraglio, S.K.T.; Silva, B.; Bergamo, G.; Brugnerotto, P.; Gonzaga, L.V.; Fett, R.; Costa, A.C.O. An overview of physicochemical characteristics and health-promoting properties of honeydew honey. Food Res. Int. 2019, 119, 44–66. [Google Scholar] [CrossRef]
- Vasić, V.; Durdića, S.; Tostia, T.; Radoičićb, A.; Lušićc, D.; Milojković-Opsenicaa, D.; Tešića, Z.; Trifkovića, J. Two aspects of honeydew honey authenticity: Application of advance analytical methods and chemometrics. Food Chem. 2020, 305, 125457. [Google Scholar] [CrossRef] [PubMed]
- Borawska, A. Regional honeys in Poland in 2010–2015. In Proceedings of the 2018 International Conference “ECONOMIC SCIENCE FOR RURAL DEVELOPMENT” No 47, Jelgava, LLU ESAF, 9–11 May 2018; pp. 443–452. [Google Scholar] [CrossRef]
- Available online: https://uk.trade.gov.pl/en/polskatastesgood/120821,podkarpacie-honeydew-honey-podkarpacki-miod-spadziowy.html (accessed on 15 December 2021).
- Majtan, J.; Majtanova, L.; Bohova, J.; Majtan, V. Honeydew honey as a potent antibacterial agent in eradication of multi-drug resistant Stenotrophomonas maltophilia isolates from cancer patients. Phytother. Res. 2011, 25, 584–587. [Google Scholar] [CrossRef] [Green Version]
- Vallianou, N.G.; Gounari, P.; Skourtis, A.; Panagos, J.; Kazazis, C. Honey and its Anti-Inflammatory, Anti-Bacterial and Anti-Oxidant Properties. General Med. 2014, 2, 1000132. [Google Scholar] [CrossRef] [Green Version]
- Samarghandian, S.; Farkhondeh, T.; Samini, F. Honey and Health: A Review of Recent Clinical Research. Pharmacogn. Res. 2017, 9, 121–127. [Google Scholar] [CrossRef]
- Dżugan, M.; Tomczyk, M.; Sowa, P.; Grabek-Lejko, D. Antioxidant Activity as Biomarker of Honey Variety. Molecules 2018, 23, 2069. [Google Scholar] [CrossRef] [Green Version]
- Dżugan, M.; Grabek-Lejko, D.; Swacha, S.; Tomczyk, M.; Bednarska, S.; Kapusta, I. Physicochemical quality parameters, antibacterial properties and cellular antioxidant activity of Polish buckwheat honey. Food Biosci. 2020, 34, 100538. [Google Scholar] [CrossRef]
- Silva, B.; Biluca, F.C.; Gonzaga, L.V.; Fett, R.; Dalmarco, E.M.; Caon, T.; Costa, A.C.O. In vitro anti-inflammatory properties of honey flavonoids: A review. Food Res. Int. 2021, 141, 110086. [Google Scholar] [CrossRef]
- Majtan, J.; Bohova, J.; Garcia-Villalba, R.; Tomas-Barberan, F.A.; Madakova, Z.; Majtan, T.; Majtan, V.; Klaudiny, J. Fir honeydew honey flavonoids inhibit TNF-α-induced MMP-9 expression in human keratinocytes: A new action of honey in wound healing. Arch. Dermatol. Res. 2013, 305, 619–627. [Google Scholar] [CrossRef]
- Mayer, A.; Slezak, V.; Takac, P.; Olejnik, J.; Majtan, J. Treatment of non-healing leg ulcers with honeydew honey. J. Tissue Viability 2014, 23, 94–97. [Google Scholar] [CrossRef]
- Majtanova, N.; Vodrazkova, E.; Kurilova, V.; Horniackova, M.; Cernak, M.; Cernak, A.; Majtan, J. Complementary treatment of contact lens-induced corneal ulcer using honey: A case report. Contact Lens Anterior Eye 2015, 38, 61–63. [Google Scholar] [CrossRef]
- McLoone, P.; Warnock, M.; Fyfe, L. Honey: A realistic antimicrobial for disorders of the skin. J. Microbiol. Immun. Inf. 2016, 49, 161–167. [Google Scholar] [CrossRef] [Green Version]
- Seraglio, S.K.T.; Valese, A.C.; Daguer, H.; Bergamo, G.; Azevedo, M.S.; Nehring, P.; Gonzaga, L.V.; Fett, R.; Costa, A.C.O. Effect of in vitro gastrointestinal digestion on the bioaccessibility of phenolic compounds, minerals, and antioxidant capacity of Mimosa scabrella Bentham honeydew honeys. Food Res. Int. 2017, 99, 670–678. [Google Scholar] [CrossRef] [PubMed]
- Madejczyk, M.; Barałkiewicz, D. Characterization of honey from different areas of Poland by their physicochemical parameters and trace elements. Ecol. Chem. Eng. A 2008, 15, 675–682. [Google Scholar]
- Rybak-Chmielewska, H.; Szczesna, T.; Waś, E.; Jaśkiewicz, K.; Teper, D. Characteristics of Polish unifloral honeys IV. Honeydew honey, mainly (Abies alba L.). J. Apic. Sci. 2013, 57, 51–59. [Google Scholar] [CrossRef] [Green Version]
- Persano Oddo, L.; Piro, R. Main European unifloral honeys: Descriptive sheets. Apidologie 2004, 35, 38–81. [Google Scholar] [CrossRef]
- Abramovič, H.; Jamnik, M.; Burkan, M.L.; Kač, M. Water activity and water content in Slovenian honeys. Food Control. 2008, 19, 1086–1090. [Google Scholar] [CrossRef]
- Tarapatskyy, M.; Sowa, P.; Zaguła, G.; Dżugan, M.; Puchalski, C. Assessment of the Botanical Origin of Polish Honeys Based on Physicochemical Properties and Bioactive Components with Chemometric Analysis. Molecules 2021, 26, 4801. [Google Scholar] [CrossRef]
- Kaczmarek, A.; Muzolf-Panek, M.; Tomaszewska-Gras, J.; Konieczny, P. Predicting the botanical origin of honeys with chemometric analysis according to their antioxidant and physicochemical properties. Polish J. Food Nutr. Sci. 2019, 69, 191–201. [Google Scholar] [CrossRef]
- Baloš, M.; Popov, N.; Vidakovic Knezevic, S.; Ljubojević, D.; Pelić, M.; Mihaljev, Ž.; Jakšić, S. Electrical conductivity and acidity of honey. Arch. Vet. Med. 2018, 11, 91–101. [Google Scholar] [CrossRef]
- Dżugan, M.; Grabek-Lejko, D.; Sidor, E.; Tomczyk, M. The impact of ultrasound decrystallization on enzymatic, antioxidant and antibacterial properties of honey. Innov. Food Sci. Emerg. Tech. 2021, 71, 102709. [Google Scholar] [CrossRef]
- United States Department of Agriculture. United States Standards for Grades of Extracted Honey. 1985. Available online: https://www.ams.usda.gov/sites/default/files/media/Extracted_Honey_Standard%5B1%5D.pdf (accessed on 15 December 2021).
- Juszczak, L.; Socha, R.; Roznowski, J.; Fortuna, T.; Nalepka, K. Physicochemical properties and quality parameters of herbhoneys. Food Chem. 2009, 113, 538–542. [Google Scholar] [CrossRef]
- Nayik, G.A.; Nanda, V. Physico-Chemical, Enzymatic, Mineral and Colour Characterization of Three Different Varieties of Honeys from Kashmir Valley of India with a Multivariate Approach. Pol. J. Food Nutr. Sci. 2015, 65, 101–108. [Google Scholar] [CrossRef] [Green Version]
- Marghitas, L.A.; Dezmirean, D.; Adela, M.; Otilia, B.; Laura, L.; Stefan, B. Physico-chemical and bioactive properties of different floral origin honeys from Romania. Food Chem. 2009, 112, 863–867. [Google Scholar] [CrossRef]
- Dinkov, D. A scientific note on the specific optical rotation of three honey types from Bulgaria. Apidologie 2003, 34, 319–320. [Google Scholar] [CrossRef] [Green Version]
- Chudzinska, M.; Baralkiewicz, D. Estimation of honey authenticity by multielements characteristics using inductively coupled plasma-mass spectrometry (ICP-MS) combined with chemometrics. Food Chem. Toxicol. 2010, 48, 284–290. [Google Scholar] [CrossRef]
- Terrab, A.; Gonzalez, A.G.; Díez, M.J.; Heredia, F.J. Mineral content and electrical conductivity of the honeys produced in Northwest Morocco and their contribution to the characterisation of unifloral honeys. J. Sci. Food Agr. 2003, 83, 637–643. [Google Scholar] [CrossRef]
- Commission Regulation (EC) No 1881/2006 of 19 December 2006 setting the maximum levels for certain contaminants in foodstuffs. Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2006:364:0005:0024:EN:PDF (accessed on 15 December 2021).
- Dżugan, M.; Wesołowska, M.; Zaguła, G.; Kaczmarski, M.; Czernicka, M.; Puchalski, C. Honeybees (Apis mellifera) as a biological barrier for contamination of honey by environmental toxic metals. Environmen. Monit. Assess. 2018, 190, 101. [Google Scholar] [CrossRef] [PubMed]
- Tomczyk, M.; Zaguła, G.; Puchalski, C.; Dżugan, M. Transfer of Some Toxic Metals from Soil to Honey Depending on Bee Habitat Conditions. Acta Univ. Cibiniensis S. E Food Tech. 2020, 24, 49–59. [Google Scholar] [CrossRef]
- Hernández, O.; Fraga, J.M.G.; Jiménez, A.; Jiménez, F.; Arias, J. Characterization of honey from the Canary Islands: Determination of the mineral content by atomic absorption spectrophotometry. Food Chem. 2005, 93, 449–458. [Google Scholar] [CrossRef]
- Bobis, O.; Marghitas, L.; Rindt, J.K.; Niculae, M.; Dezmirean, D. Honeydew Honey: Correlations Between Chemical Composition, Antioxidant Capacity and Antibacterial Effect. Lucr. Ştiinţifice Zooteh. Şi Biotehnol. 2008, 41, 271–277. [Google Scholar]
- Stojković, M.; Cvetković, D.; Savić, A.; Topalić-Trivunović, L.; Velemir, A.; Papuga, S.; Žabić, M. Changes in the physicochemical, antioxidant and antibacterial properties of honeydew honey subjected to heat and ultrasound pretreatments. J. Food Sci. Technol. 2021, 58, 2555–2566. [Google Scholar] [CrossRef] [PubMed]
- Wilczyńska, A. Phenolic content and antioxidant activity of different types of Polish honey–a short report. Pol. J. Food Nutr. Sci. 2010, 60, 309–313. [Google Scholar]
- Chis, A.M.; Purcarea, C.; Dzugan, M.; Teusdea, A. Comparative Antioxidant Content and Antioxidant Activity of Selected Romanian and Polish Honeydew Honey. Rev. Chim. (Bucharest) 2016, 67, 214–218. [Google Scholar]
- Tomczyk, M.; Tarapatskyy, M.; Dzugan, M. The influence of geographical origin on honey composition studied by Polish and Slovak honeys. Czech. J. Food Sci. 2019, 37, 232–238. [Google Scholar] [CrossRef]
- Gośliński, M.; Nowak, D.; Kłębukowska, L. Antioxidant properties and antimicrobial activity of manuka honey versus Polish honeys. J. Food Sci. Technol. 2020, 57, 1269–1277. [Google Scholar] [CrossRef]
- Srećković, N.; Mihailović, V.; Katanić-Stanković, J. Physico-chemical, antioxidant and antimicrobial properties of three different types of honey from Central Serbia. Kragujev. J. Sci. 2019, 41, 53–68. [Google Scholar] [CrossRef]
- Rybak-Chmielewska, H. High performance liquid chromatography (HPLC) study of sugar composition in some kinds of natural honey and winter stores processed by bees from starch syrup. J. Apic. Sci. 2007, 51, 23–38. [Google Scholar]
- Islam, M.K.; Sostaric, T.; Lim, L.Y.; Hammer, K.; Locher, C. Sugar Profiling of Honeys for Authentication and Detection of Adulterants Using High-Performance Thin Layer Chromatography. Molecules 2020, 25, 5289. [Google Scholar] [CrossRef] [PubMed]
- Bogdanov, S.; Martin, P. Honey Authenticity: A Review. Mitt. Lebensm. Hyg. 2002, 93, 1–20. [Google Scholar]
- Gemeda, T.K.; Li, J.; Luo, S.; Yang, H.; Jin, T.; Huang, J.; Wu, J. Pollen trapping and sugar syrup feeding of honey bee (Hymenoptera: Apidae) enhance pollen collection of less preferred flowers. PLoS ONE 2018, 13, e0203648. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cavia, M.M.; Fernández-Muiño, M.A.; Gömez-Alonso, E.; Montes- Pérez, M.J.; Huidobro, J.F.; Sancho, M.T. Evolution of fructose and glucose in honey over one year: Influence of induced granulation. Food Chem. 2002, 78, 157–161. [Google Scholar] [CrossRef]
- Alghamdi, B.A.; Alshumrani, E.S.; Saeed, M.S.B.; Rawas, G.M.; Alharthi, N.T.; Baeshen, M.N.; Helmi, N.M.; Alam, M.Z.; Suhail, M. Analysis of sugar composition and pesticides using HPLC and GC–MS techniques in honey samples collected from Saudi Arabian markets. Saudi J. Biol. Sci. 2020, 27, 3720–3726. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.H.; Nam, M.S. Classification of honeydew and blossom honeys by principal component analysis of physicochemical parameters. Korean J. Agr. Sci. 2020, 47, 67–81. [Google Scholar] [CrossRef]
- Socha, R.; Juszczak, L.; Pietrzyk, S.; Gałkowska, D.; Fortuna, T.; Witczak, T. Phenolic profile and antioxidant properties of Polish honeys. Int. J. Food Sci. Technol. 2011, 46, 528–534. [Google Scholar] [CrossRef]
- Can, Z.; Yildiz, O.; Sahin, H.; Akyuz Turumtay, E.; Silici, S.; Kolayli, S. An investigation of Turkish honeys: Their physico-chemical properties, antioxidant capacities and phenolic profiles. Food Chem. 2015, 180, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Halouzka, R.; Tarkowski, P.; Zeljkovic, S.Ć. Characterisation of Phenolics and other Quality Parameters of Different Types of Honey. Czech. J. Food Sci. 2016, 34, 244–253. [Google Scholar] [CrossRef] [Green Version]
- Makowicz, E.; Jasicka-Misiak, I.; Teper, D.; Kafarski, P. HPTLC fingerprinting—Rapid method for the differentiation of honeys of different botanical origin based on the composition of the lipophilic fractions. Molecules 2018, 23, 1811. [Google Scholar] [CrossRef] [Green Version]
- Jasicka-Misiak, I.; Makowicz, E.; Stanek, N. Chromatographic fingerprint, antioxidant activity, and colour characteristic of polish goldenrod (Solidago virgaurea L.) honey and flower. Eur. Food Res. Technol. 2018, 244, 1169–1184. [Google Scholar] [CrossRef]
- Stanek, N.; Jasicka-Misiak, I.; Samples, H. HPTLC Phenolic Profiles as Useful Tools for the Authentication of Honey. Food Anal. Methods 2018, 18, 2979–2989. [Google Scholar] [CrossRef] [Green Version]
- Recklies, K.; Peukert, C.; Kölling-Speer, I.; Speer, K. Differentiation of Honeydew Honeys from Blossom Honeys and According to Their Botanical Origin by Electrical Conductivity and Phenolic and Sugar Spectra. J. Agric. Food Chem. 2021, 69, 1329–1347. [Google Scholar] [CrossRef]
- Azevedo, M.S.; Valentim-Neto, P.A.; Seraglio, S.K.T.; da Luz, C.F.P.; Arisi, A.C.M.; Costa, A.C.O. Proteome comparison for discrimination between honeydew and floral honeys from botanical species Mimosa scabrella Bentham by principal component analysis. J. Sci. Food Agric. 2017, 97, 4515–4519. [Google Scholar] [CrossRef]
- Polish Regulation: Ordinance of the Minister of Agriculture and Rural Development from January 14, 2009 on the Methods of Analysis Related to the Evaluation of Honey. Available online: http://isap.sejm.gov.pl/isap.nsf/download.xsp/WDU20090170094/O/D20090094.pdf (accessed on 12 December 2021). (In Polish)
- Harmonised Methods of the International Honey Commission (IHC). Available online: http://www.bee-hexagon.net/en/network.htm (accessed on 15 December 2021).
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]



| Honey Sample | Water Content [%] | pH | Acidity [mval/kg] | Conductivity [mS/cm] | HMF [mg/kg] | Diastase Numer [DN] | Colour [mm Pfund] | Specific Rotation [[α]D 20] | |
|---|---|---|---|---|---|---|---|---|---|
| Honeydew | 1 | 16.6 ± 0.4 | 4.91 ± 0.01 | 41.9 ± 1.9 | 1.234 ± 0.001 | 3.21 ± 0.27 | 14.03 ± 0.11 | 128 ± 1.4 | +16.25 ± 0.08 |
| 2 | 16.2 ± 0.1 | 4.88 ± 0.02 | 41 ± 0.6 | 1.414 ± 0.001 | 2.94 ± 0.03 | 16.26 ± 0.13 | 114 ± 2.1 | +17.92 ± 0.25 | |
| 3 | 15.3 ± 0.3 | 4.78 ± 0.01 | 47.25 ± 0.25 | 1.392 ± 0.002 | 17.76 ± 0.35 | 15.13 ± 0.12 | 137 ± 0.7 | +16.67 ± 0.17 | |
| 4 | 15.4 ± 0.3 | 5.04 ± 0.05 | 51.2 ± 0.3 | 1.333 ± 0.001 | 6.53 ± 0.05 | 13.46 ± 0.10 | 127 ± 2.8 | +19.17 ± 0.17 | |
| 5 | 14.6 ± 0.3 | 5.07 ± 0.06 | 40.4 ± 1.0 | 1.262 ± 0.004 | 2.11 ± 0.14 | 14.00 ± 0.11 | 125 ± 0.7 | +25.00 ± 0.33 | |
| 6 | 17.3 ± 0.1 | 4.56 ± 0.04 | 49.75 ± 0.68 | 1.176 ± 0.003 | 6.71 ± 0.01 | 15.55 ± 0.12 | >150 | +9.17 ± 0.08 | |
| 7 | 16.7 ± 0.3 | 4.38 ± 0.06 | 65.5 ± 1.3 | 1.053 ± 0.001 | 11.24 ± 0.10 | 15.55 ± 0.12 | >150 | +2.50 ± 0.25 | |
| 8 | 17.9 ± 0.0 | 4.52 ± 0.03 | 54.9 ± 1.7 | 1.302 ± 0.001 | 5.98 ± 0.18 | 13.24 ± 0.10 | >150 | +3.33 ± 0.17 | |
| 9 | 17.5 ± 0.2 | 4.52 ± 0.04 | 49.6 ± 1.4 | 1.169 ± 0.005 | 6.17 ± 0.07 | 11.55 ± 0.16 | >150 | +7.92 ± 0.25 | |
| 10 | 17.3 ± 0.1 | 4.50 ± 0.02 | 51.75 ± 0.55 | 1.204 ± 0.004 | 6.08 ± 0.01 | 11.12 ± 0.14 | >150 | +10.83 ± 0.08 | |
| 11 | 17.0 ± 0.1 | 4.85 ± 0.05 | 37.7 ± 0.3 | 1.267 ± 0.001 | 1.43 ± 0.26 | 10.02 ± 0.18 | 136 ± 1.4 | +21.67 ± 0.08 | |
| 12 | 19.1 ± 0.1 | 4.40 ± 0.04 | 51.65 ± 0.35 | 1.181 ± 0.001 | 4.96 ± 0.10 | 15.01 ± 0.10 | >150 | +5.83 ± 0.17 | |
| 13 | 18.1 ± 0.1 | 4.36 ± 0.05 | 49.1 ± 0.3 | 1.087 ± 0.001 | 6.41 ± 0.84 | 11.01 ± 0.20 | 146 ± 1.4 | +7.92 ± 0.08 | |
| 14 | 18.1 ± 0.1 | 4.47 ± 0.04 | 43.15 ± 0.65 | 1.028 ± 0.005 | 2.79 ± 0.01 | 8.92 ± 0.19 | >150 | +8.33 ± 0.08 | |
| min | 14.60 | 4.36 | 37.70 | 1.028 | 1.43 | 8.92 | 114 | +2.50 | |
| max | 19.10 | 5.07 | 65.50 | 1.414 | 17.76 | 16.26 | >150 | +25.00 | |
| mean | 16.94 | 4.66 | 48.20 a | 1.222 a | 6.02 a,b | 13.20 b | 140 a | +12.32 a | |
| SD | 1.24 | 0.25 | 7.19 | 0.118 | 4.22 | 2.31 | 12.3 | 7.05 | |
| Heather | 16.60 ± 0.2 | 4.35 ± 0.05 | 31.20 ± 0.03 a,b | 0.771 ± 0.01 b | 0.94 ± 0.01 b | 28.25 ± 0.25 a | 57 ± 2.8 b | −30.42 ± 0.18 b,c | |
| Acacia | 17.95 ± 0.3 | 4.37 ± 0.01 | 6.95 ± 0.02 c | 0.355 ± 0.01 c | 4.50 ± 0.10 b | 15.16 ± 0.16 b,c | 10 ± 0.7 c | −43.33 ± 0.42 b | |
| Multifloral | 17.00 ± 0.2 | 4.55 ± 0.01 | 14.85 ± 0.03 b,c | 0.153 ± 0.01 c | 10.17 ± 0.07 a,b | 19.35 ± 0.14 b,c | 27 ± 2.1 c | −19.58 ± 0.11 c | |
| Buckwheat | 18.60 ± 0.2 | 3.99 ± 0.05 | 48.65 ± 0.04 a | 0.311 ± 0.01 c | 16.82 ± 0.03 a | 20.89 ± 0.13 c | >150 a | −17.92 ± 0.29 c | |
| Rape | 18.90 ± 0.1 | 4.42 ± 0.01 | 18.50 ± 0.07 b,c | 0.208 ± 0.01 c | 1.60 ± 0.08 b | 18.20 ± 0.11 b,c | 39 ± 1.4 b,c | −23.33 ± 0.33 c | |
| Honey Sample | Bioelements [mg/kg] | Toxic Metals [mg/kg] | Total [mg/kg] | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| K | P | S | Mg | Ca | Na | Mn | Fe | Cu | Zn | Sr | Al | Ni | As | Pb | Cd | |||
| Honeydew | 1 | 2634 | 448.5 | 140.6 | 34.14 | 16.09 | 16.44 | 5.902 | 2.674 | 1.613 | 0.660 | 0.035 | 28.9 | 0.756 | 0.741 | 0.045 | 0.026 | 3331 |
| 2 | 2889 | 546.1 | 98.7 | 40.74 | 30.68 | 8.19 | 5.602 | 1.815 | 1.856 | 0.867 | 0.068 | 17.3 | 1.412 | 0.766 | 0.027 | 0.037 | 3643 | |
| 3 | 2861 | 496.0 | 109.1 | 56.05 | 33.28 | 9.17 | 8.559 | 2.301 | 1.612 | 0.868 | 0.074 | 21.7 | 1.353 | 0.769 | 0.030 | 0.038 | 3602 | |
| 4 | 2951 | 526.3 | 141.1 | 36.13 | 12.87 | 9.93 | 6.222 | 2.692 | 1.871 | 0.805 | 0.035 | 31.8 | 1.097 | 0.780 | 0.034 | 0.030 | 3722 | |
| 5 | 2680 | 520.4 | 140.8 | 34.83 | 11.21 | 11.33 | 6.270 | 2.941 | 1.779 | 0.681 | 0.033 | 33.6 | 0.950 | 0.776 | 0.039 | 0.029 | 3445 | |
| 6 | 2386 | 496.2 | 134.4 | 65.10 | 38.77 | 11.81 | 7.898 | 2.370 | 1.680 | 1.882 | 0.096 | 27.1 | 0.978 | 0.776 | 0.050 | 0.041 | 3175 | |
| 7 | 2089 | 643.7 | 107.6 | 40.17 | 42.19 | 13.39 | 5.176 | 2.135 | 1.326 | 0.881 | 0.108 | 13.8 | 0.494 | 0.759 | 0.036 | 0.041 | 2960 | |
| 8 | 2813 | 538.0 | 103.6 | 38.22 | 82.07 | 14.04 | 5.282 | 4.659 | 1.552 | 1.009 | 0.127 | 17.8 | 1.381 | 0.763 | 0.085 | 0.027 | 3621 | |
| 9 | 2560 | 631.7 | 110.9 | 45.88 | 44.75 | 12.67 | 7.670 | 2.480 | 1.553 | 1.261 | 0.122 | 18.7 | 0.975 | 0.793 | 0.033 | 0.045 | 3439 | |
| 10 | 2461 | 633.3 | 105.7 | 45.22 | 45.15 | 12.18 | 7.500 | 3.142 | 1.530 | 1.249 | 0.123 | 18.1 | 0.958 | 0.746 | 0.028 | 0.046 | 3335 | |
| 11 | 2692 | 591.8 | 130.4 | 34.08 | 21.08 | 8.93 | 6.986 | 3.406 | 1.606 | 1.005 | 0.069 | 33.5 | 0.733 | 0.765 | 0.105 | 0.026 | 3526 | |
| 12 | 2494 | 444.7 | 81.9 | 33.53 | 115.78 | 13.81 | 5.470 | 1.880 | 1.170 | 1.227 | 0.154 | 13.0 | 0.810 | 0.753 | 0.017 | 0.019 | 3208 | |
| 13 | 2301 | 809.0 | 127.1 | 45.76 | 28.52 | 15.57 | 9.942 | 3.327 | 1.371 | 0.878 | 0.064 | 19.8 | 0.529 | 0.738 | 0.039 | 0.023 | 3363 | |
| 14 | 2207 | 452.5 | 107.9 | 36.36 | 31.27 | 10.65 | 9.046 | 7.075 | 1.816 | 9.184 | 0.065 | 24.8 | 0.988 | 0.781 | 0.120 | 0.035 | 2899 | |
| Min | 2088 | 444.7 | 81.9 | 33.53 | 11.21 | 8.19 | 5.18 | 1.81 | 1.17 | 0.66 | 0.03 | 13.00 | 0.49 | 0.74 | 0.02 | 0.02 | 2899 | |
| Max | 2950 | 809.0 | 141.1 | 65.10 | 115.78 | 16.44 | 9.94 | 7.08 | 1.87 | 9.18 | 0.15 | 33.57 | 1.41 | 0.79 | 0.12 | 0.05 | 3722 | |
| Mean | 2572 | 555.58 | 117.14 | 41.87 | 39.55 | 12.00 | 6.97 | 3.06 | 1.60 | 1.60 | 0.08 | 22.84 | 0.96 | 0.76 | 0.05 | 0.03 | 3376 | |
| SD | 259.5 | 97.21 | 18.01 | 9.07 | 27.60 | 2.47 | 1.47 | 1.38 | 0.20 | 2.15 | 0.04 | 6.92 | 0.28 | 0.03 | 0.03 | 0.01 | 249 | |
| %VC | 10.09 | 17.50 | 15.37 | 21.67 | 69.78 | 20.61 | 21.16 | 45.01 | 12.56 | 134.02 | 45.05 | 30.27 | 29.28 | 3.87 | 62.73 | 25.61 | 7.38 | |
| F-value | 168.8 | 5069 | 5524 | 144.6 | 2142 | 50.3 | 2254 | 32.2 | 440.9 | 316993 | 267.2 | 4327 | 8390 | 0.8 | 145.4 | 172.4 | ||
| p-value | <0.001 | 0.612 | <0.001 | |||||||||||||||
| Honey Sample | TPC [mg GAE/kg] | FRAP [mg TE/kg] | DPPH [mg TE/kg] | ABTS [mg TE/kg] | |
|---|---|---|---|---|---|
| Honeydew | 1 | 834.08 ± 22.06 | 462.30 ± 11.52 | 249.69 ± 18.42 | 1606.96 ± 35.70 |
| 2 | 635.42 ± 7.49 | 377.08 ± 15.74 | 228.80 ± 18.61 | 1367.68 ± 32.14 | |
| 3 | 737.35 ± 13.39 | 465.59 ± 14.38 | 256.46 ± 17.46 | 1538.11 ± 53.66 | |
| 4 | 853.42 ± 10.13 | 518.28 ± 15.82 | 262.28 ± 7.06 | 1693.43 ± 37.43 | |
| 5 | 769.35 ± 9.25 | 475.06 ± 10.11 | 255.87 ± 13.11 | 1600.24 ± 69.67 | |
| 6 | 1153.27 ± 25.31 | 609.67 ± 33.13 | 251.48 ± 21.59 | 1993.16 ± 66.97 | |
| 7 | 913.69 ± 13.96 | 523.22 ± 22.59 | 230.82 ± 19.28 | 1750.52 ± 29.45 | |
| 8 | 1289.43 ± 34.91 | 738.11 ± 37.22 | 280.57 ± 8.77 | 2222.37 ± 35.37 | |
| 9 | 942.71 ± 17.08 | 522.81 ± 19.46 | 238.30 ± 16.04 | 1748.85 ± 55.28 | |
| 10 | 988.10 ± 13.53 | 578.38 ± 31.48 | 242.45 ± 9.70 | 1782.43 ± 30.83 | |
| 11 | 860.12 ± 33.76 | 549.42 ± 30.19 | 263.47 ± 4.11 | 1653.97 ± 59.48 | |
| 12 | 990.33 ± 76.28 | 615.43 ± 30.31 | 260.86 ± 12.21 | 1959.58 ± 29.60 | |
| 13 | 844.49 ± 18.25 | 462.30 ± 28.28 | 209.92 ± 14.70 | 1593.52 ± 53.28 | |
| 14 | 948.66 ± 15.82 | 568.50 ± 14.93 | 258.84 ± 5.30 | 1779.07 ± 26.35 | |
| min | 635.42 | 377.08 | 209.92 | 1367.68 | |
| max | 1289.43 | 738.11 | 280.57 | 2222.37 | |
| mean | 911.46 a | 533.30 a | 249.27 a | 1734.99 a | |
| SD | 163.42 | 88.25 | 21.43 | 212.05 | |
| VC% | 17.93 | 16.55 | 8.60 | 12.22 | |
| F-value | 140.83 | 53.32 | 6.19 | 85.56 | |
| p-value | <0.001 | <0.001 | <0.001 | <0.001 | |
| Heather | 548.12 ± 10.10 c | 204.32 ± 7.64 b | 70.01 ± 4.72 c | 646.02 ± 6.63 c | |
| Acacia | 110.37 ± 4.73 d | 32.25 ± 2.41 b | 15.66 ± 8.92 d | 141.72 ± 18.95 d | |
| Multifloral | 249.01 ± 12.66 c,d | 128.58 ± 8.03 b | 42.84 ± 2.77 c,d | 443.19 ± 19.52 c,d | |
| Buckwheat | 1574.40 ± 50.86 b | 644.39 ± 5.03 a | 311.01 ± 23.76 b | 2560.50 ± 26.99 b | |
| Rape | 206.35 ± 6.67 c,d | 87.27 ± 4.53 b | 26.46 ± 6.74 c,d | 385.03 ± 18.69 c,d | |
| Rf | Colour | Honeydew (Band Present as a Percentage of All Samples) | Heather | Acacia | Multifloral | Buckwheat | Rape | |
|---|---|---|---|---|---|---|---|---|
| UV 366 | VIS | |||||||
| 0.069 | orange | grey | 100% | + | + | + | + | + |
| 0.121 | blue | - | 64% | - | - | + | - | + |
| 0.265 | pale orange | grey | 93% | + | - | + | + | - |
| 0.320 | yellow | orange | 43% | + | - | + | - | + |
| 0.336 | orange | - | 36% | - | - | - | - | + |
| 0.378 | yellow | yellow | 86% | + | - | + | + | + |
| 0.486 | orange | orange | 71% | - | - | + | + | + |
| 0.515 | green/yellow | violet | 57% | - (orange) | + | + | + | + |
| 0.524 | pink | - | 43% | - | - | - | + | - |
| 0.552 | orange/yellow | - | 64% | + | - | + | + | + |
| 0.563 | blue | - | 79% | - | - | - | - | - |
| 0.576 | - | pink | 100% | + | - | + | + | + |
| 0.646 | - | violet | 100% | - | - | - | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tomczyk, M.; Bocian, A.; Sidor, E.; Miłek, M.; Zaguła, G.; Dżugan, M. The Use of HPTLC and SDS-PAGE Methods for Coniferous Honeydew Honey Fingerprinting Compiled with Mineral Content and Antioxidant Activity. Molecules 2022, 27, 720. https://doi.org/10.3390/molecules27030720
Tomczyk M, Bocian A, Sidor E, Miłek M, Zaguła G, Dżugan M. The Use of HPTLC and SDS-PAGE Methods for Coniferous Honeydew Honey Fingerprinting Compiled with Mineral Content and Antioxidant Activity. Molecules. 2022; 27(3):720. https://doi.org/10.3390/molecules27030720
Chicago/Turabian StyleTomczyk, Monika, Aleksandra Bocian, Ewelina Sidor, Michał Miłek, Grzegorz Zaguła, and Małgorzata Dżugan. 2022. "The Use of HPTLC and SDS-PAGE Methods for Coniferous Honeydew Honey Fingerprinting Compiled with Mineral Content and Antioxidant Activity" Molecules 27, no. 3: 720. https://doi.org/10.3390/molecules27030720
APA StyleTomczyk, M., Bocian, A., Sidor, E., Miłek, M., Zaguła, G., & Dżugan, M. (2022). The Use of HPTLC and SDS-PAGE Methods for Coniferous Honeydew Honey Fingerprinting Compiled with Mineral Content and Antioxidant Activity. Molecules, 27(3), 720. https://doi.org/10.3390/molecules27030720