Insights into Structure and Biological Activity of Copper(II) and Zinc(II) Complexes with Triazolopyrimidine Ligands
Abstract
:1. Introduction
2. Results and Discussions
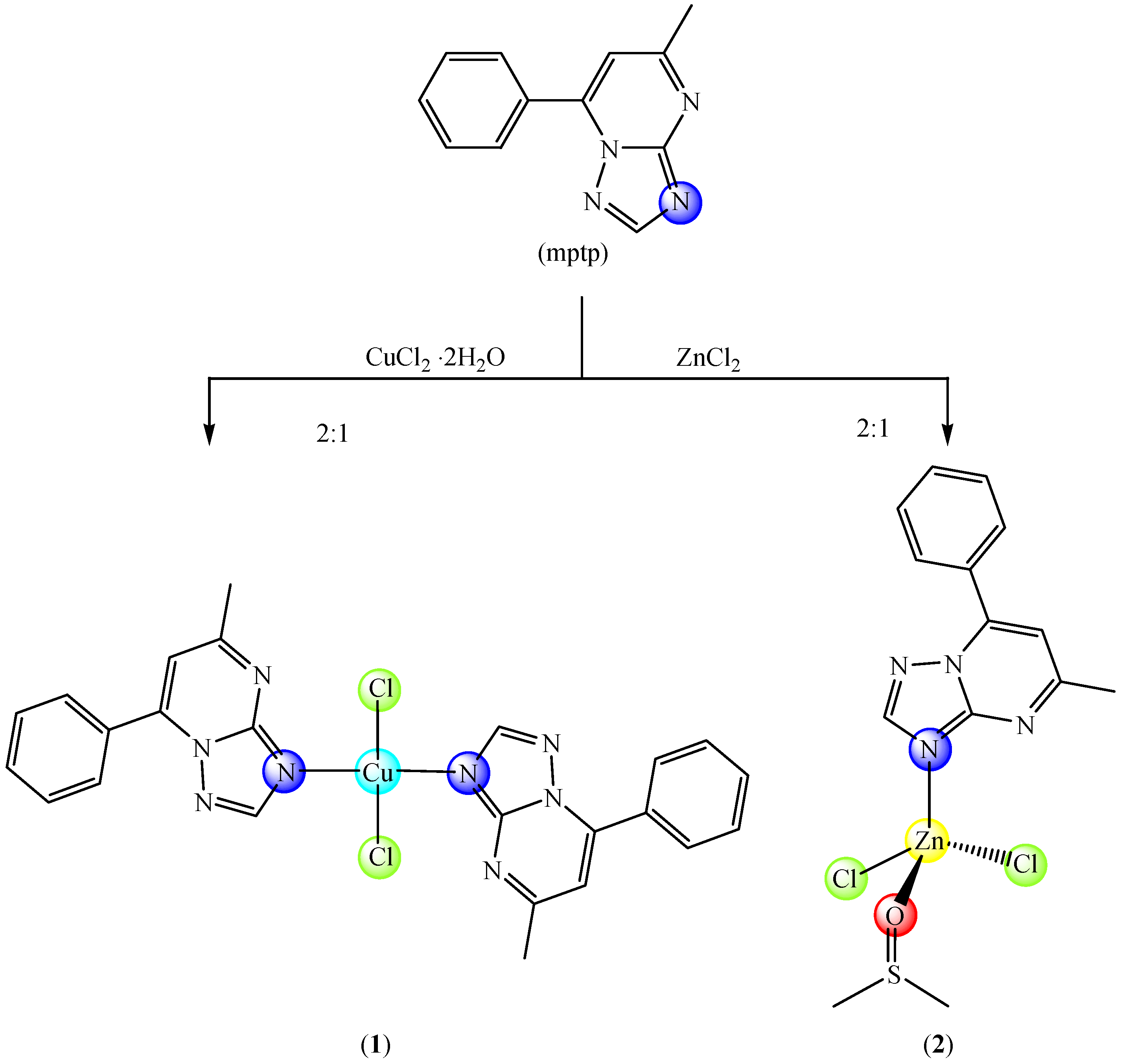
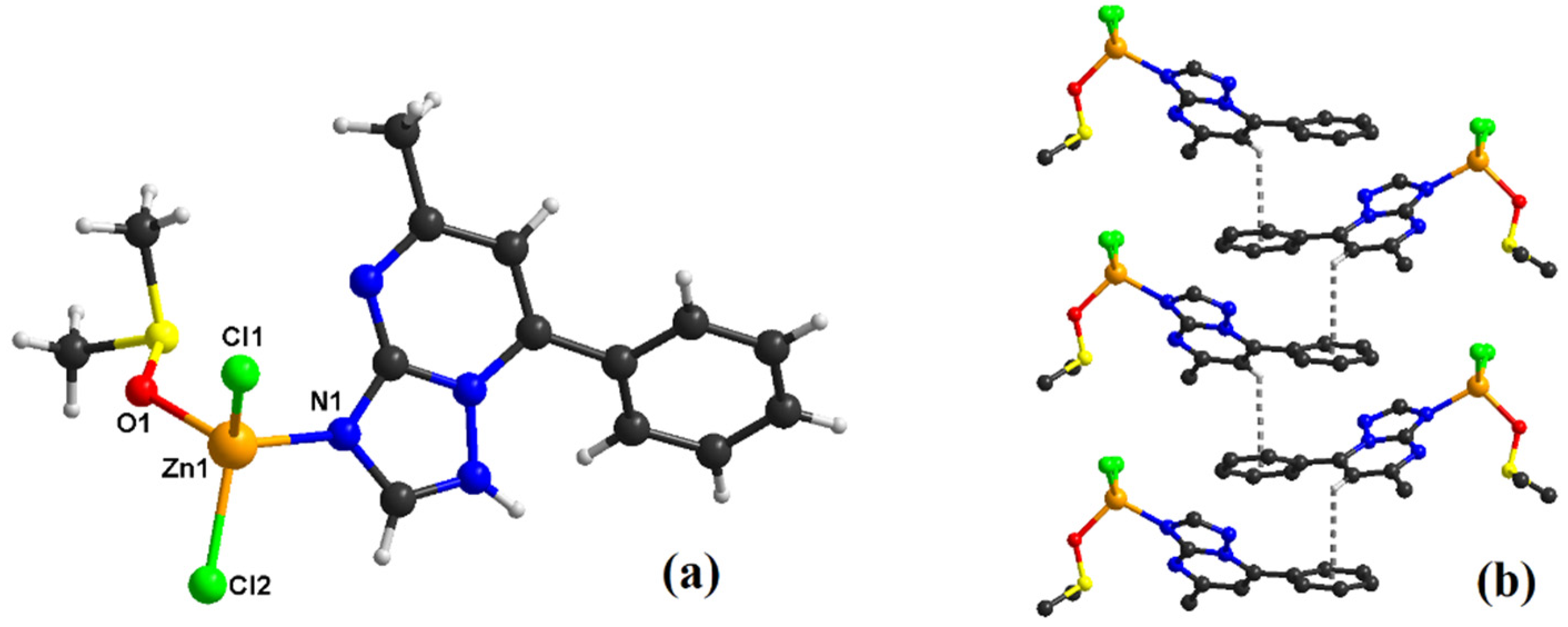
2.1. Description of the Crystal Structure of Complexes
2.2. Physico-Chemical Characterization of Complexes
2.2.1. FT-IR Spectra
2.2.2. UV-Vis Spectra
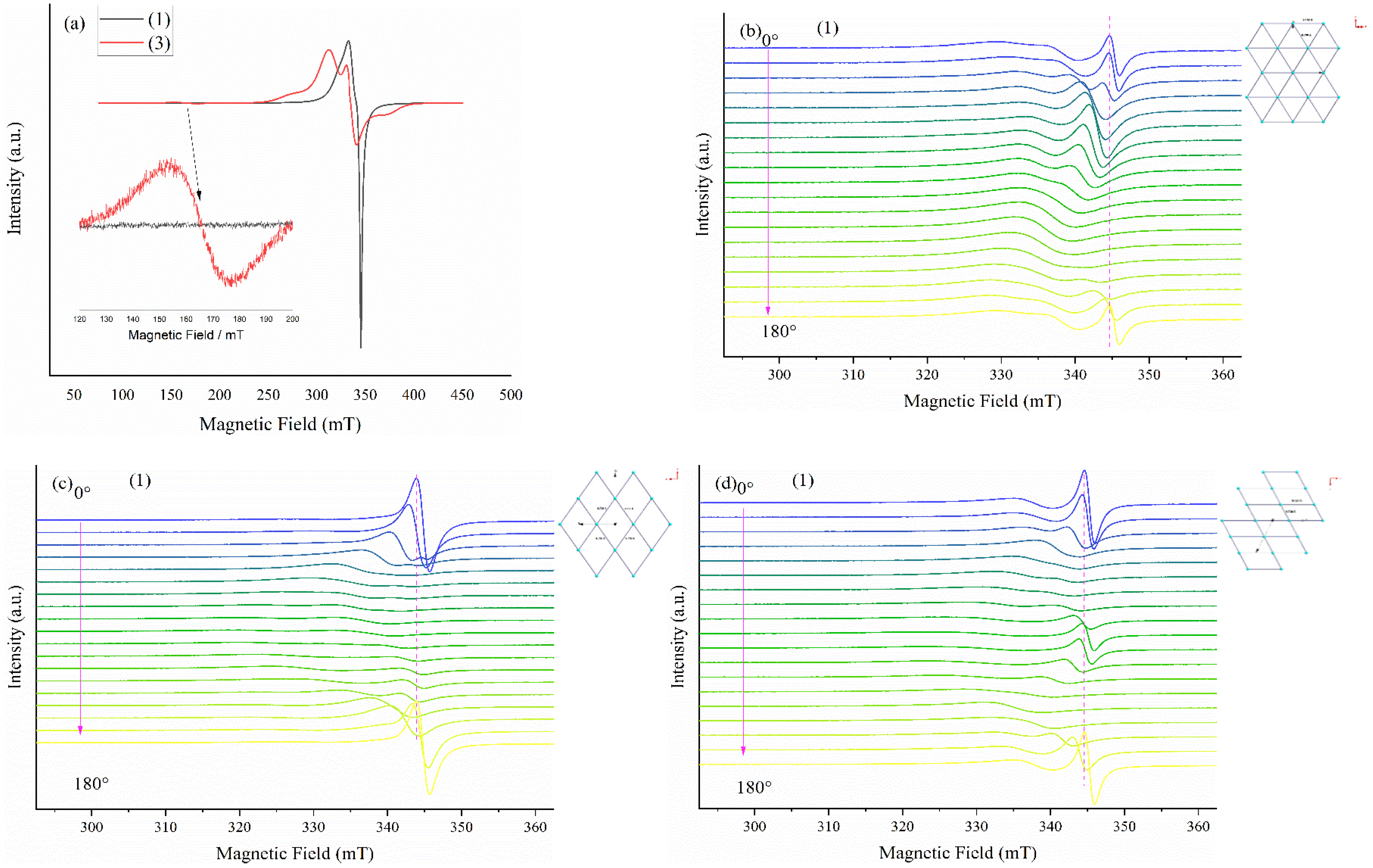
2.2.3. EPR Spectroscopy
Solid State EPR Spectroscopy
Solution EPR Spectroscopy
2.2.4. NMR Spectroscopy
2.2.5. Voltammetric Studies
2.3. Complexes Interactions with Cells and Biological Species
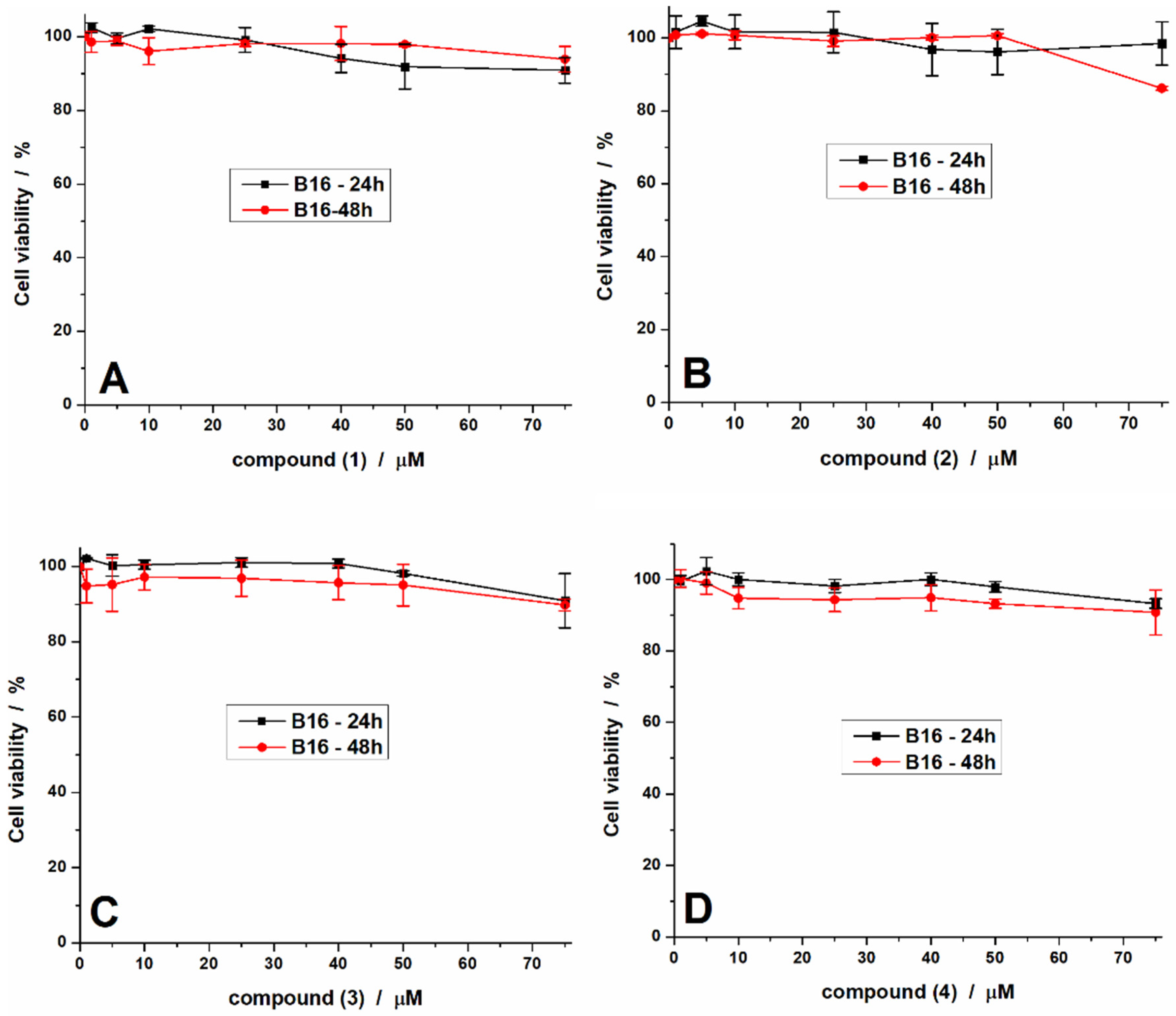
2.3.1. Antiproliferative Activity
2.3.2. Microbiological Activity
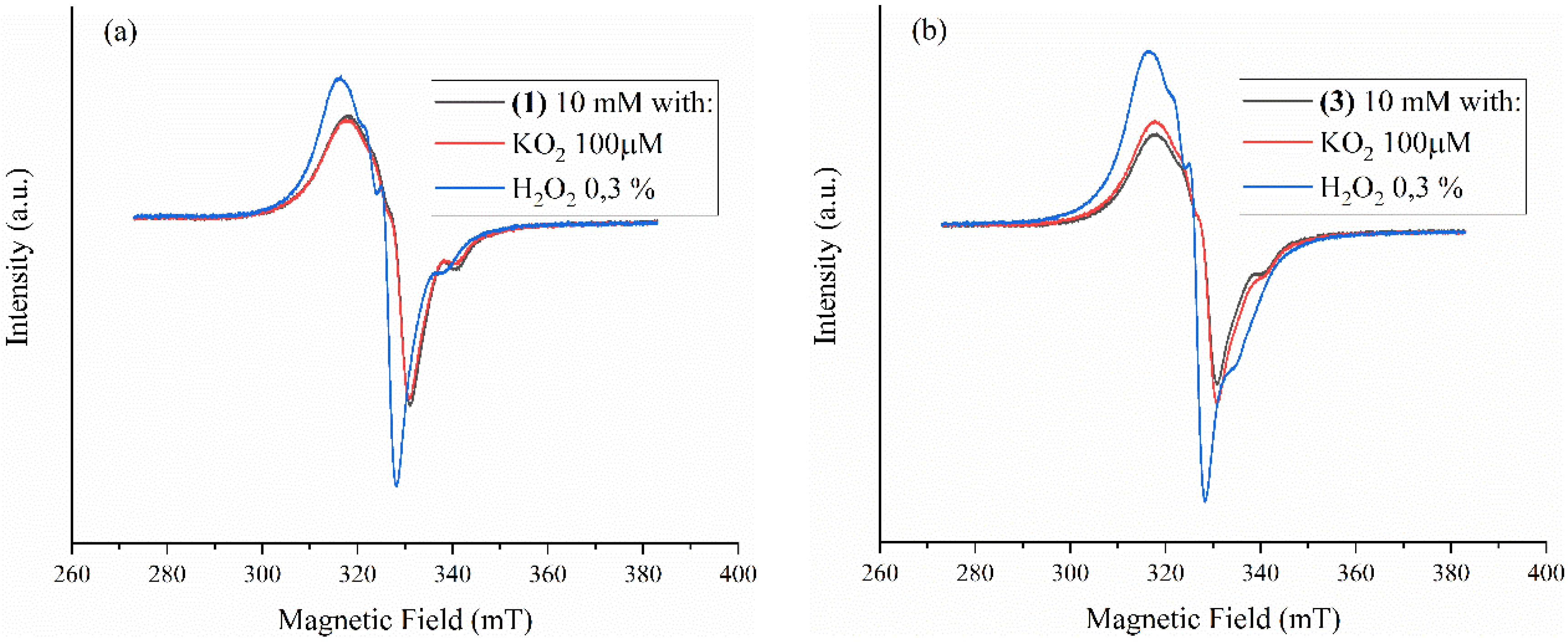
2.4. Complexes Interaction with ROS
2.5. Complexes Interaction with DNA
3. Materials and Methods
3.1. Reagents
3.2. Physical Measurements
3.3. Synthesis and Characterization of the Complexes
3.4. Biological Characterization of Compounds
3.4.1. Screening of the Antimicrobial Properties
3.4.2. In Vitro Cytotoxicity Assay
Cell Culture Conditions
Cell Viability Assay
Phalloidin Staining and Cell Imaging
3.4.3. Interaction with Biological Species
Superoxide Scavenging Ability
Fluorescence of λ-DNA/Ethidium Bromide Adduct
Nuclease-Like Activity Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- De Oliveira, D.M.P.; Forde, B.M.; Kidd, T.J.; Harris, P.N.A.; Schembri, M.A.; Beatson, S.A.; Paterson, D.L.; Walker, M.J. Antimicrobial resistance in ESKAPE pathogens. Clin. Microbiol. Rev. 2020, 33, e00181-19. [Google Scholar] [CrossRef] [PubMed]
- Novais, C.; Freitas, A.R. Transmission of antibiotic resistant bacteria and genes: Unveiling the jigsaw pieces of a one health problem. Pathogens 2020, 9, 497. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Qin, L.; Chen, W.; Chen, Q.; Sun, J.; Wang, G. Novel strategies to improve tumour therapy by targeting the proteins MCT1, MCT4 and LAT1. Eur. J. Med. Chem. 2021, 226, 113806. [Google Scholar] [CrossRef] [PubMed]
- Peng, K.; Liang, B.-B.; Liu, W.; Mao, Z.-W. What blocks more anticancer platinum complexes from experiment to clinic: Major problems and potential strategies from drug design perspectives. Coord. Chem. Rev. 2021, 449, 214210. [Google Scholar] [CrossRef]
- Bertini, I.; Grey, H.B.; Stiefel, E.I.; Valentine, J.S. (Eds.) Biological Inorganic Chemistry. Structure and Reactivity; University Science Books: Sausalito, CA, USA, 2007. [Google Scholar]
- Liang, J.; Sun, D.; Yang, Y.; Li, M.; Li, H.; Chen, L. Discovery of metal-based complexes as promising antimicrobial agents. Eur. J. Med. Chem. 2021, 224, 113696. [Google Scholar] [CrossRef]
- Riduan, S.N.; Zhang, Y. Recent Advances of Zinc-based Antimicrobial Materials. Chem. Asian J. 2021, 16, 1–9. [Google Scholar] [CrossRef]
- Krasnovskaya, O.; Naumov, A.; Guk, D.; Gorelkin, P.; Erofeev, A.; Beloglazkina, E.; Majouga, A. Copper Coordination Compounds as Biologically Active Agents. Int. J. Mol. Sci. 2020, 21, 3965. [Google Scholar] [CrossRef]
- Wehbe, M.; Leung, A.W.; Abrams, M.J.; Orvig, C.; Bally, M.B. A Perspective—Can copper complexes be developed as a novel class of therapeutics? Dalton Trans. 2017, 46, 10758–10773. [Google Scholar] [CrossRef]
- Wang, T.; Guo, Z. Copper in Medicine: Homeostasis, Chelation Therapy and Antitumor Drug Design. Curr. Med. Chem. 2006, 13, 525–537. [Google Scholar] [CrossRef]
- Zaki, M.; Arjmand, F.; Tabassum, S. Current and future potential of metallo drugs: Revisiting DNA-binding of metal containing molecules and their diverse mechanism of action. Inorg. Chim. Acta 2016, 444, 1–22. [Google Scholar] [CrossRef]
- Orlov, A.P.; Orlova, M.A.; Trofimova, T.P.; Kalmykov, S.N.; Kuznetsov, D.A. The role of zinc and its compounds in leukemia. J. Biol. Inorg. Chem. 2018, 23, 347–362. [Google Scholar] [CrossRef] [PubMed]
- Shobha Devi, C.; Thulasiram, B.; Aerva, R.R.; Nagababu, P. Recent Advances in Copper Intercalators as Anticancer Agents. J. Fluoresc. 2018, 28, 1195–1205. [Google Scholar] [CrossRef] [PubMed]
- Gałczyńska, K.; Drulis-Kawa, Z.; Arabski, M. Antitumor Activity of Pt(II), Ru(III) and Cu(II) Complexes. Molecules 2020, 25, 3492. [Google Scholar] [CrossRef] [PubMed]
- Porchia, M.; Pellei, M.; Del Bello, F.; Santini, C. Zinc Complexes with Nitrogen Donor Ligands as Anticancer Agents. Molecules 2020, 25, 5814. [Google Scholar] [CrossRef]
- Singh, N.K.; Yadav, P.N.; Kumbhar, A.A.; Pokhrel, Y.R. Anticancer potency of copper(II) complexes of thiosemicarbazones. J. Inorg. Biochem. 2020, 210, 111134. [Google Scholar] [CrossRef]
- Salas, J.M.; Caballero, A.B.; Esteban-Parra, G.M.; Mendez-Arriaga, J.M. Leishmanicidal and Trypanocidal Activity of Metal Complexes with 1,2,4-Triazolo[1,5-a]pyrimidines: Insights on their Therapeutic Potential against Leishmaniasis and Chagas Disease. Curr. Med. Chem. 2017, 24, 2796–2806. [Google Scholar] [CrossRef]
- Badea, M.; Uivarosi, V.; Olar, R. Improvement in the Pharmacological Profile of Copper Biological Active Complexes by Their Incorporation into Organic or Inorganic Matrix. Molecules 2020, 25, 5830. [Google Scholar] [CrossRef]
- Lelièvre, P.; Sancey, L.; Coll, J.-L.; Deniaud, A.; Busser, B. The Multifaceted Roles of Copper in Cancer: A Trace Metal Element with Dysregulated Metabolism, but Also a Target or a Bullet for Therapy. Cancers 2020, 12, 3594. [Google Scholar] [CrossRef]
- Łakomska, I.; Fandzloch, M. Application of 1,2,4-triazolo[1,5-a]pyrimidines for the design of coordination compounds with interesting structures and new biological properties. Coord. Chem. Rev. 2016, 327–328, 221–241. [Google Scholar] [CrossRef]
- Rostas, A.M.; Badea, M.; Ruţă, L.L.; Farcaşanu, I.C.; Maxim, C.; Chifiriuc, M.C.; Popa, M.; Luca, M.; Čelan Korošin, N.; Cerc Korošec, R.; et al. Copper(II) complexes with mixed heterocycle ligands as promising antibacterial and antitumor species. Molecules 2020, 25, 3777. [Google Scholar] [CrossRef]
- Méndez-Arriaga, J.M.; Oyarzabal, I.; Martín-Montes, Á.; García-Rodríguez, J.; Quirós, M.; Sánchez-Moreno, M. First example of antiparasitic activity influenced by thermochromism: Leishmanicidal evaluation of 5,7-dimethyl-1,2,4-triazolo[1,5-a]pyrimidine metal complexes. Med. Chem. 2020, 16, 422–430. [Google Scholar] [CrossRef] [PubMed]
- Caballero, A.B.; Rodríguez-Dieguez, A.; Quiros, M.; Salas, J.M.; Huertas, O.; Ramírez-Macías, I.; Olmo, F.; Marín, C.; Chaves-Lemaur, G.; Gutierrez-Sánchez, R.; et al. Triazolopyrimidine compounds containing first-row transition metals and their activity against the neglected infectious Chagas disease and leishmaniasis. Eur. J. Med. Chem. 2014, 85, 526–534. [Google Scholar] [CrossRef] [PubMed]
- Méndez-Arriaga, J.M.; Rodríguez-Diéguez, A.; Sánchez-Moreno, M. In vitro leishmanicidal activity of copper (II) 5,7-dimethyl-1,2,4-triazolo[1,5-a]pyrimidine complex and analogous transition metal series. Polyhedron 2020, 176, 114272. [Google Scholar] [CrossRef]
- Méndez-Arriaga, J.M.; Oyarzabal, I.; Escolano, G.; Rodríguez-Diéguez, A.; Sánchez-Moreno, M.; Salas, J.M. In vitro leishmanicidal and trypanocidal evaluation and magnetic properties of 7-amino-1,2,4-triazolo[1,5-a]pyrimidine Cu(II) complexes. J. Inorg. Biochem. 2018, 180, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Méndez-Arriagaa, J.M.; Esteban-Parraa, G.M.; Juárezb, M.J.; Rodríguez-Diégueza, A.; Sánchez-Morenoc, M.; Isac-Garcíad, J.; Salas, J.M. Antiparasitic activity against trypanosomatid diseases and novel metal complexes derived from the first time characterized 5-phenyl-1,2,4-triazolo[1,5-a]pyrimidi-7(4H)-one. J. Inorg. Biochem. 2017, 175, 217–224. [Google Scholar] [CrossRef]
- Esteban-Parra, G.M.; San Sebastián, E.; Cepeda, J.; Sánchez-Gonzálezc, C.; Rivas-García, L.; Llopis, J.; Aranda, P.; Sánchez-Moreno, M.; Quirós, M.; Rodríguez-Diéguez, A. Anti-diabetic and anti-parasitic properties of a family of luminescent zinc coordination compounds based on the 7-amino-5-methyl-1,2,4-triazolo[1,5-a]pyrimidine ligand. J. Inorg. Biochem. 2020, 212, 111235. [Google Scholar] [CrossRef]
- Boutaleb-Charki, S.; Marín, C.; Maldonado, C.R.; Rosales, M.J.; Urbano, J.; Guitierrez-Sánchez, R.; Quirós, M.; Salas, J.M.; Sánchez-Moreno, M. Copper (II) Complexes of [1,2,4]Triazolo[1,5-a]Pyrimidine Derivatives as Potential Anti-Parasitic Agents. Drug Met. Lett. 2009, 3, 35–44. [Google Scholar] [CrossRef]
- Caballero, A.B.; Marín, C.; Ramírez-Macías, I.; Rodríguez-Diéguez, A.; Quirós, M.; Salas, J.M.; Sánchez-Moreno, M. Structural consequences of the introduction of 2,2′-bipyrimidine as auxiliary ligand in triazolopyrimidine-based transition metal complexes. In vitro antiparasitic activity. Polyhedron 2012, 33, 137–144. [Google Scholar] [CrossRef]
- Calu, L.; Badea, M.; Cerc Korošec, R.; Bukovec, P.; Daniliuc, C.C.; Chifiriuc, M.C.; Măruţescu, L.; Ciulică, C.; Şerban, G.; Olar, R. Thermal behaviour of some novel biologically active complexes with a triazolopyrimidine pharmacophore. J. Therm. Anal. Calorim. 2017, 127, 697–708. [Google Scholar] [CrossRef]
- Olar, R.; Calu, L.; Badea, M.; Chifiriuc, M.C.; Bleotu, C.; Velescu, B.; Stoica, O.; Ioniţă, G.; Stanică, N.; Silvestro, L.; et al. Thermal behaviour of some biologically active species based on complexes with a triazolopyrimidine pharmacophore. J. Therm. Anal. Calorim. 2017, 127, 685–696. [Google Scholar] [CrossRef]
- Badea, M.; Calu, L.; Celan Korosin, N.; David, I.G.; Chifiriuc, M.C.; Bleotu, C.; Ioniţă, G.; Silvestro, L.; Maurer, M.; Olar, R. Thermal behaviour of some biological active perchlorate complexes with a triazolopyrimidine derivative. J. Therm. Anal. Calorim. 2018, 134, 665–677. [Google Scholar] [CrossRef]
- Ruta, L.L.; Farcasanu, I.C.; Bacalum, M.; Răileanu, M.; Rostas, A.M.; Daniliuc, C.G.; Chifiriuc, M.C.; Măruțescu, L.; Popa, M.; Badea, M.; et al. Biological activity of triazolopyrimidine copper(II) complexes modulated by an auxiliary N-N-chelating heterocycle ligands. Molecules 2021, 26, 6772. [Google Scholar] [CrossRef] [PubMed]
- Salas, J.M.; Romero, M.A.; Rahmani, A.; Faure, R. Dichlorobis(5,7-dimethyl[1,2,4]triazolo[1,5-a]pyrimidine-N3)zinc(II). Acta Cryst. 1994, C50, 510–512. [Google Scholar] [CrossRef]
- Nakamoto, K. Infrared and Raman Spectra of Inorganic and Coordination Compounds, Part B, Applications in Coordination, Organometallic, and Bioinorganic Chemistry, 6th ed.; John Wiley & Sons: Hoboken, NJ, USA, 2009; pp. 107–110. [Google Scholar]
- Lever, A.B.P. Inorganic Electronic Spectroscopy; Elsevier: Amsterdam, The Netherlands, 1986; pp. 555–572. [Google Scholar]
- Łakomska, I.; Fandzloch, M.; Popławska, B.; Sitkowski, J. Platinum(II) complexes with 5,7-disubstituted-1,2,4-triazolo [1,5-a]pyrimidines: Spectroscopical characterization and cytotoxic activity in vitro. Spectrochim. Acta Part A Mol. Biomol. Spectroscop. 2012, 91, 126–129. [Google Scholar] [CrossRef]
- Wada, A.; Jitsukawa, K.; Masuda, H. Superoxide Disproportionation Driven by Zinc Complexes with Various Steric and Electrostatic Properties. Angew. Chem. 2013, 125, 12519–12523. [Google Scholar] [CrossRef]
- Ruan, B.-F.; Liang, Y.-K.; Liu, W.-D.; Wu, J.-Y.; Tian, Y.-P. Synthesis, characterization, and antitumor activities of two copper(II) complexes with pyrazole derivatives. J. Coord. Chem. 2012, 65, 2127–2134. [Google Scholar] [CrossRef]
- Mariani, D.; Ghasemishahrestani, Z.; Freitas, W.; Pezzuto, P.; Costa-da-Silva, A.C.; Tanuri, A.; Kanashiro, M.M.; Fernandes, C.; Horn, A., Jr.; Pereira, M.D. Antitumoral synergism between a copper(II) complex and cisplatin improves in vitro and in vivo anticancer activity against melanoma, lung and breast cancer cells. Biochim. Biophys. Acta 2021, 1865, 129963. [Google Scholar] [CrossRef]
- Fujita, K.; Kim, Y.H.; Kanai, O.; Yoshida, H.; Mio, T.; Hirai, T. Emerging concerns of infectious diseases in lung cancer patients receiving immune checkpoint inhibitor therapy. Respir. Med. 2019, 146, 66–70. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, T.; Zhang, X.; Si, X.; Wang, H.; Zhang, J.; Huang, H.; Sun, X.; Wang, J.; Wang, M.; et al. Opportunistic infections complicating immunotherapy for non-small cell lung cancer. Thoracic Cancer 2020, 11, 1689–1694. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, W.Y.; Wu, C.; Yu, J. The role of gut microbiota in cancer treatment: Friend or foe? Gut 2020, 69, 1867–1876. [Google Scholar] [CrossRef]
- Nham, E.; Huh, K.; Cho, S.Y.; Chung, D.R.; Peck, K.R.; Lee, N.Y.; Kang, C.I. Characteristics and Clinical Outcomes of Extended-Spectrum beta-lactamase-producing Klebsiella pneumoniae Bacteremia in Cancer Patients. Infect. Chemother. 2020, 52, 59–69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Regiel-Futyra, A.; Dąbrowski, J.M.; Mazuryk, O.; Śpiewak, K.; Kyzioł, A.; Pucelik, B.; Brindell, M.; Stochel, G. Bioinorganic antimicrobial strategies in the resistance era. Coord. Chem. Rev. 2017, 351, 76–117. [Google Scholar] [CrossRef]
- Lepecq, J.B.; Paoletti, C. A fluorescent complex between ethidium bromide and nucleic acids: Physical—Chemical characterization. J. Mol. Biol. 1967, 27, 87–106. [Google Scholar] [CrossRef]
- Jiang, Q.; Xiao, N.; Shi, P.; Zhu, Y.; Guo, Z. Design of artificial metallonucleases with oxidative mechanism. Coord. Chem. Rev. 2007, 251, 1951–1972. [Google Scholar] [CrossRef]
- Zareia, L.; Asadia, Z.; Dusekb, M.; Eignerb, V. Homodinuclear Ni (II) and Cu (II) Schiff base complexes derived from o-vanillin with a pyrazole bridge: Preparation, crystal structures, DNA and protein (BSA) binding, DNA cleavage, molecular docking and cytotoxicity study. J. Photochem. Photobiol. A Chem. 2019, 374, 145–160. [Google Scholar] [CrossRef]
- Fischer, G. 1,2,4-Triazolo[1,5-a]pyrimidines. Adv. Heterocycl. Chem. 1993, 57, 81–138. [Google Scholar]
- Stoe & Cie; Stoe & Cie: Darmstadt, Germany, 2002.
- Sheldrick, G.M. SHELXL-97, Program for Crystal Structure Refinement; University of Göttingen: Göttingen, Niemcy, 1998. [Google Scholar]
- Prodan, A.M.; Iconaru, S.L.; Chifiriuc, C.M.; Bleotu, C.; Ciobanu, C.S.; Motelica-Heino, M.; Sizaret, S.; Predoi, D. Magnetic Properties and Biological Activity Evaluation of Iron Oxide Nanoparticles. J. Nanomater. 2013, 2013, 893970. [Google Scholar] [CrossRef] [Green Version]
- Bilcu, M.; Grumezescu, A.M.; Oprea, A.E.; Popescu, R.C.; Mogoșanu, G.D.; Hristu, R.; Stanciu, G.A.; Mihailescu, D.F.; Lazar, V.; Bezirtzoglou, E.; et al. Efficiency of Vanilla, Patchouli and Ylang Ylang Essential Oils Stabilized by Iron Oxide@C-14 Nanostructures against Bacterial Adherence and Biofilms Formed by Staphylococcus aureus and Klebsiella pneumoniae Clinical Strains. Molecules 2014, 19, 17943–17956. [Google Scholar] [CrossRef] [Green Version]
- Gheorghe, I.; Tatu, A.L.; Lupu, I.; Thamer, O.; Cotar, A.I.; Pircalabioru, G.G.; Popa, M.; Cristea, V.C.; Lazar, V.; Chifiriuc, M.C. Molecular characterization of virulence and resistance features in Staphylococcus aureus clinical strains isolated from cutaneous lesions in patients with drug adverse reactions. Rom. Biotechnol. Lett. 2017, 22, 12321–12327. [Google Scholar]
- Ferreira, B.J.; Brandão, P.; Meireles, M.; Martel, F.; Correia-Branco, A.; Fernandes, D.M.; Santos, T.M.; Félix, V. Synthesis, structural characterization, cytotoxic properties and DNA binding of a dinuclear copper(II) complex. J. Inorg. Biochem. 2016, 161, 9–17. [Google Scholar] [CrossRef]
- Ma, T.; Xu, J.; Wang, Y.; Yu, H.; Yang, Y.; Liu, Y.; Ding, W.; Zhu, W.; Chen, R.; Ge, Z.; et al. Ternary copper(II) complexes with amino acid chains and heterocyclic bases: DNA binding, cytotoxic and cell apoptosis induction properties. J. Inorg. Biochem. 2015, 144, 38–46. [Google Scholar] [CrossRef] [PubMed]




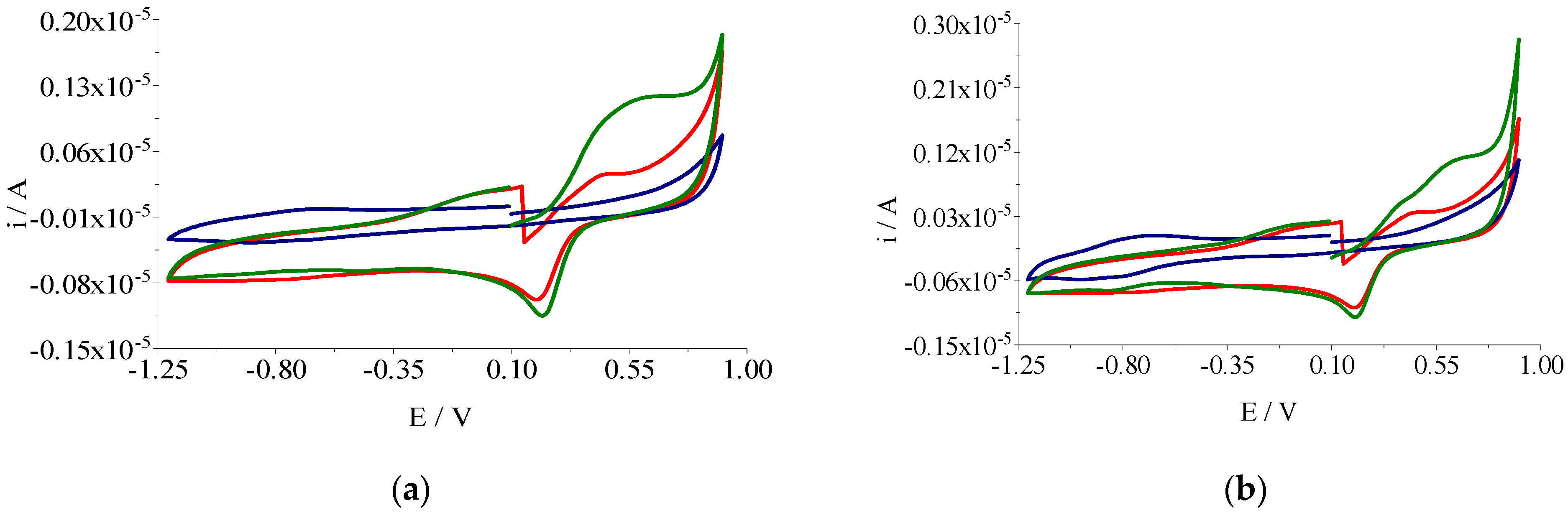
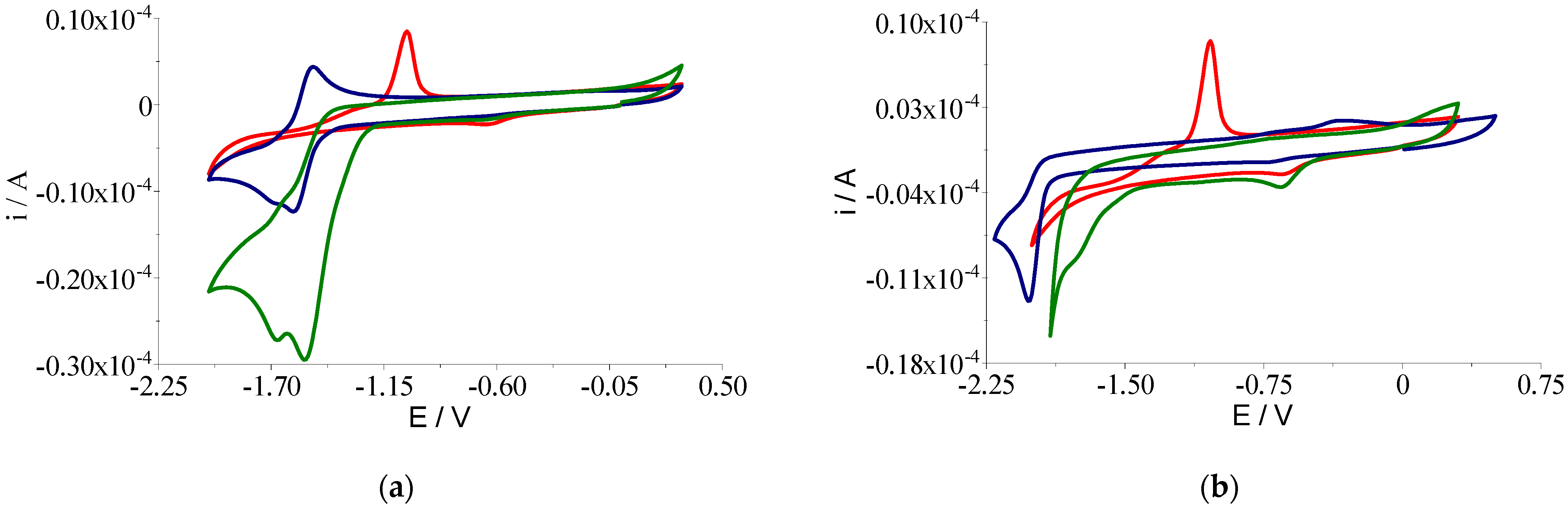



| Empirical Formula | C12H11ClCu0.5N4 (1) | C14H17Cl2N4OSZn (2) |
|---|---|---|
| Formula weight | 278.48 | 425.64 |
| Temperature/K | 293(2) | 293(2) |
| Crystal system | monoclinic | monoclinic |
| Space group | P21/n | P21/n |
| a/Å | 9.1524(12) | 13.1535(8) |
| b/Å | 14.0262(14) | 7.6713(4) |
| c/Å | 10.3212(13) | 18.0452(8) |
| α/° | 90 | 90 |
| β/° | 115.602(15) | 93.025(5) |
| γ/° | 90 | 90 |
| Volume/Å3 | 1194.9(3) | 1818.31(16) |
| Z | 4 | 4 |
| ρcalcg/cm3 | 1.542 | 1.555 |
| μ/mm−1 | 1.166 | 1.767 |
| F(000) | 570.0 | 868.0 |
| Radiation | (λ = 0.71073) | (λ = 0.71073) |
| 2Θ range for data collection/° | 5.252 to 60.824 | 3.932 to 61.588 |
| Reflections collected | 4901 | 9717 |
| Independent reflections | 2570 [Rint = 0.0254, Rsigma = 0.0368] | 4416 [Rint = 0.0312, Rsigma = 0.0451] |
| Data/restraints/parameters | 2570/0/161 | 4416/0/211 |
| Goodness-of-fit on F2 | 1.074 | 1.071 |
| Final R indexes [I ≥ 2σ (I)] | R1 = 0.0442, wR2 = 0.1356 | R1 = 0.0386, wR2 = 0.0897 |
| Final R indexes [all data] | R1 = 0.0513, wR2 = 0.1418 | R1 = 0.0643, wR2 = 0.0975 |
| Largest diff. peak/hole/e Å−3 | 0.66/−0.73 | 0.31/−0.52 |
| (1) | (2) | ||||
|---|---|---|---|---|---|
| Cu1 | Cl1 1 | 2.2583(8) | Zn1 | Cl2 | 2.2133(8) |
| Cu1 | Cl1 | 2.2583(8) | Zn1 | Cl1 | 2.2234(9) |
| Cu1 | N1 | 1.986(2) | Zn1 | N1 | 2.033(2) |
| Cu1 | N1 1 | 1.986(2) | Zn1 | O1 | 1.9921(18) |
| N3 | N2 | 1.370(3) | S1 | C4 | 1.769(4) |
| N3 | C2 | 1.378(3) | S1 | C6 | 1.767(3) |
| N3 | C7 | 1.372(3) | S1 | O1 | 1.5224(19) |
| C3 | C2 | 1.339(4) | N1 | C12 | 1.352(3) |
| C3 | C4 | 1.330(4) | N1 | C14 | 1.351(3) |
| N2 | C1 | 1.314(4) | N2 | N3 | 1.366(3) |
| N1 | C2 | 1.341(3) | N2 | C14 | 1.303(3) |
| Compound | M-Ntpds | M-Cl | Ref. |
|---|---|---|---|
| (1) | 1.986(2) | 2.2583(8) | This paper |
| (2) | 2.033(2) | 2.2133(8) 2.2234(9) | This paper |
| (3) | 1.9997(15) 2.005(15) | 2.2831(6) 2.3993(6) | [22] |
| (4) | 2.020(2) 2.058(2) | 2.197(1) 2.2499 (8) | [34] |
| Compound | Epa1 (V) | Epc1 (V) | Epc2 (V) | Epa2 (V) | E1/2 (V) * (Cu(III)/Cu(II)) |
|---|---|---|---|---|---|
| [Cu(DMSO)6]Cl2 | +0.421 | +0.200 | −0.766 | −0.061 | +0.311 |
| mptp | - | - | −0.705 | −0.796 | |
| (1) | +0.482 | +0.220 | - | - | +0.351 |
| dmtp | - | - | −0.876 | −0.705 | |
| (3) | +0.583 | +0.200 | −0.846 | - | +0.391 |
| Compound | Epc1 (V) | Epc2 (V) | Epa1 (V) | Epa2 (V) |
|---|---|---|---|---|
| [Zn(DMSO)4]Cl2 | −0.654 | - | −1.037 | - |
| mptp | - | −1.591 | 1.501 | - |
| (2) | −0.654 | −1.531 | - | - |
| dmtp | −0.725 | −2.014 | −1.924 | −0.342 |
| (4) | −0.644 | −1.742 | - | - |
| Bacterial Strain | mptp | (1) | (2) | CuCl2·2H2O | ZnCl2 | dmtp | (3) | (4) |
|---|---|---|---|---|---|---|---|---|
| K. pneumoniae 134202 | 2.38 | 0.23 | 0.15 | 5.87 | 7.35 | 3.42 | 0.14 | 0.07 |
| P. aeruginosa 27853 | 4.76 | 0.45 | - | 5.87 | 7.35 | 6.85 | 0.28 | 1.12 |
| B. subtilis 6633 | 2.38 | 0.23 | 0.29 | 5.87 | 7.35 | 3.42 | 0.14 | 0.14 |
| C. albicans 22 | - | - | 0.29 | 5.87 | 7.35 | 3.42 | 0.07 | 0.29 |
| Average MIC value | 3.173 | 0.303 | 0.243 | 5.870 | 7.350 | 4.277 | 0.158 | 0.405 |
| Bacterial Strain | mptp | (1) | (2) | CuCl2·2H2O | ZnCl2 | dmtp | (3) | (4) |
|---|---|---|---|---|---|---|---|---|
| K. pneumoniae 134202 | 2.38 | 0.11 | 0.04 | >5.87 | 7.35 | 3.42 | 0.07 | 0.07 |
| P. aeruginosa 27853 | 4.76 | 0.45 | - | 0.36 | 0.45 | 6.85 | 0.28 | 1.12 |
| B. subtilis 6633 | 2.38 | 0.23 | 0.29 | 5.87 | 7.35 | 3.42 | 0.14 | 0.14 |
| C. albicans 22 | - | - | 0.29 | 5.87 | 7.35 | 3.42 | 0.07 | 0.29 |
| Average MBEC value | 3.173 | 0.263 | 0.207 | 4.492 | 5.625 | 4.277 | 0.140 | 0.405 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Argăseală, A.; Maxim, C.; Badea, M.; Ioniță, L.; Chifiriuc, M.C.; Rostas, A.M.; Bacalum, M.; Răileanu, M.; Ruţă, L.L.; Farcaşanu, I.C.; et al. Insights into Structure and Biological Activity of Copper(II) and Zinc(II) Complexes with Triazolopyrimidine Ligands. Molecules 2022, 27, 765. https://doi.org/10.3390/molecules27030765
Argăseală A, Maxim C, Badea M, Ioniță L, Chifiriuc MC, Rostas AM, Bacalum M, Răileanu M, Ruţă LL, Farcaşanu IC, et al. Insights into Structure and Biological Activity of Copper(II) and Zinc(II) Complexes with Triazolopyrimidine Ligands. Molecules. 2022; 27(3):765. https://doi.org/10.3390/molecules27030765
Chicago/Turabian StyleArgăseală, Aura, Cătălin Maxim, Mihaela Badea, Larisa Ioniță, Mariana Carmen Chifiriuc, Arpad Mihai Rostas, Mihaela Bacalum, Mina Răileanu, Lavinia L. Ruţă, Ileana C. Farcaşanu, and et al. 2022. "Insights into Structure and Biological Activity of Copper(II) and Zinc(II) Complexes with Triazolopyrimidine Ligands" Molecules 27, no. 3: 765. https://doi.org/10.3390/molecules27030765
APA StyleArgăseală, A., Maxim, C., Badea, M., Ioniță, L., Chifiriuc, M. C., Rostas, A. M., Bacalum, M., Răileanu, M., Ruţă, L. L., Farcaşanu, I. C., Iorgulescu, E. E., & Olar, R. (2022). Insights into Structure and Biological Activity of Copper(II) and Zinc(II) Complexes with Triazolopyrimidine Ligands. Molecules, 27(3), 765. https://doi.org/10.3390/molecules27030765










